HAL Id: hal-02486422
https://hal.archives-ouvertes.fr/hal-02486422
Submitted on 26 Jun 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Ion and solvent exchange processes in PGA/PAH
polyelectrolyte multilayers containing ferrocyanide.
Raphael Zahn, Fouzia Boulmedais, János Vörös, Pierre Schaaf, Tomaso
Zambelli
To cite this version:
Raphael Zahn, Fouzia Boulmedais, János Vörös, Pierre Schaaf, Tomaso Zambelli. Ion and solvent exchange processes in PGA/PAH polyelectrolyte multilayers containing ferrocyanide.. Journal of Physical Chemistry B, American Chemical Society, 2010, 114 (11), pp.3759-3768. �10.1021/jp9106074�. �hal-02486422�
Ion and solvent exchange processes in PGA/PAH
polyelectrolyte multilayers containing ferrocyanide
Raphael Zahn †, Fouzia Boulmedais ‡, János Vörös †, Pierre Schaaf ‡ and Tomaso Zambelli *†
†
Laboratory of Biosensors and Bioelectronics, Institute for Biomedical Engineering, University and ETH Zürich, Switzerland.
‡
Centre National de la Recherche Scientifique, Institut Charles Sadron, 23 rue du Loess, BP 84047,67034 Strasbourg Cedex 2,
Author to whom correspondence should be addressed. Email: zambelli@biomed.ee.ethz.ch
RECEIVED DATE (to be automatically inserted)
We investigated ion exchange processes in Poly(L-glutamic acid)/Poly(allylamine)hydrochloride (PGA/PAH) polyelectrolyte multilayers containing ferrocyanide using electrochemical quartz crystal microbalance and infrared spectroscopy in attenuated total reflection. Reduction/oxidation of the ferrocyanide caused a reversible swelling of the film. We showed that the electrochemical swelling of this multilayer system depends on the ionic properties of the contacting buffer. A model was developed to explain the influence of ionic strength, pH value, and the charge of the counter ions in the buffer on the swelling behavior, by relating the swelling of the multilayer to the exchange of counter ions and water molecules between the buffer and the multilayer. Changing the salts in the buffer, while maintaining the same ionic strength, showed that the swelling of the multilayer is related to the counter ions’ molecular mass, hydration properties, and binding strength to PAH. The hydration efficiency of different monovalent anions follows the Hofmeister series, decreasing from kosmotropic ions to
chaotropic ones. In contrast, the strong binding affinity of divalent anions causes them to diverge from the Hofmeister series and to release ferrocyanide from the multilayer.
Keywords. QCM-D, multilayer swelling, Hofmeister series, hydration number, counter ion exchange
1. Introduction
Since their discovery by Decher’s group2,3 Layer-by-Layer (LbL) assembly of polyelectrolyte multilayers (PEMs) have become a popular tool for preparing functionalized surfaces. This method of coating is based on the alternate deposition of polyanion and polycation chains onto a charged surface. The build-up process is simple, and with such cyclic depositions, one can achieve multilayer films on almost any kind of charged surface using not only polyelectrolytes, but also different types of inorganic and organic building blocks. Nanoparticles, nanotubes and nanowires, proteins, nucleic acids and DNA, viruses etc. have all been successfully used as components to prepare LbL films.4 Aside from the building blocks, the physiochemical properties of the film also depend strongly on the deposition conditions (pH,5,6 solvent quality,7 temperature,8 etc.).
Due to their ability to condensate along polyelectrolyte chains,9-11 the concentration of ions in the buffer is known to have a strong influence on the thickness7,12 and the morphology13 of the multilayers. Recent studies have shown that not only the concentration but also the counter ion species used during the build-up effects the layer properties like mechanical stiffness,14 swelling15 or permeability to an electroactive probe. 16 The interaction between the macromolecules and different ion species has often be explained by the so called Hofmeister series:17-20 The Hofmeister series21 ranks ions by their ability to structure water from kosmotropic ions (exhibiting strong interactions with water molecules) to chaotropic ions (breaking the water structure). Although recent efforts are trying to link the Hofmeister series to molecular properties like the polarizability of the counter ions,18 their hydration number22 or their hydration entropy,23 it should be emphasized that the series should be considered a phonological ranking that is still lacking in complete understanding.
The hydration properties of PEM have received considerate attention in the last years. The hydration water plays an important role for the local structure and the dynamics of the multilayer.24,25 It influences the mechanical and chemical properties of the multilayer and is thus important for possible applications ranging from cell sheet engineering26-28 to ion separation membranes29-31. Notably the exchange of counter ions between buffer solutions and the multilayer to tune its hydrophobicity /hydrophilicity seem to be promising.20,32
Being able to choose from a large variety of polymer building blocks and different salts allows one to tune the interactions between polyelectrolytes and counter ions in PEMs. This makes it possible to construct highly ion-selective membranes,33-35 or “ion trapping” films.36 Data of ion and water transport through the membranes are usually obtained by characterizing ion fluxes using infrared spectroscopy,20,22 or electrochemistry.37,38 Another possibility to characterize ionic interactions within the PEM is to look at thickness changes due to swelling of the layers upon rehydration. This is commonly done using ellipsometry,19,39-41 atomic force microscopy,5,39 or quartz crystal microbalance.14-16
Conformational changes of polymers in a film usually result in thickness variations or changes in the mechanical properties. This kind of film response cannot be easily probed with electrochemical characterization techniques, since they are only sensitive to charge distributions and interactions among charged molecules. Therefore, instruments combining electrochemistry with other measuring techniques are advantageous. Recently, Schmidt et al. used electrochemical quartz crystal microbalance (EC-QCM), electrochemical atomic force microscopy and nanoindentation to characterize mechanical changes occurring in nanocomposite films consisting of linear poly(ethyleneimine) and Prussian Blue nanoparticles.42 Calvo’s group has assembled multilayers with osmium bipyridyl complexes covalently attached to cationic poly(allylamine) (PAH-Os). They used EC-QCM as well as electrochemical ellipsometry to measure ion and solvent exchange in the multilayers.43-45 Upon oxidation of the osmium, they observed a swelling of the multilayer44,45 and an increase of its viscoeleastic loss modulus,46 which was interpreted as uptake of counteranions and water.47 Based on their experiments, they developed a
theory describing the effect of bulk pH, salt concentration and applied electrode potential on the molecular organization of the film.48-50 In their measurements, they showed also that the amount of swelling of the electroactive PEMs depends highly on the kind of counter ion used.45 They successfully relate the swelling amplitude induced by specific counter ions to the partial osmotic pressure created by these ions in the film.
