Genet. Res., Camb. (1994), 64, pp. 107-114 With \ text-figure Copyright © 1994 Cambridge University Press 107
The HSR on chromosome 1 of the house mouse, Mus
domesticus: distribution and frequency in Switzerland
ROLAND HUBNER12•*, TIZIANO MADDALENA1, JEREMY B. SEARLE2f
AND PETER VOGEL1
1 Institut de zoologie et d'ecologie animate (IZEA), Universite de Lausanne, Lausanne, Switzerland 2
Department of Zoology, University of Oxford, Oxford, UK (Received 26 May 1994)
Summary
A total of 357 house mice {Mus domesticus) from 83 localities uniformly distributed throughout Switzerland were screened for the presence of a homogenously staining region (HSR) on
chromosome 1. Altogether 47 mice from 11 localities were HSR/ + or HSR/HSR. One sample of 11 individuals all had an HSR/HSR karyotype. Almost all mice with the variant were collected from the Rhone valley (HSR frequency: 61 %) and Val Bregaglia (HSR frequency: 81 %). For samples from most of the area of Switzerland, the HSR was absent. There was no strong association between the geographic distribution of the HSR and the areas of occurrence of metacentrics. However, at Chiggiogna the HSR was found on Rb (13). Possible explanations for the HSR polymorphism are discussed.
1. Introduction
The chromosomes of drug-resistant cell lines and tumour cells sometimes contain segments with uni-form G-band staining of medium intensity and variable size, known as homogeneously staining regions (HSRs). In 1982, Adolph discovered an HSR that can be part of an individual's regular karyotype and can be transmitted in the germ line (Traut et al. 1984). This HSR is located between bands C5 and D on chromosome 1 in the house mouse from western Europe {Mus domesticus; also known as M. musculus
domesticus). There is a polymorphism for presence or
absence of this HSR within wild populations of M.
domesticus, so that individuals can be classified as
HSR/HSR, + /HSR or + / + . The HSR is widespread in western Europe and is particularly associated with populations characterized by metacentric chromo-somes (Table 1). In Asia and eastern Europe, populations of the house mouse M. musculus (also known as M. musculus musculus) may also be characterized by an HSR polymorphism. In this case an HSR-bearing chromosome 1 has two homo-geneously staining blocks (Volobouev, 1983), a
con-* Corresponding author. Present address: Lab. Biochimie (B6), Institut de Chimie, Universite de Liege, Sart-Tilman, B-4000 Liege, Belgium.
t Present address: Department of Biology, University of York, York, U.K.
dition generated from the ancestral single-banded HSR-bearing chromosome by a paracentric inversion (Agulnik et al. 19906; Winking et al. 1991a). Molecular studies have revealed that a 'long-range repeat' family of ca. 50 copies on a normal chromo-some 1 are present in ca. 800 copies on an HSR-bearing chromosome 1 (Purmann et al. 1992; Plass et
al. 1992). These repeats contain two coding regions of
1-3 and 4-5 kb length (Eckert et al. 1991), one of which has similarities to the human nuclear autoantigen SplOO (Hubner, 1992). The amplified genes of the HSR are actively transcribed (Eckert et al. 1991).
The HSR in house mice is relatively easily scored, and therefore amenable to population genetic and biogeographical analysis. The presence of an HSR can generally be detected in G-banded or conventionally stained chromosomes, as the chromosome 1 tends to be about a third longer than normal. However, in common with other HSRs, there is length variability and individuals are best karyotyped using G-banding, to avoid missing individuals with a small HSR. The high-copy cluster of repeated sequences in the house mouse stains positively after C-banding, though less darkly than the pericentromeric heterochromatin (Adolph, 1982; Traut et al. 1984).
