Publisher’s version / Version de l'éditeur:
Polymer Electrolyte Fuel Cells 10, pp. 855-865, 2010-10-10
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1149/1.3484579
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at Properties of melt-extruded vs. solution-cast proton exchange membranes based on PFSA nanocomposites
Mokrini, A.; Raymond, N.; Theberge, K.; Robitaille, L.; Del Rio, C.; Ojeda, M. C.; Escribano, P. G.; Acosta, J. L.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=6541715f-1ab3-4ded-afe0-3b0dec270f64 https://publications-cnrc.canada.ca/fra/voir/objet/?id=6541715f-1ab3-4ded-afe0-3b0dec270f64
Properties of Melt-Extruded vs. Solution-Cast Proton Exchange Membranes Based on PFSA Nanocomposites
A. Mokrini*a, N. Raymonda, K. Thebergea, L. Robitaillea, C. Del Riob, M.C. Ojedab, P.G. Escribanob, J.L. Acostab.
a
Industrial Materials Institute – National Research Council of Canada. 75, De Mortagne, Boucherville (QC) J4B6Y4 Canada
b
Instituto de Ciencia y Tecnología de Polímeros - Consejo Superior de Investigación Científica. C/ Juan de la Cierva 3, 28006 Madrid, Spain
This study examined the relationships between structure/morphology and properties of series of composite proton exchange membranes prepared using two processing techniques; solution-casting and melt-extrusion. Three families of inorganic fillers with different aspect ratios and specific areas have been used; spherical 60 nm SiO2 nanoparticles, fiber-like sepiolite clay,
and non-structured mesoporous silica. The results show that composite PEMs present in general higher water uptake than reference membranes; water uptake increases with the specific area of the filler, and translate to lower volume change and higher dimensional stability for the fillers with low specific area. Solution cast membranes show in general higher water uptake than extruded ones that translates to lower volume change and higher dimensional stability of extruded PEMs. Finally conductivity results show that at high levels of hydration, solution-cast membranes have in general higher conductivities than extruded samples, while at high temperature and reduced relative humidity conditions, melt extruded samples show higher conductivities.
Introduction
Hybrid membranes are commonly generated by in-situ formation of the inorganic material within an ionomer preformed membrane or polymer solution, or by addition of an inorganic component to a polymer solution or dispersion followed by solution casting. Melt-extruded proton exchange membranes (PEMs) are a new class of membrane materials with different morphologies and desirable properties for fuel cell technology. Composites proton exchange membranes have been identified to improve the performance at elevated temperature and low relative humidity operation conditions (1-4). Thus, despite the extensive R&D efforts in both new polymers and hybrid systems, the ideal material has yet to emerge, and hybrid membranes investigated to date tend to be lacking in one or more of the properties required for practical applications including proton conductivity, permeation, swelling or mechanical stability (3). There is increasing evidence that the main advantage conferred by the presence of an inorganic component, in terms of both conductivity and fuel cell performance at higher temperature and lower RH, and MEA durability and lifetime, lies to the higher water retention properties of the inorganic components, but mostly to the improvement in mechanical properties (5). Through collaboration between NRC (Canada) and CSIC (Spain), this work examines the effect of the processing conditions on the morphology and properties of series of hybrid
PEM materials. Several nanocomposite PEMs have been prepared using different families of functional inorganic fillers, silica nanoparticles, clay, and non-ordered mesoporous silica. These fillers present a broad range of aspect ratios and specific surface areas. Series of composite membranes were prepared using two different processes, solution-casting and melt-extrusion.