To investigate the interactions between polymers and counter ions in PEMs, Calvo’s group and others51-55 used electrochemically active multilayers in which the redox sites are grafted to one of the polyions. The novelty of our work is that we use a system where the redox sites are not covalently bound to a polymer, but self-incorporate into the PEM as counter ions: we use ferrocyanide (Fe(CN)64-)
as redox ions and mutilayers built up from Poly(L-glutamic) acid (PGA) and Poly(allylamine hydrochloride) (PAH). Fe(CN)64- diffuses through multilayers constituted of these polymers. Once in
the film, it is trapped and cannot easily diffuse out any more.56,57 The ferrocyanide in the film stays electrochemically active and was previously used for electrocatalytic sensing of ascorbic acid.58 Grieshaber et al. showed that the PEM swells reversibly upon oxidation of the Fe(CN)64- ions in the
film.59 This was attributed to the uptake of counter anions into the film to compensate the loss of charge of the redox probe.
The purpose of this article is to enlighten the complex interactions between PGA/PAH multilayers, ferrocyanide ions and other counter ions. Since our redox sites are not bound to a polymer, but are “free”, we can use them for two purposes: (1) as electrochemical probes and (2) as multivalent counter ions with two different valency states.
(1) Using Fe(CN)64- as electrochemical probe confined into PGA/PAH films, we studied the influence
of counter ion species, counter ion valency, ionic strength and pH value on the cyclic voltammograms and the swelling behavior of the multilayers. Thereby, we obtained information about ion and solvent exchange in the multilayers.
(2) By means of Fourier transformed infrared spectroscopy (FTIR), we probed the interaction of specific counter ions with the multilayers and showed that when confined in the film, Fe(CN)64- ions can
replace polyions in the multilayers, but can be replaced themselves by other counter ions.
The swelling of the multilayer depends on several parameters of the counter ions in use, ranging from charge and to their hydration properties. We extended Calvo’s model of the electrochemical swelling to our case and succeeded in relating the amplitude of the swelling to the hydration properties of the counter anions. The findings are discussed in the light of the Hofmeister series.
2. Materials and Methods
Materials. All chemicals were used as received unless otherwise specified. The following polymers
were used: Polyethyleneimine (branched) (PEI, Sigma Aldrich 408727, MW = 25 000); Poly(L-glutamic acid) (PGA, Sigma Aldrich 408727, MW = 15 000 – 50 000); Poly(allylamine hydrochloride) (PAH, Sigma Aldrich 283 215, MW = 70 000). Buffers were prepared with ultrapure water (Milli-Q gradient A 10 system, Millipore Corporation) and filtered (0.2 µm) prior to use. All buffers contained 10 mM 4-(2-hydroxyethyl)piperazine-1-ethane-sulfonic acid (HEPES, Fluka Chemie GmbH, Switzerland) as well as 100 mM of counter ion salt (if not stated differently). For the FTIR spectroscopy measurements D2O (deuterium oxide 99 % D, AS-A339, Aldrich) based buffers were used. Salts were
purchased from Sigma Aldrich: potassium chloride (KCl), potassium bromide (KBr), potassium trifluoroacetate (KTFAc), potassium metaborate (KBO2), tetrabutylammonium chloride (TBACl),
sodium chloride (NaCl), sodium fluoride (NaF), sodium phosphate dibasic (Na2HPO4), sodium sulphate
(Na2SO4), sodium citrate tribasic (Na3Cit), calcium chloride (CaCl2), magnesium chloride hexahydrate
(MgCl2), ammonium chloride (NH4Cl) and potassium hexacyanoferrate(II) trihydrate (ferrocyanide,
Fe(CN)64-).
Film preparation. The films were built up by an alternate adsortion of positively and negatively
charged polymers onto a gold surface. PEI was used as an initial layer followed by five alternating depositions of PGA and PAH, always separated by a rinsing step. The temperature during the assembly
was 25 °C. All polymers were used at a concentration of 1 mg/ml in a buffer of pH 7.4, which contained 100 mM KCl. Adsorption steps were 5 minutes long, rinsing steps 2 minutes. In the final step, 10 mM Fe(CN)64- in 100 mM KCl buffer were adsorbed for 10 minutes. If not stated differently all
measurements were carried out at pH 7.4 in a buffer containing 10 mM HEPES and 100 mM of counter ions. For FTIR spectroscopy measurements, all buffers were adjusted to a pD of 7.9.
