Publisher’s version / Version de l'éditeur:
Journal of the American Ceramic Society, 92, 1, pp. 204-208, 2009-01-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1111/j.1551-2916.2008.02839.x
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
The interaction of methylene blue dye with calcium-silicate-hydrate Beaudoin, J. J.; Patarachao, B.; Raki, L.; Alizadeh, R.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=fc2f5348-d964-4f64-901a-3351a7f6a5d7 https://publications-cnrc.canada.ca/fra/voir/objet/?id=fc2f5348-d964-4f64-901a-3351a7f6a5d7
T he int e ra c t ion of m e t hyle ne blue dye w it h c a lc ium
-silic at e -hydrat e
N R C C - 5 1 1 7 9
B e a u d o i n , J . J . ; P a t a r a c h a o , B . ; R a k i , L . ; A l i z a d e h , R .J a n u a r y 2 0 0 9
A version of this document is published in / Une version de ce document se trouve dans: Journal of the American Ceramic Society, 92, (1), pp. 204-208, DOI:
10.1111/j.1551-2916.2008.02839.x
The material in this document is covered by the provisions of the Copyright Act, by Canadian laws, policies, regulations and international agreements. Such provisions serve to identify the information source and, in specific instances, to prohibit reproduction of materials without written permission. For more information visit http://laws.justice.gc.ca/en/showtdm/cs/C-42
Les renseignements dans ce document sont protégés par la Loi sur le droit d'auteur, par les lois, les politiques et les règlements du Canada et des accords internationaux. Ces dispositions permettent d'identifier la source de l'information et, dans certains cas, d'interdire la copie de documents sans permission écrite. Pour obtenir de plus amples renseignements : http://lois.justice.gc.ca/fr/showtdm/cs/C-42
The Interaction of Methylene Blue Dye with Calcium-Silicate-Hydrate
James J. Beaudoin, Bussaraporn Patarachao, Laila Raki and Rouhollah Alizadeh Institute for Research in Construction, National Research Council,
Ottawa, Ontario, Canada, K1A0R6
*Corresponding author: Tel. 613-993-6749; fax: 613-954-5984 E-mail address: jim.beaudoin@nrc.ca
Abstract
The interaction of Methylene Blue (MB) dye solution with various C-S-H preparations (C/S ratio = 0.60 to 1.80) was investigated. Experiments were also performed by treating the C-S-H in lime-saturated water and distilled water. The effects of MB on the X-ray basal-spacing and the degree of polymerization were determined using low angle X-ray diffraction and 29Si MAS NMR spectroscopy. An interaction mechanism for C-S-H-MB using a ‘bridging’ model for the C-S-H nanostructure is proposed. The model is
consistent with the XRD and 29Si MAS NMR results.
Introduction
The development of cement-based nanocomposites involving the interaction of an organic phase with calcium silicate hydrate (C-S-H) continues to be an active area of cement science research [1-4]. In this context some of the organic phases that have been investigated include: poly(vinyl alcohol), (PVA) ; poly(acrylic acid), (PAA);
poly(diallydimethylammonium chloride, (PDC); hexadecyltrimethylammonium chloride (HDTMA);poly(ethylene glycol), (PEG); silylated poly(vinyl pyrolidone), (PVP).
Nanostructural studies of C-S-H are of general interest as substituted forms of C-S-H comprise the principal binding phase in hydrated Portland cement. The long-term stability of C-S-H in various environments to which cement-based materials are exposed is relevant to sustainability issues on a global basis. An important objective of C-S-H modification with organic molecules is to enhance durability and engineering performance. There is currently a paucity of data to measure the success of these goals although there is some indication that PVP significantly improves flexural strength in C-S-H-PVP nanocomposites [4].
The significance of this research rests in the possibility of improving the performance of cement-based materials and their sustainability in aggressive environments through nanostructural modification of the primary binding phase, (C-S-H). The ability of organic molecules to mimic bridging tetrahedra in the silicate chain structure of C-S-H has been reported [1-4]. Understanding the nature and behavior of these modified nanostructures
will enable us to evaluate their possible role in potentially enhancing the service life and mechanical design parameters of new building materials. For example, pressurized helium gas could be used as a probe to assess the accessibility of aggressive ions to interlayer regions in the C-S-H nanostructure. Mechanical property data should facilitate the evaluation of the effectiveness of the C-S-H–organic molecule interaction for product development. There is a large exciting literature for the interaction of Methylene blue dye and layered silicates e.g. clays as a strong candidate for studies of the interaction of organic molecules with C-S-H.
