AJH 1991; 4:438-443
In Vitro Effects of DuP 753, a Nonpeptide
Angiotensin II Receptor Antagonist, on Human
Platelets and Rat Vascular Smooth Muscle Cells
Michel Burnier, Gabriel Centeno, Eric Grouzmann, Philippe Walker, Bernard Waeber, and
Hans R. Brunner
T h e s e experiments w e r e d e s i g n e d to assess the ability of the n e w n o n p e p t i d e a n g i o t e n s i n II antag onist D u P 753 to i n h i b i t the b i n d i n g and, particu larly, to antagonize the cellular response to a n g i o t e n s i n II i n h u m a n platelets a n d primary cultures of rat aortic s m o o t h m u s c l e cells (SMC). T h e b i n d i n g of 1 2 5I - a n g i o t e n s i n II w a s c o m p e t i t i v e l y i n h i b i t e d
b y D u P 753 w i t h a 50% b i n d i n g i n h i b i t i o n (IC^) of 5 to 6 X 1 0 "8 m o l / L i n platelets and 1 X 1 0 "8 m o l / L
i n vascular S M C as compared to an I C ^ of 5 to 7.5 X 1 0 ~9 m o l / L w i t h n o n l a b e l e d a n g i o t e n s i n II. In v a s
cular S M C , D u P 753 c o m p l e t e l y a b o l i s h e d the ef
fects of a n g i o t e n s i n II o n 4 5C a C l2 efflux a n d 4 5C a C l2
uptake. Moreover, i n t h e s e latter cells, D u P 753 p r e v e n t e d the a n g i o t e n s i n II but not the v a s o p r e s s i n i n d u c e d increase i n cytosolic calcium. T h e s e results demonstrate that D u P 753 competes w i t h a n g i o t e n s i n II b i n d i n g to its receptor i n b o t h a n i m a l a n d h u m a n cells and s e l e c t i v e l y blocks the cellular re s p o n s e to a n g i o t e n s i n II. A m J H y p e r t e n s 1991;4:438-443
KEY W O R D S : D u P 753, a n g i o t e n s i n II, antagonist, platelets, s m o o t h m u s c l e cells.
B
lockade of t h e renin-angiotensin system (RAS) h a s b e c o m e in recent years a very effective a p p r o a c h for t h e t r e a t m e n t of h y p e r t e n s i o n a n d congestive h e a r t f a i l u r e .1 - 4 Today, t h e cascadeof t h e RAS leading to t h e generation of angiotensin II (Ang II) a n d finally to t h e activation of t h e angiotensin II receptor can b e i n t e r r u p t e d at different sites either b y inhibition of r e n i n or angiotensin converting e n z y m e (ACE) or by direct competition w i t h t h e b i n d i n g of a n giotensin II to its r e c e p t o r .5 - 7
Historically, saralasin, a p e p t i d e analog of A n g II, w a s t h e first p o t e n t a n d specific receptor antagonist to b e u s e d to block t h e activity of t h e R A S .7 This d r u g allowed
From the Hypertension Division and Cardiovascular Research Group, University Hospital, Lausanne, Switzerland.
This work w a s supported by the S w i s s National Foundation (No. 3.816-0.87) and the Cardiovascular Research Group Foundation.
These results were presented at the American Society of Hyperten sion annual meeting, May 18 to 2 1 , 1990.
Address correspondence and reprint requests to Michel Burnier, M D , Division d'Hypertension CHUV, 1011 Lausanne, Switzerland.
