Publisher’s version / Version de l'éditeur:
Journal of Power Sources, 202, pp. 269-275, 2012-03-15
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/j.jpowrsour.2011.11.047
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Investigation of CrSi2 and MoSi2 as anode materials for lithium-ion
batteries
Courtel, Fabrice M.; Duguay, Dominique; Abu-Lebdeh, Yaser; Davidson,
Isobel J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=3a1e8729-81d6-4410-bc97-45d36fd026a1
https://publications-cnrc.canada.ca/fra/voir/objet/?id=3a1e8729-81d6-4410-bc97-45d36fd026a1
ContentslistsavailableatSciVerseScienceDirect
Journal
of
Power
Sources
j ou rn a l h o m e pa g e :w w w . e l s e v i e r . c o m / l o c a t e / j p o w s o u r
Investigation
of
CrSi
2
and
MoSi
2
as
anode
materials
for
lithium-ion
batteries
Fabrice
M.
Courtel, Dominique
Duguay, Yaser
Abu-Lebdeh
∗, Isobel
J.
Davidson
NationalResearchCouncilCanada,1200MontrealRoad,Ottawa,OntarioK1A0R6,Canada
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received7October2011 Receivedinrevisedform 14November2011 Accepted15November2011 Available online 25 November 2011
Keywords: CrSi2 MoSi2 Silicide Li-ionbatteries Anodematerial
a
b
s
t
r
a
c
t
WereportonthesuitabilityofthetwometalsilicidesMoSi2andCrSi2 asanodematerialsforLi-ion
batteries.Thetwomaterialsweresynthesizedbyhighenergyballmillingofthecorrespondingmetals andX-raydiffractionwasusedtofollowtheevolution/formationofthecrystallinephases.Thein-house preparedpowdershaveshownbetterbatteryperformancethancommercialpowders(as-receivedor ball-milled).Weobservedthat20hwerenecessaryfortheformationofMoSi2whereas100hwereneeded
fortheformationoftheCrSi2powder.Thebestbatteryperformancewasobtainedwiththesynthesized
CrSi2powderthathasatheoreticalcapacityvalueof469mAhg−1.Thispowderprovidedcapacitiesof
340,262,167,110,and58mAhg−1atC/12,C/6,C/2,C,and2C,respectively.Cyclingat60◦Cshowed
highercapacityvalues,butwithafasterfadeofthesevalues.
Crown Copyright © 2011 Published by Elsevier B.V. All rights reserved.
1. Introduction
Mostcommerciallithium-ionbatteriesusegraphiticcarbonas theanodematerialdue toitslow cost,longcyclelife,andvery stablecapacity[1].However,thereversibleelectrochemical inter-calationoflithiumionsinitsstructureleadstotheintercalationof onelithiumpersixcarbons(LiC6)thatresultsincapacities theo-reticallylimitedtoonly372mAhg−1.Siliconhasbeenstudiedfor quiteawhileasanodematerial.Siliconisabundant,quite inexpen-sive,non-toxic,andprovidesaveryhightheoreticalcapacityover 3500mAhg−1(Si+3.75Li++3.75e−
↔SiLi3.75)[2].Howeverit suf-fersfromlargestructuralvolumechangesduringcharge/discharge cyclingofthebatteryreaching300–400%[1,3,4].Thisgivesriseto mechanicalstressesthatleadtocracksandeventual disintegra-tionoftheelectrodeandafailureofthebattery[5].Intermetallic compounds,suchassilicidesM–Si(withM:Mg[6–8],Mn[2,9], Mo[10],Fe[9,11],Ni[12],Nb,Ta, V,Ca,Cr[13,14],and Ti)[1]
werefirstreportedbyAnaniandHugginsasanodematerialsfor Li-ionbatteries[14],especiallyMg2Si[6]andCrSi2[14].Since,more workhave been performedon siliconand silicon-metalalloys; onthisregard,twointerestingreviewshaverecentlybeen pub-lishedonthesubjectbyParketal.[15]andLarcheretal.[2].The insituformationoftheinactivemetalcompoundactsasamatrix that mitigates the volume change. Mg2Si has an anti-fluorine structurethatwasshowntoaccommodateLi-insertionaccording
∗ Correspondingauthor.Tel.:+16139494184;fax:+16139900347.
