Lactobacillus rhamnosus GG increases COX 2 expression and
PGE
2secretion in colonic myofibroblasts via a MyD88 dependent
mechanism during homeostasis
Gabriela Uribe1,*, Romain Villéger1,*, Philippe Bressollier3, Rachel N. Dillard1, Daniel L.
Worthley4, Timothy C. Wang5, Don W. Powell1, Maria C. Urdaci3,#, and Irina V. Pinchuk1,2,#
1Department of Internal Medicine, University of Texas Medical Branch, Galveston TX, USA 2Microbiology and Immunology, University of Texas Medical Branch, Galveston TX, USA 3Laboratoire de Microbiologie, Bordeaux Sciences Agro, University of Bordeaux, UMR 5248 Gradignan, FR
4Cancer Theme, University of Adelaide and SAHMRI, Adelaide, AUS
5Department of Medicine, Columbia University Medical Center, New York, NY, USA
Abstract
Prostaglandin E2 (PGE2) plays a critical role in intestinal mucosal tolerance and barrier integrity.
Cyclooxygenase-2 (COX-2)-dependent PGE2 production involves mobilization of arachidonic
acid (AA). Lactobacillus rhamnosus GG (LbGG) is one of the most widely used probiotics reported to colonize the colonic mucosa. LbGG contributes to the protection of the small intestine
Correspondence: Iryna V. Pinchuk, PhD, University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555-0655, ivpinchu@utmb.edu, Phone: 409-772-7409, Fax: (409) 772-0655.
*These authors contributed equally to the work on this manuscript #M.C.U. and I.V.P. share senior authorship.
Disclosures: No conflict of interest exist.
Author’s contribution to the manuscript preparation. Gabriela Uribe, BS, graduate student:
study concept and design; acquisition of data; analysis and interpretation of data; manuscript preparation & revision for important intellectual content.
Romain Villéger, PhD:
study concept and design; acquisition of data; analysis and interpretation of data; manuscript preparation & revision for important intellectual content
Philippe Bressollier, PhD:
acquisition of data; analysis and interpretation of data. Rachel N. Dillard, BS, medical student:
acquisition of data; analysis and interpretation of data. Daniel L. Worthley, PhD:
acquisition of data; generation of transgenic mouse model. Timothy C. Wang, MD:
acquisition of data; generation of transgenic mouse model. Don W. Powell, MD:
study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Maria C. Urdaci, PhD:
study concept and design; acquisition of data; study supervision; analysis and interpretation of data; manuscript preparation & revision for important intellectual content.
Irina V. Pinchuk, PhD:
HHS Public Access
Author manuscript
Cell Microbiol
. Author manuscript; available in PMC 2019 November 01.Published in final edited form as:
Cell Microbiol. 2018 November ; 20(11): e12871. doi:10.1111/cmi.12871.
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
against radiation injury through the repositioning of mucosal COX-2 expressing cells. However, it is unknown if LbGG modulates PGE2 production in the colonic mucosa under homeostasis and the
major cellular elements involved in these processes. Colonic epithelial and CD90+ mesenchymal stromal cells, also known as (myo)fibroblasts (CMFs), are abundant innate immune cells in normal colonic mucosa able to produce PGE2. Herein, we tested the hypothesis that under colonic
mucosal homeostasis LbGG modulates the eicosanoid pathway resulting in increased PGE2
production in both epithelial and stromal cells. Among the five tested human colonic epithelial cell lines, only exposure of Caco-2 to LbGG for 24 h led to the mobilization of arachidonic acid (AA) with concomitant increase in the components within the leukotriene and COX-2 dependent PGE2
pathways. By contrast, CMFs isolated from the normal human colonic mucosa responded to LbGG with increased expression of COX-2 and PGE2 in the prostaglandin pathway, but not 5-LO
in the leukotriene pathway. Oral gavage of C57BL/6 mice for 5 days with LbGG (5×108 CFU/ dose) increased COX-2 expression in the colonic mucosa. The majority of cells upregulating COX-2 protein expression were located in the colonic lamina propria, and co-localized with α-SMA+ cells corresponding to the CMF phenotype. This process was MyD88 dependent, since silencing of MyD88 expression in CMFs abrogated LbGG-induced upregulation of COX-2 in culture and in vivo. Taken together, our data suggests that LbGG increases release of COX-2 mediated PGE2, contributing to the maintenance of mucosal homeostasis in the colon and CMFs
are among the major contributors to this process.
Introduction
Prostaglandins (PGs) are important lipid mediators that regulate numerous physiological and pathophysiological processes in the gut (Fujii et al., 2016). During homeostasis, PGs have a protective role in the gastrointestinal (GI) mucosa and are critical to the maintenance of mucosal epithelial barrier integrity (Morteau, 2000). Prostaglandin E2 (PGE2) is involved in
the regulation of vascular permeability and promotion of epithelial cell proliferation (Eberhart & Dubois, 1995; Stenson, 2014). PGE2 is also required for the suppression of
acute colitis and protection against mucosal damage (Jiang et al., 2006; Miyoshi et al., 2017).
Despite its beneficial effects, inducible PGE2 also contributes to chronic inflammation and
cancer. During Inflammatory Bowel Disease (IBD), high levels of PGE2 exacerbates the
inflammatory processes (Morteau, 2000; Sheibanie et al., 2007). Therefore, there is a dichotomy in PGE2-mediated action: while this molecule contributes to the chronicity of GI
inflammatory responses and cancerogenesis, it is also critical to gut mucosal homeostasis. The initial step in the biosynthesis of inducible PGs results from the liberation of AA from membrane glycerophospholipids by phospholipase A2 (PLA2). Released AA is metabolized
into prostaglandin (PG) G2 and then to PGH2 by two major cyclooxygenase (COX)
isoforms, COX-1 or COX-2 (Smith, Urade, & Jakobsson, 2011). PGH2 is finally converted
by cell- and tissue-specific prostanoid synthases to various bioactive prostaglandins,
particularly PGE2 (Kudo & Murakami, 2005). COX-1 is constitutively expressed to maintain
normal housekeeping functions, while COX-2 is known to be induced in response to GI
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
inflammation and injury (Iyer, Srivastava, Dev, Dastidar, & Ray, 2009; Smith, Garavito, & DeWitt, 1996).
Several intestinal mucosal cells are reported to produce COX-2-dependent PGE2 during
injury, inflammation, and cancer (Fukata et al., 2006). In contrast, cells of mesenchymal origin are known to produce PGE2, not only during inflammation and cancer, but also in GI
homeostasis (Powell, Pinchuk, Saada, Chen, & Mifflin, 2011; Reisinger et al., 2017; Zhu, Hua, & Lance, 2003). Among such cells, colonic CD90+ (myo)fibroblasts (CMFs) are abundant innate immune cells in the normal GI mucosa that have recently emerged as key contributors to GI mucosal homeostasis (Beswick et al., 2014; Pinchuk et al., 2011; Pinchuk, Mifflin, Saada, & Powell, 2010; Powell et al., 2011). Following epithelial injury, intestinal (myo)fibroblasts are reported to sense inflammatory signals and activate COX-2 dependent PGE2 production, contributing to the regeneration of the epithelium (Roulis et al., 2014).
