An Agonist-Antagonist Myoneural Interface for Proprioception from a
Neurally-Controlled Prosthesis
byTyler R. Clites
B.S. Engineering Sciences Harvard University, 2014SUBMITTED TO THE DEPARTMENT OF HEALTH SCIENCES AND TECHNOLOGY IN PARTIAL FULFULLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
DOCTOR OF PHILOSOPHY IN MEDICAL ENGINEERING AND MEDICAL PHYSICS AT THE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2018
@ Tyler R. Clites. All rights reserved.
The author hereby grants to MIT permission to reproduce and distribute paper and electronic copies of this thesis document in whole or in part.
Signature redacted
AuthorTyler R. Clites Department of Health Sciences and Technology May 15, 2018
Signature redacted
Certified byHugh M. Herr, PhD Director, MIT Center for Extreme Bionics and Biomechatronics Group Professor of Media Arts and Sciences
Signature redacted
Accepted by
Emery N. Brown, MD, PhD irector, Harvard-MIT Program in Health Sciences and Technology ProfessL Computational Neuroscience and Health Sciences and Technology
MASSACHUSETTS INSTITUTE
OF TECHNOLOGY 1
JUN 11 2018
LIBRARIES
An Agonist-Antagonist Myoneural Interface for Proprioception from a
Neurally-Controlled Prosthesis
by
Tyler R. Clites
Abstract
Humans have the ability to precisely sense the position, speed, and torque of their body parts. This sense is known as proprioception, and is essential to human motor control. In the many attempts to create human-mechatronic interactions, there is still no robust, repeatable methodology to reflect proprioceptive information from a synthetic device onto the nervous system. As a solution to this shortcoming, I present the agonist-antagonist myoneural interface (AMI). The AMI is comprised of 1) a surgical construct made up of two muscle-tendons - an agonist and an antagonist - surgically connected in series so that contraction of one muscle stretches the other, and 2) a bi-directional efferent-afferent neural control architecture. The AMI preserves dynamic muscle relationships that exist within native anatomy, thereby allowing proprioceptive signals from biological sensors within both muscles to be communicated to the central nervous system. Each AMI is designed to send control signals to one joint of a prosthesis, and to provide proprioceptive feedback pertaining to the movement of that joint. The doctoral work presented in this thesis constitutes the pre-clinical and early clinical validation of the AMI. The AMI concept is first described and validated in small (murine) and large
(caprine) pre-clinical models. A detailed surgical methodology for implementation of the AMI during primary below-knee amputation is then described and evaluated in three human patients. Characterization of independent neural control of prosthetic joint position and impedance is presented for one AMI patient, as compared to a group of four persons with traditional amputation. Data are shown evidencing improved volitional control over the prosthesis in the AMI patient, as well as an emergence of natural reflexive behaviors during stair ambulation that do not exist in the traditional amputation cohort. These results provide a framework for
reconsidering the integration of bionic systems with human physiology.
Thesis Supervisor: Professor Hugh M. Herr, PhD
Director, MIT Center for Extreme Bionics and Biomechatronics Group Professor of Media Arts and Sciences
The following committee has approved this thesis:
Signature redacted
Committee Chair_____________Robert Langer, ScD David H. Koch Institute Professor
Signature redacted
Thesis ReaderTs Edward Boyden, PhD Associate Professor of Biological Engineering and Brain and Cognitive Sciences
Thesis Reader
Signature redacted
Elazer Edelman, MD, PhD Thomas D. and Virginia W. Cabot Professor of Health Sciences and Technology Professor of Medicine at Harvard Medical School Senior Attending Physician, Coronary Care Unit at the Brigham and Women's Hospital
Acknowledgments
It is a privilege for me to express here my gratitude for all those who have played such a major role in my tenure as a doctoral student.
First and foremost, I must thank Professor Hugh Herr for providing me with the support and guidance necessary to thrive in graduate school. Hugh, your vision for the future has been the driving force behind so much good. It has been an honor to work with you and learn from you over these past several years. Thank you for taking the time out of your busy life to spend with a young graduate student, and for furnishing both the professional and personal mentorship that have been so important to me through my time at MIT.
Next, I must thank Dr. Matthew Carty. In addition to your academic leadership, you have been a close mentor and friend through both wonderful and difficult times. Thank you for being such a presence in my life; it has meant more than you know. I'll never forget the boneless rats and magic goats.
Dr. Anthony Zorzos, I have rarely laughed so hard as we did. You were an anchor through the rough patches, when nothing quite worked. Thanks for telling me that I was exceptional - it was in part because of you that I believed it.
Jim Ewing, no one has given as much of themselves to this project as you have. I have loved working so closely with you, and learning from you. Thank you for everything.
I thank my thesis committee for helping me to tell this story. Professors Langer, Boyden, and Edelman, your input was instrumental in crafting the narrative that makes up the body of this dissertation. Thank you.
Biomech, there are too many of you to thank, so I'll call out just a few. Sengeh, thank you for opening my door to this world. Luke and Elliott, I wouldn't be where I am without your early guidance. Roman, thanks for always telling me you are super busy before we talk about our feelings for an hour. Shriya, what can I say? You're a rock star. Lindsey, you are the engine that makes all of this work. To the rest of Biomech, keep changing the world. You are all awesome!
I'd also like to thank all those with whom I have collaborated closely. Allison, thanks for teaching
me the value of keeping good notes, and for the career and life advice you shared with me. Jenny, Morgan, and the rest of the DCM staff, your hard work and patience with me have made this work possible. To the surgeons Rickard, Paul, Richard, Simon, Jason, and Jonathon, thank you for your insight, and for reminding me that translation and patient care are paramount in all that we do. Laurie and the rest of the HST community, thank you for building a home for me. To the undergraduate ranks, thank you for putting up with my imperfections, and for the
phenomenal work that each of you produced. I'd also like to thank the patients who were willing to give of their time and resources to be a part of this work; we could not do this without you.
I'll conclude where this all began, by recognizing the enormous contributions of my family. Mom
and Dad, thank you for your unwavering support. You have always believed that I could change whatever world I wanted to change. Jess, you and I go way back. I am consistently in awe of your creative brilliance. Thank you for always being proud of me!
Finally, I dedicate this thesis to my beautiful wife, Kendall. You are my everything. Thank you for believing in me, even in those moments when I didn't believe in myself. Forever and always.