Experimental
Inorganic fillers preparation
Spherical silica nanoparticles. Silica nanoparticles 60nm in diameter used in this work were kindly provided by Dr. G. Diaz from NRC-IMI (Canada) and were synthesized via the sol-gel method. Summarizing the protocol, ethanol (100 %) and ammonium hydroxide (28 %) were stirred together in a closed container for 15 min. MilliQ water was added and the solution thoroughly mixed for another 15 min. Tetraethyl orthosilicate (TEOS) (99.99 %, Aldrich) was added and the reaction mixture was allowed to proceed at room temperature for at least 8 hours under constant stir rate of 300 rpm. The concentrations of reagents used to prepare 60nm nanoparticles are: 91.4 vol.% ethanol, 3.4 vol.% ammonium hydroxide, 1.5 vol.% MilliQ water, and 3.8 vol.% TEOS. The nanoparticles were recovered after a series of precipitations with a centrifuge and resuspensions under sonication in fresh solvent (ethanol). Centrifugation is normally carried at 9000 rpm for 45 min.
Non-ordered mesoporous silica. Mesoporous silica used in this work was kindly provided by Dr. F. Sanchez from CSIC-IQOG (Spain). High specific surface mesoporous silica (900-1100 m2/g) was prepared by sol-gel method according to the procedure described by Reale et al. (6). Non-ordered mesoporous pure silica materials were obtained from tetramethoxysilane (TMOS) as silica precursor, in a short-chain aliphatic alcohol dissolution, in presence of ammonium fluoride NH4F as condensation catalyst.
Hydrolysis and condensation of the silicon precursor were carried out under vigorous stirring at room temperature. The nature of short-chain aliphatic alcohol modulatess the size and distribution of pores in the resulting particles and after several preliminary attempts 2-propanol was selected in this work. The obtained gel was aged at 35-50ºC for 24 hours to remove the remaining free alcohol, washed with diethyl ether and finally dried at 90 ºC under efficient vacuum for 24-48 hours.
Sepiolite clay. Sepiolite is a natural mineral clay, a complex of magnesium silicate with a typical formula Mg4Si6O15(OH)2·6H2O. The one selected for this work is
in a fibrous form (Figure 1) and was used without any further treatment. Hybrid PEMs preparation
Table I lists the compositions of the samples prepared by solution-casting and melt-extrusion.
Melt extrusion. Nanocomposite PEMs were prepared using melt-processing technologies, by blending the inorganic nanoparticles and an extrusion grade of Nafion®. Thermolastic perfluorinated precursor resins in the sulfonyl fluoride (-SO2F) form
purchased from Ion-Power was used (Nafion R1000, theoretical IEC = 1 meq.g-1, measured IEC= 0.854 meq.g-1). Melt processing of nanocomposites was carried out at 240 °C, using a 5 cc micro-extruder (DSM-Explore) equipped with a film line. The die used for thin film preparation has an opening gap of 0.1 mm, and a width of 3.5 cm. The
screws RPM, the calender rolls speed and torque were varied to achieve the required thickness. The strips of composite membranes obtained had a final width of approximately 2.5 to 3 cm and a thickness in the range of 25 to 50 microns. The former hybrid membranes were converted to the acid form prior to characterization. The protocol used for conversion of melt extruded composite membranes from sulfonyl fluoride form to the acid form includes three chemical treatment steps:
1. Hydrolysis reaction where the film is converted to the salt form (K+), this step involves hydrolysis in a solution of KOH/DMSO/DI water. (15 % KOH / 35 % DMSO / 50 % de-ionized (DI) water at 80 °C for one hour)
2. Acid conversion process where the hydrolyzed polymer is converted to the acid (H+) form by exchanging the K+ for H+ ions using a solution of nitric acid (HNO3).
3. Activation by alternating treatments with solutions of H2O2 and H2SO4, and boiling
in water.
Solution casting. Casting procedure was carry out by addition of the required amount of filler to Nafion solution (Fluka 5 %, 1100 EW) and ethylene glycol (EG)(1.5 equiv compared to Nafion). EG was used to increase the viscosity of the mixture and thus avoiding filler aggregation and sedimentation. The solution was stirred for 30 min and then ultrasonicated during 10 min to achieve an optimum dispersion of the filler. After casting in petri-dishes, the solvents were evaporated in an oven at high temperature (130 ºC) under vacuum. The resulting membranes were activated as follows: immersion in boiling 3 % H2O2 for 1 hour; boiling 10 % H2SO4 for 1 hour and finally boiling H2O for 1
hour. Thicknesses of solution-cast membranes were comprised in all cases between 60-80 microns.
