HAL Id: hal-01666265
https://hal.archives-ouvertes.fr/hal-01666265
Submitted on 18 Dec 2017
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Evidence for a Cr metastable phase as a tracer in
DLI-MOCVD chromium hard coatings usable in high
temperature environment
Alexandre Michau, Francis Maury, Frederic Schuster, Raphael Boichot, Michel
Pons
To cite this version:
Alexandre Michau, Francis Maury, Frederic Schuster, Raphael Boichot, Michel Pons. Evidence for a
Cr metastable phase as a tracer in DLI-MOCVD chromium hard coatings usable in high temperature
environment. Applied Surface Science, Elsevier, 2017, 422, pp.198-206. �10.1016/j.apsusc.2017.05.253�.
�hal-01666265�
Evidence
for
a
Cr
metastable
phase
as
a
tracer
in
DLI-MOCVD
chromium
hard
coatings
usable
in
high
temperature
environment
Alexandre
Michau
a,
Francis
Maury
a,∗,
Frederic
Schuster
b,
Raphael
Boichot
c,
Michel
Pons
caCIRIMAT,CNRS/INPT/UPS,4alléeE.Monso,31030Toulousecedex4,France bCEASaclay,DFP/DPg,91191GifSurYvette,France
cUniversityGrenobleAlpes,SIMAP,CNRS,38000Grenoble,France
Keywords: Chromiumcoatings MOCVD Crmetastablephase Structuraltransformation Tracer Hardcoatings
a
b
s
t
r
a
c
t
Crdepositsarewidelyusedasprotectivecoatingsbutmultifunctionalperformancesarerequiredinharsh environmentsmotivatingresearchonnewprocesses.MOCVDofCrmetalcoatingswascarriedoutby directliquidinjection(DLI)ofauniquesolutioncontainingbis(ethylbenzene)chromiumasmetalsource andthiophenolasinhibitorofcarbideformation.Alowamount(<6%)ofthemetastabled-Crphasewas foundembeddedinthestablea-Crphase.Theformationofthismetastablephaseoriginatesfromboth thelowdepositiontemperature(<723K)andtheuseofthiophenol.ItwasnotreportedunderotherCVD conditions.Densecoatingsweredepositedbyimplementingamultilayergrowthmode.Suchcoatings exhibitahighnanohardnessofabout17GPa.Thed-Crmetastablephaseundergoesanirreversible struc-turaltransformationtobcc-Crabove723K.Themechanicalpropertiesofcoatingsarenotaffectedbythe structuraltransformationbecauseofthesimilarityoftheircrystallographicstructures(bothcubic),their densityveryclose(avolumecontractionofonly0.4%duringthetransformation)anditslowcontent.This metastablephaseisasignatureoftheDLI-MOCVDprocessanditcanbeusedasatracerforCrcoatings operatinginhightemperatureenvironmentwithoutlossofthebasicproperties.
1. Introduction
Inordertogrowprotectivemetallicchromiumcoatings,wet routesgenerallyusealotoftoxicchemicalsincludingchromicacid solutionsbasedonhexavalentchromium[1–3]whichisnow for-biddenbyREACHregulation.Severalalternativesareinvestigated usingtrivalentchromium[4,5]buttheyshouldalsoresultina reg-ulationban.
Attheopposite,itispossibletodepositchromiumcoatingsby PVDprocesses ascathodicmagnetronsputtering [6,7],cathodic arcevaporation[8],hollowcathodedischarge[9]andhighpower impulsemagnetronsputtering(HIPIMS)[10]withoutusing danger-ouschemicals.Themaindrawbacksofthesephysicalmethodsare theircomplexityforthedepositiononlargepieces,therelatively lowgrowthratesand finallythefactthattheyareline-of-sight deposition techniques, which excludes conformal and uniform coatingsonthreedimensionalobjects.
∗ Correspondingauthorat:CIRIMAT,ENSIACET,4,alléeEmileMonso,BP44362, 31030,Toulousecedex4,France.
E-mailaddress:francis.maury@ensiacet.fr(F.Maury).
CVDprocessesarewellsuitedforconformalgrowthoncomplex shapes.Theirdrawbackcomesfromthehighworking tempera-turesusingtraditionalhalidesprecursors.Forinstance,Crmetal coatingsaredepositedbypackcementationabove1300K[11–13]. However,byusingmetalorganiccompounds(MOCVD),the depo-sitiontemperatureissignificantlydecreased,forinstancebelow 773KforCr-basedcoatingsdependingontheprecursor[14,15], andevenat523Kusingreactivetetra-alkylchromiumcomplexes
[16].Asaresult,thinfilmdepositiononawidevarietyofsubstrate materialsispossiblewithoutstructuralanddimensionalchanges. InspiteofaC-richvaporphaseoriginatingfromtheorganicligands oftheprecursor,metallicCrthinfilmscanbedepositedbyMOCVD usinginhibitorsofcarbonincorporationandcarbideformationas chlorinated[14,17–20]andsulfur-containingderivatives[21].
Anevenmoresurprisingchallengewastofindthatwhenthe emergingtechnologyofdirectliquidinjection(DLI)isimplemented for supplying a cold-wall reactor operating under atmospheric pressure with high vapor flow rates, the carbon incorporation inhibitorswerestilleffectivewhilelargeamountsofhydrocarbon solvent were injected with theprecursor [22–24]. Indeed DLI-MOCVDisanalternativeprocesswheretheprecursorisdilutedor dissolvedinaliquidorganicsolvent.Thissolutionistheninjected
asapulsedsprayinaflashvaporizationchamberand the reac-tivegasphaseistransportedbyacarriergastotheCVDreactor. Thistechniquegenerateshighlystableandcontrolledvaporflow ratesoftheprecursorleadingtorelativelyhighgrowthrateatlow temperature(<773K).Anappropriatechoiceofprecursorprovides highyields[15].Thus,DLI-MOCVDprocessforCrdepositionhas beensuccessfulusingC6Cl6[22–24]andC6H5SH[24]asinhibitors
andbis(arene)chromiumasmetalsource.
Themicrostructuralcharacteristicsandthereforetheproperties ofchromiumcoatingsdependonboththeprocessesusedandthe depositionconditions.Therearetwodifferentcrystallinephases ofchromiuminstandardconditions.Thefirstonecorrespondsto thestablebcccubicstructurewithIm-3m(229)spacegroupand 2.88Åcellparameter(PDF00-006-0694),notedbcc-Cr(ora-Cr). ThesecondoneisaprimitivecubicmetastablephasewithPm-3n (223)spacegroupand4.59Åcellparameter(PDF00-019-0323)
[25,26],notedd-Cr.
Excepthigh-temperatureCVDprocessesthatproduce thermo-chemical diffusion coatings,mostof theabove cited processes, includingDLI-MOCVD,lead totheformationofa-Cr thin films, althoughthegrowthconditionsarefarfromthermodynamic equi-librium. Theprocesses leadingtothegrowthof themetastable phase are rare. Evaporation and condensation of chromium in argonatlowpressures[25–27]ledtotheformationofcrystalline metastabled-Cr.Thesameauthorsreportedthestructural trans-formationofthemetastablephasetothestableoneabove723K.