Repositioning of COX-2-expressing MSC-like cells in the intestinal mucosa has been shown to have a radioprotective effect and contribute to wound healing (Brown et al., 2007; Riehl, Foster, & Stenson, 2012).
The GI tract, and in particular the colon, is highly populated by the microbiota, which is important to the maintenance of homeostasis. The microbiota also strongly influences immune responses to injury, justifying current interest in microbiota-based probiotic therapies for several GI pathologies (Scaldaferri et al., 2013). However, little is known about how COX-2/PGE2 is regulated by the normal gut microbiota under homeostasis. The effect
of probiotics on the regulation of inducible PGE2 has been mostly studied in culture using
cancer cell lines (Otte et al., 2009);(Simeoli et al., 2015).
Lb. rhamnosus GG (LbGG), is one of the most widely used probiotic strains. It demonstrates potent immunomodulatory activity and is able to at least temporarily colonize the human colonic mucosa (Segers & Lebeer, 2014). LbGG has been reported to induce COX-2 protein expression in T84 colonic epithelial cancer cell lines (Korhonen, Kosonen, Korpela, & Moilanen, 2004). It has also been demonstrated that LbGG may enhance healing of gastric ulcers in rats via increased synthesis of PGE2 (Lam et al., 2007) and contribute to the
protection against radiation injury in the small intestine of mice through repositioning of COX-2 expressing cells (Ciorba et al., 2012). However, how LbGG modulates the
COX-2/PGE2 pathway in the normal colonic mucosa and which cells in the mucosa are the
primary site of COX-2 induction by this bacteria, remains unknown.
In this study, we tested the hypothesis that under colonic homeostasis LbGG enhances AA metabolism, expression of COX-2, and PGE2 secretion in both epithelial and stromal
compartments of the colonic mucosa. We observed that among five neoplastic colonic epithelial cell lines exposed to LbGG, only stimulation of Caco-2 resulted in increased expression of components within the overall eicosanoid pathway. Moreover, LbGG stimulated the COX-2/PGE2 pathway but not the leukotriene pathway in primary human
CMFs isolated from the normal colonic mucosa, and this process was MyD88-dependent. Using a murine model, we observed that oral administration of LbGG led to an increase in the both AA and COX-2 in the mouse colonic mucosa. The increase in COX-2 protein expression was seen in the mucosal lamina propria, mostly co-localized with CMFs, and this
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
process depends on MyD88 expression by CMFs. Taken together, our data suggests that, under homeostasis, LbGG is capable of regulating mucosal levels of COX-2-dependent PGE2 in a MyD88-dependent manner and that stromal CMFs are implicated in this process.
Experimental Procedures
AntibodiesRabbit polyclonal anti-human COX-2, rabbit polyclonal anti-phospho-5-Lypoxygenase (5-LO) and cPLA2 rabbit polyclonal antibodies (Abs), were purchased from Cayman Chemical
(CA, USA), R&D (MN, USA), and Cell Signaling (MA, USA), respectively. Rabbit monoclonal anti-mouse COX-2 monoclonal Abs, mAbs (clone SP21) was purchased from Thermo Fisher Scientific (MA, USA). Unconjugated mouse anti-human COX-2 mAbs (clone 33) was purchased from BD (CA, USA) and labeled using Zenon Mouse IgG labeling kit was purchased from Invitrogen (CA, USA). Rabbit monoclonal anti-β-actin mAbs (clone RM112) was purchased from Millipore (MS, USA). Precision plus Dual Color standard, Western C standard, and StrepTactin-HRP Conjugate were purchased from BioRad, (CA, USA.). Fluorochrome-conjugated murine anti–α-smooth muscle actin (α-SMA; clone 1A4) monoclonal mAb was purchased from Sigma-Aldrich (St. Louis, MO).
Bacterial strain and culture conditions
Lactobacillus rhamnosus GG (LbGG) was isolated from probiotic Culturelle®, which was purchased from iHealth Inc. (Cromwell, CT, USA). Additionally, the following collection of strains were used in this study: Lactobacillus casei ATCC 334, Lactobacillus paracasei 20006, Lactobacillus plantarum LR3, Lactobacillus acidophilus 42, and Lactobacillus brevis 1 (LMBA laboratory, Bordeaux). Lactobacillus strains were grown in De Man, Rogosa and Sharpe (MRS) medium (BD, CA, USA) at 37°C under aerobic conditions.
Cells
Human colonic epithelial cell lines Caco-2, HT-29, HCT-116, LS-174T, and SW-480 were purchased from ATCC (Manassas, VA, USA) grown in MEM culture medium (Gibco, CA, USA) supplemented with 10% fetal calf serum (Sigma), 100 Tg/ml streptomycin, 100U/ml penicillin, 2 mM L-glutamine and 1 mM non-essential amino acids. For CMF isolation, full thickness fresh human colonic mucosa samples were obtained from discarded surgical resections under UTMB approved IRB protocol #99-061. CMFs were isolated from the normal margin of mucosal colonic tissue of patients undergoing colectomy for colon cancer as described previously and routinely used in our laboratory (Saada et al., 2006); (Johnson et al. 2016). The purity of isolated CD90+ CMFs (98-99%) was confirmed by flow cytometry, as previously described (Saada et al., 2006). Studies were performed with primary CMF isolates at passages 4-10 and cultured as described previously (Saada et al., 2006).
Silencing of MyD88 gene in CMFs
Primary human CMFs lacking MyD88 expression were generated in our lab using Stealth™ siRNA probes (Invitrogen, CA). Negative siRNA controls with appropriate GC content were included in each experiment. An optimal concentration of each siRNA (0.3 nM) was used
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
for each transfection. Transfection of primary cells was performed using Nucleofector™ technology (Amaxa Biosystems, MD, USA) according to the manufacturer’s instructions.
Bacteria:cell co-cultures
Human epithelial cell lines or primary human CMF isolates were seeded in 6 well plates and monitored until 95% confluency, then LbGG was added at 1:10 cell to bacteria ratio. In some experiments epithelial cell lines were grown on permeable membranes in transwells in order to form polarized cell monolayers, prior exposure to LbGG. In these experiments, 12 h cultures of LbGG growing in MRS broth were harvested and washed once with PBS, pH 6.8 by centrifugation at 503 × g, 4°C for 10 min. After washing the pellet, bacteria were resuspended in MEM medium and added to cell cultures as described above. For the transwell cell culture system, LbGG were added to the apical compartment of the epithelial cell monolayer.