TABLE I. Composition of studied membranes and specific area of selected inorganic fillers.
Sample Inorganic Filler Loading
(wt%)
Filler Specific area (m2/g) Nafion - 0 - 5wt % SiO2-60nm SiO2-60nm 5 135 5wt% Sepiolite Sepiolite 5 600-650 5wt% Mesop-SiO2 Mesop-SiO2 5 900-1100 Characterization
Scanning electron microscope (SEM) images of the fillers were obtained using a Hitachi S-4700 instrument, on platinum-sputtered samples at a 10 kV acceleration voltage.
Transmission electron microscopy (TEM) was used to examine the morphology of nanocomposite membranes. The samples were prepared by placing the thin films into epoxy resin. The cured epoxies containing the membranes were then microtomed at room temperature into thin slices of 50-80 nm using a diamond knife. TEM of ultrathin sections of the polymer/silica hybrid samples were obtained with a Philips CM 200 instrument with an acceleration voltage of 200 kV.
Water uptake (WU) was determined by equilibrating the membranes in deionised water at room temperature after the activation treatment. The membranes were then removed from the water container, quickly dry wiped and immediately weighed. Subsequently, they were dried overnight under vacuum at 80 °C temperature and weighed again. The water content was calculated from the weight difference of the
membrane (in H+ form) in its hydrated and dry state. Three measurements were carried out for each formulation.
Wet mass - Dry mass
Water Uptake (%)= ×100
Dry mass
[1]
Volume change was determined by measuring the thickness, width and length of wet (water equilibrated) and dried membranes (in H+ form). Dimensions of dried membranes were determined by drying them in a vacuum oven at 80 °C overnight. Four measurements were carried out for each formulation.
Volume of wet membrane - Volume of dry membrane
Volume Change (%)= ×100
Volume of dry membrane [2]
Conductivity at reduced humidity: A Bekktech sample punch was used to cut a piece from the membranes for testing. The dimensions of the samples to be tested were approximately 5 mm x 25 mm. In-plane proton conductivities were measured using a Solartron Impedance Analyser 1260 and a Bekktech in-plane conductivity cell with platinum electrodes. A strip of membrane (in H+ form) was set between 2 platinum (Pt) electrodes and an alternating current was passed through the plane of the sample. The cell was placed in an ESPEC-SH261 environmental chamber with controlled humidity and temperature. Nyquist plots between 5 MHz to 10 Hz were collected and membrane resistances were extrapolated by fitting the semi-circle part of the data to Randles equivalent circuit. Proton conductivities were calculated from the equation:
L σ=
R×A [3]
where σ is proton conductivity, L is the distance between the Pt electrodes, R is membrane resistance and A is the cross-sectional area of the strip. The samples were allowed to equilibrate in the chamber measuring the conductivity every 4 minutes. The membranes generally equilibrate after 30 min to 1 hour. The conductivity is calculated from the average measurement of the equilibration plateau.
Thermal stability of nanocomposite membranes was studied using a TA Instruments TGAQ500 Thermogravimetric Analyzer. Samples were heated at a rate of 10ºC.min-1 in air atmosphere.