TheaimofthispaperistostudyCrcoatingsdepositedat temper-aturesbelow723Kinahot-wallDLI-MOCVDreactor.Weshowthey havetheparticularityofcontainingsmallamountsofmetastable d-Crphaseembeddedina-Crpolycrystallinematrix.Furthermore, theseCrcoatingsaresupersaturatedwithcarbonwhichgivesthem unusualmetallurgicalproperties.Theresultinghighnanohardness makesthemgoodcandidatesashardprotectivecoatings.Thiswork alsodemonstratesthatforapplicationsinharshenvironments,if theoperatingtemperatureexceeds 723K, themetastable phase irreversiblytransformsintothestablebccphase,actingasatracer withoutlossofthebasicpropertiesofthecoating.
2. Experimental 2.1. Depositionprocess
DepositionswerecarriedoutbyDLI-MOCVDin ahorizontal, hot-wall,pyrextubularreactor(300mmlongand24mminternal diameter)withanisothermalzonearound150mm.Si(100)and stainlesssteel(304L)plateswereusedassubstratesandplacedona planarhorizontalsubstrate-holderintheisothermalzone.Thetotal pressurewaskeptconstantat6.7kPaandthegrowthtemperature wasfixedateither673(400◦C)or723K(450◦C).
Commercial BEBC, bis(ethylbenzene)chromium (from Strem Chemicals,CAS12212-68-9,infactamixtureof[(C2H5)xC6H6-x]2Cr
where x=0–4)wasusedaschromiumprecursor. Itis aviscous liquidthatwasmixedwithanhydroustoluene(99.8%from Sigma-Aldrich,CAS108-88-3)at3×10−1molL−1(4gofBEBCin50mL oftoluene).Asulfur-containinginhibitorofcarbonincorporation, thiophenol,C6H5SH(fromSigma-Aldrich,purity>99%),was
intro-ducedintothissolutionwithamolerationthiol/nBEBC=2%togrow
preferentiallyCrmetalcoatingsinsteadofchromiumcarbide coat-ings.
The liquid solution containing both the precursor and the inhibitorwasinjectedinaflashvaporizationchamber(473K)at 0.9mLmin−1 byappropriately tuninginjection parameters
(fre-quencyandopeningtime).Nitrogenwasusedascarriergaswith a500sccmflowrateandwasheatedat453Kbeforeenteringthe flashvaporizationchambertopreventcondensation.
Table1
GrowthconditionsofCrmetalcoatingsbyDLI-MOCVDinahorizontalhot-wall reactorusinganinjectionofasinglesolutionofBEBCandthiophenolintoluene,as chromiumsourceandinhibitorofcarbideformation,respectively.
Coatingtype Monolayer Multilayers (9layers) Depositiontemperature(K) 673;723 673;723 Totalpressure(kPa) 6.7 6.7 CarriergasN2(sccm) 500 500
Solvent Toluene Toluene BEBCconcentration(mol/L) 3.5×10−1 3.5×10−1
Thiophenolconcentration(mol/L) 7.0×10−3;3.5×10−2 7.0×10−3
Injectionfeedrate(mL/min) 0.9 0.4 Injectionfrequency(Hz) 10 3 Openingtimeofinjector(ms) 0.5 0.5 Growthduration(min) 60 140
Inafirstmode,coatingsweredepositedasmonolithiclayers, namelymonolayers,bykeepingconstantalltheconditionsduring thedepositionrun.Howeverthefirstanalysesrevealeda colum-narmorphologywithahighporositybetweenthecolumnswhich is not suitable for protective coatings. In order toincrease the compactness,inaseconddepositionmode,thecoatingswere struc-turedinmultilayersbystoppingtheprecursorinjectionfor5min every15minwithoutsignificanttemperaturechangeorventing. Thetemporaryshutdownofthegrowthcreatedcleaninterfaces. Atthebeginningofeachinjectionperiodanewnucleationstep andcrystalgrowthoccurredandwasstoppedbeforethe forma-tionofthecolumns,i.e.after15min,whichlimitsthethicknessof individuallayersandhindersthedevelopmentofcolumns.Thus, densemultilayercoatingswith9layerswereproduced. Character-izationswillbeshownforbothtypesofcoatings.Table1detailsthe experimentaldepositionconditions.
2.2. Coatingcharacterization
The morphology of the Cr coatings were characterized by scanning electron microscopy (SEM; Leo-435VP) and their microstructurewasstudiedbyElectronBackScatteredDiffraction (NordlysNanoEBSDDetectorwiththeAZtecHKLsoftwareinstalled ona SEM-FEGJEOLJSM-7100TTLS LV)and TEM(JEOLJEM2100 equippedwitha200kVFEG).Theircrystallinestructureand ther-malstabilitywereanalyzedbyXRDandin-situXRDvstemperature under Ar atmosphere, respectively (Bruker D8-2 diffractometer equippedwithagraphitemonochromator;Bragg-Brentano con-figuration;CuKaradiation).
Thefilmcompositionwasanalyzedbyelectronprobe micro-analysis(EPMA;CamecaSXFive,15kVand20nA)andthechemical environmentofeachelementofthecoatingwasinvestigatedusing anXPSspectrometer(ThermoScientificK-Alpha),equippedwith a monochromaticAlX-raysourceanda low energyAr+ gun(1
keV) forsurfacecleaning anddepthprofileanalysis.Rutherford BackscatteringSpectrometry(RBS;1.5MeVH+beam;detectionat
160◦)wasusedtoestimatethedensityofcoatings.
3. Resultsanddiscussion
3.1. Depositionofthebcc-Crstablephase
ManyCVDreactorconfigurationsand sizes,aswellas depo-sitionconditionshavebeenpreviouslytestedtodepositmetallic Cr [14,17–24]. These studies on MOCVD and DLI-MOCVD pro-cesses are summarized in Table 2. In the case of DLI-MOCVD process,thepresenceofalargeamountofhydrocarbonsolvent vaporisworthnoting.Experimentalsetsinvolving(i)additionof areactivegas(H2)totheinertcarriergas(N2),(ii)hot-walland
Table2
ExperimentalconditionsusedforCrdepositionbyMOCVDandDLI-MOCVD.Bis(benzene)chromium(BBC)andbis(ethylbenzene)chromium(BEBC)wereusedasprecursors andeitherC6Cl6orC6H5SHwereusedasinhibitorsofcarbideformation.
Process Reactora Carboninhibitor BBC BEBC
Atmb P(kPa) T(K) Ref. Atmb P(kPa) T(K) Ref.