Western Blot analysis
Western blot (WB) analysis were performed as previously described (Saada et al., 2006). Briefly, human cells in culture were washed with ice-cold PBS and lysed in Laemmli sample buffer. While murine tissue samples were homogenized in 5 μL lysis buffer (Cell Signaling Technology) per mg tissue and homogenized. 10 Tg of protein per sample were used for WB analysis. Expression differences are shown as adjusted density following normalization to β-actin. The Image Lab software version 5.2.1 was used to calculate adjusted density. Adjusted density values for samples were calculated by dividing the relative density of each sample lane by the relative density of the untreated control for the same lane.
Arachidonic acid and PGE2 determination
Arachidonic acid (AA) production was determined in human cell extracts by gas
chromatography as previously described by a method adapted from (Lepage & Roy,1986). Briefly, 10 μL of 10 mg/mL heptadecanoic acid (C17) were added to 10 million cells (pellet) as an internal standard. Lipids were extracted according to the method by (Folch et al.,1957) by homogenization of the pellet with 2:1 chloroform-methanol mixture (v/v) to a final dilution 20-fold the volume of the tissue sample. The extract was centrifuged and the supernatant was vacuum-dried before solubilization with methanol/benzene/acetyl chloride (4/1/0.5 v/v/v). After 1 h incubation at 100 °C, 2.5 mL of 6% potassium carbonate solution (w/v) was added to the mixture and centrifuged for 10 min at 3500 rpm. 1 μL of the upper phase was injected on Innowax column (L = 30 m, internal diameter = 0.25 mm, stationary phase thickness = 0.25 μm, S&W Scientific, USA) with a 2 mL/min helium flow.
Chromatographic analysis was performed on a gas chromatography-mass spectrometry system QP2010 series (Shimadzu, Japan). Injection was assessed in split mode (division 1:20) at 250 °C and the column temperature fixed at 180 °C. AA was identified by comparison of relative retention times and was quantified after establishment of an arachidonic acid standard curve and comparison with the internal standard (Sigma, MO, USA). AA concentrations from murine tissue samples were also measured using the AA ELISA Kit (TSZ ELISA, USA). PGE2 concentrations were determined using the
Prostaglandin E2 Parameter Assay ELISA Kit (R&D systems) according to the manufacturer’s protocol.
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Confocal microscopy
Frozen murine colon tissue sections were fixed in 1% paraformaldehyde for 20 minutes at room temperature, blocked with normal rabbit serum (1:10 in PBS) and murine serum (1:10 in PBS) for 15 min at room temperature. Sections were incubated overnight at 4°C with anti-murine COX-2 mAbs (clone33) conjugated with AF®647 (1 μg/mL). In some experiments, sections were then stained with AF® 488 conjugated anti-α-SMA mAbs (clone IA4), anti-murine CD11c mAbs (clone N418), and/or anti-anti-murine F4/80 mAbs (clone BM8) for 2 hrs at room temperature. Each staining step was followed by six washes with PBS with Ca
++/Mg++. The sections were then mounted in SlowFade® Gold antifade reagent with DAPI
(Invitrogen, CA, USA). Confocal microscopy was performed with a Zeiss LSM510 META laser scanning confocal microscope (Carl Zeiss, Thornwood, NY).
Lactobacillus rhamnosus (LbGG) in vivo treatment
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston. C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). In the colonic mucosa, α-SMA is specifically expressed by MFs. Tamoxifen-inducible MyD88 floxed mice specific to α-SMA+ cells were generated in our lab (MF-MyD88 KO). Previously generated Acta2-CreERT mice (Grcevic et al., 2012; Worthley et al., 2015) expressing CreERT under the α-SMA promoter (Acta-2) were crossed with MyD88fl/fl mice (The Jackson Laboratory). The presence of the transgenes was controlled as previously described by using standard PCR protocols as previously described (Beswick et al., 2014; Worthley et al., 2015). Control mice negative for Acta2-Cre but positive for MyD88fl/fl recombination derived from at least five backcrossing with C57BL/6 animals were used in this study in order to have similar microbiota composition. Deletion of MyD88 was induced by intraperitoneal (i.p.) injection of tamoxifen (TMX, 0.5 mg/mice for 4 days, total injection volume/animal is 100 TL). All mice (female, 6-12 weeks of age) were housed under pathogen-free conditions. Three days after the last TMX injection, animals received 5×108 CFU/dose of lyophilized LbGG in suspension in PBS or PBS only for 5 days. Colonic tissue samples were then harvested for further analysis.
Statistical analysis
Unless otherwise indicated, the results were expressed as the mean ± SEM of data obtained from at least three independent experiments done with duplicate sets in each experiment. For experiments comparing the results between two groups, Student’s t-test was used. If
necessary, the Wilcoxon signed-ranks test was used. For multiple group comparison, one– way ANOVA with post-hoc Tukey’s Honestly Significant Difference (HSD) test was used. Values of p < 0.05 were considered statistically significant.
RESULTS
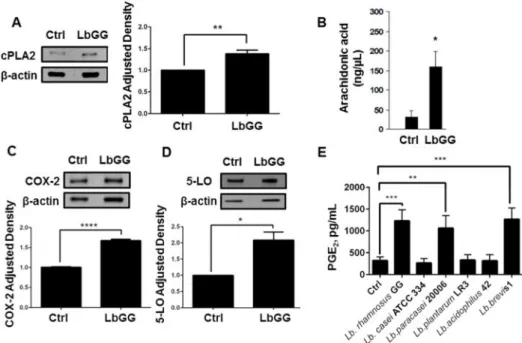
LbGG induces several components of AA pathway in Caco 2 epithelial cell line
The PGE2 synthesis pathway is initiated by AA release from the cleavage of membrane
phospholipids by cPLA2. Therefore, we first evaluated the levels of cPLA2 and its
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
downstream product AA in Caco-2 colonic epithelial cell line cultured with LbGG. We observed an increase in cPLA2 protein expression and AA production in response to 24 h stimulation with LbGG (Figure 1A–B). AA is known to be metabolized by two inducible pathways: COX-2 mediated production of prostaglandins and synthesis of leukotrienes through 5-LO (Stenson, 2014). Thus, we next evaluated the response of these two downstream enzymes after stimulation by LbGG. Western blot analysis demonstrated that COX-2 and 5-LO protein levels were increased in Caco-2 cells upon stimulation with LbGG, suggesting that there is no apparent preferential selection for either pathway (Figure 1C–D). In order to determine if the PGE2 synthesis pathway is equally inducible by different
Lactobacillus probiotic strains, or specific to LbGG, we compared PGE2 protein levels in
Caco-2 cells after incubation with 6 different lactobacilli strains (Figure 1E). PGE2
production was induced by only specific strains: LbGG, Lb. paracasei 20006 and Lb. brevis 1, thereby confirming our interest in LbGG as a potent inducer of PGE2.