Contents
Chapter 1: Introduction ... 10
Overview and Specific Aims ... 11
Background and Significance ... 12
Am putation Standard of Care... 12
State of the Art in Peripheral Nerve Interfacing ... 12
Prosthetic Control Paradigms... 13
Proprioception...13
Agonist-antagonist Myoneural Interface (AM I) ... 14
Supplemental Technologies... 15
Osseointeg ration ... 15
Sonom icrometery ... 16
Dissertation Sum m ary ... 16
Chapter 2: Demonstration of the Agonist-antagonist Myoneural Interface in a Murine M odel ... 18
Rationale and Study Design ... 19
Materials and Methods ... 20
Anim als and Measurement System Preparation... 20
Validation of Acquisition System ... 20
Experim ental Paradigms... 22
Native Architecture ... 22
AM I Architecture... 22
Data Storage and Processing ... 23
R e s u lts ... 2 4 Validation of Acquisition System ... 24
Closed-loop M uscle State Control... 25
Afferent Stretch Response ... 26
Discussion...27
Chapter 3: Scaling of the AMI to a Caprine Model... 30
Rationale ... 31
Materials and Methods ... 31
Anim als and Study Design ... 31
Preparation of Im planted Electronics... 33
S u rg e ry ... 3 4 Data Collection and Processing ... 38
Term inal Surgery and Gross Tissue Exam ination ... 39
R e s u lts ... 3 9 Below-Knee Surgical Model ... 39
Above-Knee Surgical Model... 40
Discussion...42
Chapter 4: Surgical Implementation of the AMI in a Primary Transtibial Operation ...44
Rationale ... 45
Materials and Methods ... 45
Patient Selection and Study Design ... 45
Surgical Technique ... 46 Postoperative Recovery ... 49 Data Collection ... 51 EMG ... 51 Ultrasound...51 R e s u lts ... 5 1 Clinical O utcomes ... 51 Ultrasound ... 53 E M G ... 5 4 Discussion ... 55
Chapter 5: Proprioception from an Advanced Prosthetic Limb...57
Rationale ... 58
Materials and Methods ... 58
Study Design ... 58
Subject Selection, Surgery, and Rehabilitation... 58
Surface Electrode Placement and EMG Processing ... 59
Efferent Control Architecture ... 59
Fine-wire Electrode Placement and Stim ulation ... 60
Closed-loop Torque Control... 62
Ultrasound Image Processing ... 63
Prosthetic Hardware ... 63
Data Collection, Processing, and Statistical Analysis ... 63
R e s u lts ... 6 4 AM I Surgical Construction ... 64
Control Architecture: Prosthesis-not-in-the-loop... 65
Independent Control of Joint Position and Im pedance... 68
Control Architecture: Prosthesis-in-the-loop ... 71
Characterization of Perception ... 72
Closed-loop Torque Control... 72
Descriptions of the Control Experience ... 74
Discussion ... 74
Chapter 6: Reinventing Am putation ... 78
Historical Context ... 79
Case for Change ... 80
Review of New Surgical Strategies... 81
Proposed Approach to Reinventing Limb Amputation ... 83
Implications and Conclusions ... 84
Bibliography...86
Appendix A: Surgical Protocols... 95
Rat Surgical Protocol... 95
Below-knee Goat Protocol...95
Above-knee Goat Protocol (with Osseointegrated Im plant)... 96
Human Below-knee Surgical Protocol... 97
Appendix B: W iring for Caprine Osseointegrated System ... 99
Prepare the Mold ... 99
Prepare W ires for W rapping ... 99
Sonom icrometry Crystals ... 99
Epimysial Electrodes... 99
Mold the W ire Bundle into a Medusa Head... 99
W rap Distal Portion of Lead Bundle ... 101
Prepare W ire Ends for Solder ... 103
Appendix C: Prosthesis Controller Code ... 104
user-m n-MIT_2DoFAnkle_v .h ... 104
user-m n-MIT_2DoF_Ankle vi.c ... 107
Chapter 1: Introduction
Overview and Specific Aims
Despite recent advances in the development of prosthetic devices, current limb prostheses fall short of replicating the control experience afforded by biological limbs. Several obstacles have prevented the development of a fully extrinsic neural controller that allows the user's nervous system to retain direct command of a robotic lower extremity. Non-invasive neural sensors are not capable of providing a control system that is sufficiently stable to enable sustained direct control (1, 2). Another issue derives from an inability to provide meaningful afferent feedback from the prosthesis to the user. Without such feedback, direct neural control is unlikely to be a realistic possibility (3). Unfortunately, translation of externally-produced sensory data from prostheses into biologically compatible feedback that is perceived by the amputee as normal proprioception has, to date, been unsuccessful. Prior efforts to reproduce proprioception have proven difficult due primarily to their uniform neglect to incorporate the end terminal biological mechanotransducers innate to the biological human limb. With this limitation, external or implantable nerve stimulators with a limited range of capabilities have, to date, been unable to accomplish high-resolution artificial proprioceptive sensibility.
The objective of the work presented in this dissertation is the development of a novel amputation paradigm and implant architecture that leverages existing end organs and their natural neural pathways to create an efferent-afferent prosthetic control system with bi-directional neural communication of prosthetic joint position, speed, and torque. The
fundamental hypothesis explored herein is that such a system is capable of improving function during tasks relevant to daily living, as well as embodiment, or the degree to which patients feel connected to their prosthetic device. To test this hypothesis, the current thesis addresses the following specific aims:
Aim 1. To demonstrate the Agonist-antagonist Myoneural Interface (AMI) in a murine model: In the standard clinical amputation paradigm, residual musculature is fixed isometrically, which precludes realistic fascicle strains during muscle contraction. Furthermore, an antagonist muscle is not stretched when the innervated agonist contracts, eliminating realistic spindle fiber feedback from the antagonist. I this aim, I explore a surgical framework capable of providing proprioceptive sensory feedback from an external prosthesis, and validate the neural competence of this framework in murine models. I also explore the capacity of functional electrical stimulation (FES) to modulate afferent sensation within the AMI construct. Experiments addressing this aim are described in Chapter 2.
Aim 2. To explore scaling of the AMI architecture to a larger animal (caprine) model: Having demonstrated in Aim 1 the potential of the AMI to provide graded afferent feedback along native neural pathways, I seek to develop this architecture in a larger animal model, the scale of which is surgically relevant to human subjects. To this end, I describe two single-animal studies in African pygmy goats. In each single-animal I vary the specifics of the surgical geometry, allowing for a simulation of both a transtibial and a transfemoral amputation. A system of chronically-implanted passive electronic hardware provide the basis for longitudinal monitoring of the efficacy of each strategy. Experiments addressing this aim are described in Chapter 3.
Aim 3. To assess the ability of an integrated AMI-driven efferent-afferent control system to improve function in a human subiect having a lower extremity amputation:
Building on the data collected in Aim 2, I describe implantation of the AMI system in three human subjects needing an elective unilateral transtibial amputation. For one patient, I implement a biophysical control model for efferent control of a powered ankle-foot prosthesis with two actuated degrees of freedom (ankle plantarflexion and dorsiflexion, as well as subtalar inversion and eversion), and integrate this controller with an FES-based force feedback paradigm. This integrated system provides a framework for evaluation of the impact
of the AMI on functional performance in tasks relevant to daily activity. Experiments addressing this aim are described in Chapters 4 and 5.