E-mailaddress:Yaser.Abu-Lebdeh@nrc-cnrc.gc.ca(Y.Abu-Lebdeh).
to the following path: Mg2Si+2Li+2e→Li2MgSi+Mg, followed by reaction of Mg with lithium toform an MgLi alloy [14,16]. However, other groups reported the following reaction path-way [7,8]: Mg2Si+3Li+3e→2MgLi+SiLi. Initial capacities are typically usually high but the reversible capacity is quite low. It has also been reported that lithium could be inserted into the structure of CrSi2, but is limited by slow kinetics at room temperature[17].Nevertheless, usinga composite of CrSi2 and lithium,Weydanzetal.reportedinitialdischargecapacities rang-ingfrom650to800mAhg−1,dependingontheLi:CrSi
2ratioused
[17].
Wedecidedtoinvestigatethebatteryperformanceofpristine CrSi2(withoutlithiumadditive)atroomtemperature(R.T.)andat 60◦C.Wealsoreportonthebatterybehaviorofitstwincompound MoSi2asacomparison[10].Thesetwopristinesilicideshavenever beeninvestigated asanodematerialsfor Li-ionbatteries. Based onAnaniandHugginswork[14]weestimatedtheoretical capac-itiesof804mAhg−1 forMoSi
2 and580mAhg−1 forCrSi2.These values arein therange of capacities expectedfor futureanode materialsin cathode-limitedbatteries as calculated byAppleby etal.[4].
Hereinwe reportthe investigationofsynthesized and com-merciallyavailableCrSi2andMoSi2.Thepowdersweretestedas receivedandalsoball-milledviahighenergyball-milling(HEBM) at different times. In addition, a comparison with synthesized CrSi2andMoSi2powderspreparedbyHEBMwasalsoperformed. Lithiumbatteries (half-cells)wereassembled withthedifferent materials and tested galvanostatically at differentrates and by cyclicvoltammetry.
0378-7753/$–seefrontmatter.Crown Copyright © 2011 Published by Elsevier B.V. All rights reserved. doi:10.1016/j.jpowsour.2011.11.047
270 F.M.Courteletal./JournalofPowerSources202 (2012) 269–275
2. Experimental
2.1. Materials
Chromiumsilicide(CrSi2,230mesh,99+%),molybdenum(Mo, 1–2m,>99.9%),andsilicon(Si,325mesh,99%)werepurchased from Sigma–Aldrich. Molybdenum silicide (MoSi2, 99.5%) and chromium(Cr,<10m,99.8%)werepurchased fromAlfaAesar. CarbongraphiteE-KS4andSuperScarbonwerepurchasedfrom LonzaG+T(Switzerland)andTimcal (Switzerland),respectively. Sodiumcarboxymethylcellulose(NaCMC,viscosity42.0mPas)was purchased from Calbiochem and used as a 5wt% solution dis-solvedindouble distilledH2O.Sodiumcarboxymethyl cellulose (NaCMC)wasusedasabinderinthisworkinsteadofconventional PVDFdue toitssuperiorperformance withsilicon-based anode materials.
2.2. Synthesis
CrSi2andMoSi2powderswerefirstusedasreceivedandthen ball-milledfor20husingHEBMtechnique.A50mLtungsten car-bidevial(8004,Spex)andthreetungstencarbidebeads(10.75g each,Spex)wereusedalongwithapowder/ballratioof1/3.2.CrSi2 andMoSi2 werealsosynthesizedviathesameHEBMtechnique usingthesamepowder/ballratio.Thesynthesiswasperformed usingMo,CrandSipowdersasstartingmaterialsmixedinaM/Si atomicratioof 1/2.Themixturewasball-milleduntiltheright phasewasobtained,whichtook100hforCrSi2and20hforMoSi2. HEBMwasperformedusingaSPEX8000ballmiller,andthevials weresealedunderargonatmospherebeforemilling.