Among the five colonic epithelial cell lines tested, Caco 2 was the only cell line responding
to LbGG stimulation with COX 2 expression and PGE2 production
To determine the generalizability of the observation that LbGG stimulates epithelial COX-2- mediated PGE2 release, we investigated the effect of this probiotic on several colonic
neoplastic epithelial cell lines: Caco-2 cells, HT-29 (ATCC HTB-38™), LS-174T (ATCC CL-188™), HCT116 (ATCC CCL-247™), and SW480 (ATCC CCL-228™. Additionally, HCT116 (ATCC CCL-247™) and SW480 (ATCC CCL-228™) are reported to be low/ negative for inducible COX-2 expression (Buecher, 2005). Using WB analysis, we observed that only Caco-2 out of all tested epithelial cell lines responded to LbGG stimulation with the upregulation of COX-2 protein expression (Figure 2A) and significant upregulation of secreted PGE2 (Figure 2B). Further, cell lines from the above experiments that are known to
express inducible COX-2, but did not have increased COX-2 protein levels in response to LbGG stimulation, were polarized and subsequently stimulated with LbGG at the apical compartment of the epithelial cell monolayer. This did not result in an increase of COX-2 protein expression at 24 hours after exposure to LbGG (Supplementary figure 1).
Surprisingly, stimulation of the Caco-2 epithelial cell line, which did show up regulation of COX-2 with LbGG, did not demonstrate a significant change in COX-2 mRNA levels (see Supplementary figure 2). This suggest that LbGG may regulate COX-2 protein expression via post-transcriptional or post translational modifications.
LbGG stimulates the increase in COX 2 and PGE2 within the eicosanoid pathway but not 5
LO in human colonic myofibroblasts (CMFs)
CMFs are located just beneath the epithelial basement membrane and are an abundant cell phenotype in the colonic lamina propria (Saada et al., 2006). CMFs are known to be the major producers of COX-2-dependent PGE2 in the colonic mucosa (Mifflin et al., 2002).
Thus, we determined the contribution of CMFs to LbGG-induced PGE2 synthesis. For this
purpose, normal primary human CMF isolates were co-cultured with LbGG. We observed that 24h treatment with LbGG led to a significant increase in both cPLA2 and COX-2 protein levels in CMFs (Figure 3A-B). In contrast, 5-LO enzyme production is slightly decreased (Figure 3C). Moreover, CMFs responded to stimulation by LbGG with an
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
increase in PGE2 production (Figure 3D). These results suggest that LbGG preferentially
induced COX-2 and PGE2 rather than 5-LO mediated leukotriene synthesis in CMFs.
LbGG induced COX 2 upregulation in CMFs is MyD88 dependent
Because LbGG is reported to protect the murine intestinal epithelium from radiation injury through a MyD88/COX-2-dependent mechanism (Ciorba et al., 2012), we analyzed whether the MyD88 adaptor is required for LbGG-induced modulation of COX-2 in human CMFs. Using a siRNA approach, we demonstrated that silencing of the myd88 gene in CMFs abrogated the LbGG induced upregulation of COX-2 protein expression (Figure 4). These data suggest that LbGG-induced increase of COX-2 in human CMFs likely involves signaling through Toll-Like Receptors (TLRs) that requires MyD88.
LbGG activates the AA metabolic pathway in vivo
LbGG has been shown to up-regulate COX-2 and PGE2 in murine models of intestinal
injury. However, little is known about the effect of lactobacilli on this pathway under colonic homeostasis. Our results obtained with normal human CMFs strongly suggest that under homeostasis CMFs respond to LbGG with an increase in AA mobilizing enzyme cPLA2 and
COX-2 protein expression. Next, we determined whether LbGG can stimulate AA release and upregulate COX-2 during colonic homeostasis in vivo. For this purpose, 5×108 CFU/ dose of LbGG were administrated daily by oral gavage to C57BL/6 mice for 5 days and AA and COX-2 levels were measured in the colonic mucosa. We observed that LbGG
administration resulted in an increase in colonic mucosal levels of AA (Figure 5A) and an increase in total colonic mucosal COX-2 protein expression (Figure 5B). Using COX-2 specific immunostaining followed by confocal microscopy, we confirmed results obtained by WB and observed a significant increase in COX-2 expression (in red) in the colonic mucosa of mice treated with LbGG when compared to the control group (Figure 5D). Interestingly, the LbGG-induced increase of COX-2 is mostly observed in the mucosal lamina propria, while only sparse and an occasional increase in COX-2 expression was seen in the colonic epithelium.
CMFs are a major component of the colonic mucosal lamina propria (Powell et al., 1999; Powell et al., 2011; Saada et al., 2006). To clarify our in vitro and in vivo observations, we determined whether CMFs are among the major cells that increase COX-2 expression in response to LbGG administration in vivo. Mouse colonic mucosa sections were stained with anti-α-smooth muscle actin (α-SMA) mAbs that identify the activated subset of CMFs (shown in green) and anti-COX-2 mAbs (red). We observed that a significant fraction of the COX-2- expressing cells are α-SMA+ (Figure 5C, orange-yellow color formation on the merged images) in the lamina propria of the LbGG treated group. This suggests that, during homeostasis, CMFs are important contributors to LbGG-induced COX-2 expression in the colonic mucosa.
MyD88 signaling in α SMA+ CMFs is required for COX 2 production in response to LbGG in
vivo.
Our experiments in culture demonstrated that LbGG induced the upregulation of COX-2 in CMFs in a MyD88 dependent manner. This observation raised the question whether MyD88
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
is required for COX-2 expression in CMFs in response to LbGG in vivo. In the colonic lamina propria, α-SMA is specifically expressed by myofibroblasts. Therefore, we generated an α- SMA-specific tamoxifen-inducible conditional KO mouse selective for MyD88 (MF-MyD88 KO). The specificity of (MF-MyD88 deletion within the α-SMA+CMFs, as well as retained MyD88 expression in other innate immune cells (such as CD11c+ dendritic cells, F4/80+ macrophages) within the lamina propria was confirmed by confocal microscopy analysis (Supplementary figure 3). Additionally, we did not observe any change in MyD88 expression in the epithelial compartment of the murine colonic mucosa, which was identified by location (first layer from lumen) (Supplementary figure 3).
Using immunostaining followed by multi-color confocal microscopy in this animal model, we observed that deletion of MyD88 within the CMFs resulted in an overall reduction in COX-2 protein expression (in red) within the mucosal stroma in situ (Figure 6A–B). This was further confirmed by western blot analysis of total colonic mucosal tissue (Figure 6C). Finally, these results taken together with our data with human cultured CMFs, suggest that under homeostasis, administration of LbGG may induce mobilization of AA and increase in COX-2 level in a MyD88-dependent manner.