MOCVDc hw C 2Cl6;C6Cl6 – – – – nr nr 573–748 [17] MOCVDc hw (C 6H5CH2)2S;(C6H5)2S – – – – vacuum 1.3×10−3 573–723 [21] MOCVD cw C6Cl6 H2 0.8 673 [14] – – – – MOCVD hw C6Cl6 H2 0.4–2.0 573–673 [14,18,19] H2 0.8 623 [18]
DLI-MOCVD cw C6Cl6 Tol;Cyclo 6.7;101 753–793 [22,23] – – – –
DLI-MOCVD hw C6Cl6 Tol 6.7 748 [24] – – – –
DLI-MOCVD hw C6H5SH Tol 6.7 748 [24] – – – –
DLI-MOCVD hw C6H5SH Tol 6.7 723 Thiswork Tol 6.7 673;723 Thiswork acwmeanscold-wallverticalreactorandhwmeanshot-wallhorizontaltubularreactor.
bAtmmeansatmosphere;TolandCyclomeantolueneandcyclohexaneusedassolventstoinjecttheprecursorsolution.
c Theprecursorused,namelyBarkhoswasamixtureofbis(arene)chromiumslightlydifferentfromtheBEBC(nrmeansnotreported).
orbis(ethylbenzene)chromium(BEBC)asbis(arene)chromium(0) precursor,(iv)differentcarbideinhibitors,(v)totalpressurerange fromatmospherictolowpressure(0.4kPa),and(vi)growth tem-peraturerangefrom573to793Kwerestudied.Themainreason toexploretheeffectsofalltheseconditionscomesfromthekey roleplayedbysurfacechemistryforthecompetitivedepositionof metallicCrandcarbides.Obviouslythissurfacechemistrycanbe influencedbythegasphasechemistrywhichcanbedependenton experimentalconditionsandreactorconfigurations.
So far, MOCVD and DLI-MOCVD processes only led to the growthofsingle-phasepolycrystallinebcc-Crcoatings.Thisisthe stablebody-centered cubicstructure of themetal(spacegroup Im-3m).This common phase was obtainedby adding a chlori-nated[14,17,22,24]orasulfur-containing[21,24]inhibitortothe bis(arene)chromiumprecursor. The present work complements thesepreviousstudiesandisdistinguishedonseveralpoints:(i) theDLI-MOCVDprocesswasused,(ii)withBEBCandC6H5SHin
auniquesolutioncontainingboththeprecursorandtheinhibitor, (iii)H2wasnotaddedinthereactivegasphase,(iv)alargerCVD
reactorwasused,and(v)operatingtemperatureswerelowerthan 723K,asspecifiedinTable2.
Amonolayercoatingdepositedaccordingtomode1at723Kby DLI-MOCVDonsiliconsubstrateexhibitstypicallyacolumnarand porouschromiumstructure.Itisgenerallycomposedofthestable bcccrystallinephase.Noevidenceofcarbideformationwasfound byXRD.TheSEMmicrographofFig.1ashowsa5.7mmthickcoating
consistingofdisjointcolumnsperpendiculartothesurfaceofabout 1mmwidegrownfromadenser1mmthicksub-layerwherethe columnsarenotyetformed.ThecorrespondingXRDpatterninthe 2urange35–80◦exhibitsthetwomainpeaksofthestablebcc-Crat
44.4◦(110)and64.6◦(200).TheKa2contributioncanalsobeseen
asashoulderofthe(200)Crpeak,aswellastheKbcontributionfor themostintense(110)Crpeak(Fig.1b).Thisstablebcc-Crphase exhibitsapreferentialorientationofthegrowthalongthe[110] directionwithatexturecoefficientof1.8whichisconsistentwith acolumnarmorphology.
Asexpected,thecoatingsdepositedaccordingtothemode2 have a multilayer structure with a greater compactness and a smoothersurfacemorphologycomparedwithmonolayercoatings.
Fig.2showsacross-sectionviewofacleavedsampleonSisubstrate grownat673K.Itconsistsof9layerswithclearinterfacesandthe totalthicknessis3.4mm.Theaveragegrainsizeislowerandless dispersedthanformode1;typicallyoftheorderofmagnitudeof thethicknessoftheindividuallayers(≤380nm).
Theinterfacesareclearbetweeneachlayerbecausethestopping oftheinjectionlastedlongenough(5min)tomakethetransient effects negligible and to suppress any nutrient species for the growthofthecoating.Indeed,theevacuationofthegaseous reac-tantsisrapidunderatotalpressureof6.7kPaandmaintainingthe carriergasflowrate(500sccm)duringthestoppingoftheprecursor injectionensuresanefficientpurgeofthereactor.
Fig.1.(a)SEMmicrographofacross-sectionand(b)correspondingXRDpatternofamonolayerCrcoatingdepositedonSisubstratebyDLI-MOCVDaccordingtomode1 (723K;6.7kPa;inhibitorC6H5SH).Thecoatingissingle-phasedandexhibitsatexturedbcc-Crstructure.
Fig.2. SEMmicrographofthecross-sectionofatwo-phasemultilayercoating grownat673Kand6.7kPaonSisubstrate(accordingtomode2)showingthe9 layerscomposingitanditshighcompactness.
Aboutthetransienteffectsinducedbythesemultilayer deposi-tionconditions,transientchangescanbeexpectedfortemperature and pressure.However thetotal pressureis independently and automaticallyregulatedatafixedvalueanditdoesnotaffectthe microstructureofcoatingswhenitisvariedinanarrowrange.Asa result,thetransientchangesofpressureinducedbythisstepwise deliveryofprecursorareinsignificant.Regardingthesubstrate tem-perature,thetemperatureprofilecalculatedinthis3-dimensionnal reactorshowedacoldfingerattheentranceofthereactoroverafew centimetersoriginatingfromtheinjectionofcoldervaporphase (473K).Thestoppingoftheinjectionoftheprecursorsolutioninthe toluenedecreasesthetotalflowrateofthegasphasebyabout30% (about750sccmcomparedto500sccmwithandwithout precur-sorinjectionrespectively)andconsequentlysubstantiallyreduces thelengthofthistemperaturegradientzoneattheentrance.This transienteffectonthetemperatureisnotharmfulsinceitexpends theisothermalzoneofthereactor.
Bycombining theatomic densitymeasuredbyRBSwiththe thicknessofthecoating(2mm)measuredbySEManditsatomic compositionmeasuredbyEPMA,adensityof7.7±0.6g/cm3 was found.Usuallythedensityofcoatingsislowerthanthatofbulk materials. Despite a significant experimental error,the density measuredisofthesameorderofmagnitudeasthatofbulk bcc-Cr(7.19g/cm3).ThisconfirmsthehighdensityofDLI-MOCVDCr
multilayercoatingsgrownaccordingtomode2.
ChemicalanalysesofpreviousMOCVDandDLI-MOCVDcoatings typicallyrevealedalowcarboncontent(from3to8at.%)[14,22,24]
andaslightcontaminationbyoxygenbecauseofaresidualoxygen inthereactororimpuritiesintheprecursorandinhibitor. Depend-ingonthegrowthconditions,coatingsalsocontainasmallamount ofchlorine(<1at%)orsulfur(<3at%)originatingfromtheinhibitors ofcarbideformation.