There have been two published studies that specifi-cally address between-site variation in frequency of the HSR in M. musculus. Winking et al. (1991a) studied 157 mice over a small geographic area (the
R. Hiibner and others 108
Table 1. Chromosomal characteristics of the 28 populations of Mus domesticus where the chromosome 1 HSRs
have been found
Locality
Swabia (Southern Germany) Altdorf Hohenentringen Holzelfingen Rottenburg Schwalldorf Trasenberg Wendelsheim Bichishausen Blonried
Hard bei Haigerloch Indelhausen
KreBbach Odenwaldstetten Trochtelfingen near Barcelona (Spain)
San Martino Sarroca near Pavia (Northern Italy)
Cascina Chiarello Grisons (Switzerland) Mutten Vicosoprano Tunisia Monastir Sidi-Bouzid Belgium Jesus-Eick
Caithness and Sutherland (Scotland) Ribigill Achiemore Bunahoun Thrumster Mains of Olrig Seater Argentina Posadas Min. 2n 38 38 38 38 38 38 38 36 36 36 36 36 36 36 31 38 38 39 21 (XO) 40 40 40 36 36 36 33 32 40 Metacentrics 4-12 4 1 2 4 1 2 4 1 2 4 1 2 4 1 2 4 1 2 412/1314 412/1113 4-12/?? 412/810/1314 4-12/5-15 412/515/810 412/515 4-14/5-15/6-10/9.11/1213 1617 4 1 2 10-11 Ml/2-16/3-12/4-6/5-14/7-18/8-9/ 10-17/1315 — — — 4-10/912 4-10/9-12 410/912 4-10/613/9-12/11-14 4-10/6-13/9-12/11-14 — Reference c c, e k c c c, e c c c k c, e c c, e c b, c, e d, e a, e h, k f f g j i j j i 1
Key to references: (a) Gropp et al. 1972; (b) Adolph & Klein, 1981; (c) Adolph, 1982; (d) Gropp et al. 1982; (e) Traut et al. 1984; (f) Said et al. 1986; (g) Bauchau et al. 1989; (h) Winking et al. 1991 b; (i) Searle, 1991; (j) Searle et al. 1993; (k) Agulnik et al. 1993c; (1) Gimenez & Bidau, 1993.
Baltic island of Gland) and Sabantsev et al. (1993) studied 284 mice over a huge continental expanse (Siberia and middle Asia). From these studies and others on M. domesticus (see references in Table 1) it is clear that there is considerable variation in HSR frequency between particular well-studied sites. Thus, at Tomsk (Western Siberia, n =.37), Ottenby (Oland,
n = 76), site OK in Novosibirsk (Western Siberia, n =
44) and Hohenentringen (Southern Germany, n = 44), HSR frequencies were 0%, 4 % , 51 % and 76%, respectively. However, it is not clear whether this merely reflects site-by-site variation or whether there are clear regional differences in the frequency of the HSR. In this paper we report a study that addresses this issue in M. domesticus. By collecting house mice uniformly throughout Switzerland we have been able to examine the regional pattern in HSR frequency within an area of ca. 40000 km2.
The study also examines the degree to which the HSR is associated with metacentrics in M. domesticus. It is possible that the HSR has tended to be found in areas with metacentrics (Table 1) because cyto-geneticists tend to concentrate their activity in such places. Switzerland is divided into substantial areas with or without metacentrics (Hiibner, 1992) so, given our uniform coverage, we can produce the first unbiased appraisal of the degree of association between these two types of chromosome markers.
2. Materials and methods
Both adult and immature M. domesticus were collected from farms and other buildings throughout Switzerland at various times of the year during the period 1988-91.
Chromosome 1 HSR in Swiss house mice 109
Chromosome preparations were made from bone marrow cells according to the method of Ford (1966). The mice were injected with 004% Colcemid (001 ml per gram body weight), 1 h before they were killed. For each individual, one slide was stained by the C-banding method of Sumner (1972) and 2-3 slides were exposed to the G-banding treatment of Seabright (1971) or Evans (1986). For the C-banding prepara-tions, at least 10 well-spread metaphase cells were inspected to make chromosome counts and to de-termine presence/absence of the HSR. For the G-banded preparations, 20 metaphases were analysed to confirm presence of the HSR and identity of any metacentric.
3. Results
Altogether 357 mice from 83 localities in Switzerland and adjacent regions were karyotyped (Table 2, Fig. 1). Fifty-five localities were characterized by 1-9 metacentrics (Table 2). Forty-seven mice from 11 localities were either HSR/+ or HSR/HSR (Table 3) (and the overall frequency of the HSR variant was 10% among the mice examined).