DISCUSSION
It is well established that COX-2 mediated PGE2 production has a dual effect: while it
promotes tolerogenic responses under homeostasis and wound healing (Jiang et al., 2006; Miyoshi et al., 2017), it is also a critical contributor to the progression of colon cancer (Adegboyega et al., 2004; Morteau, 2000; Sheibanie et al., 2007). Despite the exponential growth in the marketing of probiotic LbGG, little is known about how these bacteria modulate colonic PGE2 under homeostasis. In the present study, we demonstrated that
LbGG may increase colonic COX-2 mediated PGE2 production under homeostasis and that
CMFs are among the major cells contributing to this process.
Induction of COX-2-dependent PGE2 synthesis involves mobilization of AA via cPLA2
activity. However, the mechanisms whereby probiotic bacteria modulate this process are unknown. Using Caco-2, a human neoplastic epithelial cell line, and primary cultures of normal human colonic mucosal CMFs, we demonstrated that LbGG induces mobilization of AA likely through the increased concentration of cellular cPLA2 protein. While this has not been reported previously for probiotic bacteria, Valera et al. (Valera et al., 2007) observed that bacterial peptidoglycan, a major component of the Gram positive bacterial cell-wall, can induce AA mobilization in human polymorphonuclear leukocytes.
Our data also demonstrated that LbGG-mediated mobilization of AA in Caco-2 cells, results in the increase of several components in the eicosanoid pathway. This resulted in the upregulation of COX-2, PGE2, and 5-LO protein. 5-LO production is likely to increase the
production of inflammatory mediator leukotriene B4 (LTB4). While no literature is available
regarding the direct effect of lactobacilli on 5-LO/LTB4 by epithelial cells, induction of
LTB4 by bacterial peptidoglycan in polymorphonuclear leukocytes has been previously
reported (Valera et al., 2007). Thus, LbGG-mediated increase of phosphorylated 5-LO in Caco-2 cells is likely to involve the peptidoglycan component of LbGG. Additionally, we
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
found that in contrast to Caco-2 cells, stimulation of CMFs with LbGG resulted in a slight reduction of phosphorylated 5-LO. Others have reported that oral administration of Lb. acidophilus L10, but not Bifidobacterium lactis L94 or Lb. casei L26, resulted in decreased colonic expression of 5-LO-mediated LTB4 in a rat model of colitis (Otieno & Shah, 2007).
Taken together, these data suggest that modulation of the 5-LO/LTB4 pathway by probiotics
is cell and bacterial strain specific.
A similar conclusion can be applied to LbGG-induced increase in COX-2 and PGE2 in
epithelial cells. LbGG induces COX-2-mediated PGE2 production in T84 (Korhonen et al.,
2004) and Caco-2 (our data) epithelial cell lines. By contrast, stimulation of Caco-2 cells or intestinal epithelial organoids derived from chicken embryos with Lb. acidophilus strain did not result in significant upregulation of PGE2 (Pierzchalska et al., 2017; Resta-Lenert &
Barrett, 2002). In our study, we found that three out of six tested Lactobacillus strains induced PGE2 production in Caco-2 epithelial cells. Further studies are required to
definitively elucidate why there is variation in the induction of the COX-2-dependent PGE2
between different strains of the Lactobacillus genus.
Another interesting observation was that, out of four tested intestinal epithelial cell lines that are reported to express COX-2, only Caco2 cells respond to LbGG with increased COX-2 and PGE2 production. These observations are in agreement with in vivo data, where we did
not observe significant increase in COX-2 induction within the epithelium in response to LbGG. However, our data does not exclude that Tuft epithelial cells may respond to LbGG with induction of COX-2. These sparse specialized epithelial cells are reported to express COX-2 in the normal intestinal epithelium (Gerbe, van Es et al. 2011). In contrast to the epithelial cell lines tested, all normal human CMF isolates uniformly responded to LbGG stimulation with an increase in cPLA2, COX-2 and PGE2 upregulation.
Our in vivo study confirmed our observation in culture showing that oral gavage of healthy adult mice with LbGG for five days results in increased levels of AA and COX-2 protein expression in the colonic mucosa. Thus, our studies demonstrate that LbGG-mediated induction of these molecules also occurs under homeostasis. Previously the effect of lactobacilli on upregulation of COX-2 and PGE2 has been demonstrated in animal models of
injury. For example, the use of Lb. fermentum ZYL0401 in an LPS-induced hepatic injury model resulted in the increased ileal expression of COX-2 and production of PGE2 (Jin et
al., 2015). LbGG-induced COX-2-expression protected the intestinal epithelium in mice against radiation injury (Ciorba et al., 2012). Interestingly, these authors did not observe an increase in COX-2 expressing cells, but rather demonstrated repositioning of lamina propria COX-2 expressing mesenchymal stem-like cells closer to the epithelium. Mesenchymal stem cells are progenitors of CMFs, but do not express α-SMA. While our study does not exclude modulation of LbGG-induced COX-2 in mesenchymal stem cells during injury, we have shown that under homeostasis, a significant part of the increase in COX-2 expression is associated with differentiated α-SMA+ CMFs. We previously observed that lack of TLR/ MyD88 dependent signaling in CMF abrogates inflammatory responses in vivo (Beswick et al., 2014). In the present study, we demonstrate that deletion of MyD88 in human CMFs in cultures and murine α-SMA+ CMFs in vivo decreases LbGG-induced mucosal COX-2 levels. While it remains to be evaluated whether subepithelial CMFs come in direct contact
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
with the LbGG in the non-injured epithelium (homeostasis), it is likely that LbGG secreted compounds and/or bacteria cell wall components do make contact with CMFs. In fact, Ciorba et al (Ciorba et al., 2012) showed that oral administration of LbGG condition media is sufficient to induce the COX-2 mediated intestinal radioprotective effect in vivo. Further, we have shown that oral gavage of LbGG in normal mice increases COX-2 expression within CMFs in the lamina propria.
In this work, we highlighted the ability of the probiotic LbGG to induce COX-2-dependent PGE2 production in the whole colonic mucosa and in subepithelial (myo)fibroblasts through
a MyD88-dependent mechanism. Taken together with the previously described functions of PGE2 in the colonic mucosa, our data suggests that probiotic treatment with LbGG could
participate in the maintenance of epithelial barrier integrity and mucosal tolerance through increased release of COX-2-mediated PGE2 by mucosal (myo)fibroblasts. Further, the fact
that some colon carcinoma epithelial cells can respond to LbGG with tumorigenic PGE2
secretion, suggests that probiotics should be used with caution in patients with known colorectal cancer.
Supplementary Material
Refer to Web version on PubMed Central for supplementary material.
