HAL Id: tel-01680009
https://tel.archives-ouvertes.fr/tel-01680009
Submitted on 10 Jan 2018
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Role of the SUMO pathway in Acute Myeloid Leukemias
response to treatments
Hayeon Baik
To cite this version:
Hayeon Baik. Role of the SUMO pathway in Acute Myeloid Leukemias response to treatments. Human health and pathology. Université Montpellier, 2017. English. �NNT : 2017MONTT016�. �tel-01680009�
Délivré par
l’Université de Montpellier
Préparée au sein de l’école doctorale
CBS2
Sciences Chimiques et Biologiques pour la SantéEt de l’unité de recherche
IGMM CNRS UMR5535
Institut de Génétique Moléculaire de Montpellier
Spécialité
Biologie-Santé
Présentée par
Hayeon BAIK
Soutenue le 29 Juin 2017 devant le jury composé de
Dr. Valérie Lallemand-Breitenbach
Directrice de Recherche, INSERM, Paris
Rapporteur Président du jury
Pr. Stefan Müller
Professeur, Université de Francfort
Rapporteur
Dr. Jean-Emmanuel Sarry
Chargé de Recherche, INSERM, Toulouse
Dr. Guillaume Bossis
Chargé de Recherche, CNRS, Montpellier
Dr. Marc Piechaczyk
Examinateur
Examinateur
Directeur de Thèse
Role of the SUMO pathway
in Acute Myeloid Leukemias response
to treatments
Acknowledgements
I dedicate this thesis to my Heavenly Father, for all the blessings he has bestowed upon me.
Foremost, I would like to acknowledge the members of the jury for evaluating my work and for coming to Montpellier: Valérie Lallemand-Breitenbach and Stefan Müller as ‘rapporteurs’, and Jean-Emmanuel Sarry as ‘examinateur’.
I would like to express my sincerest gratitude to Dr. Marc Piechaczyk, my thesis director, for having accepted me in his laboratory, for his strong support, and his precious discussions.
Sincere thanks go to Dr. Guillaume Bossis, for his sage advice, mentoring, and for his daily support. He made my Ph.D. experience productive and stimulated me to continue in research. It has been truly a privilege for me to have Marc and Guillaume as my supervisors in a project, which I really enjoyed.
I am also grateful to Dr. Jean-Emmanuel Sarry and Dr. Martin Villalba for their valuable advices and careful guidance in my annual thesis committee meeting. I also thank Dr. Jean-Emmanuel Sarry for his deep engagement, his contribution to in vivo experiments and for having accepted me in his laboratory to follow these experiments.
Great thanks to the “Oncogenesis and Immunotherapy” team. I was very happy to work with each of you. Your dedication, insightful criticism and help were so precious for me. Particular thanks go to Julie and Mathias for their contribution to my project. I wish to also express my appreciation to all members of the IGMM for being part of a wonderful working environment. In my daily work I have been really blessed with friendly and kind members of the IGMM.
I am also very indebted to Tamara and Claire for their sincere sharing, laughing, crying in times of difficulties and happiness. I would also like to thank Sung-Muk Choi and Young-Suk Kim for their encouragements and prayers.
Many people contributed to this thesis both for its scientific aspects as well as in daily life and I express my gratitude to them.
Words cannot express the feeling, love and thankfulness I have for my family who supported and encouraged me during all my studies in France and in Madagascar. Finally, thank you Stephan, for your special dedication and support during my last year of PhD. Your presence was countlessly important.
Table of Contents
! !"#$%&'()*(+($,-! "! ,!.'(/%0/"%$,($,-! #! '1-,/%0/01*23(-! $! '1-,/%0/,!.'(-! %&! 43(!+.'(! %%! '! '()*+,-.)'+(! %"! %! /01! %#! "#"! $%&'()!*+'(,%-%.+/./!($0!)+12+'.(! "3! "#"#"!*4567898:4;:;! "3! "#"#<!=>?;74@;!8A!B:AA4@4C7:67:8C;! "D! "#"#E!)4?F45:6! "G! "#<! (=1,+!'H+)%.0!)+12+'.(! <I! "#<#"!.CJ:B4CJ4!6CB!58@76>:7K! <I! "#<#<!(J?74!5K4>8:B!>4?F45:6! <"! "#<#E!(')!J>6;;:A:J67:8C! <"! "#<#E#6! L(M!J>6;;:A:J67:8C! <"!"#<#E#N! =K78O4C47:J!J>6;;:A:J67:8C! <E!