Electrochemical quartz crystal microbalance with dissipation monitoring (EC-QCM-D).The
buildup and electrochemical studies of PGA/PAH films were performed on a QE 401 instrument respectively with a QFM 401 module and a QEM 401 module from Q-sense AB (Gothenburg, Sweden). The QCX 301 gold crystals (Q-Sense AB, Gothenburg, Sweden) were cleaned before surface preparation by immersion in a 2 % (w) SDS solution (>30min) followed by rinsing with Milli-Q water and drying in a stream of nitrogen gas. Before mounting in the flow chamber, the crystals were treated with UV/ozone for 30 min. A volume of 0.5 mL of temperature-equilibrated polyelectrolyte or buffer was injected into the QCM-D cells in order to study the PEM buildup by continuously recording the sets of resonance frequencies and dissipation factors (3rd, 5th, 7th 9th and 11th overtones). For the electrochemical measurements, the gold crystals with the adsorbed PEM film were quickly transferred to the QEM 401 module assuring that the PEM did not dry.
The QEM 401 cell represents a conventional three-electrode setup for electrochemical measurements with a platinum counter electrode and a gold working electrode (gold coated sensor surface). Potentials were measured against a saturated Ag/AgCl (3M KCl) reference electrode (66-EE009 a premium "no-leak" Ag/AgCl reference electrode, Cypress systems, Chelmsford, MA, US). An IPS Jaissle PGU10V-1A-IMP-S potentiostat/galvanostat (Jaissle Elektronik GmbH, Germany) was used for the cyclic voltammetry (CV) measurements applying a scan rate of 50 mV/s.
After transferring to the QEM 401 cell, all the PEM films were exposed to a ferrocyanide solution (10 mM Fe(CN)64- in 10 mM Hepes, 100 mM KCl, pH 7.4) for 10 min followed by a rinsing step with
the standard buffer (10 mM Hepes, 100 mM KCl, pH 7.4). To measure the swelling/contraction of the PEM film, three potential scans from 0 mV to 600 mV were performed at a scan rate of 50 mV/s. The
resulting cyclic voltammograms and corresponding QCM-D changes in frequency and dissipation were simultaneously recorded. Only the values obtained from the last scanning cycle were analyzed.
The percentage of swelling was determined by dividing the frequency and dissipation change caused by electrochemical swelling by the overall change in frequency or dissipation that was observed from the multilayer build-up. After one swelling/contraction measurement in our standard buffer (100 mM KCl, pH 7.4), the buffer was exchanged to one having a different counter ion species, molarity or pH. A swelling/contraction measurement was then performed. A typical EC-QCM-D experiment is shown in figure 3A. The swelling/contraction was consistently observed for all measured harmonics. Only the 3rd harmonic is shown to avoid graphs with too many superposed curves. Between two buffer rinses with different ionic strengths, we always measured with the standard buffer. If the swelling ratio, measured in the standard buffer, was comparable before and after measuring in another buffer, with one different parameter, we assumed that changing this parameter did not have an irreversible influence on the multilayer structure. Further measurements are then performed on this PEM film. If the swelling ratio in the standard buffer was significantly reduced after a parameter variation (e.g. after the use of phosphate containing buffer, see figure S-3 in the in the supplementary information (SI), a new PGA/PAH multilayer was used for the next measurement.
The swelling/contraction procedure, involving several buffer-rinsing steps, was repeated several times using the same PEM film. In this case, the swelling ratio and the transferred charge decreased slowly. However, when no buffer was injected between the potential scans, the swelling ratio and charge transfer remained nearly constant. The decrease in swelling ratio after several rinsing step can be attributed to Fe(CN)64- ions being electrochemically passivated,57 or being washed-out of the
multilayers upon each buffer injection. With fewer ions available for oxidation and reduction, the swelling is reduced. Therefore, we corrected for this washing-out effect by comparing the measurement done in the standard buffer after each parameter variation to the one recorded in the standard buffer at the beginning of the experiment. The applied correction was usually between 4% and 8% of the value of
the swelling amplitude. If the necessary correction exceeded 12% of the value of the measurement, the data point was excluded from the data evaluation.
Fourier transform infrared spectroscopy (FTIR) FTIR spectra were performed in attenuated total
reflection (ATR) mode with a Vertex 70 (Bruker, Germany). For these experiments D2O was used as
solvent instead of water because the amide I band of the polypeptide is affected by the strong water band absorption around 1643 cm-1 (O-H bending), whereas the corresponding vibration in D2O is
around 1209 cm-1.60 The penetration depth of the infrared radiation was calculated to be 1.26 μm at 1550 cm.1 and is thus well above the films thickness which was found to be in the order of 100 nm (see AFM micrograph, figure S-1 in the SI). Films were deposited on a trapezoidal ZnSe crystal (Graseby-Specac, Orpington, U.K.) located on the bottom of a flow cell (Graseby-(Graseby-Specac, Orpington, U.K.) by allowing each polyelectrolyte solution to circulate over the substrate for 5 min. Two polyelectrolyte adsorption steps were separated by a buffer rinse for 10 min. Polyelectrolyte and buffer circulation was allowed through Tygon tubing by means of a peristaltic pump. The infrared spectra of the film were acquired during each buffer rinsing step in total attenuated reflection mode by accumulating 128 interferograms at a resolution of 2 cm-1. The signal transmitted through the ZnSe crystal was collected by deuterated triglycine sulfate (DTGS) detector. During the deposition of the PEI-(PGA-PAH)5
multilayer film, the absorption spectrum was calculated as -log(Tfilm/Tbuffer), where Tfilm and Tbuffer
represent respectively the transmission of the film adsorbed on ZnSe substrate in contact with buffer and the buffer in contact with the native ZnSe crystal. After its build-up and its characterization, the PEI-(PGA-PAH)5 film was put in contact with a ferrocyanide containing solution (10 mM Fe(CN)64- in
10 mM HEPES, 100 mM KCl containing buffer) for 10 min. Then the spectrum of Fe(CN)64- containing
PEI-(PGA-PAH)5 film was acquired in presence of KCl containing buffer before its exposition to
different ions.