Acknowledgments
Grant support: NIDDK R01DK10-150
Abbreviations
LbGG Lactobacllus rhamnosus GG
COX-2 Cyclooxygenase-2
MyD88 Myeloid differentiation factor-88
CMFs CD90+ myofibroblasts/fibroblasts
PGE2 Prostaglandin E2
AA Arachidonic Acid
cPLA2 Cytosolic phospholipase A2
TLRs Toll-like receptor
PGs Prostaglandins
GI Gastrointestinal
IBD Inflammatory Bowel Disease
5-LO 5-Lypoxygenase
α-SMA α-smooth muscle actin
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
LTB4 Leukotriene B4
MRS De Man Rogosa and Sharpe
WB Western blot
TMX Tamoxifen
i.p intraperitoneal injection
HSD Tukey’s Honestly Significant Difference
MF MyD88 KO Tamoxifen-inducible MyD88 floxed mice specific to α-SMA+ cells
References
Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res. 2004; 10(17):5870– 5879. DOI: 10.1158/1078-0432.CCR-0431303 [PubMed: 15355919]
Beswick EJ, Johnson JR, Saada JI, Humen M, House J, Dann S, Pinchuk IV. TLR4 Activation Enhances the PD-L1–Mediated Tolerogenic Capacity of Colonic CD90+Stromal Cells. The Journal of Immunology. 2014; 193(5):2218–2229. DOI: 10.4049/jimmunol.120-441 [PubMed: 25070848] Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS.
Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. The Journal of clinical investigation. 2007; 117(1):258–269. DOI: 10.1172/jci29159 [PubMed: 17200722]
Buecher B, Bouancheau D, Broquet A, Bezieau S, Denis MG, Bonnet C, Heymann MF, Jarry A, Galmiche JP, Blottière HM. Growth Inhibitory Effect of Celecoxib and Rofecoxib on Human Colorectal Carcinoma Cell Lines. Anticancer Research. 2005; 25(1A):225–233. [PubMed: 15816542]
Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, Stenson WF. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012; 61(6):829–838. DOI: 10.1136/gutjnl32011-300367 [PubMed: 22027478] Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995; 109(1):
285–301. doi.org/10.1016/0016-5085(95)90296-1. [PubMed: 7797026]
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry. 1957; 226(1):497–509. [PubMed: 13428781]
Fujii S, Suzuki K, Kawamoto A, Ishibashi F, Nakata T, Murano T, Watanabe M. PGE2 is a direct and robust mediator of anion/fluid secretion by human intestinal epithelial cells. Sci Rep. 2016; 6:36795.doi: 10.1038/srep36795 [PubMed: 27827428]
Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Abreu MT. COX-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006; 131(3):862–877. DOI: 10.1053/j.gastro.2006.06.017 [PubMed: 16952555]
Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Sylvie Robine S, Jay P. Distinct ATOH1 and Neurog-requirements define tuft cells as a new secretory cell type in the intestinal epithelium. The Journal of Cell Biology. 2011; 192(5):767–780. [PubMed: 21383077]
Grcevic D, Pejda S, Matthews BG, Repic D, Wang L, Li H, Kalajzic I. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012; 30(2):187–196. DOI: 10.1002/stem.780 [PubMed: 22083974]
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Iyer JP, Srivastava PK, Dev R, Dastidar SG, Ray A. Prostaglandin E2 synthase inhibition as a therapeutic target. Expert Opinion on Therapeutic Targets. 2009; 13(7):849–865. DOI: 10.1517/14728220903018932 [PubMed: 19530988]
Jiang GL, Nieves A, Im WB, Old DW, Dinh DT, Wheeler L. The Prevention of Colitis by E Prostanoid Receptor 4 Agonist through Enhancement of Epithelium Survival and Regeneration. The Journal of pharmacology and experimental therapeutics. 2006; 320(1):22–28. DOI: 10.1124/jpet. 106.111146 [PubMed: 17008451]
Jin P, Chen Y, Lv L, Yang J, Lu H, Li L. Lactobacillus fermentum ZYL0401 Attenuates
Lipopolysaccharide-Induced Hepatic TNF-α Expression and Liver Injury via an IL-10- and PGE2-EP4-Dependent Mechanism. PloS one. 2015; 10(5):e0126520.doi: 10.1371/journal.pone.0126520 [PubMed: 25978374]
Johnson P, Beswick EJ, Chao C, Powell DW, Hellmich MR, Pinchuk IV. Isolation of CD 90+ Fibroblast/Myofibroblasts from Human Frozen Gastrointestinal Specimens. JoVE. 2016; (107):e53691–e53691. [PubMed: 26863470]
Korhonen R, Kosonen O, Korpela R, Moilanen E. The expression of COX2 protein induced by Lactobacillus rhamnosus GG, endotoxin and lipoteichoic acid in T84 epithelial cells. Letters in Applied Microbiology. 2004; 39(1):19–24. DOI: 10.1111/j.1472-3765X.2004.01531.x [PubMed: 15189283]
Kudo I, Murakami M. Prostaglandin E synthase, a terminal enzyme for prostaglandin E2 biosynthesis. International journal of biochemistry and molecular biology. 2005; 38(6):633–638.