The interaction of methylene blue dye (MB) with smectites and other layered silicates has been studied extensively [5,6]. It has been demonstrated that adsorption and intercalation of MB occurs and that techniques such as differential thermal analysis (DTA) can be used to rapidly characterize and speciate different clay minerals [7].The authors, in this study, have investigated the interaction of MB with a suite of C-S-H preparations (C/S ratios of 0.60 to 1.80) in an attempt to determine the nature of the interaction and to provide a simple means of characterizing different types of C-S-H. The authors employed X-ray diffraction and
29
Si MAS NMR techniques for this purpose and found evidence to suggest that MB
interaction modifies the nanostructure of C-S-H as observed through changes in the degree of polymerization and changes in the X-ray basal-spacing due to drying. The changes to the degree of polymerization appear to be consistent with those observed for other C-S-H- polymer interactions [3].
Modified C-S-H Nanostructures
The nanostructure of C-S-H has in recent years been studied extensively. Cong and Kirkpatrick have given an excellent review highlighting the similarities of C-S-H structure to those of tobermorite and jennite [8]. Their work extensively utilizes 29Si MAS NMR spectroscopy. The principle and general application of this technique are explained elsewhere [9]. Typical NMR spectra contain one or more peaks (at resonance frequencies) each corresponding to structural sites for the observed nucleus (element specific) with different chemical shifts. The NMR frequencies are difficult to measure accurately and the frequencies are normally reported as chemical shifts, expressed in ppm, relative to an experimentally useful standard. The 29Si MAS NMR chemical shifts for all silicates become more negative (more shielded) with increasing polymerization. The polymerization is characterized by the Qn nomenclature where Q indicates a silicon atom on a tetrahedrally coordinated site and n indicates the number of bridging oxygens per tetrahedron. The Si sites of the primary cement phases have Q0 polymerization. The spectra of most C-S-H samples contain peaks for only Q1 and Q2 sites. Some samples at C/S ratios less than 0.9 yield a peak for Q3 sites indicating possible cross-linking. Quartz has Q4 polymerization.
Cong and Kirkpatrick’s 29Si MAS NMR spectroscopy work has shown that the ratio of Q2/Q1 sites decreases with increasing C/S ratio. At low C/S ratios (C/S < 1.00) the Q1 peak is generally absent and Q2 increases as C/S ratio decreases. At C/S ratio ≤ 0.80 a small Q3 peak sometimes appears indicating cross linking of silicate chains. In accord
with Cong and Kirkpatrick, Merlin et. al. note that a transition in the C-S-H nanostructure occurs at a C/S ratio= 1.00 [8-11]. A new type of C-S-H with a lower degree of
polymerization of the silicate chains appears. The frequency of structural defects e.g. missing bridging silica tetrahedra increases. The higher the C/S ratio, the larger is the number of these defects, and the shorter the average chain length.
A brief comment on the work of Merlin et. al., Franceschini et. al. and Matsuyama and Young is germane [11,12,3]. Merlin et. al. report that neither the intercalation of polymer in pre-formed C-S-H nor the intercalation occuring during insitu formation by pozzolanic reaction or precipitation were found to be effective [11] . They cite evidence that the polymer affects the stacking order of the layers and that it is adsorbed in
significant amounts on the surface or in the void space left by the layer stacks.