2.3. Characterization
PowderX-raydiffractionwascarriedoutusingaBrukerAXSD8 diffractometerwithaCuK␣sourcewithastepsizeof0.03◦ and anacquisitiontimeof1sperstep.Thepatternswereanalyzedby theRietveldrefinementmethod[18]usingthesoftwareTOPAS4 fromBrukerAXS[19].Thecrystallitesizesweredeterminedusing thefundamentalparameterapproachmethoddevelopedbyCheary etal.[20].Powderswerealsocharacterizedbyscanningelectron microscopy(SEM) usinga JEOL 840A.Battery cycling was car-riedoutonhalf-cellsusing2325-typecoincellsassembledinan argon-filledglovebox. Capacitymeasurementswereperformed bygalvanostaticexperimentscarriedoutonamultichannelArbin batterycycler.Theworkingelectrodewasfirstcharged(lithiated) downto5mVversusLi/Li+atdifferentC-ratesandthendischarged (delithiated)upto2VversusLi/Li+.Thevaluesofthecell parame-ters,thecrystallitesizes,andthecompositionpercentagesaregiven withanerrorvaluebetweenbrackets.
Theworkingelectrodeswerepreparedasfollows:theactive materialwasmixedwith5wt% SuperScarbon,5wt% Graphite and10wt%binder.Theelectrode filmsweremadebyspreading ontoahighpuritycopperfoilcurrentcollector(cleanedusinga 2.5%HClsolutioninordertoremovethecopperoxidelayer)using anautomateddoctor-bladeandthendriedovernightat85◦C in aconvectionoven.Individualdiskelectrodes(Ø=12.5mm)were punchedout, dried at 80◦C under vacuumovernight and then pressedunderapressureof0.5metricton.Electrodesweremade of3–4mgofactivematerial.Alithiummetaldisk(Ø=16.5mm) wasusedasanegativeelectrode(counterelectrodeandreference electrode).70Lof a solutionof1MLiPF6 inethylene carbon-ate/dimethylcarbonate(EC:DMC,1:1,v/v)wasusedaselectrolyte andspreadoveradoublelayerofmicroporouspropylene separa-tors(Celgard3501,thickness=30m,Ø=2.1mm).Thecellswere assembledinanargon-filleddrygloveboxatroomtemperature.
3. Resultsanddiscussion
3.1. X-raydiffraction
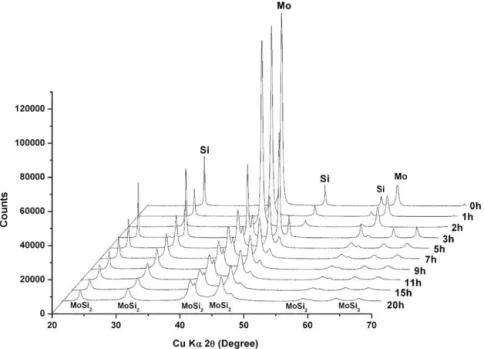
XRD hasbeen performed on the MoSi2 and CrSi2 commer-cialpowders,as-receivedand ball-milledinordertofollowthe decreaseofthecrystallitesizes.X-raypatternswerealsorecorded duringtheHEBMstepsoftheMo–SiandCr–Simixtures.
Fig.1showstheXRDprofilesofcommercialMoSi2asa func-tionof HEBM time. Using theRietveld refinementmethod,the composition(inwt%)oftheas-receivedcommercialMoSi2 pow-derwasof:93.3(1)%MoSi2,4.9(1)%Mo5Si3,and0.8(6)%Mo.The MoSi2 phase exhibits a tetragonal I4/mmm structure with cell parameterof:a=3.20508(3) ˚Aandc=7.84638(9) ˚A,anda crystal-litesizeof306(14)nmwascalculated.Thesecondarytetragonal
I4/mcmMo5Si3 phasehascellparameters ofa=9.6462(6) ˚A and c=4.9064(5) ˚A andcrystallitesizesof210(100)nm.After20hof HEBMadecreaseofMoSi2contentandanincreaseoftheMo5Si3 phase have been observed. The powder is then composed of 70.7(1)%MoSi2, 28.1(1)%Mo5Si3, and 1.2(4)% Mo.Asexpected, a decrease inthe crystallitesizesto 173(10)nmfor MoSi2 and 5(2)nm for Mo5Si3 hasbeen observed. Fig.2 shows theX-ray patterns of the Mo–Si mixture HEBM from 0hto 20h. Before theHEBM started, as expected,a mixture of elemental molyb-denum and silicon wasobserved. Afteronly 3h of HEBM, the tetragonalMoSi2 phasestartedappearing alongwiththe disap-pearanceoftheSipeaksandthedecreaseoftheMopeakintensity, asobservedbyMaetal.[10].After20hofHEBM,thein-house preparedpowderiscomposedof93.2%MoSi2(twodifferent crys-tallinestructures)and6.8%Mo.ThefirstMoSi2identifiedphaseis thetetragonalI4/mmmstructurethatrepresents71.4%,withcell parametersof:a=3.2032(2) ˚Aandc=7.8658(7) ˚A,andcrystallite sizesof13(18)nm.Thesecond identifiedMoSi2 structure hasa P6222spacegroup,represents21.8%andhascellparametersof:
a=4.704(6) ˚Aandc=6.094(4) ˚A,andcrystallitesizesof10(8)nm. Smallercrystallitesizesareobservedforthein-housesynthesized MoSi2comparedtothecommercialHEBMMoSi2powder.