Differential Scaning Calorimetry (DSC) technique was used to study the state of water in the composite membranes. For melt extruded samples, a TA Instruments Thermal Analyzer was used, every sample previously equilibrated in water was quickly wiped and hermetically sealed in a pan, rapidly cooled inside the DSC chamber at -95 ºC and then heated to 100 ºC using a scan rate of 15 ºC/min. For hybrid solution cast membranes, a Mettler 400 DSC was used. A wet sample was hermetically sealed, rapidly cooled inside the DSC chamber at -50 ºC and then heated at 50 ºC using a heating rate of 15 ºC/min. Freezable water percentage is determined from the melting enthalpy peak observed at around 0 ºC in the DSC curves using the following equation: (334 j/g being the enthalpy of fusion of pure water)
DSC sample ΔH (mj) Freezable Water(%)= /334(j/g) ×100 W (mg) ⎧⎛ ⎞ ⎫ ⎪ ⎪ ⎜ ⎟ ⎨⎜ ⎟ ⎬ ⎪⎝ ⎠ ⎪ ⎩ ⎭ [4]
The thickness of the membranes was measured with a high precision Mitutoyo Gauge which applies a constant pressure of 3.5 Newtons to the sample using a non-rotating stem was used. Rated accuracy is ± 3 microns.
Results and discussion
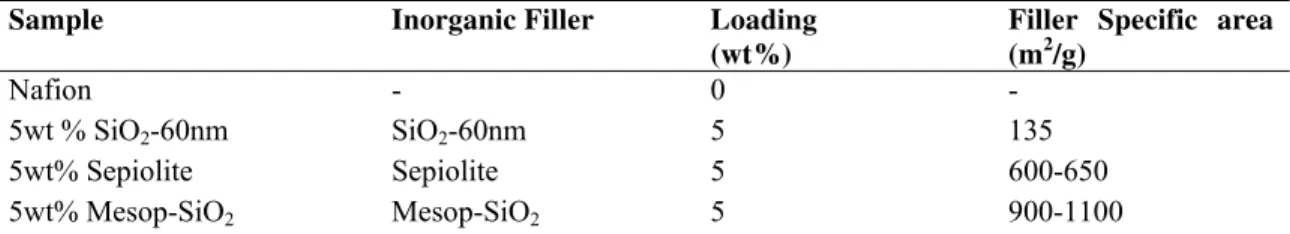
The inorganic fillers used for this comparative study have been characterized by SEM to determine their morphology. Figure 1 shows SEM micrographs obtained; sample SiO2
-60nm shows monodisperse spherical nanoparticles with a diameter around 60 nm and a surface area of 135 m2/g (determined from BET analysis), Sepiolite sample shows a very high aspect ratio fiber-like morphology with 50-60 nm width and few microns length (600-650 m2/g surface area), while mesoporous silica sample shows large non-ordered micro-particles with a layered structure and a very high surface area of 900-1100 m2/g. The composition of the studied hybrid membranes are shown in Table 1.
Figure 1. SEM micrographs on selected inorganic fillers.
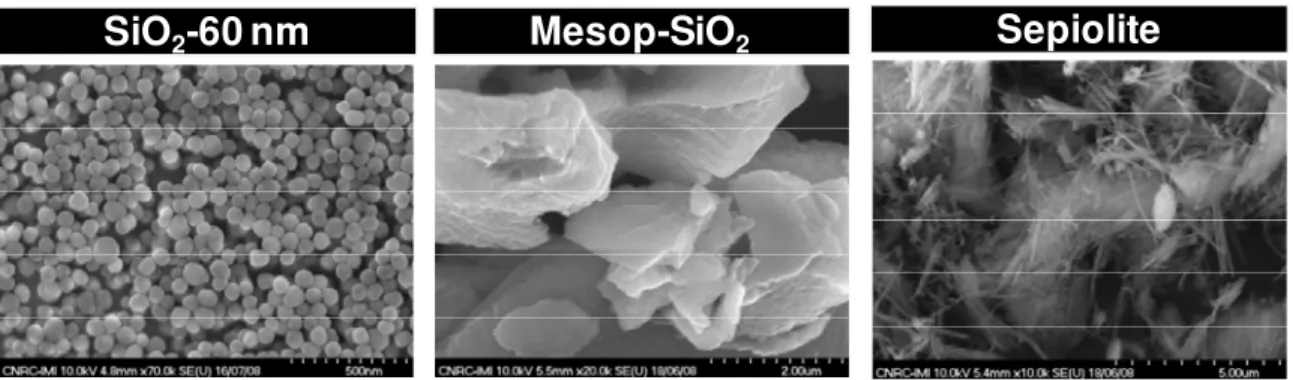
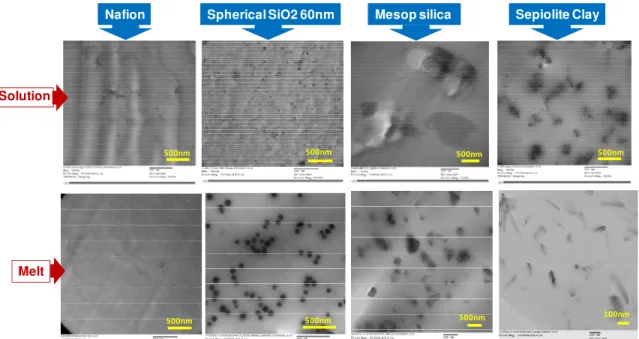