An understanding of the nanostructure of C-S-H is relevant to a study of its interaction with organic molecules. Although there are relatively few publications on this topic there is divided opinion as to the structural role of the organic species.Arguments have been made by Franceschini et. al. that suggest that intercalation of silylated polymers into the interlayer regions of C-S-H does not occur [12].Matsuyama and Young concluded that the addition of a polymer such as PAA results in lengthening of the chains (C/S ratio = 1.30, observed increase in the intensity of the Q2 peak) without any change in the C/S molar ratio [3]. This result can be interpreted in terms of the polymer molecule occupying space created by the defective silicate chain. These authors proposed a structure for the C-S-H-PVA nanomaterials where PVA molecules occupied spaces
between dimers in the silicate layer. It is likely that the operative interaction mechanism is dependent on C-S-H preparation conditions (pozzolanic or precipitation method) and whether the interaction with the polymer occurs during insitu formation or with pre-formed C-S-H. . Intercalation of MB and other possibilities e.g. surface adsorption and interactions at C-S-H surface defect sites are discussed in this paper.
Experimental
C-S-H Preparation
C-S-H samples with C/S ratios of 0.60,0.80,1.00,1.20,1.40,1.50,1.60,1.65, and 1.80 were prepared.Stoichiometric amounts of CaO and amorphous silica mixed with water at a water/solids ratio of about 11.80 were used to prepare samples with C/S ratio varying from 0.60 to 1.60.The preparations with C/S ratios 1.65 to 1.80 were prepared with stoichiometric amounts of CaO and amorphous silica corresponding to initial C/S ratios of 1.70 and 2.00.These samples contained significant amounts of free lime and the C/S ratios were corrected accordingly. The CaO was produced using precipitated calcium carbonate heated at 900ºC for 24h. The CaO was purged with nitrogen gas and stored in a desiccator until required. The amorphous silica (Cabosil) was heated at 110ºC to dry the material thoroughly. The reactants for producing C-S-H were placed in high density polyethylene (HDPE) bottles that were continuously rotated for periods up to one year. The reaction temperature was 23ºC. The material was then filtered to remove excess water and dried under vacuum for 4 days. The resulting products were placed in HDPE
bottles, purged with nitrogen gas and stored until further use. Samples of the various C-S-H preparations were put aside for immediate characterization using thermogravimetric analysis (TGA) and X-ray diffraction (XRD) methods.
Characterization of C-S-H
TGA: The various C-S-H samples (10 mg) were placed in a TAQ 600 TGA instrument
and heated at rate of 10ºC per min. from room temperature to 1050ºC. The analysis was conducted in a nitrogen environment. The thermogravimetric curves (mass loss versus temperature) for the C-S-H preparations (C/S ratio of 0.80 to 1.60) were quantitatively and qualitatively similar to that reported for the C-S-H gel [13]. The mass loss in the region 400º-600ºC was very small for the latter. An even smaller loss was observed for the C-S-H used in this study, suggesting that the residual amount of Ca(OH)2 is small or
negligible. Constitutional water loss likely contributes to the small mass loss in this region. This is not the case for the preparations having initial C/S ratios of 1.70 and 2.00 (corrected to C/S ratios of 1.65 to 1.80) due to the free lime that they contained.
NMR: 29Si MAS NMR spectra were recorded at 39.7MHz on a TecMag Apollo
spectrometer using a 7mm Doty probe. Chemical shifts are reported relative to an external tatramethylsilane reference sample (δ = 0.0 ppm). Samples were spun in 7mm zirconia rotors at rates ranging between 3 and 6 kHz, and a 7 μs 90ο pulse was used. The final spectra took up to 48h to obtain.
XRD: The XRD measurements were performed with a Scintag XDS 2000 diffractometer
using CuKα radiation. Characterization of the C-S-H was carried out in the range 5º<2θ<60º using a continuous scan rate of 2º/min. For the tube, a scatter slit width of 4 mm and a divergence slit width of 2 mm were used, respectively. The scatter and receiving slit widths for the detector were 0.5 mm and 0.3 mm, respectively. A
background correction was performed on the XRD patterns. The X-ray patterns indicated the presence of the primary peaks previously reported for C-S-H [14]. Scans in the range 5º <2θ<15º were used to follow changes in the 002 basal spacing. A step size of 0.03º at 5s intervals was used. C-S-H powder samples were covered by Mylar film in order to prevent any humidity change during the XRD analysis. This was particularly important for establishing the basal-spacing versus weight-loss curves (from 11%RH) in the first series of experiments. The change in the 002 basal spacing was also determined using the first drying condition of the samples as the reference condition in the second series of tests involving C-S-H –MB interaction. The second dry state was used for the
experiments involving resaturation with MB. Further details of these experiments will follow in the results and discussion section.