t h e crucial role of t h e RAS in t h e d e v e l o p m e n t a n d m a i n t e n a n c e of h y p e r t e n s i o n in a n i m a l m o d e l s8-9 a n d in
h u m a n forms of h y p e r t e n s i o n to b e d e m o n s t r a t e d .1 0 - 1 2
T h e clinical use of this receptor antagonist, h o w e v e r , h a s b e e n limited by its lack of oral bioavailability, its short d u r a t i o n of action, a n d b y its i n h e r e n t agonistic a c t i v i t y .1 0 - 1 2
Recently, several n o n p e p t i d e imidazole A n g II recep tor antagonists h a v e b e e n p r o d u c e d .1 3 1 4 These com
p o u n d s exhibit a h i g h affinity for A n g II b i n d i n g sites in r a t s .1 5 Moreover, t h e y h a v e b e e n s h o w n to decrease
blood pressure in animal m o d e l s of h y p e r t e n s i o n partic ularly in those associated w i t h a n increased activity of t h e R A S .1 5 1 6 D u P 753 is o n e of these n e w orally active
A n g II receptor antagonists t h a t effectively inhibit Ang II b i n d i n g in v i t r o1 5 1 7 a n d exert functional A n g II a n t a g o
n i s m in rabbit a o r t a .1 7 Oral administration of D u P 753 to
renal h y p e r t e n s i v e rats causes a d o s e - d e p e n d e n t de crease in blood p r e s s u r e .1 5 - 1 8 In h e a l t h y h u m a n volun
teers, D u P 753 given orally abolishes t h e blood pressure r e s p o n s e to exogenous A n g I I .1 9
AJH-MAY 1991-VOL 4, NO. 5, PART 1 I N V I T R O EFFECTS O F D u P 753 439
T h e p r e s e n t experiments w e r e designed to s t u d y t h e ability of D u P 753 to inhibit t h e b i n d i n g of A n g II in h u m a n platelets m u c h as it does in rat vascular s m o o t h muscle cells. In addition, t h e capacity of D u P 753 to a n t a g o n i z e t h e cellular calcium r e s p o n s e to A n g II w a s e v a l u a t e d in p r i m a r y cultures of rat aortic s m o o t h m u s cle cells.
M E T H O D S
B i n d i n g S t u d i e s Angiotensin II b i n d i n g w a s per formed b o t h o n freshly isolated h u m a n platelets a n d p r i m a r y cultures of rat aortic s m o o t h muscle cells (SMC).
Angiotensin II Binding in Human Platelets Blood w a s
d r a w n from t h e forearm veins of h e a l t h y volunteers a n d anticoagulated w i t h s o d i u m citrate. Platelets w e r e t h e n isolated as described b y Le Q u a n S a n g a n d D e v y n c k .2 0Platelet-rich p l a s m a w a s w a s h e d twice at r o o m t e m p e r a ture in M e d i u m 199 containing 5 m m o l / L EDTA, 0 . 2 % b o v i n e s e r u m a l b u m i n (BSA), 10 m m o l / L HEPES a n d 1 m g / m L bacitracin ( p H 7.4, 22 °C) (assay buffer). T h e platelets w e r e c o u n t e d automatically a n d t h e v o l u m e w a s adjusted to yield a concentration of 1 06 cells///L.
Binding studies w e r e performed as described b y M a n n et a l .2 1 In brief, platelets w e r e i n c u b a t e d for 3 h w i t h
0.1 n m o l / L 1 2 5I - a n g i o t e n s i n II in a final v o l u m e of
100 ßL at 3 7 ° C in a s h a k i n g w a t e r b a t h . Incubations w e r e s t o p p e d b y dilution w i t h assay buffer at 4 ° C a n d centrifugation at 10000 g for 5 m i n . T h e s u p e r n a t a n t w a s discarded a n d t h e platelets w a s h e d twice in m e d i u m 199 at 4 ° C . Radioactivity w a s c o u n t e d in a y-counter. Nonspecific b i n d i n g w a s assessed b y a d d i n g u n l a b e l e d angiotensin II at 1 0 "6 m o l / L . Displacement
of specifically b o u n d 1 2 5I - a n g i o t e n s i n II w a s performed
with increasing concentrations of A n g II, D u P 753 (Du P o n t d e N e m o u r s , Wilmington, DE) a n d a n o n r e l e v a n t peptide ( m o r p h i n e m o d u l a t i n g peptide, M M P ) .