In all HSR/HSR and HSR/+ individuals, the chromosomal position of the HSR was confined to the region between bands C5 and D on chromosome 1, as expected for M. domesticus (Traut et al. 1984). No major size variation was detected; the length of the HSR was about 20-25 % of the length of chromosome 1 (estimated from photographs). The individual from Chiggiogna (Table 3) had a rather larger HSR than normal (about 30% of chromosome 1). In this case, chromosome 1 was part of a huge metacentric chromosome, Rb (1-3), that was very conspicuous in chromosome spreads.
There is a striking pattern of geographic distribution of the HSR within Switzerland. Almost all individuals with this variant were collected from the Rhone valley (Valais) and Val Bregaglia (Table 3, Fig. 1). The Rhone valley is within the western part of Switzerland, which is characterized by mice with the standard In = 40, all-telocentric karyotype. Among the 32 mice (5 sites) sampled from the Rhone valley the frequency of the HSR variant was 61 %. Val Bregaglia is within the eastern part of Switzerland, dominated by populations with metacentric chromosomes. In Val Bregaglia itself chromosome number varied between 2« = 34-40 with individuals homozygous or heterozygous metacentric for arm combinations Rb (4-12), Rb (10-11) and Rb (16-17), or homozygous telocentric (Table 3). This valley is at the contact between two major karyotypic groups: the Rb (412) cluster to the north of the Alps and the Rb (1617) cluster to the south (Hiibner 1992). Among the 18 mice (5 sites) sampled from Val Bregaglia the frequency of the HSR variant was 81 %. There was no clear environmental peculiarity associated with populations characterized by the HSR
and the mice in these populations were as healthy as those without the HSR. HSR mice were not found preferentially on farms where rodenticides were used. 4. Discussion
In Swiss house mice there is no particular tendency for the HSR and metacentrics to occur together. One of the two geographic regions where the HSR is common is an area where no metacentrics have been found (the Rhone valley) and in most of the places within Switzerland where metacentrics occur no HSRs have been found (Fig. 1).
Therefore, it must be suspected that the apparent geographic association between the HSR and meta-centrics, as revealed in Table 1, does not have a biological basis but rather reflects biased sampling. In addition to the Rhone valley, there appear to be at least two other places within the range of M.
domesticus where the HSR is found well outside the
distribution of metacentrics: Sidi-Bouzid in Tunisia (Said et al. 1986) and Posadas in Argentina (Gimenez & Bidau, 1993). Also, although the HSR is found throughout the range of M. musculus (Winking et al. 1991 a, Sabantsev et al. 1993), metacentrics are only known from one locality in that semispecies (Zima et
al. 1990).
How do we explain the presence of the HSR in wild populations of the house mouse? The fact that the HSR represents an amplification of a sequence with similarity to an immunological gene (Hiibner, 1992) brings to mind the possibility that the HSR has a role in resistance to pathogens. Clearly, pathogens are sometimes distributed patchily over the range of the species; so the high frequency of the HSR in some regions and not others in Switzerland could be compatible with such a role.
Unfortunately, there have been no studies on pathogen resistance of HSR individuals. However, other aspects of fitness have been studied. The viability and fertility of HSR mice have been examined under laboratory and field conditions. The work of Agulnik
et al (19936) suggests that HSR/HSR M. musculus
may have low viability and fertility. However, they were working with animals of mixed laboratory mouse-wild mouse background, and Winking et al. (1991a) found no such effects with equivalent wild-strain animals. Also, Winking et al. (19916), working with both M. domesticus and M. musculus, have found HSR/ + individuals no less fertile than + / + animals.