The doctoral work described in this dissertation presents a clear trajectory to clinical
implementation of a fully integrated neuromechanical prosthetic system with bi-directional neural communication. This thesis provides insight into novel surgical techniques and amputation paradigms, highlights the importance of biomimetic efferent control schema and reliable afferent feedback to bionic limb function, and elucidates the central nervous system's ability to adopt a bionic limb in the presence of physiologically relevant neural communication.
Background and Significance
Amputation Standard of Care
The clinical standard-of-care for limb amputation surgery has not changed in almost two centuries, and is not currently optimized to facilitate neural integration with bionic limbs. In a typical amputation surgery, muscle tissues in the residual limb are configured isometrically to create padding for a prosthetic socket (4), severing the dynamic relationship between agonist-antagonist muscle pairs and limiting the ability of the muscle spindles and Golgi tendon organs within these tissues to communicate meaningful information to the central nervous system. Muscles, tendons, skin, bones, and other tissues distal to the amputation site are typically discarded, despite their potential capacity to contribute to reconstruction of the amputated residuum. Nerves that cross the amputation boundary are cut under tension, then buried into fat tissue or deep in the residuum in an effort to prevent neuroma formation (5); while sometimes effective in preventing neuropathic pain, this technique creates a hurdle for neural interfaces due to the limited longitudinal viability of direct contact between synthetic interfaces and peripheral nerve tissues, especially for neural recording (1, 6). These procedures date back to the Civil War era, and are designed to prepare the residuum for fitting of a prosthetic socket (4). State- of-the-art approaches to peripheral nerve interfacing have been developed within the bounds of this dated amputation paradigm, working to extract efferent signals from and reproduce afferent signals in transected peripheral nerves that are buried deep within the residual tissues (1, 2).
State of the Art in Peripheral Nerve Interfacing
Many attempts have been made to overcome the limitations of state-of-the-art amputation surgical techniques and commercial prosthetic systems. Direct stimulation of upstream peripheral nerves through implantable electrodes has shown great promise in restoring cutaneous touch perception, and, in some cases, isolated kinesthetic sensations (7-13). However, due in part to a mismatch between the complexity of proprioceptive afferent signaling and the relatively low resolution and precision of even the best implantable stimulation
methodologies, none of these approaches are engineered to provide, with high-probability, stable and natural proprioceptive percepts. Vibration-induced illusory kinesthesia (e.g. 12) has been explored as a means of providing joint state information through activation of cutaneous stretch receptors; unfortunately, translation of this approach has been a major hurdle. Targeted muscle reinnervation (TMR), which leverages the regenerative properties of nerve to
reinnervate muscles within the residual limb, expands the controllability of myoelectric prostheses and limits pathological neurology (15-17). Although this approach addresses the poor nerve treatment that is typical of amputation, it requires cannibalization of healthy muscle to provide reinnervation targets. Regenerative peripheral nerve interfaces (RPNls) provide an alternative strategy in which free muscle grafts are coapted to the distal ends of transected nerves (18-20). Regenerative peripheral nerve interfaces have emerged as a means of stifling neuroma formation, preventing phantom pain, increasing the number of independent neural control targets, and conveying cutaneous sensory information (20, 21). However, neither TMR
nor RPNIs restore dynamic agonist-antagonist muscle relationships, limiting the ability of patients to receive proprioceptive feedback from a prosthetic limb.
Prosthetic Control Paradigms
The majority of today's robotic lower-extremity prostheses do not communicate with the nervous system. Instead, they these devices on an array of intrinsic sensors to infer user intent and inform motor output (22-25). Neural control is sought as a means of increasing versatility of prosthetic devices. Surface electromyography (sEMG) has been used in upper-extremity prosthesis control for decades (26-29) and serves as the primary extrinsic control input to the majority of robotic upper-extremity prostheses. More recently, the desire to make lower-extremity prostheses that can adapt to terrain in a biomimetic way has spurred interest in using neural signals as a control input. Unfortunately, the neural signals associated with ambulation for persons with traditional amputation are complicated by inconsistency (30) and unintended co-contraction (31). As a means of mitigating these complications, several researchers have developed pattern-recognition based approaches of classifying user intent (32-34). However, these approaches are unable to fully replicate biological control because the number of possible output states is limited by the number of classes in the learning model. Other methodologies attempt direct proportional sEMG control. In an acute study, Huang et al. demonstrated a single subject's ability to walk with a symmetric gait at a single speed over level ground using single-muscle sEMG as a proportional torque control signal (35). This experiment was carried out in a controlled laboratory environment, and did not evaluate volitional free-space control or
adaptation to variations in ground surface.
Proprioception
Proprioception is the sense of the relative spatial positioning of one's body parts, and of the amount of force exerted on the environment (1). It is essential to motor control, functional joint stability, and gait adaptation (36, 37). Although neuroscience research has documented the contributions of various biological mechanoreceptors to proprioceptive sensation, including joint receptors (e.g. (38-40)) and cutaneous sensory receptors (e.g. (41-44)), evidence points to muscle spindle afferents as predominant mediators of joint kinesthesia (45-47). It is well understood that muscle spindle afferents discharge proportionally to changes in muscle length and velocity (e.g. (48-51)). Primary spindle afferent response is best characterized by ramp-and-hold stretches, with discharge rates proportional to stretch velocity and magnitude. Secondary spindle afferent response is largely proportional to stretch magnitude, with a lower dynamic sensitivity (e.g. (52-55)). Although muscle afferents represent only a portion of the larger proprioceptive system, studies in vibratory-induced illusory kinesthesia have indicated that preservation of functional muscle stretch relationships is sufficient to promote sensations of joint kinesthesia (e.g. (14, 56, 57)). This assertion is further bolstered by the preservation of
kinesthesia in patients having orthopedic joint replacement surgery (58), during which the physiological joint capsule in its entirety was removed. The same phenomenon has been observed in patients having lost neural connectivity to cutaneous and joint capsule receptors secondary to severe dorsal column injury, who report intact position and movement sensibility (59, 60). For a complete discussion of the roles of the various afferent receptors in
proprioception, the reader is referred to (45).