Angiotensin II Binding in Vascular Smooth Muscle Cells
Primary cultures of rat aortic s m o o t h muscle (6th to 7th passage) cultured in Dulbecco's Modified Eagle M e d i u m (DMEM) w i t h 1 5 % fetal calf s e r u m w e r e u s e d for these studies. Binding w a s performed 3 to 5 d a y s after plating t h e cells o n 3.5 c m dishes. At confluence, t h e cells w e r e w a s h e d w i t h t h e assay buffer ( M e d i u m 199 containing 0 . 2 % BSA, 1 m g / m L bacitracin, a n d 10 m m o l / L HEPES). They w e r e t h e n i n c u b a t e d for 1 h at 3 7 ° C w i t h 0.01 n m o l / L 1 2 5I - a n g i o t e n s i n II. T h e experiments w e r e t e r m i n a t e d b y aspirating t h e s u p e r n a tant a n d w a s h i n g t h e cells three times w i t h t h e b i n d i n g buffer. T h e cells w e r e r e m o v e d from t h e plates w i t h a lysis buffer containing 0 . 1 % s o d i u m dodecyl s u l p h a t e (SDS), 2 % N a2C 03 a n d 0.1 Ν N a O H a n d scraping w i t h
a " r u b b e r p o l i c e m a n . " Radioactivity w a s c o u n t e d in a y-counter. Displacement curves w e r e performed w i t h
increasing concentrations of u n l a b e l e d angiotensin II, D u P 753, or saralasin.
C a l c i u m S t u d i e s i n V a s c u l a r S m o o t h M u s c l e C e l l s Primary cultures of rat aortic S M C (7th to 10th passage) g r o w n to confluence in D M E M w e r e u s e d to s t u d y t h e ability of D u P 753 to interfere w i t h t h e cellular r e s p o n s e to A n g II.
45Ca2+ Efflux T h e culture m e d i u m w a s r e m o v e d b y aspi ration a n d t h e cells w a s h e d twice w i t h a physiologic saline solution (PSS) a n d l o a d e d w i t h 5 //Ci of 4 5C a C l2 in
1 m L P S S at 3 7 ° C for 3 h . After loading, t h e cultures w e r e rapidly rinsed five times a n d a n o t h e r 1 m L of fresh PSS w a s a d d e d . T h e s u p e r n a t a n t w a s r e m o v e d a n d r e placed w i t h 1 m L PSS every 30 sec for 6 m i n . T h e direct effects of A n g II or D u P 753 w e r e e v a l u a t e d b y a d d i n g t h e p e p t i d e or t h e d r u g at 3 m i n . In s o m e experiments, p r e t r e a t m e n t w i t h D u P 753 w a s d o n e b y a d d i n g various concentrations of t h e antagonist in t h e P S S buffer con tinuously from time 0. T h e a m o u n t of 4 5C a2 + lost from
t h e cells in 12 consecutive 30 sec intervals a n d t h e a m o u n t r e m a i n i n g inside t h e cells at t h e e n d of t h e 6 m i n experiment w a s m e a s u r e d b y liquid scintillation counting. T h e results are expressed as t h e p e r c e n t a g e of t h e total 4 5C a2 + activity released in t h e s u p e r n a t a n t d u r
ing a 30 sec time interval.
