Publisher’s version / Version de l'éditeur:
ASTM Bulletin, 238, pp. 61-63, 1959-05
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Measurement of surface moisture
Sereda, P. J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=af74f371-07b7-4e25-9892-95a444d857bc
https://publications-cnrc.canada.ca/fra/voir/objet/?id=af74f371-07b7-4e25-9892-95a444d857bc
Measurement of Surface Moisture
Second Progress Report
P. J. SEREDA
Reprinted from ASTM Bulletin No. 238 May 1959, Pp. 61-63
S E C O N D PROGRESS REPORT1
Measurement of Surface Moisture
By P. J. SEREDA
This is the second progress report on measurement of surface moisture a n d zinc were exposed side by side with
carried out at the Division of Building Research, National Research Coun- those of platinum and steel on the same
cil, Canada, a project initiated by the Task Group on Measurement of At- corrosion rack. Each clement con-
mospheric Factors of ASTM Committee B-3 on Corrosion of Non-Ferrous sisted of two cells, one o n each face
Metals and Alloys. This second report describes further development of representing the grouncl~vard and the
instrumentation to record the time-of-wetness on exposed metal speci- mens. Limits of sensitivity of this instrumentation are given.
A
METHOD of detecting surfacenloisture on steel panels exposed to the atmosphere was described in the first report on this work. T h e principle of operation of a detecting element con- sisting of a cell of platinum and steel was discussed, and graphs were in- cluded to show typical performance
-
NOTE:-DISCUSSION OF THIS PAPER IS INVITED, either for publication or for the attention of the author. Address all communications to ASTM Headquarters, 1916 Race St., Philadelphia 3, Pa.
1 See P. J. Sereda, "Measurenlent of Sur- face M o i s t u r e A Progress Report," ASTM BULLETIN, NO. 228, February, 1958, p. 53 ( T P 59) for the First Progress Report.
characteristics. The design of the element and associated instrumentation were also described.
In the first report i t mas stated t h a t elements consisting of platinum and zinc showed considerable promise. This present report includes t h e results obtained with this type of element and sho\vs t h a t i t is superior to t h e platinum and steel element described in the first report.
T h e following data were obtained a t the Ottawa site during t h e period from September, 1957 to June, 1958. For comparison, elements of platinum
P. J. SEREDA, associate research officer, Division of Building Research, National Research Council,Ottawa, has been engaged since 1950 in the study of the behavior of water in porous systems including methods of detecting and measuring the presence of water on surfaces of materials.
M a y 1 9 5 9
.A S T M B U L L E T I N
(TP 129)
6 1
Authorized Reprint from the Copyrighted ASTM BULLETIN No. 238, May, 1959 Published by the American Society for Testing Materials, Philadelphia 3, Pa.
12
1 1 1 1 1 1 1 1 1 1 ~ ~ ) l ~ 1 ~ , I
-70 66 6 3 5 6 8 6 4 6 3 62 5 9 5 5 8 5 5 7 5 5 5 5 4 5 53 5 2 5 8 5 6 9
P a n e l Temperoture ,deg Fohr
1 I 1 1 1 l 1 ~ ~ ~ ~ ~ ~ ) ~ , ~ ~ , 55 58 5962 62 62 6 0 5 8 5 8 5857 57 56 55 56 56
Dew Polnt Temperoture o f Air , d e g F o h r
Air Ternperoture ,deg F o h r
, ,
72 70 68 69 69 67 67 65 64 62 61 59 58 57 58 60, , ,
,
,
,
,
,
,
,
,
I I I I I 1 0 --
4 2 --