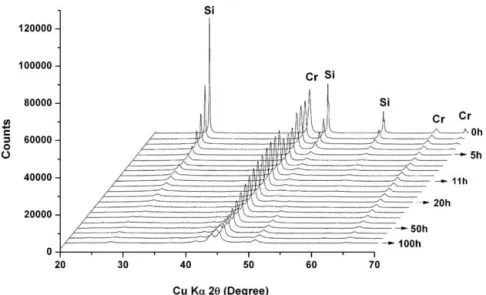
Fig.3showstheXRDprofilesofcommercialCrSi2asafunction ofball-millingtime.ByRietveldrefinement,thecalculated com-positionoftheas-receivedcommercialCrSi2powderwas99.75% CrSi2 and 0.25% CrSi. The CrSi2 phase exhibits a P6422 struc-turewithcellparametersof:a=4.42784(5) ˚Aandc=6.3726(1) ˚A, andusingthefundamentalparameterapproachmethodcrystallite sizesof187(63)nmwerecalculated.After20hofHEBM,theCrSi2 crystallitesizehasdecreasedto7(1)nmwithcellparametersof
a=4.4284(5) ˚Aandc=6.380(2) ˚A;thetwosecondaryphases,CrSi andSiwerenolongervisible.Thein-housepreparedCrSi2powder tookalongertimetopreparethantheMoSi2 powder.Thephase startedappearingafter20hofHEBM,along withthe disappear-anceofthechromiumphase.After100hofHEBM,thechromium phase isat a minimumlevel. Thepowder isthen composedof 65.1%CrSi2,27.2%Cr5Si3,and7.7%Cr.TheCrSi2powdershowed smallcrystallitesizeof9(3)nm,similarlyfortheCr5Si3 powder at9(4)nmFig.4.
3.2. Scanningelectronmicroscopy
Fig.5showstheSEMmicrographsofthethreeCrSi2powders andthreeMoSi2powders.AsshowninFig.5aandc,theas-received commercialCrSi2andMoSi2showsmoothparticlesrangingfrom 5to10minsize.After20hofHEBM, adecreaseinthe parti-clesizewasnoticedforbothpowders.AsshownbyFig.5bandd sizesnowrangeatandbelow5m.CrSi2powdermadeby100hof HEBMdirectlyfromaCr–Simixtureshowsasmallandan homo-geneoussizedistribution,around2mwithaggregatesizesupto
Fig.1. XRDpatternsofcommercialMoSi2duringtheHEBMsteps.
Fig.2.XRDpatternsoftheMo–SimixtureduringtheHEBMsteps.
6–7m.TheMoSi2powderpreparedthesamewaywith20hof HEBMshowsparticlesizesaround1m.
3.3. Batterycycling
Allsixpowdershavebeentestedasanodematerialsin half-cells using lithium metalas a counter and reference electrode. TheC-rate and theoretical capacities werecalculated usingthe workperformedbyAnani andHuggins[14] whoestimatedthe lithium storage capacities of several silicides,including molyb-denum andchromiumsilicides.Capacityvalues ofthedifferent molybdenum and chromium silicides indentified by XRD have been calculated using the formula reported in reference [21]: Cap. (mAhg−1)=(96,500×numberof Li+ exchanged)/(molecular weightofthesilicide×3.6).Thecapacityvalues,rangingfrom279 to804mAhg−1,aresummarizedinTable1.