TEM technique represents a powerful analysis tool to examine the morphology of the polymer nanocomposites. The hydrophobic/hydrophilic phase separation within the ionomer, dispersion of the filler as well as the presence of agglomerated clusters or defects can be assessed. High resolution TEM images of Pb2+ stained Nafion membranes prepared by solution-casting and melt-extrusion are presented in Figure 2. The fine phase separation of hydrophilic and hydrophobic domains characteristic of PFSA ionomers is visible in both cases. For solution-cast film, ordered and aligned ionic domains agglomerated in dark spheres ranging from 3 to 10 nm in diameter embedded in a pale background of hydrophobic fluoropolymers can be observed. The very clear regions could suggest free volume left by solvent evaporation. In the case of melt processed films, ionic domains are smaller and uniform (4 to 6 nm), less ordered, and more interconnected.
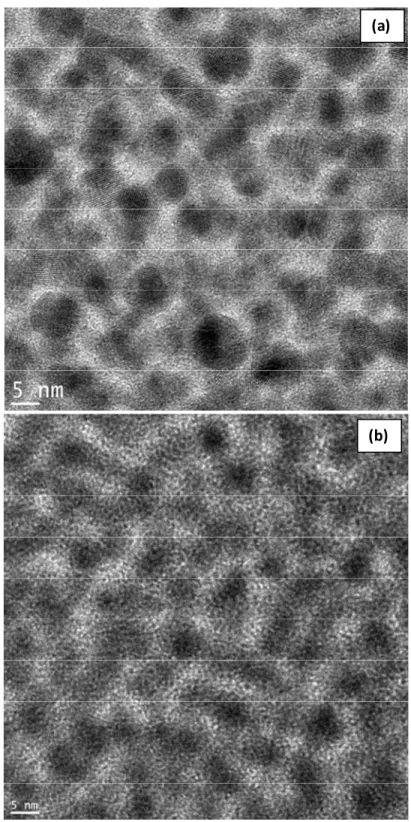
Figure 3 shows representative unstained TEM micrographs for the series of hybrid membranes studied as well as cast and melt reference Nafion membranes without fillers. Nanocomposites with 5 wt% SiO2-60nm particles typically showed a very good
and efficient dispersion of nanoparticles with both preparation processes, individual nanoparticles can be distinguished. Sepiolite clay seems to be much more exfoliated with melt processing technique, where individual platelets are observed, while slightly larger agglomerates can be observed for solution cast membranes, but still well dispersed. However, solution-cast hybrid membrane with 5 wt% mesoporous silica shows very poor dispersion and micron size large particles can be distinguished, while counter-rotation
screw configuration used for melt-extruded sample provides a high shear stress that reduces the particle size and allows a much more dispersive mixing. These differences in morphology are expected to have an impact on the other properties of PEMs.