Also worth noting are the recent segregation data collected by Ruvinsky and colleagues on M. musculus. They found HSR/+ females to show meiotic drive for the HSR, with this meiotic drive conditional on the karyotype of the fertilizing sperm (Agulnik et al. 1990 a, 1993 a, b; see also Pomiankowski & Hurst 1993). If such meiotic drive currently occurs in nature, or has occurred in the past, it wouid be an important factor in understanding the geographic distribution
R. Hubner and others 110 Table 2. Chromosome number (2n) and number of different metacentrics
in samples of house mice from Switzerland and adjacent areas. The female from Ecublens had an XO karyotype
Number of individuals Number of different Code Locality 2n Females Males metacentrics
AB AG AL A T AR BA BW BM BE BO BR BG BU CA CE CG Cl CL CO CM CV D N D E D M D O EC EL ER ES Fl FL FO G W GA G R GS GU HE HU H G HT KA KU LA LI LU M N M D MA Abtwil Agno Altdorf I Altdorf II Araschgen Bassins Basel - W Bauma Bellinzona Bois de Chenes Brederis (Austria) Brig Buchs Chamoson Cheiry Chiggiogna Chiasso Claro Colombier La Combaz d'Aarau Couvet Donath Domat/Ems I Domat/Ems 11 Dornbirn (Austria) Ecublens Elm Erstfeld I Erstfeld 11 Fiez Filisur La Fontaine Gahwil Grandson Griisch Gstaad Guntmadingen Heimenschwand Hiinikon Hugstetten I (Germany) Hugstetten II Kau Kiissnacht Lausanne Liestal Lumino Mannedorf Malans-Dorf Malans 24 24 32 31 28 40 40 39 24 24 40 22 40 22 40 40 31 24 26 40 40 40 37 36 35 31 30 29 28 27 40 39 24 32 31 34 40 32 40 24 40 28 27 26 40 24 40 24 38 37 36 22 24 40 39 38 26 28 24 26 26 1 1 0 0 0 1 6 1 2 2 1 2 1 5 1 3 1 9 1 3 0 4 0 6 1 1 0 8 2 3 0 1 0 1 2 0 2 0 9 1 3 1 3 2 5 0 0 5 1 2 0 1 3 1 1 0 5 1 1 0 3 0 0 2 1 1 2 1 0 1 5 0 7 0 0 3 4 0 1 2 2 1 4 1 0 0 0 1 5 0 0 3 0 2 1 0 1 4 1 2 2 0 0 3 2 2 1 1 1 1 0 1 0 2 0 2 1 2 1 0 1 2
Chromosome 1 HSR in Swiss house mice
Table 2 {cont.)
I l l
Number of individuals
Code Locality 2n Females Males
MI MO MR MU NY OL PR PV PO QU RI RO RU SW SM SE SP SA ST
ss
SNsu
TH TR TI VC VT VE VI VS VP WO YV ZO Milchbuck Montbovon Morzine (France) Miistair Nyon Olivone Preonzo Preverenges Promontogno Quartino Riehen Romoos Riittenen Schnottwil Schmiedtrued Sementina Spreitenbach St Aubin Steinegg St Sulpice Susten Surava Thayngen Trin Tiengen (Germany) Villa di Chiavenna I (Italy) Villa di Chiavenna II (Italy) Verbier Vicosoprano Visperterminen Vicosoprano II Worb Yverdon Zofingen 24 2 40 2 40 3 40 3 40 1 24 1 28 3 40 2 38 4 24 5 39 1 26 2 40 1 39 0 38 3 40 0 26 1 24 0 24 3 40 8 22 2 40 8 40 5 32 4 22 1 38 1 35 0 34 1 33 1 35 1 34 1 35 1 34 1 40 1 39 1 38 2 40 1 40 1 40 3 40 0 26 1 1 1 2 2 2 2 2 1 1 4 0 2 0 1 5 2 1 2 1 3 0 8 3 6 3 1 1 1 0 1 1 1 1 4 0 1 3 0 0 1 1 Number of different metacentricsand frequency of the HSR in the house mouse. However, once again, this work has involved indi-viduals with mixed laboratory mouse-wild mouse genetic background and it remains to be seen whether it has relevance to wild house mice. Even if meiotic drive for the HSR can be demonstrated in wild M.
musculus, it may relate to the chromosome 1
para-centric inversion (Agulnik et al. 1993 a) which does not occur in M. domesticus.