Thetheoreticalcapacityvaluesofthesixinvestigatedpowders havebeencalculatedaccordingtothecompositionobtainedvia theRietveldrefinementmethodappliedtotheXRDpatterns.The capacityvaluessummarizedinTable2donottakeintoaccount a possibleamorphous silicon phase thatwas notdetectableby XRD.
Fig.6showstheperformanceandtheratecapabilityof com-mercialMoSi2,as-receivedandHEBMfor20h,andin-housemade MoSi2 electrodes. After20 cyclesat C/12, the previously men-tionedpowdersshowedcapacitiesof100,115and135mAhg−1, Table1
Theoreticalcapacityvaluesofthedifferentmolybdenumandchromiumsilicides.
Silicide MoSi2 Mo5Si3 CrSi2 CrSi Cr5Si3
Lithiumstored[14] 4.56 5.88 2.34 0.93 4.32
272 F.M.Courteletal./JournalofPowerSources202 (2012) 269–275
Table2
Compositionandtheoreticalcapacityvaluesofcommercial(as-receivedandHEBM)andin-housesynthesizedmolybdenumandchromiumsilicides.
Silicide MoSi2commercial
as-received MoSi2commercial 20hHEBM MoSi2in-house 20hHEBM CrSi2commercial as-received CrSi2commercial 20hHEBM CrSi2in-house 100hHEBM CompositionfromRietveldrefinement
(wt%)
93.3MoSi2 70.7MoSi2 93.2MoSi2 99.75CrSi2 93.53CrSi2 65.1CrSi2
4.9Mo5Si3 28.1NMo5Si3 6.8Mo 0.25CrSi 6.47Cr Cr5Si3
Mo 1.2Mo 7.7Cr
Capacity(mAhg−1) 764 647 749 579 542 469
respectively,whichislowerthangraphite(330mAhg−1atC/12). AccordingtoHuggins,theexpectedcapacitiesare764,647, and 749mAhg−1, respectively. The lower capacities could not be attributedtopoorelectronic conductivityas itis reported that MoSi2isagoodelectricalconductorof3.5×104Scm−1[22,23]but mostprobablytopoorionicconductivityasitisalsopossiblethat thelithiumdiffusioncoefficientisverylowatR.T.Another possi-bilityisthatMoSi2mightnotbeveryreactivetowardslithium,as pointedoutbyDahnetal.formostsilicides[9].Fig.6alsoreports theratecapabilityofthesethreeelectrodes,thatareobviouslyalso
verylow.Howeverthepowdermadein-houseprovidedthebest capacityvalues.
InordertoimprovethelithiummobilityinMoSi2,ahalf-cell madeofthein-housepreparedpowderhasbeencycledat60◦C. Itshowedahighfirstdischargecapacityof450mAhg−1 andan irreversiblecapacityof235mAhg−1,whichrepresentsabout65% ofthefirstdischargecapacity.After20cycles,theelectrode has acapacity ofonly75mAhg−1.AsreportedbyFleischaueretal., thecapacityfadeforsilicon-richsilicidescouldberelatedtothe increaseddegreeofLi15Si4crystallizationathighertemperatureas
Fig.3. XRDpatternsofcommercialCrSi2duringtheHEBMsteps.
Fig.5.SEMmicrographsof(a)as-receivedcommercialCrSi2,(b)commercialCrSi2after20hofHEBM,(c)synthesizedCrSi2,(d)as-receivedcommercialMoSi2,(e)commercial
MoSi2after20hofHEBM,and(f)synthesizedMoSi2.