In this work, the two types of coating deposited according to modes 1 (monolayer) and 2 (multilayer) using thiophenol as inhibitor of carbon incorporation have similarcompositions whichareingoodagreementwithpreviousstudies[14,22,24].For instance,EPMAanalysisofmultilayercoatingsdepositedat673and 723Kunderlowpressure(6.7kPa)andusingamoleratio thiophe-nol/BEBCequalsto2%intheinjectedsolutionhavethecomposition Cr0.92C0.04O0.03S0.01.
Fig.3.XRDpatternsofatwo-phasecoatingcomprisingthemetastabled-Crphase andthestablebcc-Crphase(topandblackpattern;multilayercoating)andofastable bcc-Crmonolayercoating(bottomandgreypattern)depositedbyDLI-MOCVDon Sisubstrate,respectivelyat673Kand723K(otherconditions:6.7kPa;C6H5SH).
3.2. Evidenceofthemetastablephaseı-Cr
ForallpreviousMOCVDfilmsdepositedat623and673Kwith C6Cl6asinhibitor,thestablebcc-Crphasewastheonlyone
identi-fiedbyXRD[14,18,19].ThesameresultwasfoundbyDLI-MOCVD usingC6Cl6inthetemperaturerange748–793K[22–24].The
coat-ingsgrownat748Kusingthiophenolasinhibitoralsoexhibita single-phase bcc structure [24]. In the present work, thin film growthbyDLI-MOCVDusingthiophenolat673and723Kallowed toobservetheformationoftwocrystallinephases:thestable bcc-Croneandthemetastabled-Crphasewhichhasacubicstructure (space group Pm-3n) witha larger latticeparameter of 4.59Å. TheXRDpatternofapartiallymetastablecoatinggrownat673K accordingtomode2(multilayers)isshowninFig.3andcompared withapurestablebcc-Crmonolayercoatinggrownat723K(mode 1).
For multilayer coatingsdeposited at 673K, both stable and metastablephasesareidentified,whateverthesubstrateused(Si orstainlesssteel).Thestablebcc-Crphaseshowsapolycrystalline structureconsistingofwellcrystallizedgrainswhoseaveragesize islargeenough(>300nm)nottoinduceameasurablewideningof theXRDpeaks.Onthecontrary,theXRDpeaksofthemetastable d-CrphasehavelargeFWHMwhichreflectsthenanometricaverage sizeofthecrystallites,estimatedat17and28nmusingtheScherrer equationfromthepeaks(211)and(200),respectively.
FromtherelativeintensityofexperimentalXRDpeaks(Ihkl)of
Fig.3comparedtothoseofPDFfilesofrandomlyorientedcoatings (I0
hkl),atexturecoefficient(Thkl)forbothphaseswascalculated
accordingtoThkl=(Ihkl/I0hkl)/[n·S(Ihkl/I0hkl)],wherenisthenumber
ofdiffractionpeaksconsideredinagivenangularrange. Contrar-ilytocoatingsgrowninthemonolayermode,multilayercoatings grownat673Kdonotexhibitpreferentialorientationforthe dom-inantbcc-Crphase;T110=0.9comparedto1.8forthemonolayer
coating.Thisisdirectlytheresultofthemultilayerstructurewhich hindersthecolumnargrowth,decreasestheaveragegrainsizeand subsequentlyleadstoahigherdensityofcoatings.Forthis two-phase coating, the metastable phase d-Cr exhibits a noticeable preferentialorientationinthe[211]directionwithanestimated
Fig.4. InsituXRDofapartiallymetastablechromiummonolayercoatingat dif-ferenttemperaturesunderAratmosphere(depositionat673Kand6.7kPaonSi substrate).Arrowsshowdisappearanceofthemetastabled-Crphase.Thesample washeatedwithatemperaturerampof1Kmin−1anditwasmaintained35minat
eachtemperaturetorecordthepattern.
texturecoefficientof1.8despitethelowintensityofthediffraction peaks.
TheXRDpeaksofthemetastablephasearealwayslessintense thanthoseofthestablephase,indicatingsmallamounts.Fromthe ratiooftherelativeXRDintensityofthemostintensepeaksofd-Cr (211)andbcc-Cr(110),andsoneglectingthelowtextureofthe metastablephase, acoarseproportionofd-Crwasestimatedat about6%.Thismethodsuffersfromtwoapproximations;(i)the lowtextureofd-Croverestimatestheintensitythatshouldbe con-sideredford-Cr(211)and(ii)thecrude intensityofbcc-Cr(110) isalsooverestimatedbecauseofoverlapwiththed-Cr(210)peak. Interestingly,bothapproximationscompensatewhatmakessense tothemethod.
InsituXRDanalysesallowedustheverificationofthethermal stability,inargonatmosphere,ofthemetastablephaseembedded inthestablebccmatrix.ThearrowsonXRDpatternsofFig.4show thecompletetransformationofthemetastabled-Crphaseintothe stablebcc-Cronebeyond723K,ingoodagreementwithliterature data[25–27].Tracesofchromiumoxideappearabove823K,mainly duetotheresidualoxygenpartial pressurein theXRDanalysis chamber.Onreturningtoroomtemperature,theXRDpatternis thesameasat873Kconfirmingthatthed-Cr→a−Cr transforma-tionisirreversible.FromthisseriesofXRDpatternsrecordedinsitu onadiffractometerdifferentfromthatofFig.3,themean crystal-litesizeestimatedwiththed-Cr(211)peakis21,32and30nmat thetemperatures623,673and723Krespectively.Thesevaluesare consistentwiththatmeasuredatroomtemperaturewithdataof
Fig.3.Nosignificantvariationoftheaveragesizeofd-Cr crystal-liteswasfoundforthisheattreatment.Above723K,themetastable phasedisappears.
Noevidenceforthemetastabled-CrphasewasfoundbyTEM analysis,probablybecauseoftheverylowamountofthisphase comparedtothestableone.Anotherhypothesis isthatitcould undergoaphasetransformationundertheTEM200kVelectronic beam. Electron-beam induced phase transformation is a well-known phenomenon as reported in several papers relative to metallicalloys[28],minerals[29]oroxides[30].
Fig. 5 illustrates a TEManalysis of a two-phase monolayer coatingcharacterizedbyacolumnarmorphology.APrecisionIon PolishingSystem(PIPS)wasusedtopreparethesample,resulting inathinsliceofthecoatingapproximatelyparalleltothesurface ofthefilm.ThereforeFig.5ashowsacross-sectionofonecolumn (andapartofanotherone)perpendiculartoitslongitudinalaxis. TheinsertinFig.5bpresentsthecorrespondingelectron
diffrac-tionpattern attributedtothestablebccphase with[011]zone axis.Anexperimentallatticeparameterof2.92Åwasdeducedfrom electrondiffractionpatternsofseveralcrystallitesingood agree-ment, within2%,withthe literaturevalueof bcc-Cr(2.88Å).A high-resolutionpictureinthesameareashowninFig.5crevealsa veryhomogeneouspacking.ItsFouriertransformintheinsert(d) isinagreementwiththeexperimentalpatternin(b).Moreover, columnsobserved by TEMappear polycrystallinein agreement withthefactthatthetextureisnotveryimportantevenifitexists formonolayercoatings(Fig.1b).Thecross-sectionconfirmsthese columnshaveanaveragediameterofabout1mm,asfoundinSEM observations(Fig.1a).