C-S-H–MB Interaction
The initially dry C-S-H samples were resaturated in MB solutions having a MB
concentration of 15mg/L. MB adsorption was very rapid and equilibrium was attained in a few hours. All C-S-H samples were kept in the MB solution for 24h. Following
XRD and 29Si MAS NMR measurements were performed on both untreated samples and samples treated in MB solution.
Results and Discussion
The 29Si MAS NMR and X-ray diffraction results will be presented separately.
29
Si MAS NMR Spectroscopy
The spectra for most of the C-S-H reference samples contain only two major peaks located at about –79.5 and –85.3 ppm (Figure 2). These chemical shift values correspond to Q1 and Q2 Si sites respectively. The Q2/Q1 ratios for the different C/S ratio
preparations are qualitatively similar to those reported by Cong and Kirkpatrick [9]. The Q2/Q1 ratio exhibits a dramatic decrease at C/S ratio = 1.00 and continues to decrease as the C/S ratio increases. Saturation of C-S-H specimens with MB results in a significant increase in the Q2/Q1 ratio beginning at C/S ratio = 1.00. This increase remains
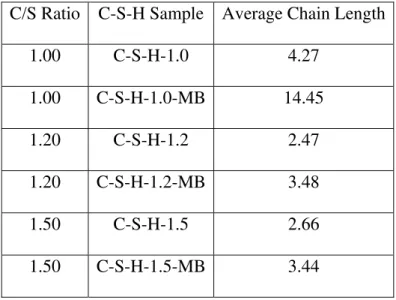
pronounced at C/S ratios of 1.20 and 1.50, though not as large. At C/S ratio=1.80 the Q2/Q1 ratio is similar for both the reference C-S-H and those saturated with MB solution. It is apparent that the treatment with MB solution increases the degree of polymerization and the silicate chain length. Estimates of the average chain length [(Q2/Q1) + 1] x 2 are given in table 1 for C-S-H preparations with C/S = 1.00, 1.20 and 1.50.
The increases in chain length following treatment in the MB solution are compatible with the observed increases in the intensity of the basal-spacing for the C/S ratio =1.00 and 1.50 preparations. It would be expected that an increase in the degree of polymerization would result in more layering of the material. This would be reflected in an increased X-ray basal-spacing intensity (see previous section on X-X-ray diffraction). This was not the case for the C/S = 1.20 preparation. It should be noted that the controls were not subjected to rewetting and second drying which undoubtedly would result in a greater shrinkage and reduced intensity (as indicated by samples resaturated in H2O and redried).
Despite this, the basal spacing intensities for the MB treated C-S-H (C/S = 1.00 and 1.50) which were subjected to second drying are much larger than the controls. Application of the model of Matsuyama and Young for C-S-H-PVA complexes provides one explanation for the observed increase in chain length [3]. It was suggested that the PVA molecule might fit into space created by defective silicate dreirketten. Grafting at sites of missing tetrahedra has been reported for silylated polymers [12]. It is known that electrons of the atoms in the vicinity of existing –O-Si-O bonds can shield the silicon nuclei resulting in a detectable chemical shift. These shifts depend on the extent and strength of shielding. A chemical shift for the silicon atom will occur in the following cases: -O-Si-O-H; -O-Si-O-Na; -O-Si-O-Si-; -O-Si-O-[Polymer]. The chemical shift is different most of the time but it is theoretically possible to have two different attachments that result in a similar chemical shift. In other words the chemical shift of Si in the vicinity of the polymer can be similar to that obtained with a Si-O-Si bond and mimic the latter i.e. a Q2 response. This would explain the increase in the Q2/Q1 ratio observed for
C-S-H-PVA by Matsuyama and Young [3]. A schematic of the C-S-H structure showing possible sites for polymer grafts is provided in Figure 4.