The fundamental motor unit to control a biological joint is an agonist-antagonist muscle-tendon pair. Such a muscle-tendon relationship allows for simultaneous control of joint state (position and speed) and impedance (stiffness and damping) for upper and lower extremity motor tasks. At least one pair of antagonistic muscles is needed for each degree of freedom of a limb in order to control joint state, torque, and impedance (61). Evidence suggests that proprioceptive feedback of joint positioning is largely dependent on the dynamic relationship between muscle
spindle afferents in agonist and antagonist muscle groups acting simultaneously on the same degree of freedom (57, 62). When a muscle on one side of a biological joint contracts (e.g. the "agonist") and moves the joint, this motion elongates the muscle ("antagonist") that is attached to the opposite side of the joint and causes the antagonist spindle afferent receptors to discharge. Similarly, if contraction of the antagonist causes the joint to rotate in the opposite direction, then the agonist will be elongated, causing the agonist spindle receptors to discharge (57). The complexity of this afferent feedback system poses a challenging hurdle for the
development of bionic limbs that benefit from bi-directional neural communication. In the current clinical amputation surgery, muscle tissues in the residual limb are configured isometrically to create padding for a prosthetic socket (4), severing the dynamic relationship between agonist-antagonist muscle pairs and limiting the ability of the muscle spindles and Golgi tendon organs within these tissues to communicate meaningful information to the central nervous system. The architecture explored in this study is designed to preserve the complex patterns of muscle receptor discharge responsible for kinesthetic sensation, by replicating basic dynamic muscle relationships.
Agonist-antagonist Myoneural Interface (AMI)
Presented as an alternative to the current state-of-the-art peripheral nerve interface
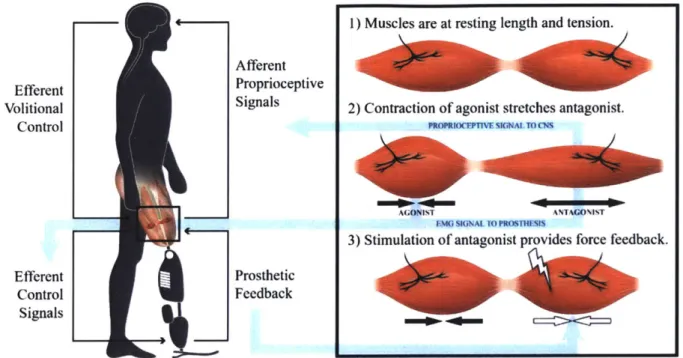
approaches, this dissertation describes an agonist-antagonist myoneural interface (AMI). An AMI is made up of an agonist and an antagonist muscle-tendon connected mechanically in series, so that when the agonist contracts, the antagonist is stretched, and vice versa (63, 64). The purpose of an AMI is to control and interpret proprioceptive feedback from a bionic joint. The AMI approximates physiological proprioceptive mechanisms by incorporating residual muscles from discrete agonist-antagonist muscle pairs (e.g., gastrocnemius and tibialis anterior) in series. Contraction of the agonist muscle via the standard motor efferent nerve activates the native contractile mechanoreceptors in the agonist muscle, as well as the native intrafusal muscle spindle stretch fibers of the mechanically-coupled antagonist muscle, both of which provide afferent proprioceptive signaling through the sensory components of their respective innervation nerves. Subsequent activation of the antagonist stimulates a complimentary stretch on the agonist; as such, the AMI has the capacity to reproduce a physiologically relevant antagonist mechanical coupling, providing non-isometric fascicle strains and agonist-antagonist fascicle state spindle feedbacks.
1) Muscles are at resting length and tension. Afferent
Efferent Proprioceptive
Volitional Signals 2) Contraction of agonist stretches antagonist.
Control PROPUWC'WMUMALTCM
A( ONIST ANTAGO%I%T
EMG SIGNAL TO PROSTH
3) Stimulation of antagonist provides force feedback.
Efferent Prosthetic
Control Feedback
Signals
Figure 1: Illustration of the AMI concept. Volitional contraction of the agonist causes stretch in the antagonist. Force feedback can be provided by stimulating the antagonist, producing a force that inhibits excursion of the agonist. Original art contributed by Stephanie Ku, figure reproduced from (65).
Supplemental Technologies
Implementation and validation of the AMI architecture is aided by several complementary
technologies that are still under development. The two most significant examples are
osseointegration and sonomicrometery.
Osseointegration
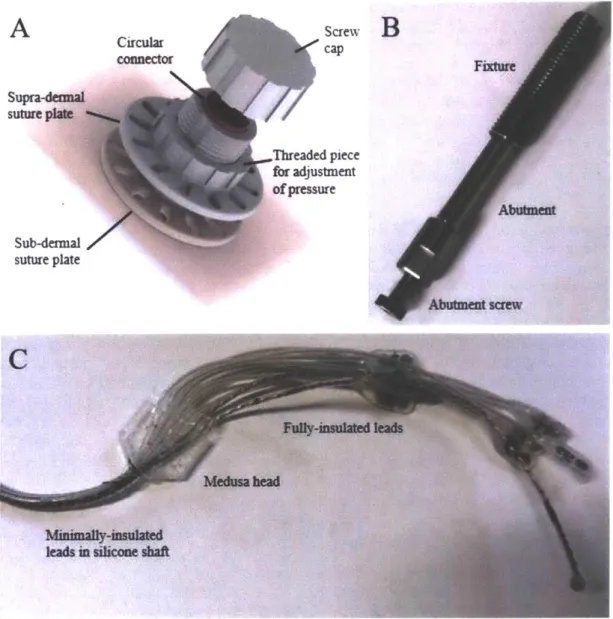
To address the shortcomings of conventional prosthetic sockets, Dr. Rickard Branemark
introduced the technology of direct skeletal attachment of limb prostheses. His product, called
Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA), is comprised of a
percutaneous implant that extends distally from a fixture placed directly into the trabecular bone
of the residual limb of a person with amputation (66-68). Recent work by Ortiz-Catalan et al. (8)
has demonstrated the long-term viability, in one subject, of an osseointegrated conduit for
bi-directional neural communication. The OPRA system described in Ortiz-Catalan's work
represents a crucial design modification from previous iterations of the OPRA; a central canal
for wiring is included in the abutment with the aim of establishing a stable means of
percutaneous electrical communication between the peripheral nervous system and the control
hardware of an external powered prosthesis. This "osseo-neural conduit" allows for direct wiring
of passive implantable electronic devices. In (8), epimysial EMG electrodes were inserted into
the dominant elbow flexors and extensors of a unilateral transhumeral amputee, and used to
control a motorized upper-limb prosthesis. In addition, a neural cuff electrode placed on the
ulnar nerve approximately 10 centimeters from the distal neuroma was stimulated to providegraded tactile feedback.
- -- - - - -~ - *1.
Muscl
T Bone
Abutment...
Figure 2: OPRA with osseo-neural conduit. Figure reproduced from (8).
Sonomicrometery
Robust measurement of muscle fascicle state is essential to a closed-loop control architecture for skeletal muscle. Muscle force production depends on fascicle length and velocity, and accurate modelling of muscle function is impossible without real-time measurements of these parameters. Absolute distance can be measured in vivo using piezoelectric "sonomicrometer"
crystals (Sonometrics, Inc.) implanted along muscle fascicles. An "emitter" crystal is stimulated,
sending an acoustic pulse through the muscle. After traveling through the muscle, this pulsecauses vibration in a "receiver" crystal, which generates a voltage in response to motion.