Lam EKY, Tai EKK, Koo MWL, Wong HPS, Wu WKK, Yu L, Cho CH. Enhancement of gastric mucosal integrity by Lactobacillus rhamnosus GG. Life Sciences. 2007; 80(23):2128–2136. DOI: 10.1016/j.lfs.2007.03.018 [PubMed: 17499310]
Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one- step reaction. Journal of lipid research. 1986; 27(1):114–120. [PubMed: 3958609]
Liu A, Claesson HE, Mahshid Y, Klein G, Klein E. Leukotriene B4 activates T cells that inhibit B-cell proliferation in EBV-infected cord blood-derived mononuclear cell cultures. Blood. 2008; 111(5): 2693–2703. DOI: 10.1182/blood-2007-08-102319 [PubMed: 18094330]
Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest. 1989; 84(5):1609–1619. DOI: 10.1172/JCI114338 [PubMed: 2553777]
Mifflin RC, Saada JI, Di Mari JF, Adegboyega PA, Valentich JD, Powell DW. Regulation of COX-2 expression in human intestinal myofibroblasts: mechanisms of IL-1-mediated induction. American journal of physiology Cell physiology. 2002; 282(4):C824–834. DOI: 10.1152/ajpcell.00388.2001 [PubMed: 11880271]
Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, Stappenbeck TS. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. The EMBO journal. 2017; 36(1):5–24. DOI: 10.15252/embj.201694660 [PubMed: 27797821]
Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, Eghtesadi S. Effects of Probiotics on Biomarkers of Oxidative Stress and Inflammatory Factors in
Petrochemical Workers: A Randomized, Double-blind, Placebo-controlled Trial. International journal of preventive medicine. 2015; 6:82.doi: 10.4103/200837802.164146 [PubMed: 26445629] Morteau O. Prostaglandins and inflammation: the cyclooxygenase controversy. Archivum
immunologiae et therapiae experimentalis. 2000; 48(6):473–480. doi.org/ 10.1007/978-94-015-9702-9_6. [PubMed: 11197601]
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)3 dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009; 15(1):42–49. DOI: 10.1038/nm.1905 [PubMed: 19098906]
Otieno DO, Shah NP. A comparison of changes in the transformation of isoflavones in soymilk using varying concentrations of exogenous and probiotic- derived endogenous beta-glucosidases. J Appl Microbiol. 2007; 103(3):601–612. DOI: 10.1111/j.136532672.2006.03245.x [PubMed: 17714393]
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Otte JM, Mahjurian-Namari R, Brand S, Werner I, Schmidt W, Schmitz F. Probiotics Regulate the Expression of COX-2 in Intestinal Epithelial Cells. Nutrition and Cancer. 2009; 61(1):103–113. DOI: 10.1080/01635580802372625 [PubMed: 19116880]
Ouwehand AC, Tiihonen K, Saarinen M, Putaala H, Rautonen N. Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: intestinal and immune
parameters. The British journal of nutrition. 2008; 101(03):367.doi: 10.1017/s0007114508003097 [PubMed: 18634707]
Pierzchalska M, Panek M, Czyrnek M, Gielicz A, Mickowska B, Grabacka M. Probiotic Lactobacillus acidophilus bacteria or synthetic TLR2 agonist boost the growth of chicken embryo intestinal organoids in cultures comprising epithelial cells and myofibroblasts. Comp Immunol Microbiol Infect Dis. 2017; 53:7–18. doi: S0147-9571(17)30043-7 [pii]10.1016/j.cimid.2017.06.002. [PubMed: 28750869]
Pinchuk IV, Beswick EJ, Saada JI, Boya G, Schmitt D, Raju GS, Powell DW. Human colonic myofibroblasts promote expansion of CD4+ CD25high Foxp3+ regulatory T cells. Gastroenterology. 2011; 140(7):2019–2030. DOI: 10.1053/j.gastro.2011.02.059 [PubMed: 21376048]
Pinchuk IV, Mifflin RC, Saada JI, Powell DW. Intestinal mesenchymal cells. Current gastroenterology reports. 2010; 12(5):310–318. DOI: 10.1007/s11894-010-0135-y [PubMed: 20690004]
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. The American journal of physiology. 1999; 277(2 Pt 1):C183–201. [PubMed: 10444394]
Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annual review of physiology. 2011; 73:213–237. DOI: 10.1146/annurev.physiol. 70.113006.100646
Reisinger KW, Schellekens DH, Bosmans JW, Boonen B, Hulsewe KW, Sastrowijoto P, Poeze M. Cyclooxygenase-2 Is Essential for Colorectal Anastomotic Healing. Annals of surgery. 2017; 265(3):547–554. DOI: 10.1097/SLA.0000000000001744 [PubMed: 27070935]
Resta-Lenert S, Barrett KE. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: role of iNOS and COX-2. Gastroenterology. 2002; 122(4):1070–1087. doi.org/10.1053/gast.2002.32372. [PubMed: 11910358]
Riehl TE, Foster L, Stenson WF. Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. Am J Physiol Gastrointest Liver Physiol. 2012; 302(3):G309– 316. DOI: 10.1152/ajpgi.00248.2011ajpgi.00248.2011 [PubMed: 22038822]
Roulis M, Nikolaou C, Kotsaki E, Kaffe E, Karagianni N, Koliaraki V, Martini E. Intestinal
myofibroblast-specific Tpl2-COX-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proceedings of the National Academy of Sciences. 2014; 111(43):E4658–E4667. DOI: 10.1073/ pnas.1415762111
Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC, Powell DW. Subepithelial Myofibroblasts are Novel Nonprofessional APCs in the Human Colonic Mucosa. The Journal of Immunology. 2006; 177(9):5968–5979. DOI: 10.4049/jimmunol.177.9.5968 [PubMed: 17056521] Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I, Gasbarrini A. Gut
Microbial Flora, Prebiotics, and Probiotics in IBD: Their Current Usage and Utility. BioMed Research International. 2013; 2013:139.doi: 10.1155/2013/435268
Segers ME, Lebeer S. Towards a better understanding of Lactobacillus rhamnosus GG - host interactions. Microbial Cell Factories. 2014; 13(Suppl 1):S7.doi: 10.1186/1475-2859-13-s1-s7 [PubMed: 25186587]
Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The Proinflammatory Effect of Prostaglandin E2 in Experimental Inflammatory Bowel Disease Is Mediated through the IL-2-3>IL-17 Axis. The Journal of Immunology. 2007; 178(12):8138–8147. DOI: 10.4049/ jimmunol.178.12.8138 [PubMed: 17548652]
Simeoli R, Mattace Raso G, Lama A, Pirozzi C, Santoro A, Di Guida F, Meli R. Preventive and therapeutic effects of Lactobacillus paracasei B21060-based synbiotic treatment on gut inflammation and barrier integrity in colitic mice. The Journal of nutrition. 2015; 145(6):1202– 1210. DOI: 10.3945/jn.114.205989. [PubMed: 25926411]
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Smith W. Prostanoid biosynthesis and mechanisms of action. American journal of physiology Renal physiology. 1992
Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and− 2. Journal of Biological Chemistry. 1996; 271(52):33157–33160. DOI: 10.1074/jbc. 271.52.33157 [PubMed: 8969167]
Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev. 2011; 111(10):5821–5865. DOI: 10.1021/cr2002992 [PubMed: 21942677]
Stenson WF. The universe of arachidonic acid metabolites in inflammatory bowel disease. Current Opinion in Gastroenterology. 2014; 30(4):347–351. DOI: 10.1097/mog.0000000000000075 [PubMed: 24837228]
Valera I, Vigo AG, Alonso S, Barbolla L, Crespo MS, Fernandez N. Peptidoglycan and mannose-based molecular patterns trigger the arachidonic acid cascade in human polymorphonuclear leukocytes. Journal of Leukocyte Biology. 2007; 81(4):925–933. DOI: 10.1189/jlb.0706451 [PubMed: 17264305]
Wang D, Mann JR, Dubois RN. The Role of Prostaglandins and Other Eicosanoids in the Gastrointestinal Tract. Gastroenterology. 2005; 128(5):1445–1461. DOI: 10.1053/j.gastro. 2004.09.080 [PubMed: 15887126]
Worthley Daniel L, Churchill M, Compton Jocelyn T, Tailor Y, Rao M, Si Y, Wang Timothy C. Gremlin 1 Identifies a Skeletal Stem Cell with Bone, Cartilage, and Reticular Stromal Potential. Cell. 2015; 160(132):269–284. DOI: 10.1016/j.cell.2014.11.042 [PubMed: 25594183]
Zhu Y, Hua P, Lance P. Cyclooxygenase-2 expression and prostanoid biogenesis reflect clinical phenotype in human colorectal fibroblast strains. Cancer Res. 2003; 63(2):522–526. [PubMed: 12543811]
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Figure 1. Lactobacillus rhamnosus
GG (LbGG) induces an increase in cPLA2, release of AA, an increase in COX-2 expression, and increased PGE2 production in Caco-2 cells. Caco-2 cells were exposed to LbGG (10
bacteria: 1 epithelial cell) for 24 h and harvested for Western blot and ELISA analysis. (A) Western blot using anti-cPLA2 revealed a significant increase in cPLA2 protein expression in Caco-2 cells following exposure to LbGG. Protein density was normalized to the housekeeping protein β-actin (relative density) and adjusted density was calculated for each sample by dividing the relative density of the experimental condition by those obtained for a control sample. (B) Arachidonic acid (AA) levels were determined by gas chromatography in Caco-2 cell. Exposure to LbGG significantly enhanced the release of AA. Western blot analysis demonstrated an increase in (C) COX-2 and (D) 5-LO protein expression following LbGG stimulation. (E) PGE2 concentrations (ELISA analysis) were measured in the
conditioned media of Caco-2 cells after exposure to different strains of lactobacilli. The results are shown as means ± SEM. Student’s t test was used to calculate the significance of the obtained results when comparing two groups (panel A, B, C, and D). One-way ANOVA test was used to analyze significance of the obtained results when comparing more than two groups (panel E). n=3, *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Figure 2.