"#<#P!(')!7@46754C7! <3! "#<#P#6! /76CB6@B!JQ4587Q4@69K! <3! "#<#P#N! 0:AA4@4C7:67:8C!7Q4@69K!R(-)S!-')T&(&UV! <D! "#<#3!$8W4>!7Q4@694?7:J6>!699@86JQ4;! <X! "#<#Y!(')!J4>>!>:C4;! EI! 2! /)*/!'(,-.3,4*3/.)'5/)'+(!+6!,'663*3()'/)'+(!'(!/01! "%! <#"! 0.LL+&+$,.(,.%$!,*+&(-H! E"! <#"#"!&47:C8:B;!6CB!&47:C8:B!6J:B!@4J4978@;! E"! <#"#<!(,&(!:C!7Q4!7@46754C7!8A!(-)! EE! <#"#E!0:AA4@4C7:67:8C!7Q4@69KZ!6!9@85:;:CO!699@86JQ!:C!(')!7@46754C7! EE! <#<! 0.LL+&+$,.(,.%$!,*+&(-.+/!1/.$[!(,&(!.$!$%$T(-)!(')! EY!
<#<#"!+AA4J7!8A!@47:C8:B;!:C!C8CT(-)!(')! EY!
<#<#<!(,&(!:C!J85N:C67:8C!\:7Q!4]:;7:CO!JQ4587Q4@69K! ED!
<#<#E!+AA4J7!8A!(,&(!:C!;94J:A:J!(')!;?N7K94;! EX!
<#E! 0+&+[1)(,.%$/!%L!(,&(T'+0.(,+0!,&($/=&.-,.%$()!(=,.^.,H!.$!(')!=+))/!($0!-+&,.$+$=+!%L!.,/!
,(&[+,.$[! EG! <#E#"!.CQ:N:7:8C!8A!&(&!6J7:W:7K!NK!>4?F45:J!8CJ89@874:C;! EG! <#E#<!+9:O4C47:J!58B:A:J67:8C;!;KC4@O:_4!\:7Q!(,&(!:C!C8CT(-)!(')!NK!J8C7@8>>:CO!&(&! ;:OC6>:CO! PI! <#E#<#6! *:;78C4!(J47K>67:8C! P<! <#E#<#N! '47QK>67:8C! P<! "! 7-0+81/)'+(! 9#! E#"! 1M.`1.,.$! P3! E#<! /1'%Z!(!-%/,T,&($/)(,.%$()!'%0.L.+&!%L!,*+!1M.`1.,.$!L('.)H! PY! E#<#"!/1'%!9@874:C! PY! E#<#<!/?58K>67:8C!4C_K54! PD! E#<#E!04;?58K>6;4! PG! E#<#P!/1'%!J8C;4C;?;!587:A! 3I! E#<#3!/1'%!.C74@6J7:CO!'87:A!R/.'V! 3"!
E#E! ,(&[+,.$[!,*+!/1'%!-(,*a(H! 3<!
E#P! &+[1)(,.%$!%L!,&($/=&.-,.%$!MH!/1'%! 3E!
E#3! /1'%!.$!=($=+&! 3D! E#3#"!/1'%!:C!J6@J:C8O4C4;:;! 3D! E#3#<!/1'%!:C!(')! YI! E#3#<#6! /?58K>67:8C!:C!(-)! YI! E#3#<#N! /1'%!:C!C8CT(-)!(')! Y"! ''! *37-1)7! :#! 0/(-7.*';)!%! :<! '''!,'7.-77'+(7! $<! '5!+)=3*!;*+>3.)7! %&#!