Acoustic signal propagation time through the muscle, the acoustic properties of which are well documented, gives an accurate dynamic representation of fascicle state. Sonomicrometry has been used successfully to measure skeletal muscle length changes in situ and during walking in cats (69), running in turkeys (70), and during ambulation across various speeds in goats (71).Dissertation Summary
The objective of the work presented in this dissertation is to describe the pre-clinical and clinical step that have been taken to validate implementation and function of the AMI architecture. The purpose of an AMI is to provide bi-directional neural communication of joint position, speed, and force between and advanced robotic prosthesis and the central nervous system.
Chapter 2 presents a series of experiments designed to validate the AMI concept in a murine model (Aim 1). Electroneurographic evidence that the AMI is capable of reproducing afferent neural signaling consistent with native proprioceptive sensation is provided. Data are presented that demonstrate the ability to modulate this afferent signal proportional to joint angle.
Chapter 3 contains a description of two case studies that explore implementation of the AMI at near-human scale in a caprine model (Aim 2). Data from these case studies indicate that the functional dynamic agonist-antagonist relationships can be preserved in AMIs constructed at the
time of primary amputation, at both the transtibial and transfemoral amputation levels. Chapter 4 discusses the first three cases in which AMIs were created in human subjects. Surgical technique is described in detail, and clinical outcomes are reported. Data are shown
demonstrating the potential of the AMI to preserve dynamic agonist-antagonist muscle relationships, and to improve volitional activation of residual musculature.
Chapter 5 describes a study in which the first AMI patient was connected to a myoelectric prosthesis. Data are presented showing that the AMI patient exhibits improved control over prosthetic joint position and impedance during volitional free-space control than persons with traditional amputation using the same prosthesis. A reemergence of reflexive behaviors during stair ambulation is demonstrated, as well as an increased capability to directly modulate prosthetic joint torque. Patient testimonials are included, which indicate that the AMI increases prosthetic embodiment.
Chapter 6 lays a framework for understanding the implications of this doctoral work in redefining amputation surgery as an alternate form of limb salvage. The chapter contains a review of the history of amputation surgery, a discussion of current advancements in amputation surgery, and a paradigm for changing the way primary amputations are performed.
The appendices contain additional detailed information about the amputation surgery and relevant experimental equipment.
Chapter 2: Demonstration of the
Agonist-antagonist Myoneural Interface in a Murine
Model
Proprioceptive mechanisms play a critical role in both reflexive and volitional lower extremity control. Significant strides have been made in the development of bionic limbs that are capable of bi-directional communication with the peripheral nervous system, but none of these systems have been capable of providing physiologically-relevant muscle-based proprioceptive feedback through natural neural pathways. In this chapter, I present the Agonist-antagonist Myoneural Interface (AMI), a surgical approach with the capacity to provide graded kinesthetic feedback from a prosthesis through mechanical activation of native mechanoreceptors within residual agonist-antagonist muscle pairs. Sonomicrometery and electroneurography measurement systems were validated using a servo-based muscle tensioning system. A heuristic controller was implemented to modulate functional electrical stimulation (FES) of an agonist muscle, using sonomicrometric measurements of stretch from a mechanically-coupled antagonist muscle as feedback. One AMI was surgically constructed in the hindlimb of each rat. The gastrocnemius-soleus complex (GSC) of the rat was cycled through a series of ramp-and-hold stretches in two different muscle architectures: native (physiologically-intact) and AMI (modified). Integrated electroneurography (iENG) from the tibial nerve was compared across the two architectures. Correlation between stretch and afferent signal demonstrated that the AMI is capable of provoking graded afferent signals in response to ramp-and-hold stretches, in a manner similar to the native muscle architecture. The response magnitude in the AMI was reduced when compared to the native architecture, likely due to lower stretch amplitudes. The closed-loop control system showed robustness at high stretch magnitudes, with some oscillation at low stretch magnitudes. These results indicate that the AMI has the potential to communicate meaningful kinesthetic feedback from a prosthetic limb by replicating the agonist-antagonist relationships that are fundamental to physiological proprioception.
Rationale and Study Design
The work presented in this chapter represents a first step toward validation of the AMI concept; the fundamental advancement presented herein is a murine model of the series coaptation of natively-innervated agonist-antagonist muscle pairs. In this animal model, I demonstrate the utility of sonomicrometery in enabling closed-loop control of muscle stretch in both the native and AMI architectures, and present neural signals indicating the potential of the AMI to provide graded muscle state feedback. Standard amputation paradigms do not allow for physiologically-relevant proprioceptive feedback because the innervated residual muscles are held isometric, and force on any given residual muscle is not modulated by an antagonist muscle. The AMI approximates physiological proprioceptive mechanisms by incorporating residual muscles from discrete agonist-antagonist muscle pairs (e.g., gastrocnemius and tibialis anterior) in series. Contraction of the agonist muscle via the standard motor efferent nerve activates the native contractile mechanoreceptors in the agonist muscle, as well as the native intrafusal muscle spindle stretch fibers of the mechanically-coupled antagonist muscle, both of which provide afferent proprioceptive signaling through the sensory components of their respective innervation nerves. Subsequent activation of the antagonist stimulates a complimentary stretch on the agonist; as such, the AMI has the capacity to reproduce a physiologically relevant agonist-antagonist mechanical coupling, providing non-isometric fascicle strains and agonist-agonist-antagonist fascicle state spindle feedbacks.
Effective implementation of the AMI as a means of communicating proprioceptive information from an external limb prosthesis to the nervous system depends primarily on two essential, non-obvious advancements. I present developments in each of these areas, which include:
1. closed-loop stimulation control with reliable, real time muscle-state feedback, and 2. a surgical geometry that provides sufficient agonist-antagonist coupling to evoke a
graded spindle response.
The study presented in this chapter was designed to validate these components, and to demonstrate efficacy of the systems-level integration thereof in a murine model. The full experimental system from this study is shown in Figure 3. A proportional-integrative controller was used to modulate stimulation of the tibialis anterior (TA) to enforce a desired lateral gastrocnemius (LG) stretch. Electroneurography (ENG) from LG motor afferents was recorded via a nerve cuff placed on the tibial nerve. LG muscle stretch was recorded via
sonomicrometery crystals, and used to inform the stimulation controller. In this chapter, I first describe a measurement validation procedure, which was carried out to ensure robustness in the ENG and sonomicrometery recording systems, as well as to establish a baseline for graded motor afferent response to LG muscle stretch. I then present results from two experimental paradigms. The first, which serves as a positive control, evaluated the afferent response of the
LG to stretch induced by FES of the antagonist TA, with both muscles in their native
orientations. The second experiment was a reproduction of the first, with a surgical modification to the muscle geometry, wherein an AMI was constructed and movement was transduced through direct mechanical coupling of the muscles.