45Ca2+ Uptake T h e culture m e d i u m w a s r e m o v e d a n d t h e cells w a s h e d several times w i t h fresh PSS. T h e cells w e r e allowed to equilibrate for 3 h in this n e w buffer. T h e y w e r e t h e n i n c u b a t e d for 5 m i n w i t h 2 //Ci 4 5C a C l2
in t h e presence or absence of a n y effector in 1 m L PSS. To terminate u p t a k e , extracellular 4 5C a C l2 w a s r e m o v e d
by w a s h i n g t h e cells rapidly five times w i t h cold P S S containing 2 m m o l / L EDTA. T h e cells w e r e lysed w i t h t h e lysis buffer described a b o v e a n d t h e intracellular radioactivity d e t e r m i n e d b y liquid scintillation count ing. P r e t r e a t m e n t w i t h D u P 753 w a s d o n e b y a d d i n g various concentrations of t h e antagonist ( 1 0 ~7 to
1 0 ~4 m o l / L ) d u r i n g t h e equilibration period. In o n e set
of experiments, t h e cells w e r e rinsed three times before t h e u p t a k e w a s started. Protein w a s d e t e r m i n e d in all studies b y t h e m e t h o d of Lowry et a l .2 2
Determination of Cytosolic Calcium [Ca]{ Confluent m o n o l a y e r cultures of rat aortic SMC w e r e h a r v e s t e d b y gently scraping t h e dish w i t h a " r u b b e r p o l i c e m a n . " T h e cells w e r e centrifuged for 5 m i n at 200 g a n d resus-p e n d e d in DMEM. Fura-2 A M (Molecular P r o b e s , Eugene, OR) w a s a d d e d at a final concentration of 2.5 / / m o l / L from a stock solution in d i m e t h y l sulfoxide. T h e cells w e r e t h e n i n c u b a t e d for 30 m i n in t h e dark at r o o m t e m p e r a t u r e . After loading, t h e cells w e r e w a s h e d once w i t h fresh culture m e d i u m a n d r e s u s p e n d e d in PSS at a concentration of 2 X 1 06 c e l l s / m L . T h e Fura-2
fluorescence w a s m e a s u r e d o n a spectrofluorometer (LS 5, Perkin Elmer, O a k b r o o k , IL). T h e cells w e r e kept in
440 B U R N I E R ET A L AJH-MAY 1991-VOL 4, NO. 5, PART 1
s u s p e n s i o n b y gentle stirring at 3 7 ° C . Stimulation of t h e cells w a s p e r f o r m e d w i t h various agents including a n giotensin II, vasopressin, or D u P 7 5 3 . M a x i m u m fluores cence w a s o b t a i n e d b y addition of Triton X a n d m i n i m u m fluorescence b y chelation of Ca w i t h a n excess of EDTA. T h e calculations of [Ca]j w e r e p e r f o r m e d accord ing to Grynkiewicz et al w i t h o u t corrections for extra cellular C a .2 3
Cytosolic calcium w a s also d e t e r m i n e d in h u m a n platelets using t h e fura-2 m e t h o d described a b o v e . For c o m p a r i s o n s o m e m e a s u r e m e n t s h a v e b e e n d o n e w i t h t h e Q u i n - 2 m e t h o d r e p o r t e d earlier.2 4
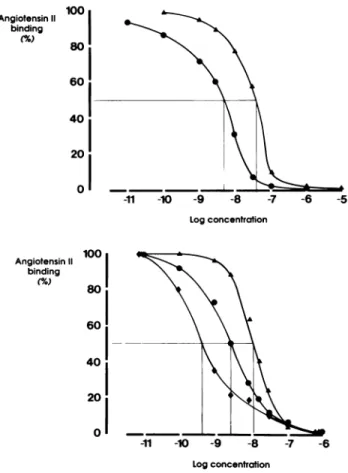
S t a t i s t i c a l A n a l y s i s Data are p r e s e n t e d as m e a n ± SEM. Statistical analysis w a s p e r f o r m e d w i t h a o n e - w a y analysis of variance. AP < .05 w a s considered signifi cant. R E S U L T S I n t e r a c t i o n w i t h A n g II B i n d i n g T h e displacement of 1 2 5I - A n g II b y cold angiotensin II or D u P 753 in h u m a n platelets is s h o w n in t h e u p p e r p a n e l of Figure 1. A n g II c o m p e t e s w i t h t h e b i n d i n g of radiolabeled A n g II w i t h a 5 0 % b i n d i n g inhibition ( I C5 0) at 7.5 X 1 0 "9 m o l / L . T h e -11 -10 -9 -8 -7 -6 -5 Log concentration Log concentration
FIGURE 1. Effects of angiotensin II, DuP 753 or saralasin on 125I-angiotensin binding to human platelets (upper panel, n = 6 in
each curve) or rat aortic smooth muscle cells (lower panel, n = 3 in each curve). · angiotensin II, A DuP 753, • saralasin.