A band decomposition of the peaks in the recorded infrared spectra was achieved by using Origin 8.0 and assuming Gaussian bands.
Atomic force microscopy (AFM) We used the Nanowizard I BioAFM (JPK Instruments, Berlin,
Germany) and the Mikromasch CSC38/noAl cantilevers in intermittent-contact mode. To observe in situ the morphology of the film, we conceived a Teflon liquid-cell to be able to fabricate and explore LbL films without a drying step.59,61 The polymers were deposited on a gold-coated (ca. 60 nm) glass slide that was cleaned applying the same procedure used for the QCM-D crystals. The supernatant of the cell (~1 ml) was exchanged using a micropipette. For imaging, the sample was positioned under the AFM block without removing the liquid from the cell. Thereby the LbL films never underwent a drying process before and during the AFM experiment.
3. Results
In this section, we use the experimental results to explain the interactions between polyelectrolyte multilayers, different counter ions, and their hydration shell.
Multilayer buildup and ferrocyanide adsorption. Figure 1 shows the build-up of PEI-(PGA-PAH)5
and the subsequent incorporation of Fe(CN)64- measured by QCM-D and FTIR spectroscopy in ATR
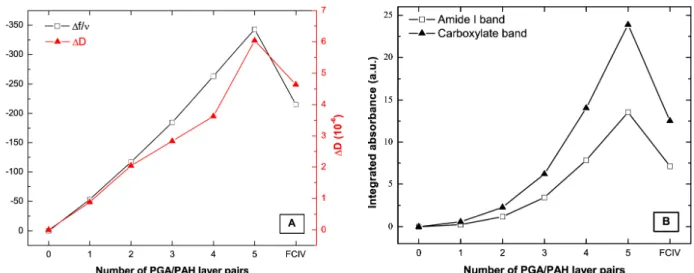
Figure 1. Buildup of PEI-(PGA-PAH)5-Fe(CN)64- showing the influence of the Fe(CN)64- adsorption
in the last adsorption step. (A) Evolution of the normalized frequency (∆f/ν) and the dissipation (∆D) measured by QCM-D. (B) Absorbance spectra of the film obtained by FTIR spectroscopy in ATR mode: The evolution of the amide I band of PGA (absorbance peak around 1635 cm-1) and its carboxylate band (around 1560 cm-1) are shown.
Figure 2. Cyclic voltammograms of Fe(CN)64-/Fe(CN)63- recorded at a scan rate of 50 mV/s on a
gold electrode in the EC-QCM-D. A clear shift in apparent redox potential is visible between the voltammogram of free ferrocyanide (red triangles) and FCIV containing PEI-(PGA-PAH)5 film
mode. Previous work has described the exponential growth of this kind of PEM under the same experimental conditions (ionic strength and temperature).58,59,62 For the first PGA/PAH bilayers, the exponential growth in the QCM-D measurement is not as pronounced as in the integrated absorbance measured by FTIR. This might be attributed to the fact that QCM-D detects layer thickness rather than deposited mass.63,64 For five bilayer depositions of PGA/PAH on a gold substrate, no homogeneous PEM is obtained, but AFM measurements show that the film consists of small, densely packed droplets that cover the whole surface of the substrate (see figure S-1 in the SI). While the amount of polymer deposited on the surface as measured in FTIR spectroscopy increases exponentially with the number of bilayers we see only an almost linear increase in the PEM thickness as measured by QCM-D. The slower increase in thickness can be explained as the droplets on the surface spread and fill the gaps. For higher layer numbers (from 8 bilayer depositions), we observed the expected exponential growth in QCM-D measurements (data not shown).
After contact with Fe(CN)64- ions and a subsequent rinsing step with buffer, the amide I (at 1635 cm-1)
and the carboxylate bands (at 1560 cm-1) decrease by about 47% in the FTIR spectra. These two absorption bands are associated with PGA.60,65 Simultaneously a strong adsorption around 2038 cm-1 appears which is attributed to Fe(CN)64- (data not shown). The QCM-D, which is sensitive to both, PGA
and PAH, detects a decrease in thickness of only 35%.
The electrochemical properties of the Fe(CN)64- containing PEI-(PGA-PAH)5 multilayers are
investigated using an EC-QCM-D cell. Comparing the cyclic voltammograms of free Fe(CN)64- in
solution (in contact with a gold electrode) and Fe(CN)64- entrapped in a PGA/PAH film (figure 2), shifts
in the oxidation peak potential (Eox), and the reduction peak potential (Ered) are visible. This shift in apparent redox potential (E1/2= 1/2Eox + 1/2Ered) towards higher potentials for entrapped ferrocyanide
results from the so-called Donnan potential. The Donnan potential (∆ΦD) is related to the number of
fixed charges in the film. These fixed charges originate from the non-compensation of charges carried by the PGA and the PAH chains during the film build-up. It is related to the redox potential of ferrocyanide in absence of the PEM (E0) by:43,49
E1/2 = E0 + ∆ΦD
Thus, changes in the charge density within the PEM can be monitored by measuring E1/2. For
PEI-(PGA-PAH)5 with Fe(CN)64-, the Donnan potential was determined to be (140 +/- 9) mV.