Afferent
dischae ro
ri a
Desired T.A Joint movent (native) or cf
LG stretch Po stimulas direct e r ripong (AMM)oht th e conttller anmtraction st t s t Fascicle
Fgrrpongation Sonoirometr
Mateial an Methodss
Measured LG Stretch
Figure 3: Experimental system overview. Stimulation of the TA was modulated by a proportional-integrative controller, with difference between desired LG stretch and sonomicrometric measurement of actual LG stretch serving as the error signal. Motion from the TA was transduced to LG stretch, and measurements of LG afferent activity and fascicle elongation were recorded. Figure reproduced from (72).
Materials and Methods
Animals and Measurement System Preparation
All
animal
care and procedures were conducted in accordance with the Guide
for
the Care and
Use of Laboratory Animals. In these acute experiments, each of 10 male 4.5-month old Lewis
rats (381 +/- 9g), were
anesthetized
under an isoflurane/oxygen gas mixture at 1-2% isoflurane.
A 5 cm incision was
made
on the lateral aspect
of the
left distal hindlimb.
The
biceps femoris
was disinserted
at
the knee, and retracted
to
reveal the common peroneal and tibial branches
of
the sciatic nerve. A custom tri-polar nerve cuff was sutured in place on the tibial branch.
Sonomicrometery has long been used in the
field
of biomechanics to measure muscle
elongation. This technology utilizes ultrasound time-of-f light measurements from a pair of
implanted piezoelectric crystals
to
provide
acirth 5-0sremnylo sutuse ankle
ngth
(69-71). A pair of 1 mm sonomicrometery crystals (Sonometrics) was inserted into the LG. The
procedure
for
this is
as
follows:
first, a pocket was created for each crystal by piercing the
epimysial fascia of the muscle with small pointed scissors. The proximal crystal was then
positioned
at the proximal musculotendous
junction
of the corresponding muscle;
the
distal
crystal was placed approximately 1 cm distal to the proximal crystal, along the muscle line of
action. Crystal alignment was manipulated to
maximize
signal-to-noise ratio, while monitoring
crystal output via the sonomicrometry amplifier (UDG, Sonometrics) and oscilloscope (TDS
2002, Tektronix)
during
implantation. The
crystals were
secured in place by closing the pockets
and suturing
the
lead wires to the muscle surface with 5-0 nylon suture. The ankle joint was
then suspended from above, via 5-0 nylon suture attached to a base-mounted spring clamp,
and the
foot was
manually cycled through its full range of motion while
sonomicrometery
data
were
recorded.
Rest position w
as eablisthe
equilibrium ankle
joint
position in the absence
of external stimulation or manipulation. The maximum and minimum distance signals from the
LG sonomicrometery crystals during
this
cycling were analyzed to establish percent stretch
metrics for the LG.
Validation of Acquisition System
The following experiment was designed to ensure robustness in the ENG and sonomicrometery
recording systems, as well as to establish a baseline for graded motor afferent response to LG
muscle stretch. In 5 animals the gastrocnemnius-soleus complex (GSC) was disinserted at the
Achilles tendon, and isolated from the surrounding musculature. The knee
joint
was then pinnedto the experimental surface, and the distal Achilles tendon was anchored to the lever arm of a
dual-mode muscle tensioning system (305C-LR, Aurora Scientific) via 5-0 nylon suture (Figure
4). Resting stretch in the instrumented GSC was adjusted by moving the lever arm until the force transducer in the muscle tensioning system showed a non-zero tension, indicating that there was no slack in the muscle. As a secondary form of validation, crystal readouts at this
position were compared to resting values from the range-of-motion trial, and small adjustments were made to the lever arm until any discrepancy was eliminated.
A series of ramp-and-hold stretches were then carried out to each of 5 stretch magnitudes: 2, 4, 6, 8, and 9 mm, which was representative of the full range of muscle stretch achieved during passive joint cycling. In an attempt to standardize dynamic response characteristics, all dynamic trajectories (the "ramps") were carried out at a constant velocity of 1 mm/sec. This velocity was selected to be comparable to the slowest values presented in the literature (73), so as to
preserve the characteristic dynamic response of the primary spindle afferents, but limit the impact of this response on the subsequent hold plateau. Stretch positions (the "holds") were maintained for 5 seconds (53, 74). A rest period of 10 seconds was allowed between
subsequent ramp-and-hold trials. During the trials, force and length data were recorded from the muscle tensioner equipment. Sonomicrometery stretch data were simultaneously recorded from the crystals within the LG. Afferent electroneurography was recorded from the tibial nerve using a tripolar cuff electrode. Leads from the two outer electrode contacts were tied together and passed to one input of the differential amplifier; the lead from the middle electrode contact was passed to the other differential input (52). Differential signals were pre-amplified (Grass P511 AC Amplifier, Grass Instruments), bandpass filtered (0.3-3 kHz), and amplified (RHD2000, Intan Technologies) before being recorded. 5 trials were performed for each stretch magnitude for each animal. System validation was based on (1) visual inspection of the ENG signal for a characteristic peak-plateau shape, (2) high fidelity of sonomicrometric measurements to ground-truth stretch measurement obtained via sensors within the muscle tensioner, and (3) a positive correlation between ENG amplitude and stretch magnitude.
Measurement Validation
Figure 4: Experimental setup for measurement validation. A muscle tensioner was used to stretch the GSC through a series of ramp-and-holds, while muscle state was recorded via sonomicrometery (white circles in the muscle tissue), and ENG was recorded via a nerve cuff on the tibial nerve. Figure reproduced from (72). Artwork: Stephanie L. Ku, 2016.
Experimental Paradigms
Native Architecture
The following experiment was designed as a positive control, to which function of the AMI
architecture was compared. In the remaining animals (n = 5), with hindlimb musculature in its
native architecture, a stimulating hook electrode was inserted into the TA muscle belly (Figure
5a). Monophasic muscle stimulation was delivered by a commercial muscle stimulator (NL800,
Digitimer), with stimulation parameters controlled digitally using a microcontroller. A heuristic
control loop was enforced, with the LG sonomicrometery signal serving as feedback. LG muscle
stretch (and consequently ankle position) was directly controlled by modulating TA stimulation
parameters. Specifically, a proportional-integrative gain architecture modulated stimulation
amplitude and pulse width within safe limits (amplitude from 0-3 mA, pulse width from 300-800
us) to minimize the difference between the desired ramp-and-hold trajectories and actual LG
stretch signals. Amplitude and pulse width were changed simultaneously and linearly, with
abrupt output saturation at maximum values. Controller gains were tuned experimentally, for
each animal, to optimize the balance between rise time and overshoot. All stimulation was
delivered at 40 Hz, to ensure a fused tetanus.