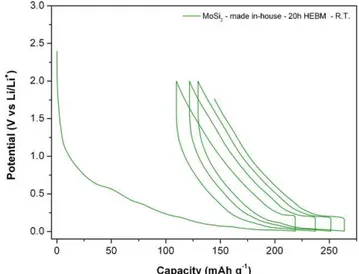
opposedtoamorphousLi15Si4whichismorereactive[24].Itcould alsobeduetothedegradationoftheelectrolyteandtheformation ofathickerSEIlayeratthesurfaceoftheparticles.Fig.7showsthe voltageprofileoftheMoSi2powderpreparedin-houseby20hof HEBM.Itshowsadischargethatstartsmostprobablywiththe for-mationofaSEIatabout1.25V.Aninsertionplateauisalsoobserved atlowpotentialatabout0.01V.Thedelithiationstartedwithashort plateauat0.2V,andthenaslope(straightline)between0.005V and2V.AccordingtoAnaniandHuggins,theadditionof molybde-numtothebinarysilicon–lithiumsystemproducesthreeadditional phasesintheternaryphasediagramMo–Si–Li:MoSi2,Mo5Si3,and Mo3Si.Asaconsequence,itgivesrisetotwoadditionalequilibria: MoSi2–Mo5Si3–SiLi3.25andMo5Si3–Mo3Si–SiLi4.4[14].Itis impor-tanttopointoutthatmolybdenumdoesnotformanalloywith lithium.Thereactionscorrespondingtotheseequilibriaareshown byEqs.(1)and(2):
20MoSi2+91Li++91e−↔4Mo5Si3+28SiLi3.25 (1) 15Mo5Si3+88Li++88e−↔25Mo3Si+20SiLi4.4 (2)
Fig.6.RatecapabilityMoSi2anodesmadefrom:as-receivedcommercialpowder
cycledatR.T.,commercialpowderball-milledfor20hcycledatR.T.,powdermade in-housevia100hofHEBMatR.T.andat60◦C.Batterieswerecycledbetween5mV
and2VversusLi/Li+atC/12,C/6,C/2,C,2CandbacktoC/12.
Chromiumisinthesamegroupasmolybdenumintheperiodic tableand sharessomephysicalandchemical properties,andits silicidecompoundsareofsimilarinterest.SimilartoMoSi2,the per-formanceofpristineCrSi2hasneverbeenreportedintheliterature, onlyWeydanzetal.reportedfirstdischargecapacitiesofa compos-iteofCrSi2andlithiumwhichvariedfrom650to800mAhg−1as afunctionoftheLi:CrSi2ratioused[17].Fig.8showsthe perfor-manceofdifferentCrSi2electrodematerials:theas-receivedand 20hball-milledcommercialpowdersandthein-housesynthesized powders.Theas-receivedpowderprovidesavery lowcapacity, around75mAhg−1.TheHEBM (20h)powdershowed enhance-ment in capacity with a first discharge of 330mAhg−1, which represents61%ofthetheoreticalcapacityandanirreversible capac-itybetweenthefirstandthesecondcycleofabout50mAhg−1(15% ofthefirstdischargecapacity).Thispowdershowedareversible capacityof225mAhg−1after20cyclesatC/12.
The in-house prepared CrSi2 powder showed better per-formance than the commercial one. When cycled at room temperature,areversiblecapacityof340mAhg−1wasobtainedfor thispowderafter20cyclesatC/12,whichisalmostasgoodasthe
Fig.7. Voltageprofileofthefirst4cyclesofMoSi2madein-houseby20hofHEBM
274 F.M.Courteletal./JournalofPowerSources202 (2012) 269–275
Fig.8.CyclingbehaviourofCrSi2anodesmadefrom:as-receivedcommercial
pow-dercycledatR.T.,commercialpowderball-milledfor20hcycledatR.T.,powder madein-housevia20hofHEBMatR.T.andat60◦
C.Batterieswerecycledbetween 5mVand2VversusLi/Li+atC/12.
reversiblecapacityofgraphiteforthesameC-rate(330mAhg−1). TheratecapabilityoftheCrSi2powderpreparedin-houseisshown inFig.9.Capacitiesof262,167,110,and58mAhg−1wereobtained atC/6,C/2,C,and2C,respectively.Whenthebatterywascycled backatC/12,theelectroderecovered94%ofitsprevious capac-ity(atC/12).Similartomolybdenum,chromiumdoesnotforman alloywithlithium,anditsadditiontothebinarysilicon–lithium systemproducesfouradditionalphasesintheternaryphase dia-gramCr–Si–Li:CrSi2,CrSi,Cr5Si3,andCr3Si,whichgaverisetothree additionalequilibria:CrSi2–CrSi–SiLi2.33,CrSi–Cr5Si3–SiLi2.33,and Cr5Si3–Cr3Si–SiLi3.25 [14].Eqs.(1)–(3)showthereactions corre-spondingtotheseequilibria:
3CrSi2+7Li++7e−↔3CrSi+3SiLi2.33 (3) 15CrSi+14Li+
+14e−
↔3Cr5Si3+6SiLi2.33 (4) 3Cr5Si3+13Li++13e−↔5Cr3Si+4SiLi3.25 (5) Weobservedthatchromiumsilicidepowdersshowedbetter per-formance than the molybdenum silicide powders. Contrary to MoSi2,CrSi2isnotaconductorbutap-typesemiconductorwith abandgaprangingfrom0.3to0.84eV[25]whichresultsinlower
Fig.9. RatecapabilityCrSi2anodesmadefrom:as-receivedcommercialpowder
cycledatR.T.,commercialpowderball-milledfor20hcycledatR.T.,powdermade in-housevia100hofHEBMatR.T.andat60◦C.Batterieswerecycledbetween5mV
and2VversusLi/Li+atC/12,C/6,C/2,C,2CandbacktoC/12.