Figure 2. TEM on lead acetate stained (left) solution cast and (right) melt extruded Nafion samples.
(a)
Figure 3. Representative TEM micrographs of melt-extruded and solution-cast membranes without and with selected fillers.
The thermogravimetric analysis of the hybrid membranes was carried out to study their degradation and assess their thermal stability. Figure 4 compares thermograms obtained for two hybrid membranes with 5 wt% Sepiolite and Mesoporous Silica preparaed by solution-casting and melt-extrusion. The results show in general a three-step degradation pattern, typical for sulfonated polymers: dehydration below 250 ºC, desulfonation from 300 to 350 ºC and degradation above 400 ºC (Figure 4). The results shows that nanocomposite membranes base on 5 wt% sepiolite clay, show similar thermal stability independently of the process of fabrication, while hybrid membranes based on 5 wt% mesoporous silica show higher thermal stability when melt extrusion is used for membrane preparation.
Figure 4. Comparison of TGA thermograms obtained for hybrid membranes with sepiolite ans sesoporous silica fillers prepared by melt-extrusion and solution-casting.
Spherical SiO2 60nm Mesop silica Sepiolite Clay
Solution Nafion Melt 500nm 500nm 500nm 500nm 500nm 500nm 500nm 100nm 0 20 40 60 80 100 25 125 225 325 425 525 625 725 825 We ig h t los s (wt % ) Temperature (oC) 5wt% Sep‐Cast 5wt% Mesop‐Cast 5wt% Sep‐Melt 5wt% Mesop‐Melt
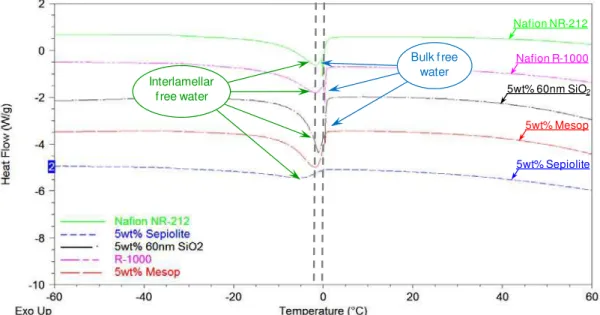
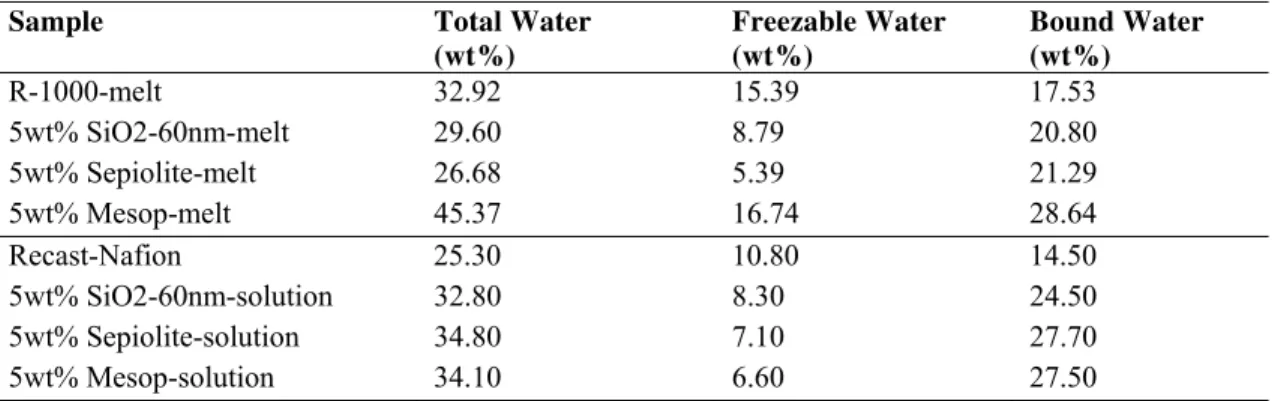
The state of water in the hybrid membranes have been studied using DSC technique and water uptake measurements (7). Proton conductivity depends on the water absorption, the hydration of the polymer and the transport of protons in PEM are known to be critical factors. The state of water in hydrated membranes can be broadly classified into two groups, which are respectively freezable water and bound water. Water equilibrated membranes have been analyzed by DSC. From the melting enthalpy peak observed in the thermograms around 0 °C, the percentage of free water can be approximated according to the equation [4] described in the experimental part. The double peak suggests two states of freezable water, free water that occurs at 0 °C, and interlamellar water at slightly lower temperature as shown in Figure 5 for melt extruded series of membranes. The melting temperature of the interlamellar water shifts to lower temperature for some membranes. The lower the melting temperature implies the increased interaction between water and the polymer matrix. The bound water in the membranes is calculated from the difference between total water and free water contents, all the results are represented in table II.