With the hindlimb suspended above the table by a suture through the ankle joint capsule, the
stimulation control system, acting on the TA, was used to perform a series of ramp-and-hold
stretches of the
GSC.
A total of 90 ramp-and-hold stretches were carried out in the 5 animals, to
various stretch magnitudes corresponding to approximately 20, 40, 60, 80, and 100 percent
maximal stretch. All controls were enforced in units of percent crystal strain, defined as the
difference between the instantaneous crystal distance and the rest crystal distance, divided by
the rest crystal distance. Dynamic trajectories were carried out at a constant velocity of 1%
crystal strain per second. Stretch positions were maintained for 5 seconds. A rest period of 10
seconds was allowed between subsequent ramp-and-hold trials. During the trials,
sonomicrometery stretch data were recorded from the crystals within the LG. Afferent neural
activity was recorded from the tibial nerve using the tripolar cuff electrode and amplifier
configuration described above, with the addition of a sample-and-hold blanking circuit after
pre-amplification. This blanking circuit was used to silence the preamplifier for a 1 ms window after
each stimulation pulse. Although this 1 ms window was sufficient to prevent amplifier saturation,
it did not entirely eliminate stimulus artifact; a 5 ms blanking window was enforced in software
during processing to ensure complete elimination of the stimulus artifact.
AMI Architecture
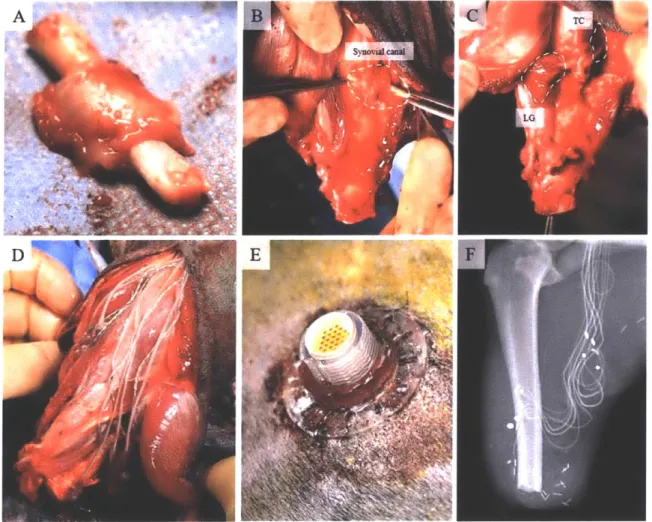
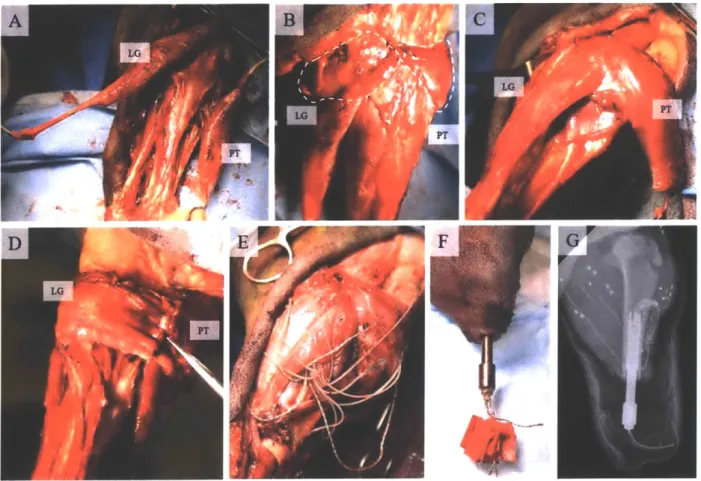
Once data collection in the native muscle geometry was complete, the AMI was constructed in
the instrumented hindlimb of each animal (Figure 5b). The distal Achilles and TA tendons were
first identified and transected near their insertions. The
GSC
and TA were then isolated from
surrounding tissues and wrapped medially in opposite directions around the tibia. The distal
tendons were coapted medially with 5-0 nylon suture, with special attention given to ensuring
that the resulting crystal signals at rest matched the rest signals from the physiological milieu.
The full surgical paradigm is shown in Figure 5.
The stimulation control system, acting on the TA, was again used to perform a series of
ramp-and-hold stretches of the GSC. A total of 60 ramp-ramp-and-hold stretches were carried out in the 5
animals, to various stretch magnitudes. Target dynamic trajectories were carried out at a
constant velocity of 1 % crystal strain per second. It should be noted here that the range of
motion for the AMI construct allowed for lower stretch magnitudes than the native architecture;
this is explored in depth in the discussion section of this chapter. Stretch positions were
maintained for 5 seconds. A rest period of 10 seconds was allowed between subsequent
ramp-and-hold trials. During the trials, sonomicrometery stretch data and afferent nerve signal were
recorded as described in 2.4.1. These data were then compared to those obtained from the
native architecture; similarity amongst the two systems would imply that the AMI architecture is
capable of replicating the physiological interaction between agonist and antagonist muscles.
(a)
Native
S TA ISOLATE / DISINSERT _ (b)AMI_
WRAP COAPTFigure 5: Experimental paradigms. With the musculature in (a) native and (b) AMI architectures, the TA was stimulated through a series of ramp-and-holds, while LG muscle state and ENG were recorded. White circles in the GSC represent sonomicrometery crystals. The bottom panel shows the surgical steps involved in building the AMI. Figure reproduced from (72). Artwork: Stephanie L. Ku, 2016.
To ensure blanking efficacy, after completion of the AMI experiment, the tibial nerve was
transected distal to the nerve cuff. A ramp-and-hold series was then performed with parameters identical to those used for the AMI experiment, and both ENG and crystal strain were recorded.
Data Storage and Processing
Force and length data from the muscle tensioner system were stored at 10 kHz, and exported from proprietary software (DMC/DMA, Aurora Scientific). Sonomicrometery and ENG data were sampled on the Intan RHD2000 at 20 kHz. All data were processed in Matlab (Mathworks, Inc.). Sonomicrometery signals were smoothed with a 0.5 s sliding window, and baseline-drift
corrected. To validate signal quality, ENG signal-to-noise ratio (SNR) was computed according to the analysis presented in (75). Specifically, mean absolute value (MAV) was defined as the arithmetic mean of the rectified ENG signal. SNR was then computed as the ratio of the average MAV during passive motion of the joint to the average value of MAV during rest. This analysis produced an SNR of 3.57dB, which is within the range of values seen for comparable trials in (75) (2.28 dB-3.82 dB). ENG signals were bandpass filtered (0.3-3 kHz, Butterworth),
normalized, digitally rectified, and then bin integrated using a moving window of 250 ms to
,
,
produce an integrated ENG (iENG) signal. Zero-velocity ENG amplitude was defined
as
aplateau-
abase,where
abase is the average baseline amplitude of the iENG signal, andaplateau
is the mean amplitude during the second half of the "hold" portion (53), as shown in
Figure 6.