During the oxidation from Fe(CN)64- to Fe(CN)63-, each ferrocyanide molecule in the PEM loses a
single electron. Therefore, the amount of ferrocyanide trapped in the film can be estimated from the transferred charge during one cyclovoltammogram. Assuming a mean thickness of 70 nm (see AFM micrograph, figure S-1 in the electronic supplementary information), the Fe(CN)64- concentration in the
film is calculated to be greater than 500 mM which is similar to the value of Hubsch et al. obtained for 10 bilayers of PGA/PAH in contact with 1 mM Fe(CN)64- solution during 10 h. 56
Counter ion dependent swelling of the multilayers: effect of ionic strength. To study the effect of
the ionic strength on the multilayer swelling, we exposed the PEM to solutions containing different concentrations of KCl prior to oxidation of the ferrocyanide in the layer. Figure 3 shows the changes in electrochemical swelling of the multilayers as measured by EC-QCM-D. The swelling was measured by frequency and dissipation changes and showed a strong increase up to 300 mM of KCl. For a higher ionic strength, no further increase in swelling ratio was detected. On the contrary, the properties of the cyclic voltammograms (peak potentials, E1/2) showed no dependency on the ionic strength (data not
shown).
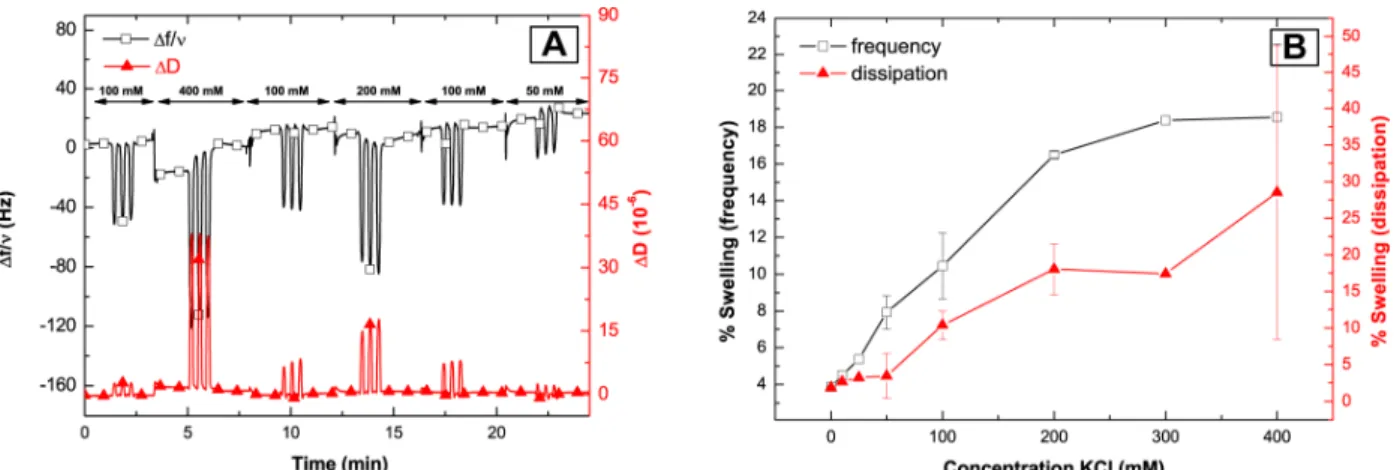
Figure 3. Influence of the ionic strength on the swelling of PEI-(PGA-PAH)5-Fe(CN)64- multilayers.
(A) In a typical EC-QCM-D measurement, KCl buffers of different molarity are injected into the flowcell one after another. For each buffer three oxidation/reduction cycles at a scan rate of 50 mV/sec are measured. Upon oxidation of the Fe(CN)64-, the multilayer is swelling resulting in a
drop in normalized frequency (∆f/ν, black squares) and an increase in dissipation (∆D, red triangles). This behavior is completely reversible and the initial frequency and dissipation values are restored upon reduction. (B) Swelling ratio in percent of the multilayer thickness. The multilayer is swelling/contracting the more, the higher the salt concentration in the buffer is. For salt concentrations higher than 300 mM no further increase is observed.
Effect of pH value. Figure 4 shows the pH of the buffer in contact with the film influences its
swelling ratio. Sufficient and comparable PEM stability is only provided for pH values between the pKa values of the used polymers.66 The pKa value for PGA is 4.3,67 the one for PAH is 8.8.68 Our
measurements were performed between pH 4 and pH 9. Since pH 4 is already below the pKa value of
PGA, we attribute the large error for these measurements to the onset of multilayer dissolution.
For increasing pH, the swelling ratio decreases nearly monotonically from 25% at pH 4 to less than 5% at pH 9.
In the corresponding cyclic voltammograms, the E1/2 remains essentially constant for increasing pH
values between 4 and 6. For pH values above the assembly pH of 7.4, a clear drop in the E1/2 is visible.
Since the area enclosed by the cyclic voltammograms does not significantly change (inset figure 4B), the charge transferred during one redox cycle is not influenced by the pH value. This indicates that no Fe(CN)64- ions are leaving the multilayer when the pH value of the buffer is changed.
Figure 4. (A) Effect of the pH on the swelling of PEI-(PGA-PAH)5-Fe(CN)64- multilayers. For
increasing pH values, less swelling of the multilayer is observed in the EC-QCM-D. The corresponding cyclic voltammograms (B) show a clear drop in the E1/2value for higher pH values.
Effect of the counter cation species and molarity. Using salts of different cations (KCl, TBACl,
NaCl, NH4Cl, CaCl2 and MgCl2) we did not observe any significant influence on either the swelling
ratio of the multilayers or the E1/2 value in the cyclic voltammograms (see figure S-2 in the SI).