b i n d i n g of 1 2 5I - A n g II is displaced b y D u P 753 w i t h an
I C5 0 of 5 to 6 X 1 0 ~8 m o l / L . In platelets, n o displace
m e n t of t h e labeled A n g II h a s b e e n o b t a i n e d with M M P , a n o n r e l e v a n t p e p t i d e . Similar results h a v e b e e n f o u n d in rat aortic SMC as s h o w n o n t h e lower p a n e l of Figure 1. In this cell type, t h e I C5 0 for cold A n g II is at
5 X 1 0 ~9 m o l / L . D u P 753 inhibits t h e A n g II b i n d i n g
w i t h a n I C5 0 of 1 X 1 0 ~8 m o l / L , w h e r e a s saralasin com
petes w i t h A n g II w i t h a higher affinity ( I C5 0 of 5 to
7 X 1 0 ~1 0 m o l / L ) .
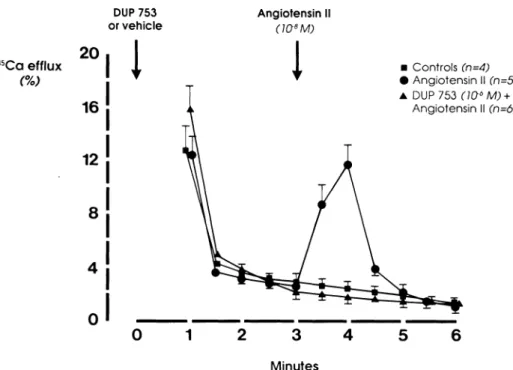
C a l c i u m S t u d i e s As s h o w n in Figure 2, angiotensin II ( 1 0 ~8 m o l / L ) induces a significant increase in 4 5C a2 +
efflux in rat aortic SMC w h e n c o m p a r e d to t h e u n treated cells. This calcium efflux is completely blocked b y p r e t r e a t m e n t w i t h D u P 753 ( 1 0- 6 m o l / L ) . D u P 753
h a s n o effect, per se, o n calcium efflux in these cells (data n o t s h o w n ) .
A significant increase in 4 5C a2 + u p t a k e is also o b
served in aortic SMC after exposure to A n g II, a n effect w h i c h can b e p r e v e n t e d b y p r e i n c u b a t i o n of t h e cultures w i t h D u P 753 (Figure 3). Interestingly, w h e n t h e cells are w a s h e d three times after incubation w i t h D u P 753, h i g h e r doses of D u P 753 ( 1 0 ~4 m o l / L instead of
1 0 "6 m o l / L ) are n e e d e d to obtain a n inhibition of t h e
A n g II effect. In these conditions, at c o m p a r a b l e molar ity ( 1 0 ~6m o l / L ) , saralasin a p p e a r s again to h a v e a
h i g h e r receptor affinity t h a n D u P 7 5 3 .
In addition to t h e inhibition of t h e A n g II-induced increases in calcium fluxes, D u P 753 also d o s e d e p e n -dently antagonizes t h e increase in cytosolic free calcium p r o d u c e d by A n g II (Figure 4). Indeed, cytosolic calcium increases from 144.3 ± 6.6 n m o l / L (n = 13) to 367.4 ± 7.2 n m o l / L (n = 8, Ρ < .001) after stimulation w i t h A n g II ( 1 0 " 7 m o l / L ) . P r e t r e a t m e n t w i t h D u P 753
( 1 0 ~4 m o l / L ) b l u n t s t h e calcium r e s p o n s e to A n g II w i t h
a [Ca]j at 169.9 ± 16 n m o l / L (P < .001 ν A n g II). W i t h a lower dose of t h e antagonist ( D u P 7 5 3 , 1 0 ~6 m o l / L ) , t h e
A n g II stimulation is only partially a t t e n u a t e d resulting in a [Ca]i at 240 ± 6.5 n m o l / L (P < .01). Again, t h a t D u P 753 h a s n o effect, p e r se, e v e n at a h i g h concentra tion ( 1 0 ~4 m o l / L ) suggests t h a t t h e c o m p o u n d h a s n o
agonistic effect. In these cells, D u P 753 does n o t affect t h e increase in cytosolic calcium i n d u c e d b y 1 0 ~7 m o l / L
vasopressin (389 ± 35 n m o l / L , η = 4).