Only Caco-2 among the five tested colonic epithelial cell lines (LS174T, SW480, HCT116, Caco-2, and HT-29) responded to LbGG with an increase in COX-2 expression and PGE2
production. The epithelial cell lines were co-cultured with LbGG (10 bacteria: 1 epithelial cell) for 24 h, and harvested for western blot analysis and ELISA. (A) Representative western blot and summary of relative density data are shown and demonstrate that LbGG induces COX-2 protein expression in Caco-2 cells but not other cell lines. (B) Caco-2, but not other tested cell lines respond to LbGG stimulation with a significant increase in PGE2
production. The results are shown as means ± SEM. The summary results shown on panel (A) and (B) are expressed as means ± SEM. One-way ANOVA was used to calculate the significance of the obtained results, three experimental repeat per tested cell line were run in duplicate (n=6), ****p<0.0001.
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Figure 3.
LbGG activated the COX-2-dependent PGE2 pathway in primary human CMFs. Primary
CMFs isolated from normal colonic mucosa were exposed to LbGG for 24h at a ratio of 10 bacteria per one CMF. Western blotting (WB) using antibodies specific to (A) cPLA2, (B) COX-2, and (C) 5-Lipoxygenase proteins demonstrated that the total cPLA2 and COX-2 levels are increased in LbGG-treated CMFs cultures compared to the untreated controls. The representative WB and summary of the adjusted density are shown. The summary of adjusted density results for each studied molecule are shown as means ± SEM. Student’s t test was used to calculate the significance of the obtained results n=3, *p<0.05; **p<0.01; ****p<0.0001. (D) PGE2 concentrations determined by ELISA were increased in the condition media of CMFs after exposure to LbGG. Student’s t test (panel A, B, C) and Wilcoxon signed ranked test (panel D) were used to calculate the significance of the obtained results. Results from three experiments running in duplicate are shown as means ± SEM, n=6, *p<0.05.
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Figure 4.
Silencing of myD88 gene expression in normal primary human isolates of CMFs inhibits the increase in LbGG-dependent COX-2 production. CMFs were transfected with MyD88 siRNA or control siRNA 10 days prior to exposure to LbGG, COX-2 expression was analyzed 24 h post LbGG exposure using western blot analysis. A representative WB and summary of WB adjusted density analysis for COX-2 expression are shown. One-way ANOVA was used to calculate the significance of the obtained results. Results are shown as means ± SEM, n=3, **p < .01 and ****p< 0.0001.
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Figure 5.
LbGG activates the arachidonic acid (AA) metabolic pathway in vivo. 5×108 CFU/dose of
the LbGG was delivered daily by oral gavage for 5 days to C57BL/6 mice. (A) AA levels in the harvested, homogenized mucosa were determined by ELISA. Exposure to LbGG significantly enhanced the release of AA in the colonic mucosa of LbGG-treated mice. The results are shown as means ± SEM, n=5, ***p< 0.001. (B) Western blot analysis
demonstrated an increase in COX-2 expression in the colonic mucosa of LbGG-treated mice. The results are shown as means ± SEM, n=6, *p<0.05. (C) Representative tissue sections of the colonic mucosa from control and LbGG treated mice were immunostained and analyzed by confocal microscopy (see Methods). Expression of COX-2 is increased within the α-SMA+ CMFs in the colonic mucosa of mice treated with LbGG. DAPI was used to stain cell nuclei (blue); activated CMFs were detected by anti–α-SMA mAb (green; clone A4);anti-COX-2 mAb (red; clone 33) was also used. A yellow-orange color on merged images indicates co-localization of α-SMA and COX-2 (indicated by arrows). Scale bar represents 20 Tm. Representative cross-sections are shown, n= 5 animals per group. (D) The summary of changes in the Corrected Total Cell Fluorescence (CTCF) fluorescence intensity from in situ COX-2 protein expression in the LbGG-treated and control murine colonic mucosa. Student’s t test was used to calculate the significance of the obtained results. The means ± SEM are shown, n=5 per group **p< 0.01.
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
A
uthor Man
uscr
ipt
Figure 6.
MyD88 signaling in α-SMA+ CMFs is required for COX-2 production in response to LbGG. 5×108 CFU/dose of the LbGG was delivered daily by oral gavage for 5 days to MF-MyD88 KO or control mice. Control mice negative for Acta2-Cre but positive for MyD88fl/fl recombination were derived from at least five backcrossing of MF-MyD88 KO with C57BL/6 animals. (A) Immunostaining of colonic mucosal tissue cross-sections was performed, followed by confocal microscopy. DAPI was used to stain cell nuclei (blue); activated CMFs were detected by anti–α-SMA mAb (green; clone A4) and the sections were stained for COX-2 with mAb (red; clone 33). Scale bar represents 20 Tm. (B) Measurement of CTCF fluorescence intensity from confocal microscopy images of COX-2 protein, and (C) western blotting using antibodies specific to COX-2, demonstrated that the total COX-2 levels increased in colonic tissue of LbGG-treated mice compared with the untreated controls. Deletion of MyD88 within CMFs reduced this response. One-way ANOVA was used to calculate the significance of the obtained results. Results are shown as means ± SEM. Four-five animals per group were used in the above experiments, *p < .05.