The salts of the monovalent cations (KCl, TBACl, NaCl, and NH4Cl) were used at a concentration of
100 mM. In the case of divalent cations (CaCl2 and MgCl2), the molarity of the anions in the buffer is
always double than that of the cations. Therefore, measurements involving divalent cations were performed using two different salt concentrations: 50 mM (corresponding to 100 mM in anion concentration) and 100 mM (corresponding to 200 mM in anion concentration, data not shown). The swelling ratio measured for a buffer containing only 50 mM in divalent cations (i.e. 100 mM of anions) was found to be equal to the swelling ratio obtained for a buffer with 100 mM in monovalent cations (and 100 mM anions). For buffers containing 100 mM of divalent cations (thus 200 mM of anions), we observed increased swelling ratio that corresponds approximately to the swelling ratio observed for a buffer containing 200 mM of KCl (see figure 3). From these observations, we conclude that only the molarity of the counter anion exerts an influence on the swelling behavior of the multilayer.
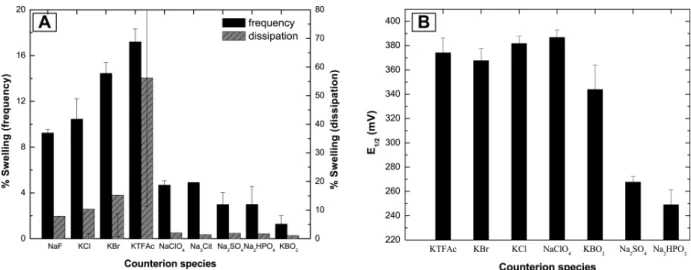
Figure 5. (A) The percentage swelling ratio of PEI-(PGA-PAH)5-Fe(CN)64- multilayers is highly
dependent on the counter anion species used. For the corresponding voltammograms (B) a clear difference in the E1/2value is only visible between monovalent and divalent anions.
Effect of the counter anion species. Using salts of different anions (KTFAc, KBr, KCl, NaClO4,
Na3Cit, Na2SO4, Na2HPO4 and KBO2 - all at a concentration of 100 mM), we found that the
electrochemical swelling behavior is strongly dependent on the anion species (figure 5A). The swelling ratios in thickness range from less than 2% for KBO2 to more than 17% for KTFAc. As shown in the
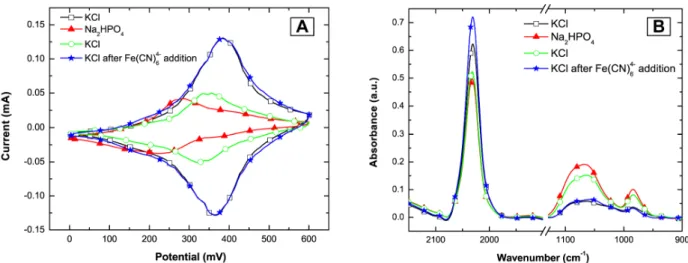
Figure 6. Influence of divalent counter cations on the Fe(CN)64- content in the multilayers. The
cyclic voltammograms (A) show that phosphate-containing buffer can wash out Fe(CN)64- ions of the
multilayers. Comparing a measurement in KCl buffer (black squares) to a subsequent measurement in Na2HPO4 buffer (red triangles) shows that the Fe(CN)64- content in the multilayers is reduced.
Rinsing with Fe(CN)64- containing KCl buffer restores the initial voltammogram (blue stars). The
corresponding FTIR spectra (B) show that the release of Fe(CN)64- from the multilayer is
accompanied by an uptake of phosphate ions. By changing the buffer from KCl (black squares) to Na2HPO4 buffer (red triangles), the peak attributed to Fe(CN)64- (2033 cm-1) decreases.
Simultaneously the two bands related to the absorbance by phosphate (950 cm-1 – 1000 cm-1 and 1000 cm-1 – 1100 cm-1) increase. After changing back to KCl containing buffer (green circles), most of the phosphate remains in the multilayer. Only after a rinse with Fe(CN)64- containing buffer (blue
previous section, changing the cation from sodium to potassium does not influence the swelling behavior or the voltammograms (see figure S-2 in the SI). Therefore, we attribute the changes in the swelling ratio only to the influence of different counter anions. The swelling depends on the valency of the counter anions. Compared to monovalent ions, for divalent and multivalent ions, we observe lower swelling ratio and a significantly reduced E1/2 value (figure 5B). For the monovalent ions (KTFAc,
KBr, KCl, NaClO4), the swelling ratio differs from ion to ion and seems to be dependent on ion-specific
properties (see discussion). In contrast, the cyclic voltammograms are identical for all monovalent anions.
The divalent anions were able to replace some of the ferrocyanide in the multilayers. This behavior is shown in the cyclic voltammograms of buffer containing Na2HPO4 (figure 6A). The area enclosed by
the cyclic voltammograms is reduced for phosphate-containing buffer and does not recover by switching to a buffer containing KCl (green circles in figure 6A). The only way to restore the voltammogram is to rinse with Fe(CN)64--containing buffer. The same behavior is observed for the swelling ratio in the
QCM-D measurements: the ratio remains reduced after a phosphate rinse and does not return to its original value until the multilayer is exposed to Fe(CN)64--containing buffer (see figure S-3 in the SI).
4. Discussion
We focused the presentation of our experimental data on the influence of the buffer properties on the electrochemical swelling of PGA/PAH multilayers film containing Fe(CN)64- ions. After a brief
discussion of the multilayer build-up and the Fe(CN)64- incorporation, we will propose a model for the
swelling of the film and discuss its validity regarding to our results.