How do our studies of M. domesticus in Switzerland add to this understanding of the HSR polymorphism in the house mouse? First, we found a high proportion of homozygotes among those individuals carrying the HSR, in support of the contention of Winking et al. (1991a) that HSR/HSR mice do not suffer great inviability. Several of our samples (including one of 11 individuals) consisted solely of HSR/HSR mice (Table
3). Secondly, our data confirm the existence of extreme between-site variation in HSR frequency, with some samples (of up to 16 individuals) with 0 % and others, as described above, with 100% (Tables 2 and 3). Thirdly, our data suggest that the HSR is absent or at very low frequency over much of Switzerland (Fig. 1). This situation may be the norm throughout the distribution of the house mouse; published reports have been biased towards information on where the HSR has been found rather than where it has been looked for and not found. Fourthly, we have shown that there are clear regional differences in the frequency of the HSR over the geographic area of Switzerland. The HSR is at high frequency in some regions and absent (or at very low frequency) in others. If the occurrence of the HSR relates to pathogen resistance, it would be important to explain
R. Hiibner and others 112
DO
Fig. 1. Collection localities of house mice from Switzerland and adjacent areas {i.e. Italian entrance of Val Bregaglia, Vorarlbeg in Austria, Freiburg area in southern Germany, Haute Savoie in France). Symbols for populations: open squares = all-telocentric (2« = 40) populations, closed circles = metacentric (In 3= 26) populations, closed triangles = metacentric (2n = 22 or 24) populations, open stars = all-telocentric populations with chromosome 1 HSR, closed stars
= metacentric populations with chromosome 1 HSR, closed star with white centre (MUTT) = Mutten population from Table 1. Two-letter codes correspond to localities given in Table 2. Shading represents a mean January temperature of below — 2 °C. Major rivers are indicated.
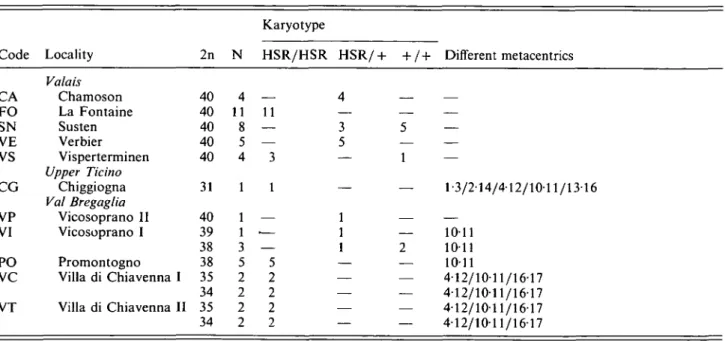
Table 3. Characteristics of the samples where the chromosome 1 HSR was encountered in Switzerland
Code Locality Karyotype 2n N HSR/HSR HSR/+ + / + Different metacentrics CA FO SN VE VS CG VP VI PO VC VT Valais Chamoson La Fontaine Susten Verbier Visperterminen Upper Ticino Chiggiogna Val Bregaglia Vicosoprano II Vicosoprano I Promontogno Villa di Chiavenna I Villa di Chiavenna II 40 40 40 40 40 31 40 39 38 38 35 34 35 34 4 11 8 5 4 1 1 1 3 5 2 2 2 2 — 11 — — 3 1 — •— — 5 2 2 2 2 1-3/214/412/10-11/1316 1011 1011 1011 4-12/10-11/1617 412/1011/1617 412/1011/1617 4-12/1011/1617
this geographic pattern in terms of the epidemiology of the disease(s).
So far, we have discussed selective factors that may
be important in explaining the HSR polymorphism. But is it possible to discount the null hypothesis that the HSR variant is selectively neutral?
Chromosome 1 HSR in Swiss house mice 113 Neutral polymorphisms are transient; given
suf-ficient time either the ancestral type or derived variant will go extinct (Kimura, 1983). The HSR poly-morphism appears to predate the separation of M. musculus and M. domesticus, which may have occurred 500000 years ago (Winking et al. 1991 b; Boursot et al. 1993). At first sight, this long survival of the polymorphism appears to argue against neutrality, but if the effective population size of the house mouse has been large since the HSR variant arose (as is probable), then survival of the polymorphism is not surprising (Kimura, 1983).