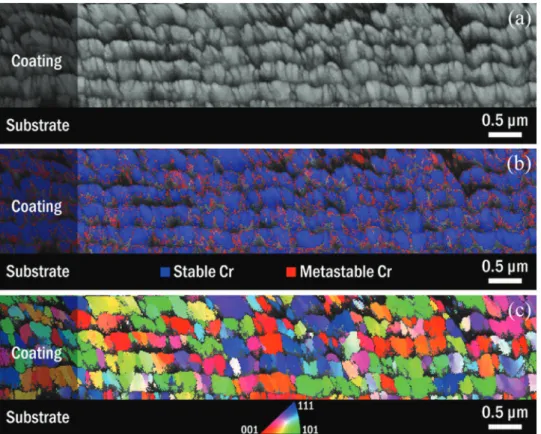
A two-phase multilayer coating comprising the metastable componentwasanalyzedbySEM,coupledtoanEBSDdetector. Itisinfactacross-sectionofthismultilayercoatingpreparedwith across-sectionpolisher(JEOLIB-19510CP)whichwasstudied.An EBSDbandcontrastexampleispresentedonFig.6a.Itissimilarto classicalsecondaryelectronsSEMimages,asshowninFig.2,except thatthecontrastmakesiteasiertoseethegrains.EBSDphase map-pingis showninFig.6b.Thestablebcc-Crandmetastabled-Cr phasesareidentifiedwithtwodistinctcolors,respectivelyblueand red.
EBSDstatisticalanalysiswasperformedonapproximately1000 grains.IdentificationofbothmetastableandstableCrphaseswas possible.Themetastabled-Crphaseproportionisaround3%.This comparesquitewellwiththe6%determinedbyXRDassumingthat bothphaseswerenottextured.Themetastablephaseisessentially foundatinterfacesofthemultilayerandatgrainboundariesofthe dominantbccphasewherethereisthehighestdensityofstructural defectsandinlowEBSDsignalareas.Itwascomplicatedto dis-cernthetwophasesbecausetheyhavethesamecompositionand bothexhibitcubicstructures(bccforthestablephaseandprimitive cubicforthemetastableone).Inthistwo-phasemultilayercoating, theaveragegrainsizeofbcc-Crisapproximately300nmwhileitis onlyafewnanometersford-Cr.Inapreviouswork,Itwasreported thattheparticlesofthismetastabled-Crphasedidnotexhibita definitecrystalshapeandtheiraveragesizewaslowerthan20nm
[26].
NospecificgrainorientationwasobservedbyEBSDanalysisfor thestablebccphase,asitcanbeseenontheinversepolefigurein
Fig.6c.Thisconfirmsthatnopreferentialorientationofthegrowth occursusingthismultilayerCVDmode.Thisresultisinverygood agreementwithXRDanalysiswhichshowedtheabsenceoftexture forthestablephasewithatexturecoefficientof0.9forthe[110] direction(Fig.3).
XPSanalyseswereperformedtostudytheenvironmentofatoms andanalyzethenatureofimpuritiesinthesecoatings.Asurvey scanafterthesurfacecleaningbyAr+sputtering,reportedas
sup-plementaryinformation,revealsthepresenceofonlyCr,O,Cand S(Fig.S1).Itisnotpossibletodistinguisha-Crfromd-Cr.Fig.7
presentsXPSregionspectraofCr2p,O1s,C1sandS2plevelsfrom atwo-phasemultilayerCrcoatingdepositedonaSisubstrate.Three differentsputteringtimesaredisplayedforeachelectroniclevel: 0s(as-deposited),120sand2012s.As-depositedsampleexhibits asurfacecontaminationcontainingchromiumoxides(Cr2p3/2at
576.6eVandO1sat530.8eV),adventitiouscarbon(C1sat284.8 and288.3eV)andsulfateanions(S2p3/2at168.7eV).
Forthelongestcleaningtimebysputtering,thiscontaminated surfaceissufficientlycleanedanddataaremorerepresentativeof thecoatingcomposition.ThemaincontributionofCr2p3/2isshifted
to574.0eVwhichischaracteristicofmetalCr,evenifanoverlap existswithasmallcontributionduetoCr Cbonds(Fig.7a).Traces ofoxygenleadtoaresponseofCr Obondsasashoulderat576.6eV forCr2p3/2andacontributiontoO1sat530.8eV(Fig.7b).This
oxy-genwasattributedtoaslightoxidationofthecoatingsinagreement withEPMAdata(3at%).Fig.7cshowscarbonincorporatedintwo
Fig.5.(a)TEMobservationofatwo-phasemonolayerCrcoating;(b)correspondingselectedareaelectrondiffractionpattern([011]zoneaxis);(c)Highresolutionmicrograph; (d)correspondingFouriertransformofthehigh-resolutionview.
Fig.6. EBSDanalysisofacross-sectionofatwo-phasemultilayercoatinggrownat673Kand6.7kPaonSisubstrate:(a)bandcontrast;(b)phasemapping;(c)inversepole figure(zaxis).
forms:asfreecarbon(C1sat284.6eV,dominantcontribution)and ascarbide(C1sat282.8eV).Bycombiningtherelativeintensityof freeCandcarbidecomponentswiththetotalCcontentdetermined byEPMA(4at%),theamountofCinthecarbideformisestimated atabout0.8at%.ThisissignificantlyhigherthanCsolubilityinCr whichwasreportedtobe<0.1at%atabout750K[31].
Aftersurfacecleaning,theS2plevel,althoughnotveryintense, givesevidenceforauniqueenvironmentintheformofS Crbonds withS2p3/2shiftedat161.6eV,withashoulderat162.8eVforS
2p1/2(Fig.7d).ThisischaracteristicofmetalsulfideorofS
solubi-lizedinthemetalstructure.Inthissample,totalScontentanalyzed byEPMAwasonly0.5at%whichishigherthanthesolubilityof sulfurinchromiumat673K[32].Consequentlyasulfide
contam-inationlikelyexistsbuttheamountistoolowtobeanalyzedby XRDorTEM.
3.3. Originofthemetastablephaseı-Cr
Themetastablephased-Crwasclearlyidentifyinsmallamount byXRDandEBSDinDLI-MOCVDCrmetalcoatingsdepositedboth inmode1(monolayer)andmode2(multilayer).Itisbetter visi-bleinmultilayercoatingsthaninmonolayerbecausethecolumnar growthand textured structure in thelattermake it difficult to observe,sincetherelativeintensityofd-CrXRDpeaksiscrushed bythatofthepreferentialorientationofa-Cr.Severalfactorsseem toinfluencetheformationofthismetastablephase.