The increase in the Q2/Q1 ratio for C-S-H-PVA preparations has also been observed by Raki et. al. for C/S ratio = 0.70 [2]. There was little expansion of the basal-spacing at this C/S ratio (hence minimal intercalation) suggesting that the increase was due to polymer-bridging at defect sites. The authors have also observed a significant increase in Q2/Q1 for C-S-H treated with hexadecyltrimethylammonium (HDTMA). It is apparent that the effect of increased silicate polymerization resulting from treatment of C-S-H with a polymer is not the exclusive domain of a single polymer. Precise descriptors of the operative mechanisms involved in the interaction of polymers with C-S-H are not yet available. Nevertheless it is expected that the engineering behavior of modified C-S-H should be enhanced and its stability improved.
The C-S-H specimens with C/S ratio= 0.60 and 0.80 have the greatest MB adsorption capacity and the highest values of nitrogen surface area (204 and 186 m2/g respectively) [16]. Adsorption of MB for the specimens having C/S≥ 1.0 is significantly less and in the following order: C/S=1.65>1.80>1.50>1.20>1.00. The nitrogen surface area values given in the same order are 49.0, 43.0, 52.0, 30.0, and 29.0 m2/g. An argument could be made that if the C-S-H preparations with C/S ratio= 1.65, 1.80 and 1.50 are considered as a group (surface area values vary in a narrow range i.e. 43.0-52.0 m2/g), that adsorption is dependent on surface area. While it would appear that surface effects related to polymer-bridging at defect sites satisfactorily account for the increased degree of polymerization,
other interaction processess e.g. intercalation of polymer between the silicate sheets can not be completely ruled out. The adsorption of MB onto C-S-H surfaces is not a
monotonically increasing function of surface area [16]. Some of the MB molecules may be present in the interlayer regions.
A C-S-H hybrid model proposed by Minet et. al. depicts a calcium silicate layer
incorporating organotriethoxy silane at the ends and in the middle of C-S-H chains [4]. The model is also compatible with an effective increase in silicate chain length and degree of polymerization. Franceschini et.al. refer to the inclusion of nanometer-wide polymer regions that covalently bond C-S-H crystallites. Their studies included C3S
hydration and C-S-H precipitation from salts with aqueous solutions containing silane grafted polymer. None of these polymer chains are believed to be present in the interlayer space.
X-ray Diffraction
It is important to recognize the moisture condition of the C-S-H prior to testing in order to interpret any changes in the basal-spacing. All C-S-H specimens were filtered to remove excess water and dried under vacuum for 4 days prior to resaturation with either distilled water, lime-saturated water or MB solution (15mg/L). The resaturation with distilled water or lime-saturated water was to ensure that effects attributed to the C-S-H-MB interaction were not due to the solvent itself. All specimens following resaturation were dried a second time employing the same procedure used for first-drying the
reference specimens. Changes in the intensity of the XRD peaks and basal-spacing resulting from resaturation in the various fluids were determined. It would be expected that any shifting of the basal-spacing would be to lower values due to drying shrinkage [15]. The first-drying effect on the basal-spacing of untreated C-S-H preparations (C/S ratio = 0.80-1.50) is shown as a function of weight loss from the 11% RH condition in Figure 1. The lower C/S ratio preparations exhibit a gradual reduction in basal-spacing with weight loss whereas the higher C/S ratio preparations exhibit a more pronounced structural collapse.
A summary of the basal-spacing changes for each C-S-H preparation (reference and treated) is presented. The XRD traces for the C/S= 0.80 and 1.50 are shown in Figure 3. The effect of second- drying the C-S-H after resaturation with distilled water, lime-saturated water and MB solution is shown by a shift of the basal-spacing to lower values for most of C-S-H preparations. It can also be stated that MB resaturation of C-S-H followed by second-drying promotes a greater degree of nanostructural collapse than water resaturation prior to drying. The effect of water or lime-saturated water on these shifts is similar. The effect appears to be most pronounced for C/S ratios of 1.00 and 1.20 (not shown in Figure 3). The intensity of the basal-spacing peak also increases
significantly for the C/S ratio = 1.00 and 1.50 preparations. The basal-spacing peak for MB treated samples is significantly broadened for the high C/S ratio preparation (C/S = 1.80, not shown in Figure 3). It is suggested that the basal-spacing peak intensity increases observed for the MB resaturated specimens may be a result of an increased degree of polymerization of the C-S-H.