Fig.10.Voltageprofileofthefirst4cyclesofCrSi2madein-houseby100hofHEBM
cycledatR.T.and60◦CatC/12.Batterieswerecycledbetween5mVand2Vversus
Li/Li+.
electricalconductivity.AccordingtoAnaniandHugginsstudy per-formedat400◦C, itis mostlyprobablethatlithiumdiffusionin CrSi2isquitelowatR.T.[17].So,inordertoimprovethelithium diffusion,ahalf-cellmadeofchromiumsilicidepreparedinhouse by100hofHEBMwascycledat60◦C.After20cyclesatC/12,this electrodeshowedacapacityof446mAhg−1,whichishigherthan thecapacityobtainedatR.T.TheratecapabilityillustratedbyFig.9, gavethefollowingperformance:382,315,263,and191mAhg−1at C/6,C/2,C,and2C,respectively.WhencycledbackatC/12,the bat-teryprovided360mAhg−1whichrepresentsabout81%ofcapacity recovery.However,arecoveryof94%wasobtainedwhenthe bat-terywascycledatR.T.,whichmeansthattheperformanceofthe batterydegradesfasterwhencycledat60◦C.AsforMoSi
2,thisis mostprobablyduetotheformationofathickerSEIthatbuildsup withcyclingathighertemperature,orduetotheincreaseddegree ofLi15Si4crystallizationat60◦CasopposedtoamorphousLi15Si4, asreportedbyFleischaueretal.[24].
Fig.10showsthevoltageprofileofchromiumsilicidepowder preparedin-houseafter100hofHEBMofSiandCrpowders.These batterieswerecycledatR.T.or60◦CatC/12between0.005Vand 2VversusLi/Li+.Thegeneralvoltageprofileisquitesimilartowhat wasobservedforMoSi2.ThebatterycycledatR.T.showsa shoul-deratabout0.5VthatcouldberelatedtotheformationoftheSEI, whereasthesameshoulderappearsatabout1.8Vforthebattery cycledat60◦C.Thepotentialthenreachedaplateauatabout0.04V atR.T.and0.17Vat60◦C.Theseplateauscorrespondtothe forma-tionofchromium-richsilicidesandtheformationofsilicon–lithium alloyssuchasSiLi3.25andSiLi2.33,accordingtoequilibriums(3)–(5). Weydanzetal.observedtheonsetofthelithiationatslightlylower voltageof300mVfortheLi–Cr–Sisystem[17].Thebatterycycledat R.T.showedafirstdischargecapacityof435mAhg−1,which repre-sents93%ofthetheoreticalcapacity.Afirstirreversiblecapacityof about50mAhg−1 (12%ofthefirstdischargecapacity)was mea-sured.The samematerial cycled at 60◦C showeda higher first dischargecapacityof870mAhg−1,whichis85%higherthanthe theoreticalcapacity thatwe calculated forthis powder.Soit is probablethattheextracapacityobtainedat60◦Ccorrespondsto theformationofathickerSEIonthesurfaceoftheCrSi2particles thatconsumesalargeamountofcharge.Thishighcapacitycame alongwithahighfirstirreversiblecapacityof285mAhg−1(33%of thefirstdischargecapacity).Thedelithiationofchromiumsilicide occursasaslope,almostastraightlinebetween0.005Vand2V forbothcyclingtemperatures.Aspreviouslymentioned,alarger irreversiblecapacity wasobservedfor thebattery dischargedat 60◦C.