14 I I I I I I I I I I I I I I I I I I 50 435 38 38 3 8 5 37 37 375 36 36.5 35 35 34 34 39 53 69 84 P a n e l T e m p e r a t u r e , d e g F o h r 1 l 1 1 1 1 1 1 1 1 1 l 1 1 1 1 1 i 40 42 40 42 4 0 4 0 39 39 38 38 37 37 36 36 37 4 0 42 45 D e w P o l n t T e m p e r o t u r e o f Air , d e g F a h r Alr T e m p e r a t u r e , d e g F a h r 1 , 1 1 1 , 1 1 1 1 1 1 1 , 1 1 1 1 ' 52 51 4 8 45 44 43 42 41 4 0 4 0 39 38 37 37 38 41 46 51Platinum and Zinc Cell
,
0.8 - - 10-
--
2 >5
0 6-
--
-
= 0 8 - .- 0-
a c 0 4 - aPlotinurn ond Steel C e l l
-
z
n 0.6 - -0 2 - Platinum and Steel Cell
-
0.4
-
0 1 6 1 7 1 8 1 9 2 o 2 1 2 2 2 3 o o o 1 o 2 o 3 o 4 o 5 o 6 ~ d ~ d 9 1 o 1 1 12 0 2 - - S e p t , I3J-
Sept ,I4 0 , T l m e , h r 17 18 19 2 0 21 22 23 00 01 0 2 03 0 4 05 06 0 7 0 8 0 9 10 1 1 12 O c t 4+act
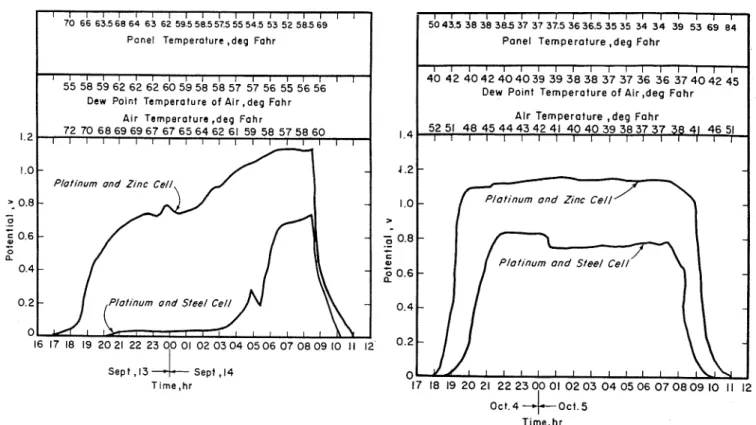
5 T ~ m e . h rFig. 1.-Record of potential during (a) light dew and ( b ) heavy dew at Ottawa.
skyward euposure. A Speedomax 12- more stable in its performance character- ferent rates of cooling corresponding t o point potentiometer recorder was used to istics over a prolonged period of opera- different amounts of water present o n record the potential developed by each tion. I n Ottawa it appears t h a t the the surface for any particular imposed cell across a series of shqnt resistances platinum and zinc element can be oper- condition.
It
is for this reason that t h e of 1, 5, and 100 megohms. ated for a t least 1 y r whereas the plati- apparent sensitivity of the cell varies num and steel element requires t h a t the under natural atmospheric conditions Results a n d Discussioncorrosion be rcmoved from because these conditions are transient When measurement of surface mois- the vicinity of the electrode every 2 and variable. I t is, therefore, difficult
ture by this method mas begun i t was months. and meaningless to assign a single value
thought t h a t the area under the It has been found t h a t the platinum for the sensitivity of t h e cell just as it is potential versus time curves would and zinc cell is more sensitive than the difficult to define the transient con- describe more accurately the corroding platinum and steel cell for the same con- ditions imposed on a panel of steel conditions than would the period in ditions of measurement when first p u t exposed to t h e outdoor atmosphere. hours during which the generation of any into service and a t temperatures above In Table
I
arc listed a selected number potential occurred. However, after freezing (Fig. 1). A t temperatures of individual periods when a potential studying the records obtained during below freezing, t h e measured potential in excess of 0.1 v mas measured. T h e 4 months it became apparent t h a t there of the platinum and steel cell decreases conditior~s of temperature and humidity was no justification for supposing t h a t markedly n ~ i t h decreasing temperature are given for the atmosphere and t h e the area under the potential versus a s was indicated in the first report,Relotlve Hurn~d~iy , per cent
time curves were more representative whereas a similar decrease in the meas-
of the conditions of wetness on the ured potential was not observed in 82 86 Above so 95 Below pallel than simply the time during which the case of the platinum and zinc Cell. Dew Point .deg Fohr Dew Point .deg Fahr
any potential was recorded. Subse- T h e sensitivity of this cell under
quent records were collected in terms of laboratory controlled conditions is 0 8 - the time-of-wetness in hours, which is shown in Fig. 2.