In h u m a n platelets, n o significant c h a n g e in cytosolic calcium w a s observed after stimulation w i t h a n g i o t e n sin II w h e n [Ca]| w a s d e t e r m i n e d w i t h t h e Fura-2 or t h e Q u i n - 2 m e t h o d s .
D I S C U S S I O N
Since stimulation of t h e angiotensin II receptor r e p r e sents t h e ultimate step in t h e physiologic activation of t h e RAS, inhibition of angiotensin II b i n d i n g b y a s p e cific receptor antagonist a p p e a r s to b e a logical a n d ef fective a p p r o a c h to decreasing t h e activity of this p o t e n t
AJH-MAY 1991-VOL 4, NO. 5, PART 1 IN VITRO EFFECTS OF DuP 753 441
DUP 753
or vehicle Angiotensin I (ΐσβΜ)
4 5C a efflux (%)
FIGURE 2. Effects of DuP 753 on
the changes in 45CaCl2 efflux in
duced by angiotensin II in rat aortic smooth muscle cells.
2 0 16 12 8
I
• C o n t r o l s (n=4) • A n g i o t e n s i n II (n=5) A DUP 753 (ia6M) + A n g i o t e n s i n II (n=6) 3 Minutesvasoconstrictor system. DuP 753 is a new nonpeptide antagonist of angiotensin ΙΙ.1 5-1 7-1 8-2 6 ln contrast to the
peptidic analogs that have been used in the past to block the RAS, this compound has a long duration of action, can be administered orally and seems to be devoid of any agonistic effect.1 5 1 7"1 9 , 2 6
The ability of DuP 753 to compete with angiotensin II at the receptor has been demonstrated in animal tissues such as rat adrenal cortical microsomes and rat aortic smooth muscle cells.1 7 In these two preparations, spe
cific angiotensin II binding was inhibited by DuP 753 with an IC5 0 of about 2 X 10~8 nmol/L. In the present
experiments, angiotensin II binding was performed
both in primary cultures of rat aortic SMC and in human platelets to assess the ability to block receptors located in animal as well as in human cell types. In our smooth muscle cells, DuP 753 inhibited the Ang II binding with an IC5 0 of 1 X 1 0 "8 mol/L, a value which is comparable
to those obtained in the above-mentioned tissues.1 7 This
new antagonist had less affinity for the angiotensin II receptor than saralasin, a finding which is also consist ent with previous observations.1 7 The antagonist dis
placed 100% of the specific binding which suggests the presence of only one receptor type in these SMC.
Today, there is still little evidence available that DuP 753 acts as a specific antagonist to angiotensin II in
Continuous exposure to
the antagonist Pre-treatment with the antagonist + washes
FIGURE 3. Effects of DuP 753 or
saralasin on the changes in 45CaCl2
uptake induced by angiotensin II in rat aortic smooth muscle cells. The cells were either continuously ex posed to the antagonist orpretreated with the antagonist and washed be fore stimulation with angtiotensin 11. " C a uptake 200 (cpm/mgprot. 1Q3) 150 100 M e a n + SEM e , ,p< 0 . 0 0 1 50 A N G II DUP 753 Saralasin (n) 107 Μ 107 Μ 106 Μ (6) (14) (11) 107 Μ 107 Μ ΙΟ"7 Μ ΙΟ6 Μ ΙΟ4 Μ ΙΟ6 Μ (9) (6) (4)
humans besides the recent observation that DuP 753 administered orally to normotensive volunteers blunts the blood pressure response to exogenous angiotensin II.1 9 The results of the present study demonstrate that
this antagonist is indeed capable of competing with the binding of angiotensin II in human cells such as plate lets. The IC5 0 for DuP 753 in platelets (5 Χ 1 0 "8 mol/L)
was again very similar to that reported in the literature for animal tissues.1 7
A physiologic action of angiotensin II on platelet func tion has still not been established although it has been suggested that angiotensin II might interfere with plate let aggregation.25 The second messenger system coupled
to the platelet angiotensin II receptor is also unknown. Using the Fura-2 method, we could not demonstrate any significant change in cytosolic free calcium after stimulation of the platelets with angiotensin II whereas a marked increase in cytosolic calcium was found after stimulation with vasopressin.2 4 A relatively low number
of angiotensin binding sites per platelet might explain the difficulty to obtain a measurable signal.2 1 It is also
possible that this receptor is not coupled to a calcium mediated pathway.