Figure 5. DSC on series of extruded nanocomposites PEMs with different surface area inorganic fillers.
The results in Table II show that solution cast hybrid membranes have higher total water content than extruded composite membranes, neat polymer show the opposite trend. The amount of bound water is higher for composite membranes in all cases, as a consequence of the incorporation of hydrophilic fillers to the polymer. Since bound water will evaporate at higher temperatures compared to free water, the results suggest that the fillers retain more water molecules in the membrane’s structure. In general, solution-cast composite membranes show higher total water content and bound water compared to melt-extruded membranes. An interesting observation is the increase in bound water while specific surface area of the filler increases.
Bulk f ree water Interlamellar f ree water Nafion NR-212 Nafion R-1000 5wt% 60nm SiO2 5wt% Mesop 5wt% Sepiolite
TABLE II. Different states of water in reference and composite PEMs determined from DSC measurements
Sample Total Water
(wt%) Freezable Water (wt%) Bound Water (wt%) R-1000-melt 32.92 15.39 17.53 5wt% SiO2-60nm-melt 29.60 8.79 20.80 5wt% Sepiolite-melt 26.68 5.39 21.29 5wt% Mesop-melt 45.37 16.74 28.64 Recast-Nafion 25.30 10.80 14.50 5wt% SiO2-60nm-solution 32.80 8.30 24.50 5wt% Sepiolite-solution 34.80 7.10 27.70 5wt% Mesop-solution 34.10 6.60 27.50
Water uptake and dry/wet volume change results of the nanocomposite PEMs are presented in Figure 6. Composite PEMs show in general higher water uptake than reference membranes, while solution cast membranes show in general higher water uptake than extruded ones. With both manufacturing processes, an increase in the water uptake increases with the specific area of the filler. These results translate to lower volume change for the fillers with low specific area. In this case the composite membranes with 60 nm silica nanoparticles, shows the better dimensional stability independently from the process used for membranes manufacturing.
Figure 6. Water uptake (left) and Dry/wet volume change (right) measured for series of solvent-cast and melt-extruded references and composite PEMs.