4. Conclusions
Inthisstudywereportedtheinvestigationofmolybdenum sili-cideandchromiumsilicideasanodematerialsforLi-ionbatteries. Thein-housepreparedpowdersshowbetterperformancethanthe ball-milledcommercialMoSi2 andCrSi2 powders.AHEBM time of20hwasnecessaryfortheappearanceoftheMoSi2crystalline phasewhereasalongertimeof100hwasnecessaryforthe for-mationoftheCrSi2crystallinephase.Chromiumsilicidepowders showedbetterperformancethanmolybdenumsilicidepowders; morespecificallythein-housepreparedchromiumsilicidepowder providedacapacityof340mAhg−1atC/12.Highercycling temper-atureshowedarapiddecadeofthecapacityduetothedegradation oftheelectrode.
Acknowledgment
ThisworkwassupportedbyNaturalResourcesCanada’sOffice ofEnergyResearchandDevelopment.
References
[1]G.-A.Nazri,G.Pistoia,LithiumBatteries:ScienceandTechnology,Kluwer Aca-demicPublisher,Boston,Dordrecht,NewYork,London,2004.
[2]D.Larcher,S.Beattie,M.Morcrette,K.Edstrom,J.-C.Jumas,J.-M.Tarascon,J. Mater.Chem.17(2007)3759–3772.
[3]J.Li,R.B.Lewis,J.R.Dahn,Electrochem.Solid-StateLett.10(2007)A17–A20. [4] U.Kasavajjula,C.Wang,A.J.Appleby,J.PowerSources163(2007)1003–1039. [5]P.B.Balbuena,Y.Wang,Lithium-IonBatteries:Solid-ElectrolyteInterphase,
ImperialCollegePress,London,2004.
[6]A.Anani,R.A.Huggins,J.PowerSources38(1992)363–372. [7]J.Santos-Pe ˜na,T.Brousse,D.Schleich,Ionics6(2000)133–138.
[8]H. Kim, J. Choi, H.-J. Sohn, T. Kang, J. Electrochem. Soc. 146 (1999) 4401–4405.
[9]M.D.Fleischauer,J.M.Topple,J.R.Dahn,Electrochem.Solid-StateLett.8(2005) A137–A140.
[10]E.Ma,J.Pagán,G.Cranford,M.Atzmon,J.Mater.Res.8(1993)1836–1844. [11] H.-Y.Lee,S.-M.Lee,J.PowerSources112(2002)649–654.
[12]Y.-N.Zhou,W.-J.Li,H.-J.Chen,C.Liu,L.Zhang,Z.Fu,Electrochem.Commun.13 (2011)546–549.
[13]H.Wang,J.-C.Wu,Y.Shen,G.Li,Z.Zhang,G.Xing,D.Guo,D.Wang,Z.Dong,T. Wu,J.Am.Chem.Soc.132(2010)15875–15877.
[14]A.Anani,R.A.Huggins,J.PowerSources38(1992)351–362.
[15] C.-M.Park,J.-H.Kim,H.Kim,H.-J.Sohn,Chem.Soc.Rev.39(2010)3115–3141. [16]T.Moriga,K.Watanabe,D.Tsuji,S.Massaki,I.Nakabayashi,J.SolidStateChem.
153(2000)386–390.
[17] W.J.Weydanz,M.Wohlfahrt-Mehrens,R.A.Huggins,J.PowerSources81–82 (1999)237–242.
[18] H.Rietveld,ActaCrystallogr.22(1967)151–152.
[19]BrukerAXS,DIFFRACPlusTOPAS:TOPAS4.2UserManual,Karlsruhe,Germany, Bruker-AXSGmbH,2008.
[20]R.W.Cheary,A.Coelho,J.Appl.Crystallogr.25(1992)109–121. [21] R.S.Treptow,J.Chem.Educ.80(2003)1015–1020.
[22]S.Köbel,J.Plüschke,U.Vogt,T.J.Graule,Ceram.Int.30(2004)2105–2110. [23]Z.Guo,G.Blugan,T.Graule,M.Reece,J.Kuebler,J.Eur.Ceram.Soc.27(2007)
2153–2161.
[24]M.D.Fleischauer,R.Mar,J.R.Dahn,J.Electrochem.Soc.154(2007)A151–A155. [25]H.N.Zhu,K.Y.Gao,B.X.Liu,J.Phys.D:Appl.Phys.33(2000)L49.