On the basis of present information, founder effects and genetic drift can explain the distribution of the HSR variant in Swiss house mice as readily as selection. House mice apparently invaded Switzerland within the last 10000 years (Auffray et al. 1990; Hiibner, 1992). If the invaders were largely + / + but with some individuals carrying the HSR variant, then as a neutral marker, the HSR would have remained at low frequency or become extinct over most of Switzerland. However, by chance, HSR mice could have come to dominate small founding populations of certain alpine valleys. The high frequency of the HSR variant in the Rhone valley and Val Bregaglia could have arisen in this fashion.
So, for M. domesticus at least, it cannot be disproved that the HSR variant is selectively neutral, even though HSR individuals have a substantial amplifica-tion of a transcribed gene. Clearly, a combinaamplifica-tion of further studies on (a) fitness and segregation, (b) molecular genetics and physiology, and (c) population genetics, are all necessary to understand this strange polymorphism.
We are grateful to the Roche Research Foundation, the University of Lausanne, the Berrow Foundation and the Royal Society of London for funding.
References
Adolph, S. (1982). Chromosomenvariationen in Mus musculus domesticus, Rutty, aus Suddeutschland. Dis-sertation, Universitat Tubingen, 86p.
Adolph, S. & Klein, J. (1981). Robertsonian variation in Mus musculus from central Europe, Spain and Scotland. Journal of Heredity 72, 219-221.
Agulnik, S. 1., Agulnik, A. I. & Ruvinsky, A. O. (1990a). Meiotic drive in female mice heterozygous for the HSR inserts on chromosome 1. Genetical Research 55, 97-100. Agulnik, S. I., Borodin, P. M., Gorlov, I. P., Ladygina,
T. Yu. & Pack, S. D. (19906). The origin of a double insertion of homogeneously staining regions in the house mouse (Mus musculus musculus). Heredity 65, 265-267. Agulnik, S. 1., Sabantsev, I. D., Orlova, G. V. & Ruvinsky,
A. O. (1993 a). Meiotic drive on aberrant chromosome 1 in the mouse is determined by a linked distorter. Genetical Research 61, 91-96.
Agulnik, S. I., Sabantsev, I. D. & Ruvinsky, A. O. (1993ft). Effect of sperm genotype on chromatid segregation in female mice heterozygous for aberrant chromosome 1. Genetical Research 61, 97-100.
Agulnik, S., Adolph, S., Winking, H. & Traut, W. (1993c). Zoogeography of the chromosome 1 HSR in natural
populations of the house mouse (Mus musculus). Hereditas 119, 39^6.
Auffray, J.-C, Vanlerberghe, F. & Britton-Davidian, J. (1990). The house mouse progression in Eurasia: a palaeontological and archaeozoological approach. Bio-logical Journal of the Linnean Society 41, 13-25. Bauchau, V., Smets, S., Viroux, M.-C, Nootens, D. & de
Caritat, A.-K. (1989). Robertsonian translocations in free-living populations of the house mouse in Belgium. Biological Journal of the Linnean Society 41, 193-201. Boursot, P., Dod, B., Dallas, J. & Bonhomme, F. (1993). Historical aspects of the radiation of the house mouse: consequences of genomic coadaptation and the formation of hybrid zones. Abstracts, Fourth Congress of the European Society of Evolutionary Biology, Montpellier, France, p. 52.
Eckert, W. A., Plass, C , Weith, A., Traut, W. & Winking, H. (1991). Transcripts from amplified sequences of an inherited homogeneously staining region in chromosome 1 of the house mouse (Mus musculus). Molecular and Cellular Biology 11, 2229-2235.
Evans, E. P. (1986). The karyotyping of mouse cells. In Manipulating the Mouse Embryo. A Laboratory Manual (ed. B. Hogan, F. Constatini and E. Lacy), pp. 221-225. Cold Spring Harbor: CSH Laboratory.