%! )=3!*+7?7-0+!/@'7!.+()*'A-)37!)+!)=3!*37;+(73!+6!/.-)3!0831+',!13-B30'/!.3117!)+! .=30+)=3*/;3-)'.!,*-C7!D0/(-7.*';)!2E! %&<! 0/(-7.*';)!2! %&$! 2! *+13!+6!(/,;=!+@',/737!'(!/.-)3!0831+',!13-B30'/7!.=30+*37'7)/(.3! %2%! 5! *363*3(.37! %2"! -2++!35! %#F! !
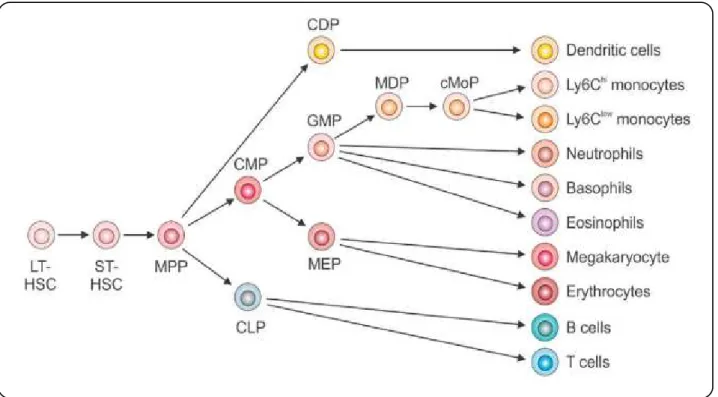
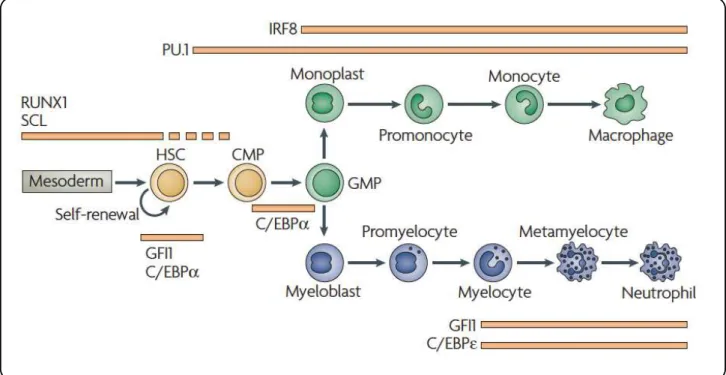
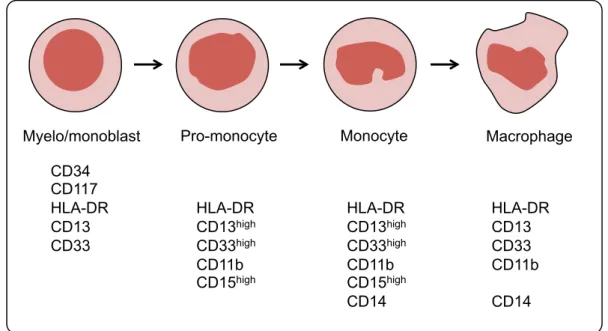
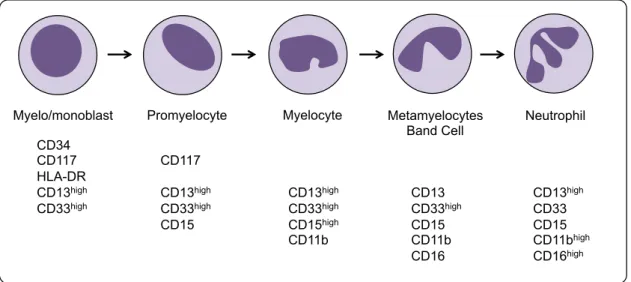
List of Figures
! L.[1&+!"Z!*+'(,%-%.+/./! "3! L.[1&+!<!Z!(!/,+-a./+!&+`1.&+'+$,!L%&!,&($/=&.-,.%$!L(=,%&/!01&.$[!'H+)%.0!! 0.LL+&+$,.(,.%$! "Y! L.[1&+!EZ!$%&'()!'%$%=H,.=!0+^+)%-'+$,!.$!M%$+!'(&&%a! "D! L.[1&+!PZ!$%&'()