It
appears t h a t thisdefined as the time when the potential, cell mill detect absorbed films of water
,,
as generated by the cell, is in excess of corresponding to about 85 per cent -0.1 v. relative humidity a t near room tempera-
The reproducibility and the degree of ture when using the p H recorder to 0 4 stability of the platinum and steel measure the potential developed across a sensing elements were dcinonstrated in the 100-megohm shunt resistance. This the first report.
It
mas pointed out t h a t potential is dependent upon the current- the rapid corrosion of the steel pro- generating capacity of the cell, and this duced voluminous corrosion products in turn is dependent upon the area of the4 3 2 1 1 i 2 3 4 1: 110 I
-
. I 5I/
/.I
-CW
- /-.<.
,1.
which interfered with prolongrd opera- electrodes covered by the electrolyte 0 4 T ~ r n e of Cool~ng 8 12 16 ,rnln 20 24 28 tion of the element through change in and the ionic composition of the electro-
sensitivity. T h e slower corrosion rate lyte. Fig. 2.-Record of potential from plati-
of zinc and t h e fact t h a t the corrosion T h e curve in Fig. 2 represents a num and zinc cell (corroded) under lab-
products were washed by the rain made transient condition, and the shape of oratory conditions. Shunt resistance =
the platinum and zinc element much this curve would be different with dif- loomegohms.
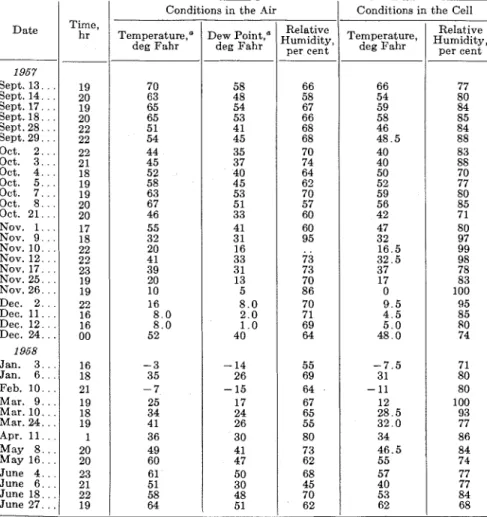
TABLE I.-CONDITIONS UNDER WHICH T H E CELL OF PLATINUM AND ZINC ON GROUNDWARD S I D E DEVELOPED 0.1 v OR MORE ACROSS A 100-MEGOHM
S H L N T RESISTANCE.
1
I
Conditions in the .4irI
Conditions in the CellSept. 13. . . Sept. 14. . . Sept. 17. . . Sept. 18. . . Sept. 28. . . Sept. 29. . . Oct. 2 . . . Oct. 3 . . . Oct. 4 . . . Oct. 5 . . . Oct. 7 . . . Oct. 8 . . . Oct. 21. . . Nov. 1 . . . Nov. 9 . . . Nov. 1 0 . . . Nov. 12. . . Nov. 17. . . Nov. 25. . . Nov. 26. . . Dec. 2. . . Dec. 1 1 . . . Dec. 1 2 . . . Dec. 24. . . 23 16 16 00 1968 Jan. 3 . . . Jan. 6 . . Feb. 1 0 . . . Mar. 9 . . . Mar. 1 0 . . . M a r . 2 4 . . . June 4 . .