It is true that typically the FWHM of an X-ray peak is used to discuss crystallinity. The use of peak intensity as a qualitative indicator, however, was chosen for the following reason. It was observed (based on NMR evidence presented in the previous section) that C-S-H treated with MB solution resulted in an increase in the degree of polymerization. This phenomenon occurred with C-S-H nanostructure consisting primarily of dimeric silicate units. It is proposed that MB molecules mimic bridging silica tetrahedra simulating longer silicate chains. This should result in the increased probability of a greater number of basal spacing reflections. It is further argued that this would be detected by an increase in the x-ray peak intensity. The X-ray evidence is, however, considered supplementary to the 29Si NMR evidence as it pertains to arguments related to degree of polymerization. It must be taken in tandem with NMR evidence.
The XRD pattern for the C-S-H with C/S ratio=1.2 (not shown) is quite different from the patterns for the other C-S-H preparations. It is possible that the greater crystallinity observed for this preparation is related to a longer reaction time (C-S-H prepared by the pozzolanic reaction of CaO and colloidal silica) i.e. for a period greater than 1 year. The C-S-H with C/S ratio=0.80 was excluded from the discussion on polymerization. The degree of polymerization of this preparation is already very high. This means that the number of ‘defects’ i.e. missing bridging tetrahedra is relatively much lower than is the case for C/S ratios of 1.0 or greater. There are, then, significantly fewer sites for MB to mimic the bridging tetrahedra. It is true that the surface area is higher than that of other preparations. The NMR shows no change in degree of polymerization as would be
expected. The MB is adsorbed on the surface in the classical manner. In summary arguments related to the degree of polymerization following treatment with MB are related to the nanostructure of the C-S-H and not directly to the surface area or the total amount adsorbed.
The bridging model (Figure 4) is consistent with both the XRD and NMR results. Briefly the model indicates the possibility for MB molecules to mimic missing bridging
tetrahedra and to increase the degree of silicate polymerization. This, it is argued, is related to the probability of an increase in the number of basal spacing reflections-hence the consistency with the XRD results. It is reiterated here that the primary evidence for an increase in polymerization is the NMR data. The XRD evidence is in itself not
Conclusions
1. The interaction of C-S-H with MB solution appears to promote a greater degree of collapse of the layered structure on drying.
2. Significant changes to the C-S-H nanostructure at C/S ratio = 1.00 occur in both the untreated and MB treated material.
3. The degree of polymerization of the silicate layers in the C-S-H structure increases significantly with MB treatment when the C/S ratio varies between 1.00 and 1.50.
4. The increase in the degree of polymerization of the C-S-H treated with MB
solution (based on 29Si MAS NMR evidence) is consistent with a model for C-S-H nanohybrids wherein polymer acts as a bridge between drierketten at defect sites. 5. The increase in degree of polymerization of C-S-H after treatment with MB solution is not unique to MB interaction and occurs with other organic molecules e.g. PVA and HDTMA.
References
[1] J. J. Beaudoin, H. Drame, L. Raki and R. Alizadeh, “Formation and Characterization of C-S-H-HDTMA Nano-Structures”, J. Mater. Res (in press).
[2] L. Raki, S.C. Mojumdar, S. Lang and D. Wang, “Spectral and Microscopic Properties of Calcium Silicate Hydrate Polymer Nanocomposites”, Proc. 12th Int. Congr. Chem. Theme ST5, Montreal, July 08, 2007.
[3] H. Matsuyama and J.F. Young, “Intercalation of Polymers in Calcium Silicate Hydrate: A New Synthetic Approach to Biocomposites”, Chem. Mater. 11, 16-19, 1999.
[4] J. Minet, S. Abramson, B. Bresson, A. Franceschini, H. VanDamme and N. Lequeux, “Organic Calcium Silicate Hybrids: A New Approach to Cement-based
Nanocomposites,” J. Mater. Chem., 16, 1379-1383, 2006.