In agreement with literature data, we found that PGA/PAH films grow exponentially with the number of deposited bilayers. In contact with Fe(CN)64- containing buffer, we verified the uptake of the redox
ions by the multilayer using FTIR spectroscopy. This incorporation is accompanied by a release of PGA visible in FTIR and a decrease in thickness measured by QCM-D. Comparing the results from QCM-D
Figure 7. Schematic representation illustrating the mechanism for electrochemical PEM swelling.
(A) Fe(CN)64- is incorporated in the multilayers by binding to the ammonium groups of PAH. (B)
Upon oxidation of Fe(CN)64- to Fe(CN)63-, negatively charged counter ions diffuse into the PEM to
reestablish electroneutrality. Depending on their mass and the size of their hydration shell, the counter ions will cause the PEM to swell by different amounts. (1) Chaotropic ions like perchlorate only have a small hydration shell causing less PEM swelling than kosmotropic ions (e.g. fluoride, 2), that are surrounded by larger amounts of water.
and from FTIR spectroscopy, we saw that approximately 47% of the PGA is released from the PEM while the thickness of the film only changes by about 35%. These results indicate that PGA in the PEM is replaced by Fe(CN)64- while most of the PAH, which is invisible by FTIR, remains in the multilayer.
This finding has not been reported before and is remarkable considering the size of a Fe(CN)64-
molecule and a PGA chain. It could have been expected, as the opposite effect – PGA replacing Fe(CN)64- – was described earlier.56 However, this observation is in contrast to our previous
observations where no PGA left the multilayers upon Fe(CN)64- incorporation.59 We probably did not
observe this effect, since the Fe(CN)64- concentration they used was one order of magnitude lower than
the one used in our experiments (1 mM vs. 10 mM). Due to this difference in Fe(CN)64- concentration,
the binding equilibrium in our previous study was possibly favoring the PGA remaining in the multilayer.
Recently, we also described the swelling/contraction effect of Fe(CN)64- containing PGA/PAH films
upon oxidation/reduction of the Fe(CN)64- ions.59 We suggested that this effect is caused by diffusion of
counter anions into the film. Here we investigate the validity of their assumption by using counter ions with different molecular properties. Calvo’s group has suggested that for PAH-Os/PVS films the redox driven swelling of the multilayer can be understood looking at the changes in total osmotic pressure for a swelling/contraction cycle.45 In contrast to Calvo’s system, our redox sites are not covalently grafted to one of the polymers. Nonetheless, we can adapt this model to explain the swelling in our system. A schematic picture of the suggested swelling mechanism is given in figure 7. When the Fe(CN)64-
contained in the multilayer is oxidized, counter anions from the buffer have to diffuse into the multilayer to compensate for missing charge. A hydration shell consisting of several water molecules accompanies each of these counter anions. This uptake of anions and water molecules results in the observed swelling phenomena. Upon reduction of Fe(CN)63-, the counter anions and their hydration
shell leave the film leading to a contraction of the multilayer back to its initial thickness.
For this model we assume that all the excess positive charge in the multilayer (created by oxidation of the Fe(CN)64- ions) is compensated by the uptake of anions. However theoretically, electroneutrality
could also be achieved by the release of cations from the multilayer. In the result section, we showed that neither the cation species used, nor its concentration had an influence on the swelling ratio or the cyclic voltammograms of Fe(CN)64- entrapped in the film. This behavior can be explained by the
positive Donnan potential of the multilayer, determined to be around 140 mV. This positive charge excess inside the film is common for PAH containing films69 and is caused by an overcompensation of the negative charges of PGA by the positively charged ammonium groups in the PAH. Due to the positive Donnan potential, we assume that there are no small cations are present in the multilayer, and only counter anions are exchanged between the film and the buffer. As a consequence to this so-called Donnan permselectivity, cation valency, species or molarity in the buffer does not influence the swelling behavior of our multilayer.
When the ionic strength of the anions in the buffer solution was increased, we observed higher swelling amplitudes of the multilayers (see figure 3). It has previously been shown that the number of water molecules associated with a counter ion in the multilayer depends on the ionic strength of the buffer solution. At higher ionic strength, more water molecules follow the counter ion as it is exchanged between the buffer and the multilayer.43,70 Thus, the additionally exchanged water molecules cause the observed increase in swelling ratio with ionic strength.
A different amount of exchanged water is also the explanation for the observed dependency of the swelling amplitude on the pH value of the buffer solution. Since the transferred charge during one redox cycle remains the same independent of the pH value (see inset in figure 4B), the number of counter anions exchanged during a swelling/contraction cycle also remains constant. Therefore, the high swelling amplitudes at low pH values must be attributed to an increased amount of exchanged water, while at high pH values the multilayer only takes up little water. Looking at the influence of the pH value on the degree of ionization of the multilayer helps understanding this phenomenon. In solution, PGA and PAH have pKa values of approximately 4.3 and 8.8 respectively.67,68 Therefore, both
polyelectrolytes are fully ionized during the assembly process at pH 7.4 (PGA is 100% ionized, PAH is 96% ionized). We can assume that during the build-up process almost all ionic groups of PGA form
ion-pairs with the charged groups of the PAH. If the pH value is lowered, PAH stays fully ionized while the degree of ionization for PGA drops to 83% at pH 5. At pH 4 less than 33% of the carboxylate groups of PAH are charged. This results in a large amount of broken ionic crosslinks and a strong increase of free ammonium groups on PAH. Since these ammonium groups are positively charged the Donnan potential is expected show a drastic increase. This is not the case, as the Donnan potential nearly remains constant between pH 4 and pH 6 (see figure 4B). From this we can deduce that counter anions enter the multilayer and form so-called “contact-ion pairs” with the positively charged free ammonium groups of PAH, thereby keeping the Donnan potential constant. If the polyelectrolyte segments are paired with salt ions instead of another polyelectrolyte the multilayer becomes more hydrophilic because the condensated counter ion screen the hydrophobic polymer backbone.13,20 This increased hydrophilicity allows the counter anions, which are exchanged during a swelling/contaction cycle, to have a larger hydration shell and thereby the swelling magnitude is increased. Changing the pH value from 7.4 to 9 the degree of ionization of PAH drops from 96% to 39%. This results in fewer free positive charges (as indicated by the reduced Donnan potential) and an increased hydrophobicity of the multilayer. Therefore less water is exchanged in a swelling/contraction cycle and the swelling magnitude is decreased.