Ford, C. E. (1966). The use of chromosome markers. In Tissue Grafting and Radiation (ed. H. S. Micklem and J. F. Loutit), pp. 197-206. New York: Academic Press. Gimenez, M. D. & Bidau, C. J. (1993). Polimorfismos de
heterocromatina constitutiva en Mus musculus domesticus. Abstracts, 24th Congreso Argentino de Genetica, Posadas, Misiones, Argentina, p. 119.
Gropp, A., Winking, H., Zech, L. & Muller, H. J. (1972). Robertsonian chromosomal variation and identification of metacentric chromosomes in feral mice. Chromosoma 30, 265-288.
Gropp, A., Winking, H., Redi, C , Capanna, E., Britton-Davidian, J. & Noack, G. (1982). Robertsonian karyotype variation in wild house mice from Rhaeto-Lombardia. Cytogenetics and Cell Genetics 34, 67-77.
Hiibner, R. (1992). Chromosomal and biochemical variation in wild mice from Switzerland: relevance for models of chromosomal evolution in European house mice. D.Phil. thesis, University of Oxford, 177p.
Kimura, M. (1983). The Neutral Theory of Molecular Evolution. Cambridge University Press.
Plass, C , Hellwig, T., Traut, W. & Winking, H. (1992). Evolution of a B2 tagged sequence from a long-range repeat family in the genus Mus. Mammalian Genome 3, 197-201.
Pomiankowski, A. & Hurst, L. D. (1993). Siberian mice upset Mendel. Nature 363, 396-397.
Purmann, L., Plass, C , Gruneberg, H., Winking, H. & Traut, W. (1992). A long-range repeat cluster in chromo-some 1 of the house mouse, Mus musculus, and its relation to a germline homogeneously staining region. Genomics 12, 80-88.
Sabantsev, I., Spitsin, O., Agulnik, S. & Ruvinsky, A. (1993). Population dynamics of aberrant chromosome 1 in mice. Heredity 70, 481-489.
Said, K , Jacquart, T., Montgelard, C , Sonjaya, H., Helal, A. N. & Britton-Davidian, J. (1986). Robertsonian house mouse populations in Tunisia: karyotypical and bio-chemical study. Genetica 68, 151-156.
Seabright, M. (1971). A rapid banding technique for human chromosomes. Lancet ii, 971-972.
Searle, J. B. (1991). A hybrid zone comprising staggered chromosomal dines in the house mouse (Mus musculus domesticus). Proceedings of the Royal Society of London B 246, 47-52.
R. Hiibner and others 114
Searle, J. B., Navarro, Y. N. & Ganem, G. (1993). Further studies of a staggered hybrid zone in Mus musculus domesticus (the house mouse). Heredity 71, 523-531. Sumner, A. T. (1972). A simple technique for demonstrating
centromeric heterochromatin. Experimental Cell Research 75, 304-306.
Traut, W., Winking, H. & Adolph, S. (1984). An extra segment in chromosome 1 of wild Mus musculus: a C-band positive homogeneously staining region. Cyto-genetics and Cell Genetics 38, 290-297.
Volobouev, V. T. (1983). Les types de polymorphisme chromosomique et role evolutif chez les mammiferes (Insectivora, Rodentia et Carnivora). These de doctorat d'etat, Universite Pierre et Marie Curie Paris VI, 205p. Winking, H., Bostelmann, H. & Fredga, K. (1991a).
Incidence of double-band HSRs in chromosome 1 of the house mouse, Mus musculus musculus, from Oland (Sweden): a population study. Hereditas 114, 111-116. Winking, H., Weigh, A., Boldyreff, B., Moriwaki, K.,
Fredga, K. & Traut, W. (19916). Polymorphic HSRs in chromosome 1 of the two semispecies Mus musculus musculus and Mus musculus domesticus have a common origin in an ancestral population. Chromosoma 100, 147-151.
Zima, J., Gaichenko, V. A., Macholan, M., Radjabli, S. I., Sablina, O. V. & Wojcik, J. (1990). Are Robertsonian variations a frequent phenomenon in mouse populations in Eurasia? Biological Journal of the Linnean Society 41, 229-233.