Incidence of cardiotoxicity with the oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil
Texte intégral
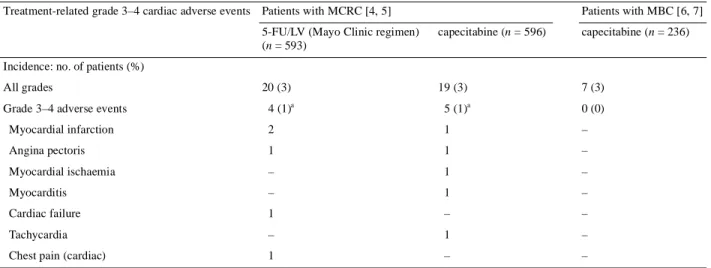
(2) 485 236 patients treated in these trials experienced serious treatment-related or fatal cardiac events. In conclusion, these data show that the incidence of cardiotoxicity in patients receiving capecitabine monotherapy is within the range that occurs with 5-FU. Physicians administering any fluoropyrimidine should be aware of the potential for cardiotoxicity and discontinue treatment promptly in patients developing clinical signs of such toxicity. E. Van Cutse m 1, P. M. Hoff 2, J. L. Blum3, M. Abt4 & B. Osterwalder 5 1. University Hospital Gasthuisberg, Leuven, Belgium (E-mail: eric.vancutsem@uz.kuleuven.ac.be); 2Centro Paulista de Oncologia, Albert Einstein Hospital, Sâo Paulo, Brazil; 3Baylor-Charles A. Sammons Cancer Center, US Oncology, Dallas, Texas, USA; 4 Biostatistics, F. Hoffmann-La Roche Ltd, Basel, Switzerland; 5 Clinical Science, F. Hoffmann-La Roche Ltd, Basel, Switzerland (E-mail: Eric.VanCutsem@uz.kuleuven.ac.be). References 1. Schnetzler B, Popova N, Collao Lamb C et al. Coronary spasm induced by capecitabine. Ann Oncol 2001; 12: 723–725. 2. Bertolini A, Fiumano M, Fusco O et al. Acute cardiotoxicity during capecitabine treatment: a case report. Tumori 2001; 87: 200–206. 3. Becker K, Erckenbrecht JF, Haussinger D et al. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 1999; 57: 475–484. 4. Hoff PM, Ansari R, Batist G et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 2001; 19: 2282–2292. 5. Van Cutsem E, Twelves C, Cassidy J et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin (Mayo Clinic regimen) in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 2001; 19: 4093–4096. 6. Blum JL, Jones SE, Buzdar AU et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999; 17: 485–493. 7. Blum JL, Dieras V, La Russo PM et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast cancer patients. Cancer 2001; 92: 1759–1768.. DOI: 10.1093/annonc/mdf108.
(3)
Figure
Documents relatifs
background Immune checkpoint inhibitor (ICI)- associated early cardiac adverse events (CAEs), mostly acute and fulminant myocarditis, have been well characterized and mainly
Purpose.The aims of the present study were (i) to investigate the impact of great age on pharmacokinetics of capecitabine and its metabolites and (ii) to evaluate
Delayed anemia assessment in patients treated with oral artemisinin derivatives for uncomplicated malaria: a pooled analysis of clinical trials data from Mali.... However,
Auprès de patients sans accès à des infirmières spécialisées, Dans d’autres pays européens. Ces avancées permettraient de connaître au mieux les besoins et attentes des
Diese können bei der Erstellung einer Selbstevaluation oder diversen Fragen eingesetzt werden.. Für die visuelle Erklärung unterstützt die Abbildung 20, die sich
Sedimentological and geochemical studies of boxcores from the Brittlestar Ridge I and Cabliers carbonate mounds, along the Moroccan Mediterranean margin, show that sediments
Older patients ( ≥65 years) receiving 5-FU/LV experienced a higher incidence of early severe toxicities (gastrointestinal toxicities, infections, neutropenia and
Byrne and Mason (Appendix A) using the motor rotor as the flywheel load and calculating the electric torque from the acceleration of the rotor appears to be the