Figure 7 shows the preliminary conductivity results obtained for two series of hybrid PEMs prepared by solution casting and melt-extrusion measured at room temperature in water, and 80 °C at two relative humidities; 50 and 30 %. It shows that at high levels of hydration (100 %), solution cast membranes have in general similar or higher conductivity than extruded samples, except for solution cast 5 wt% sepiolite membranes that shows exceptionally low conductivity at RT in water, and could not be measured at high temperature because of the weak mechanical properties. At 80 °C and 50 %RH, extruded and cast references still show similar conductivities (22 mS.cm-1), while extruded composite PEMs shows improved conductivity compared to those prepared by solution-casting, 60 nm SiO2 filler with the low aspect ratio and specific area shows the
highest conductivities for both solution-cast (21 mS.cm-1 at 80 °C/50 %RH) and melt-extruded composites (38 mS.cm-1 at 80 °C/50 %RH). At 80 °C and 30 %RH, extruded and cast references show clear differences in conductivity; 1.4 mS.cm-1 for recast Nafion
0.00 10.00 20.00 30.00 40.00 50.00 Wa te r upt a ke (%) Melt‐extruded Solution‐Cast 40 50 60 70 80 90 Vo lu m e ch an ge (%) Melt‐extruded Solution‐Cast
and 5.3 mS.cm-1 for extruded R-1000. The higher density and connectivity of hydrophilic domains in melt processed membranes suggested from TEM analysis might be an explanation. Extruded composite PEMs shows improved conductivity compared to those prepared by solution-casting, 60 nm SiO2 filler with the low aspect ratio and specific area
shows the highest conductivities for both solution-cast (4.9 mS.cm-1 at 80 °C/50 %RH) and melt-extruded composites (10.3 mS.cm-1 at 80 °C/50 %RH).
Figure 7. Ex-situ in-plane proton conductivity measured for series of solvent-cast and melt extruded PEMs at RT/in water, 80 °C/50 %RH and 80 °C/30 %RH.
Conclusions
This study examined the relationships between structure/morphology and properties of series of composite proton exchange membranes prepared using two processing techniques; solution-casting and melt-extrusion. Three families of inorganic fillers with different aspect ratios and specific areas have been used. The results suggest that for low aspect ratio nano-sized particles, both processes show efficient dispersion of the filler. For higher dimension particles, melt-extrusion provides a high shear stress that reduces the particle size and allows a much more dispersive mixing. Composite PEMs show in general higher water uptake than reference membranes, while solution cast membranes show in general higher water uptake than extruded ones. With both manufacturing processes, an increase in the water uptake increases with the specific area
0.0100 0.1000 1.0000 C o nduc ti v it y (S /c m ) Melt‐extruded Solution‐Cast RT in water 0.0010 0.0100 0.1000 C o n d u ctiv ity (S /c m ) 80°C, 50%RH 0.0001 0.0010 0.0100 0.1000 C o nduc ti v it y (S/ cm ) 80°C, 30%RH
of the filler. These results translate to lower volume change and higher dimensional stability for the fillers with low specific area and for extruded samples in general. Finally conductivity results show that at high levels of hydration, solution-cast membranes have in general higher conductivities than extruded samples, while at high temperature and reduced relative humidity conditions, melt extruded samples show higher conductivities.
Acknowledgments
The authors thank Dr. G. Diaz from IMI-NRC and Prof. F. Sanchez from IQOG-CSIC for providing functional fillers, and the NRC-CSIC Collaborative Research Program for the financial support.
References
1. K.T. Adjemian, R. Dominey, L. Krishnan, H. Ota, P. Majsztrik, T. Zhang, J. Mann, B. Kirby, L. Gatto, M. Velo-Simpson, J. Leahy, S. Srinivasan, J.B. Benziger, and A.B. Bocarsly, Chem. Mater., 18, 2238 (2006).
2. L. Wang, D. Zhao, H. M. Zhang, D. M. Xing, and B. L. Yia, Electrochem.
Solid-State Lett., 11, B201 (2008).
3. E. Burgaz, H. Lian, R.H. Alonso, L. Estevez, A. Kelarakis, E.P. Giannelis, Polymer,
50, 2384 (2009).
4. P.Y. Vuillaume, A. Mokrini, A. Siu, K. Théberge, and L. Robitaille, Europ. Polym.
J., 45 (6), 1641 (2009).
5. D.J. Jones, J. Rozière, Adv. Polym. Sci., 215, 219 (2008).
6. E. Reale, A. Leyva, A. Corma, C. Martinez, H. Garcia and F. Rey, J. Mater. Chem.,
15, 1742 (2005).
7. S.M. Javaid Zaidi, and T. Matsuura, Polymer Membranes for Fuel Cells, eds., Springer (2009).