Fig.7. XPSanalysesofatwo-phasemultilayerCrcoatingcomprisingthemetastabled-Crandthestablea-Crphasesdepositedat673K.Spectralregionsof(a)Cr2p,(b)O 1s,(c)C1sand(d)S2parereportedfordifferentAr+sputteringtimes(1keV):0s(as-deposited;blackspectrum,bottom),120s(blue,middle)and2120s(red,top).Anoffset
alongtheYaxishasbeenappliedforclarityandadeconvolutionofthemostrepresentativespectrumofthecoatingisplotted.(Forinterpretationofthereferencestocolour inthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.)
It wasnever reported in Crcoatingsgrown by MOCVD and DLI-MOCVDusing chlorinatedinhibitors[14,17–19,22–24] even fordepositiontemperatureaslow as573K.Whenusing sulfur-containinginhibitorsitwasnotmentionedbyMOCVD[21]norby DLI-MOCVD[24](Table2).Ithasbeenobservedinthisworkby DLI-MOCVDusingC6H5SHasinhibitorofcarbonincorporationonlyfor
depositiontemperature≤723K.Thisisingoodagreementwiththe factthatbeyondthiscriticaltemperatureastructuralphase trans-formationintobcc-Croccurs,asreportedby[25–27]andconfirmed byinsituXRDanalysisinthisstudy(Fig.4).
Whenthedepositioniscarriedoutat723K,theobservation ofthisphasedependsonthepositionofthesamplesina quasi-isothermalreactor,whichmeansitdependsonthelocaldeposition conditions,i.e. mainlyonthe substratetemperature.Numerical modeling(notreportedhere)showedforourDLI-MOCVD reac-torthatwhenthetemperatureofthefurnaceissetto723K,the substratetemperaturewasapproximately50Klower,i.e.around 673K.Thistemperaturedecreasecomesfromofthepresenceofa coldfingerattheentranceofthereactoroverseveralcentimeters intheaxialdirection[33].Thisisduetothelowertemperatureof thereactivegasphase(approximately473K)comingfromtheflash vaporizationchamberwhichentersthereactor.Consequently,the formationofthemetastablephaseisthenpossiblebecausethe sub-stratetemperatureislowerthanthecriticalvaluebeyondwhich thestructuraltransformationoccurs.Forthesamefurnace
tem-perature(723K),butusinganothergeometryandsizeoftheCVD reactordifferenttransportconditionshavetobeusedleadingto differenttemperaturefields.Consequently,thetemperature dif-ferencebetweenthesubstrateand thefurnaceset-pointwillbe differentthaninthisworkandthemetastablephasemaynotbe observedifthereactorismoreisothermal.Thisisconsistentwitha sharpstructuralphasetransformationatthiscriticaltemperature of723K.So,forthedepositionofmetallicCrcoatingscontaining d-Crasaminorphase,DLI-MOCVDisasuitableprocess.The sub-stratetemperaturemustbelowerthan723KandC6H5SHisthe
bestinhibitor.
Structuralphasetransformationinmetalsinducedby impuri-tiesisacommonphenomenon[34].Moreover,magneticordering of chromium is affectedby impurities (doping) which induces changesinphasetransition[35].Thiophenollikelypromotesthe formationofthemetastablephase.Sulfurimpuritiesare incorpo-ratedinthecoating(<1at.%)andtheycouldinducethestructural phasetransformationwithinCrcoatings.Thiophenolactionwas foundefficientstartingfromamoleratiothiophenol/BEBCof2% (thetestedvalueswereintherange1–10%).Foramoleratioof only1%,noinhibitoryactionwasobservedsincetheformationof chromiumcarbidesoccurredinsteadofmetallicchromium.
TherelativelylargeFWHMofXRDpeaksofd-Crphasetogether withtheEBSDanalysisgivesevidencethatthismetastablephase ispresentintheformofnanocrystallites.Thenanometricsizeof
d-Crparticlesmaybeanothercauseofitsstability.Indeedsucha nanometriceffectisknownforinstancetostabilizethemetastable anataseform of titania relative torutile although this last one isthermodynamicallythemoststablephase.Thisisbecausethe surfaceenergyofanataseissignificantlylowerleadingtostable anatasenanoparticlesbelowacriticalsizeofabout15nm[36].Our observationofd-Cronlyintheformofnanoparticlesisconsistent with[26]whereauthorsalsoreportedd-Crasnanoparticles,which couldbeexplainedbyalowersurfaceenergythanthatofbcc-Cr.
Asmentionedabovethed-Crphaseismoreeasilyseenin multi-layercoatingsthaninthemonolayercoatings.Furthermore,EBSD analyseshaveshownthatitislocatedbothattheinterfacesand grainboundaries(Fig.6).Fromfundamentalsof nucleationand crystalgrowthitcanbeassumedthatthemetastablephase depo-sitioniscontrolledbyaheterogeneousnucleationstepratherthan asteadystategrowthregimebecausethesurfaceseemstoplay animportantroleanditscrystallitesizestayslimitedatthe nano-metricscale.Thishypothesissuggeststhatitsproportionwould increasewiththenumberofgrainboundariesandinterfacesas obtainedinthemultilayercoatings.Sobyincreasingthe nanos-tructurationwhichmeansbyincreasingthenumberofindividual layersinthecoating,thenumberofbothgrainboundaries(dueto loweraveragecrystallitesize)andinterfaceswillincrease,which couldfavorthegrowthofd-Cr.Thisideahasnotyetbeenverified. FromtheXRDpatternofFig.3,theproximityofthemostintense peaksofeachphase suggeststhatanepitaxialrelationshipmay existwhichwouldfavorthenucleationofd-Cr(211)planeon a-Cr(110)andwouldexplainthepreferentialorientationofd-Crin the[211]direction.
3.4. Unusualproperties
These DLI-MOCVD Cr coatings are promising candidates as protectivemetallurgicalcoatings.Theyaredepositedatlow tem-perature(T<723K)witharelativelyhighgrowthrate(∼5mm/h). The multilayer coatings exhibit a density as high as that of bulkmetallicCr(7.19g/cm3).Forapplicationsinharsh
environ-ments,iftheoperatingtemperatureincidentallyexceeds723K,the metastablephasewillbeirreversiblytransformedintothestable bccphase(Fig.4).Accordingtotheircrystallographicstructures,the metastabled-Crphaseisslightlylessdensethana-Cr(7.16g/cm3).
Thismeansthatatthetimeofthephasetransformationtheoverall volumecontractionwillbeonly0.4%.Furthermoretheproportion ofd-Crdoesnotexceed6%inmultilayercoatingsasdeducedfrom XRDandEBSDanalyses.Thisindicatesthatitcanactasatracer likely withoutloss of thebasicproperties of thecoatings.This isparticularlyusefulinnon-destructivetestingofmanufactured structuralcomponentsprotectedwithsuchacoating.