[5] C. Breen and B. Rock, “The Competitive Adsorption of Methylene Blue onto Montmorillonite from Binary Solution with Thioflavin T, Proflavin and Acridine Yellow”, Clay Miner. 29 (12), 179-189 , 1994.
[6] K. Bergman and C. T. O’Konski, “A Spectroscopic Study of Methylene Blue Monomer, Dimer and Complexes with Montmorillonite “ J. Phys. Chem., 67,2169-2177,1963.
[7] V.S.Ramachandran, K.P.Kacker and N.K. Patwardhan, “Basic Dyestuffs in Clay Mineralogy”, Nature 191, 696, 1961.
[8] X. Cong and R. J. Kirkpatrick, “29Si and 17O MAS NMR Investigation of the Structure of Some Crystalline Calcium Silicate Hydrates”, Adv. Cem. Based Mat.,3,133-143,1996.
[9] R.J. Kirkpatrick, “Nuclear Magnetic Resonance Spectroscopy,” Chapter 6 in
Handbook of Analytical Techniques in Concrete Science and Technology, Editors V. S. Ramachandran and J. J. Beaudoin, William Andrew Publishing, pp 205-230, 2001. [10] X. Cong and R.J. Kirkpatrick, “29Si MAS NMR Study of the Structure of Calcium
Silicate Hydrate”, Adv. Cem. Bas. Mat, 3, 144-0156,1996.
[11] F. Merlin, H. Lombois, S. Joly, N. Lequeux, J.-L. Halay and H. VanDamme, “Cement-polymer and Clay-polymer nano and meso-compososites: Spotting the difference”, J. Mater. Chem., 12, 3308-3315, 2002.
[12] A. Franceschini, S. Abramson, B. Bresson, H. VanDamme, N. Lequeux, “Cement- silylated Polymers Nanocomposites”, Proc. 12th Int. Cong. Chem. Cement. Theme ST 5, Montreal, July 08-13, 2007.
[13] H.F.W. Taylor, “Proposed structure for Calcium Silicate Hydrate Gel”, J. Am. Ceram. Soc., 69 [6], 464-467, 1986.
[14] H.F.W. Taylor, “The Chemistry of Cement Hydration”, in Progress in Ceramic Science, Vol 1, 89-145, Ed. J.E. Burke, Pergamon Press, New York, 1960.
[15] R. Alizadeh, J. Beaudoin and L. Raki, “C-S-H(I)- A Nanostructural Model for the Removal of Water from Hydrated Cement Paste”, J. Amer. Ceram. Soc., 90(2), 670-672, 2007.
[16] J. Beaudoin, B. Patarachao, L. Raki and R. Alizadeh, “The Adsorption of Methylene Blue Dye by C-S-H”, submitted to Cem. Concr. Res., 2008.
Table 1 C-S-H Chain Length Before and After Treatment with MB solution
C/S Ratio C-S-H Sample Average Chain Length
1.00 C-S-H-1.0 4.27 1.00 C-S-H-1.0-MB 14.45 1.20 C-S-H-1.2 2.47 1.20 C-S-H-1.2-MB 3.48 1.50 C-S-H-1.5 2.66 1.50 C-S-H-1.5-MB 3.44
Figure Captions
Figure 1. Changes in basal-spacing (002) versus weight change on drying from 11% RH for various C-S-H preparations.
Figure 2. 29Si MAS NMR spectra for C-S-H (C/S ratio = 0.80, 1.50) treated in MB solution (15mg/L): (a) reference samples (b) treated samples.
Figure 3. Low angle X-ray diffraction traces for C-S-H preparations treated in MB solutions, saturated Ca(OH)2 solutions and distilled water. C/S ratio =(a)
0.80 and (b) 1.5.
Figure 4. Schematic of C-S-H nanostructure showing possible site for polymer grafts.
0.9 1 1.1 1.2 1.3 1.4 1.5 0 2 4 6 8 10 12 Weight Loss, % 002 basal-spacing, nm C/S:1.0 C/S:1.5 C/S:1.2 C/S:0.8 Figure 1
Figure 3(a)
C-S-H model Adsorbed Water Interlayer water Silicate tetrahedron MB Methylene Blue Figure 4