. I
23 16 18 21 19 18 19 Apr. 11 . . . May 8 . . . May 1 6 . . . T e m p e r a t ~ r e , ~ deg Fahr 1 20 20 Dew P ~ i n t , ~ deg Fahr Relative ~ ~ ~ iTemperature, d i ~ ~ , per cent deg FahrRelative Humidity,
per cent
" Rockcliffe Meteorological Station, 1% miles from test cell.
sensing element, thus shon~ing the sensitivity for detecting moisture under those conditions. T h e hourly values for air temperature and dew point tempera- ture were obtained from the Rockcliffe Meteorological Station about 1; miles from t h e location of the sensing elements so t h a t small temperature variations from values given might be expected for the exposure site. The panel temperature was measured by means of a 30-gage copper-constantan thermo- couple taped to t h e groundward face of t h e panel.
It
is believed t h a t this temperature was very close t o t h e actual panel temperature. Because of possible variations in air temperature and humidity conditions between t h e Rockcliffe Station and this site, i t is not possible to give any limits of ac- curacy for the data in Table I.It
is of interest to note t h a t the cell detected the presence of water a t a temperature much below freezing, indi- cating t h a t the electrolyte remained unfrozen a t these low temperatures.This may be accounted for by t h e presence of salts deposited from t h e atmosphere and the fact t h a t adsorbed films of water are serving a s t h e electro- lyte.
It
has been observed t h a t t h e sensi- tivity for detecting moisture is con- siderably decreased when t h e cell is connected across a shunt resistance of 1 or 5 megohms rather t h a n 100 megohms. This merely indicates the limiting current-generating capacity of the cell and emphasizes the fact t h a t the potential measuring circuit used in conjunction with this cell should have a very high impedance in order to limit the current t o less than 0.1 pa a t full potential. Otherwise the sensitivity for detecting moisture films nrill be reduced and correspondingly t h e hours of wetness detected may be reduced by a factor of 1.3 to 2, depcnding on many factors pertaining t o the transient climatic conditions.Where simplified instrumentation is required and where the resistance of t h e
A S T M B U L L E T I N
external measuring circuit is low, i t will be necessary to increase the current capacity of the detecting cells in order t o achieve the same sensitivity. This m a y be possible by providing a sensing element having a multiple electrode system. This mould increase the current capacity by virtue of multiple cells in parallel connection. Preliminary trials along these lines have been made, and results a r e promising.
Field Instrument
T h e in~portance of measuring hours of wetness during exposure of mctals to atmospheric corrosion need not be emphasized. In fact, when monthly rates of corrosion of steel were reduced t o t h e r a t e based on hours of wetness, a useful correlation with other factors mas found, which will be reported shortly. For this reason i t would be useful to develop a portable time-of- wetness instrument to be used a t the corrosion sites.
An attempt is being made to develop a field instrument which would use a simple transistor amplifier with the multiple cell sensing element described here. T h e periods of wetness corre- sponding t o the generation of a potential in excess of 0.1 v would be indicated b y means of some type of operational time recorder.
It
is important to record t h e air temperature during the period of wetness, and for this reason the time-of-wetness recorcltr may be combined with a thermograph to record both factors simultaneously.Conclusion
I n conclusion i t can be stated t h a t a method has been developed which can detect t h e presence of surface moisture on metal panels exposed to outdoor conditions. This results in the measure- ment of the period of wetness or the actual time during which metal is cor- roding while exposcd t o outdoor con- ditions. This then becomes the major factor in assessing the rate of corrosion of metals in a given atmosphere.
There is no doubt t h a t a simple, portable instrument developcd along t h e same principles xvould be invaluable a s a n aid in collecting pertinent in- formation when exposing various metals for test t o the'varying conditions of t h e atmosphere throughout t h e world. Acknowledgment:
T h e author gratefully acknowledges t h e invaluable assistance of
H.
F.Slade and S. E. Dods in setting u p the apparatus and taking t h e records.