Indeed carbon-steels and alloys undergo specific heat treatments to optimize their mechanical properties (ductility, hardness...) and theiroperating temperaturemust not exceed the temperatureof the last treatmentundergone as hardening ortemperingannealing.Beyondthecriticaltemperatureasteel structurecannotcarrytheserviceloadforwhichitwasdesigned. Thephasetransformationofd-Croccursinthetemperaturerange oftheheattreatmentsofmanysteelsandmetallicalloys,asfor instanceZircaloy-4 usedin nuclearindustrywhich willloseits metallurgicalstateascladdingmaterialabove753K[37].IfaCr coatingisdepositedbyDLI-MOCVDonthisalloy,the disappear-anceofthemetastablephased-Crwillrevealincidentalconditions inservicegreaterthan723Kwhichwillalsoaffecttheproperties of thezirconiumalloy.Obviously, theexposuretime alsoplays animportantroleinthisd-Cr/a-Crtransformation,butakinetic studyofthistransformationwasnottheprimarypurposeofthis paperanditwillbeinvestigatedshortly.
Preliminary results of mechanical properties were obtained usingananoindenter(Nanoscratchtester,CSMinstrument)for monolayer and multilayercoatings deposited onsteel at 673K usingC6H5SHasinhibitor.ThedataarereportedinTableS1
(Sup-plementary material) and are compared to previous values for similarcoatings.The nanohardness ofa columnar 3.5mmthick monolayercoatingis9.7GPa.Thisisconsistentwiththe13.0GPa found for columnarmonolayer coatingsdeposited at thesame temperaturewithC6Cl6asinhibitor[18].Moreinterestingfor
tech-nologicalapplicationisthehighernanohardnessof16.9GPafound for a 5.5mm thickmultilayer coatingthat exhibitsa high den-sity(depositedaccordingmode2).WhenCrcoatingsgrownby MOCVDandDLI-MOCVDusingC6Cl6 asinhibitoraresufficiently
dense,theyalsoexhibitacomparablehighnanohardnessof19.0
[18]and17.0GPa[22],respectively.Whatevertheinhibitorused (C6Cl6 orC6H5SH)densecoatingshaveanelasticmodulusinthe
range270–310GPawhichisclosetothe285GPaofbulkCr. Further-moretheyallexhibitacompressiveresidualstresscloseto0.6GPa (determinedfromthecurvaturechangeofsamples).
Wewillnolongercommentonthesepreliminaryproperties,but therearetwostrengthsthatmustberetained:(i)DLI-MOCVDCr metalcoatingsaremuchharderthanthosedepositedbyother tech-niques,e.g.electrodeposition[1–3]orarcevaporation[8],and(ii) thereisnosignificantdifferenceforMOCVDandDLI-MOCVD coat-ingsdependingonthenatureoftheinhibitorused,whichmeans thatthepresenceofthemetastablephased-Crdoesnot signifi-cantlyaffectmechanicalproperties.Theveryhighhardnessofthe sameorderofmagnitudeasthatofchromiumcarbidesisprobably duetocarbonsupersaturationinCrasrevealedbyXPSanalyses (Fig.7).Thefactthatthemetastablephasewouldnotaffectthe mechanicalpropertiesofthetwo-phasecoatingswouldbedueto thecubicstructureofeachcomponent,theirverysimilardensity andthesmallamountofd-Cr.
4. Conclusions
Alow amount(<6%)ofthecubicmetastabled-Crphase was foundmixedwiththestablebcc-CrphaseinDLI-MOCVDcoatings. Theformationofthismetastablephaseresultsfromboththelow temperatureofdeposition(<723K)andtheuseofthiophenolas inhibitor ofcarbide formation.It wasnot reportedunderother CVDconditions.Densecoatingsweredepositedbyimplementing amultilayergrowthmodeinordertoavoidacolumnarandporous morphology.Suchcoatingsexhibitahighnanohardnessofabout 17GPa.
Accordingtotheliterature,thismetastablephaseisirreversibly transformedintothestablebcconefrom723Kininertatmosphere. Thisstructuraltransformationshouldnotaffectthepropertiesof thecoatingduetothesimilarityoftheircrystallographicstructures (bothcubic)andtheirveryclosedensity(avolumecontractionof only0.4%atthetimeofstructuraltransformation).Thepresence ofthismetastablephaseconstitutesasignatureoftheDLI-MOCVD process.Itcanbeusedasatracerforcoatingsoperatinginhigh temperatureenvironmentwithoutlossofthebasicpropertiesof thecoatings.Thisisparticularlyusefulinnon-destructivetesting ofmanufacturedstructuralcomponents.
Acknowledgements
The authors thank Dr. Arnaud Proietti and Marie-Christine LafontfortheirhelpinEBSDandTEManalysesrespectively,and R.Laloofornanohardnessmeasurement.RBSanalysesweremade byH.GuéganinARCANE-CENBG,Gradignan,France.Thisworkwas partiallysupportedbyCNRS,CEA,INPTandtheCentreofExcellence ofMultifunctionalArchitecturedMaterials(CEMAM).
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.apsusc.2017.05.253.
References
[1]S.Hoshino,H.A.Laitinen,G.B.Hoflund,Theelectrodepositionandproperties ofamorphouschromiumfilmspreparedfromchromicacidsolutions,J. Electrochem.Soc.133(1986)681–685.
[2]N.Imaz,M.Ostra,M.Vidal,J.A.Díez,M.Sarret,E.García-Lecina,Corrosion behaviourofchromiumcoatingsobtainedbydirectandreversepulseplating electrodepositioninNaClaqueoussolution,Corros.Sci.78(2014)251–259.
[3]G.A.Lausmann,Electrolyticallydepositedhardchrome,Surf.Coat.Technol. 86–87(Part2)(1996)814–820.
[4]A.Liang,L.Ni,Q.Liu,J.Zhang,Structurecharacterizationandtribological propertiesofthickchromiumcoatingelectrodepositedfromaCr(III) electrolyte,Surf.Coat.Technol.218(2013)23–29.
[5]V.S.Protsenko,F.I.Danilov,V.O.Gordiienko,A.S.Baskevich,V.V.Artemchuk, ImprovinghardnessandtribologicalcharacteristicsofnanocrystallineCr-C filmsobtainedfromCr(III)platingbathusingpulsedelectrodeposition,Int.J. Refract.Met.HardMater.31(2012)281–283.
[6]A.Aubert,R.Gillet,A.Gaucher,J.P.Terrat,Hardchromecoatingsdepositedby physicalvapourdeposition,ThinSolidFilms108(1983)165–172.
[7]F.Cosset,G.Contoux,A.Celerier,J.Machet,Depositionofcorrosion-resistant chromiumandnitrogen-dopedchromiumcoatingsbycathodicmagnetron sputtering,Surf.Coat.Technol.79(1996)25–34.
[8]G.Cholvy,J.L.Derep,M.Gantois,Characterizationandwearresistanceof coatingsintheCr-C-Nternarysystemdepositedbyphysicalvapour deposition,ThinSolidFilms126(1985)51–60.