Angiotensin II binding in human platelets was per formed originally in an attempt to monitor the degree of in vivo inhibition in volunteers receiving DuP 753 or ally. For this purpose, we measured angiotensin II bind ing on platelets harvested before and after administra tion of the drug (Ref. 19 and unpublished results). Even at peak blockade of the blood pressure response to exog enous angiotensin II no decrease in angiotensin II bind ing was observed on the platelets of these volunteers. Our observation that DuP 753 is easily removed from the receptor at concentrations of 10~6 mol/L when the
cells are washed three times might explain why no bind ing inhibition was seen in platelets collected after admin istration of DuP 753. Most likely, the compound was
displaced from the receptor during preparation of the platelets or during the repeated washes necessary to perform the angiotensin II binding.
The results of the calcium studies indicate that DuP 753 not only competes with the binding but also antago nizes effectively and specifically the cellular response to angiotensin II. Inhibition of angiotensin II-induced 4 5Ca
efflux by DuP 753 has been shown previously in SMC and is confirmed by the present experiments.1 7 In our
hands, DuP 753 also blocked the cellular calcium uptake resulting from the angiotensin II stimulation. It is very unlikely that these effects of DuP 753 on calcium fluxes are due to a direct inhibitory effect of this compound on calcium channels. Indeed, previous studies have demon strated that DuP 753 has absolutely no affinity for cal cium channels.1 7
The measurement of free cytosolic calcium in vascular SMC is the definite way to look for an agonistic effect of the drug since cytosolic calcium is directly related to the activation of the receptor. In our aortic SMC, DuP 753 had no effect per se on cytosolic calcium suggesting no activation of the receptor by the drug itself. Moreover, our results confirm the specificity of the compound as DuP 753 blocked the angiotensin II-induced increase in cytosolic calcium but not that produced by vasopressin. This observation is in agreement with previous reports demonstrating no inhibitory activity of DuP 753 on sev eral other receptors such as αλ, vasopressin, serotonin,
histamine, acetylcholine, and bradykinin.1 7 4 8 , 2 6
Finally, one has to recall that DuP 753 is metabolized and that active metabolites with different receptor af finities might be generated. A metabolism of DuP 753 has indeed been demonstrated in vivo in the rat and in vitro using human and rat liver microsomes.2 7 All results
obtained in vitro should therefore be interpreted cautiously particularly when trying to extrapolate from in vitro to in vivo antagonistic actions of the compound.
AJH-MAY 1991-VOL. 4, NO. 5, PART 1 IN VITRO EFFECTS OF DuP 753 4 43
Taken together, the results of the present experiments confirm that DuP 753 is an effective antagonist of an giotensin II which inhibits Ang II binding to its receptor in both animal and human cells and selectively blocks the cellular response to angiotensin II.
REFERENCES
1. Gavras H, Brunner HR, Turini GA, et al: Antihyperten sive effect of oral angiotensin converting enzyme inhibi tor SQ 14225 in man. Ν Engl J Med 1978;298:991-995. 2. Brunner HR, Nussberger J, Waeber B: Effects of angio
tensin converting enzyme inhibition: a clinical point of view. J Cardiovasc Pharmacol 1985;7(suppl 4):73-81. 3. Turini GA, Brunner HR, Gribic M, et al: Improvement of
chronic congestive heart failure by oral captopril. Lancet 1979;i:1213-1215.
4. The Consensus Trial Study Group: Effects of enalapril on morality in severe congestive heart failure. Ν Engl J Med 1987;23:1429-1435.
5. Delabays A, Nussberger J, Porchet M, et al: Hemody namic and humoral effects of a new renin inhibitor enal-kiren in normal humans. Hypertension 1989;13:941-947.
6. Nussberger J, Delabays A, De Gasparo M, et al: Hemody namic and biochemical consequences of renin inhibition by infusion of CGP 38560A in normal volunteers. Hy pertension 1989;13:948-953.