10- ramp hol | - |
01
5
0
Figure 6: Typical
iENG
response during a single ramp-and-hold trial. Zero-velocity iENG amplitude
is defined as the average iENG value during the second half of the hold.
lENG
signal is normalized
to maximum zero-velocity plateau amplitude recorded for each animal. Figure reproduced from (72).
Results
Validation of Acquisition System
Figure 7 shows the results of a sample trial of the muscle tensioner experiment, which was used
to validate the sonomicrometric measurement and the ENG acquisition systems. ENG
recordings were visually inspected for the peak-plateau shape characteristic of spindle afferent
response to ramp-and-hold stretches.
To evaluate measurement accuracy, sonomicrometer distance signals were compared to
lengths enforced on the muscle tensioner system. The smoothed sonomicrometer signal was
normalized to maximum crystal strain, then scaled to fit the maximum stretch enforced on the
muscle tensioner (9 mm). A trigger pulse from the muscle tensioner to the sonomicrometery
recording system allowed for the two signals to be time-synced. A correlation analysis was then
performed on the two signals from each trial. The correlation coefficients for each trial were
averaged. The average coefficient of fit across all trials wask = .9897 + 0.0089, providing
strong evidence for crystal signal accuracy.
10 .. Muscle Tensioner - Sonomicrometery 5 2 0 -5" 2.5 -0 LA, 0 30 60
Figure 7: Results of the measurement validation experiments. lENG signal is normalized to maximum zero-velocity plateau amplitude recorded for each animal. Figure reproduced from (72). Figure 8 shows a regression analysis for normalized crystal strain values versus zero-velocity iENG amplitude. Samples were binned based on stretch magnitudes, and bin centers were regressed against zero-velocity plateau amplitude. Error bars on each point represent standard error within the bin. The coefficient of fit for the regression was .78.
R2=.78
0.5
01
~
0.50 0.5 1
Muscle stretch (normalized)
Figure 8: Regression of zero-velocity plateau amplitude versus normalized muscle stretch for the measurement validation experiments. Figure reproduced from (72).
Closed-loop Muscle State Control
Sample position tracking trajectories for the closed-loop muscle state control system are shown in Figure 9. PI gains were experimentally tuned for each animal to optimize the balance
between rise time and overshoot. In the typical trial, performance in the AMI was characterized by slower reactivity, manifested in the plots as slower rise times and fall times. Steady state error was typically indicative of either physical limitations on muscle stretch, or muscle fatigue in the stimulated muscle, and was more prevalent in the AMI trials.
100 ..- Desired stretch (a) Native - typical case
- Actual stretch
50-100
(b) Native - best case
50
-(c) AMI - typical case
50 ...
0 15 30 45
Time (s)
Figure 9: Tracking trajectories for the closed-loop muscle stimulation controller. Muscle stretch is normalized as a percentage of the maximum stretch achieved for the associated muscle
architecture. Figure reproduced from (72).
Afferent Stretch Response
Sample crystal and integrated ENG data for the native and AMI experiments are shown in Figure 1 Oa and b. To account for changes in absolute crystal distances associated with the surgical change in muscular geometry, crystal strains were normalized to the maximum values achieved for the associated animal and muscle geometry paradigm (native or AMI). iENG
values were normalized to the maximum zero-velocity iENG amplitude for each animal, because
nerve cuffs did not change position between muscle geometry paradigms. Of particular note in
these data is the preservation of characteristic peak-plateau responses to ramp-and-hold
stretches in both the native and AMI paradigms. The post-nerve-transection iENG is shown inFigure 1 Oc. The lack of neural signal present in these trials verified efficacy of the blanking
system in eliminating stimulation artifact.Regression analysis was performed in both experimental paradigms, comparing normalized
crystal strain values against zero-velocity iENG amplitude (Figure
10d).
As above, samples
were binned based on stretch values, and bin centers were regressed against discharge rates.
Error bars on each point represent standard error within the bin. Coefficients of fit in the native and AMI architectures were similar to those seen in the muscle tensioner (R2 of 0.95 and 0.80,
respectively). The slope of the regression line for the AMI case (.24) was lower than that for the
native case (.62), indicating that the native geometry was capable of provoking largerzero-velocity iENG amplitudes than the AMI geometry.
U U Cl) U 0
z
LL~ (a) Native 50 0 3 2 0 U U U Cl) 0z
rdA 0zji
LLI 15 30 45 100 (c) Nerve Cut 50 321
0 15 Time (s) 30 100 -(b) AMI 50 0 2 0 0 Is 30 45(d)
o Native o AMI 0.5 -"I 45 0 50Muscle stretch (% max)
Figure 10: Experimental results comparing performance of the (a) native, (b) AMI, and (c) severed nerve architectures. Muscle stretch is normalized as a percentage of the maximum stretch achieved for the associated muscle architecture. iENG and plateau amplitudes are normalized to maximum zero-velocity plateau amplitude recorded for each animal. Figure reproduced from (72).
Discussion
In this chapter, I present results demonstrating the potential of a novel surgical architecture to provide physiologically-relevant muscle-tendon proprioceptive feedback through native afferent channels. Specifically, two fundamental advancements were explored and validated. First, a closed-loop stimulation control system, based on reliable muscle stretch data from
sonomicrometery crystals, was shown to provide robust control of an agonist-antagonist muscle pair. Second, the AMI demonstrated the capacity to provide sufficient agonist-antagonist
coupling to evoke a graded spindle response in the antagonist's innervation nerve. This work
represents the first step toward the long-term goal of a clinically-viable amputation paradigm for direct neural interfacing with advanced limb prostheses.The AMI is designed for bi-directional neural communication with a bionic limb. The expected
use case is as follows:
1. One AMI is surgically constructed for each degree-of-freedom of the prosthesis. Muscle electrodes and sonomicrometery crystals are placed within each muscle of
each AMI. At rest, both the agonist and antagonist muscles of each AMI are at
resting length and tension.2. When joint motion is intended (either volitional or reflexive), the subject sends efferent neural commands to cause muscle contraction. EMG recorded from electrodes within the contracting muscle(s) and muscle fascicle length and contraction velocity are combined to generate a movement command to the
prosthesis. There are several potential control architectures by which this movement command could be generated; herein, I have opted to present a method involving direct position-impedance modulation. In this approach, the angular position of each
100
I
roe-..,
I i-A JaAL L.AL