[9]S.Komiya,S.Ono,N.Umezu,Hardnessandgrainsizerelationsforthick chromiumfilmsdepositedbyhallowcathodedischarge,ThinSolidFilms45 (1977)473–479.
[10]H.Högberg,L.Tengdelius,M.Samuelsson,J.Jensen,L.Hultman,b-Taanda-Cr thinfilmsdepositedbyhighpowerimpulsemagnetronsputteringanddirect currentmagnetronsputteringinhydrogencontainingplasmas,Phys.B: Condens.Matter439(2014)3–8.
[11]R.G.I.Leferink,W.M.M.Huijbregts,Chromiumdiffusioncoatingsforthe protectionoflow-alloysteelinasulphidizingatmosphere,Corros.Sci.35 (1993)1235–1242.
[12]V.A.Ravi,Packcementationcoatings,in:S.D.Cramer,B.S.CovinoJr.(Eds.), ASMHandbookVolume13A:Corrosion:Fundamentals,Testing,and Protection,ASMInternational,2003,2017,pp.763–771.
[13]A.R.Castle,D.R.Gabe,Chromiumdiffusioncoatings,Int.Mater.Rev.44(1999) 37–58.
[14]F.Maury,L.Gueroudji,C.Vahlas,Selectionofmetalorganicprecursorsfor MOCVDofmetallurgicalcoatings:applicationtoCr-basedcoatings,Surf.Coat. Technol.86–87(Part1)(1996)316–324.
[15]A.Michau,F.Maury,F.Schuster,R.Boichot,M.Pons,E.Monsifrot,Chromium CarbideGrowthatLowTemperaturebyaHighlyEfficientDLI-MOCVDProcess inEffluentRecyclingMode,Surf.Coat.Technol.(2017),submittedfor publication.
[16]F.Maury,F.Ossola,Evaluationoftetra-alkylchromiumprecursorsfor organometallicchemicalvapordepositionI:Filmsgrownusing Cr[CH2C(CH3)3]4,ThinSolidFilms207(1992)82–89.
[17]V.B.Polikarpov,A.S.Luzin,V.A.Dodonov,E.K.Klement,Chromiumfilms obtainedbypyrolysisofchromiumbisarenecomplexesinthepresenceof chlorinatedhydrocarbons,IzvestiyaAkademiiNaukSSSR20(1984) 1839–1842.
[18]F.Maury,C.Vahlas,S.Abisset,L.Gueroudji,Lowtemperaturemetallorganic ChemicalVaporDepositionroutestochromiummetalthinfilmsusing bis(benzene)chromium,J.Electrochem.Soc.146(1999)3716–3723.
[19]F.Maury,F.-D.Duminica,F.Senocq,NovelMOCVDprocessforthelow temperaturedepositionofthechromiumnitridephases,in:M.D.Allendorf, M.L.Hitchman(Eds.),CVDXV:ProceedingsoftheFifteenthInternational SymposiumonChemicalVaporDeposition,TheElectrochemicalSociety, Pennington,NJ,2000,pp.260–267.
[20]C.Vahlas,F.Maury,L.Gueroudji,AthermodynamicapproachtotheCVDof chromiumandofchromiumcarbidesstartingfromCr(C6H6)2,Chem.Vap.
Depos.4(1998)69–76.
[21]A.S.Luzin,V.B.Polikarpov,V.A.Dodonov,E.K.Klement,Chromiumfilms obtainedbypyrolysisofbis(arene)chromiumcomplexesinpresenceof sulfur-containingadditives,Zh.Prikl.Khim.61(1988)1235–1239.
[22]F.Maury,A.Douard,S.Delclos,D.Samelor,C.Tendero,Multilayerchromium basedcoatingsgrownbyatmosphericpressuredirectliquidinjectionCVD, Surf.Coat.Technol.204(2009)983–987.
[23]A.Douard,C.Bernard,F.Maury,Thermodynamicsimulationofatmospheric DLI-CVDprocessesforthegrowthofchromium-basedhardcoatingsusing bis(benzene)chromiumasmolecularsource,Surf.Coat.Technol.203(2008) 516–520.
[24]G.Boisselier,F.Maury,F.Schuster,Growthofchromiumcarbideinahotwall DLICVDreactor,J.Nanosci.Nanotechnol.11(2011)8289–8293.
[25]K.Kimoto,I.Nishida,Anelectrondiffractionstudyonthecrystalstructureofa newmodificationofchromium,J.Phys.Soc.Jpn.22(1967)744–756.
[26]I.Nishida,K.Kimoto,Crystalhabitandcrystalstructureoffinechromium particles:anelectronmicroscopeandelectrondiffractionstudyoffine metallicparticlespreparedbyevaporationinargonatlowpressures(III),Thin SolidFilms23(1974)179–189.
[27]J.Forssell,B.Persson,Growthandstructureofthinchromiumfilms condensedonultra-highvacuumcleavedNaClandKClcrystals,J.Phys.Soc. Jpn.29(1970)1532–1545.
[28]J.Reyes-Gasga,G.R.Garcia,M.Jose-Yacaman,Electron-beam-induced structuretransformationofthequasicrystallinephasesoftheAl62Cu20Co15Si3
alloy,Radiat.Phys.Chem.45(1995)283–291.
[29]K.Yin,Y.Xia,Z.Liu,J.Yin,L.Sun,Electron-beaminducedphasetransformation inb-Ag2Sethinfilms,Phys.StatusSolidi(a)209(2012)135–138.
[30]U.Golla-Schindler,G.Benner,A.Orchowski,U.Kaiser,Insituobservationof electronbeam-inducedphasetransformationofCaCO3toCaOviaELNESat
lowelectronbeamenergies,Microsc.Microanal.20(2014)715–722.
[31]W.D.Klopp,Recentdevelopmentsinchromiumandchromiumalloys,JOM21 (1969)23–32.
[32]J.Oudar,N.Barbouth,Solubilityofsulphuriniron-chromiumalloys,Scr. Metall.15(1981)41–43.
[33]A.Michau,F.Maury,F.Schuster,I.Nuta,R.Boichot,M.Pons,Chromium carbidegrowthbydirectliquidinjectionchemicalvapordepositioninlong andnarrowtubes,experiments,modelingandsimulation,submittedfor publication.
[34]R.G.Hennig,D.R.Trinkle,J.Bouchet,S.G.Srinivasan,R.C.Albers,J.W.Wilkins, Impuritiesblockthe[alpha]to[omega]martensitictransformationin titanium,Nat.Mater.4(2005)129–133.
[35]R.S.Fishman,S.H.Liu,Effectofimpuritiesonthemagneticorderingin chromium,Phys.Rev.B45(1992)12306–12318.
[36]D.A.H.Hanaor,C.C.Sorrell,Reviewoftheanatasetorutilephase transformation,J.Mater.Sci.46(2011)855–874.
[37]S.Fourgeaud,J.Desquines,M.Petit,C.Getrey,G.Sert,Mechanical characteristicsoffuelrodcladdingsintransportconditions,Packag.Transp. StorageSecur.Radioact.Mater.20(2009)69–76.