7. Pals DT, Masucci FD, Sipos F, Denning GS, Jr.: A specific competitive antagonist of the vascular action of angio tensin II. Circ Res 1971;29:664-672.
8. Brunner HR, Kirshmann JD, Sealey JE, Laragh JH: Hy pertension of renal origin: evidence for two different mechanisms. Science 1971,174:1344-1346.
9. Gavras H, Brunner HR, Vaughan ED, Jr., Laragh JH: Angiotensin-sodium interaction in blood pressure main tenance of renal hypertensive and normotensive rats. Science 1973;180:1369-1372.
10. Brunner HR, Gavras H, Laragh JH, Keenan R: Angioten sin II blockade in man by sar1-ala8-angiotensin II for
understanding and treatment of high blood pressure. Lancet 1973;ii:1045-1048.
11. Brunner HR, Gavras H, Laragh JH, Keenan R: Hyperten sion in man, exposure of the renin and sodium compo nents using angiotensin II blockade. Circ Res 1974;34(suppl l):35-43.
12. Streeten DHP, Anderson GH, Freiberg JM, Dalakos TG: Use of an angiotensin II antagonist (saralasin) in the rec ognition of angiotensinogenic hypertension. Ν Engl J Med 1975;292:657-662.
13. Furukawa Y, Kishimoto S, Nishikawa K: Hypotensive
imidazole derivatives. Issued by Takeda Chemical In dustries, Ltd., Osaka, Japan. US Patent 1982;4:340-598. 14. Wong PC, Chiu AT, Price WA, et al: Nonpeptide angio
tensin II receptor antagonists. I. Pharmacological charac terization of 2-n-butyl-4-chloro-1 -(2-chlorobenzyl)imid-azole-5-acetic acid, sodium salt (S-8307). J Pharmacol Exp Ther 1988;247:1-7.
15. Wong PC, Price WA, Chiu AT, et al: Nonpeptide angio tensin II receptor antagonists. Studies with EXP 9270 and DuP 753. Hypertension 1990;15:823-824. 16. Wong PC, Price WA, Chiu AT, et al: Nonpeptide angio
tensin II receptor antagonists. IV. EXP 6155 and EXP 6803. Hypertension 1989;13:489-497.
17. Chiu AT, McCall DE, Price WA, et al: Nonpeptide angio tensin II receptor antagonists. VII. Cellular and biochemi cal pharmacology of DuP 753, an orally active antihy pertensive agent. J Pharmacol Exp Ther 1990; 252:711-718.
18. Wong PC, Price WA, Chiu AT, et al: Nonpeptide angio tensin II receptor antagonists. IX. Antihypertensive activ ity in rats of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 1990;252:726-732. 19. Christen Y, Waeber B, Nussberger J, et al: Oral adminis
tration of DuP 753, a specific angiotensin II antagonist, to normal volunteers. Circulation (in press).
20. Le Quan Sang KH, Devynck MA: Increased platelet cy tosolic free calcium concentration in essential hyperten sion. J Hypertens 1986;4:567-574.
21. Mann JFE, Sis J, Ritz E: 125I-Angiotensin II binding to
human blood cells. J Hypertens 1985;3:131-137. 22. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein
measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-272.
23. Grynkiewicz G, Poenie P, Tsien RY: A new generation of C a+ + indicators with greatly improved fluorescence prop
erties. J Biol Chem 1985;260:3440-3450.
24. Mooser V, Burnier M, Nussberger J, et al: Effects of smok ing and physical exercise on platelet free cytosolic cal cium in healthy normotensive volunteers. J Hypertens 1989;7:211-216.
25. Poplowski A: The effect of angiotensin II on the platelet aggregation induced by adenosine-diphosphate, epi nephrine and thrombin. Experientia 1970;26:86. 26. Wong PC, Price WA, Chiu AT, et al: Nonpeptide angio
tensin II receptor antagonists. VIII. Characterization of functional antagonism displayed by DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 1990;252:719-725.
27. Du Pont Pharmaceuticals Internal Report: DuP 753, Clinical Investigators Brochure. April 1989. Wilmington, Delaware.