This hypothesis is further supported by looking at the “static”, pH-dependent swelling of the multilayer (see figure S-5 in the SI). The static swelling ratio is defined as the change in multilayer thickness in percent upon exposure to a pH different from the pH of assembly. For pH values lower than the pH of assembly a swelling of the multilayer is observed in the QCM-D data. If exposed to a pH value higher than the pH of assembly, the multilayer contracts. This effect is due to water molecules entering or leaving the multilayer, as the film becomes more hydrophilic at lower pH values or more hydrophobic at higher ones.
Considering the results described above, several parameters influence the electrochemical swelling of Fe(CN)64- containing PGA/PAH multilayers. Consequently, in order to investigate the ion exchange
processes, we have to keep the pH value and the anionic strength of the buffer solution constant and vary only the anion species, that we use as a counter ion.
The swelling ratio of the multilayers is highly dependent on the anion species used in the buffer solutions. Since the hydration number differs for every anion species, one expects that the PEM swells more if stronger hydrated anions are used. Surprisingly, arranging the anions used for the swelling experiments in the order of the Hofmeister series did not reveal any systematic behavior in their swelling ratios (see figure 5A). In particular, it was not possible to observe an increased swelling for the kosmotropic ions (large hydration shell) or a decreased swelling for the chaotropic ones (small hydration shell) as derived from figure 7. In order to see that kosmotropic ions are indeed more sufficient in wetting the multilayer the QCM-D data has to be normalized by the molecular weight of the particular counter anions in use. This procedure is justified, as we observed that the frequency and dissipation changes upon electrochemical swelling/contraction were identical for all the harmonics measured (3rd, 5th, 7th, 9th and 11th overtones, data not shown). Therefore PGA/PAH multilayers were assumed to be rigid films, which is in agreement with previous findings.62 For rigid films the Sauerbrey equation can be used to interpret the QCM-D data.71,72 This equation states a linear relation between the
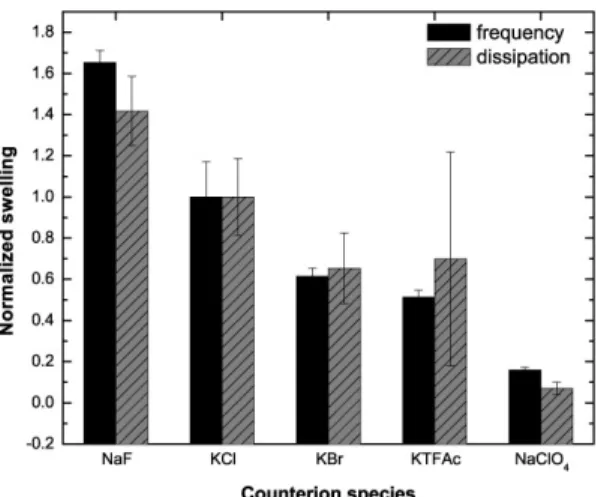
Figure 8. The normalized swelling ratio of PEI-(PGA-PAH)5-Fe(CN)64- follows the Hofmeister
mass adsorbed on the crystal and the frequency change measured. Therefore, the measured swelling rate in figure 5A corresponds to the mass uptake rather than to the number of molecules exchanged. In order to see how much water is exchanged by using different counter anions, the QCM-D data has to be normalized by their molecular weight. Figure 8 shows the changes in frequency and dissipation upon swelling divided by the molecular weight of the anion used and normalized to the swelling value of Cl-. Thus, the normalized swelling essentially corresponds to the amount of water taken up by the multilayer per exchanged anion. The dependency of the water uptake on the counter anions species follows nicely the Hofmeister series: the kosmotropic ions F- and Cl- hydrate the layer best followed by the more chaotropic anions Br-, TFAc- and ClO4-. Comparing our findings to literature data, we found that the
normalized swelling is directly proportional to the hydration number of the monovalent anions used (see table 1 and figure S-3 in the SI).
From our results we can argue that the Hofmeister series can only be used to rationalize the different swelling amplitudes for monovalent ions. Due to their high charge density, all divalent and multivalent ions are considered to be kosmotropic and thus, if they followed the model described above, they should show swelling amplitudes comparable or higher than the one measured for chloride. Yet, the contrary was observed; in our experiments the divalent and multivalent ions showed the lowest swelling ratios (see figure 5A). To understand this phenomena it has to be recalled that our electrochemically active molecules, the Fe(CN)64- ions, are not covalently bound to the polymers but are believed to form
contact-ion pairs with the positively charged free ammonium groups of PAH.73,74 If the counter ions used during the swelling experiments also have a high affinity to these ammonium groups, they can replace the Fe(CN)64- ions in the multilayer. In the results section, we showed that phosphate ions can
replace Fe(CN)63- in the multilayers when the multilayer is exposed to phosphate-containing buffer (see
figure 6). The reduced amount of electrochemically active ions in a multilayer results in fewer counter anions being exchanged during the oxidation/reduction process (since less charge has to be compensated) and thus in a smaller swelling ratio. The ion exchange process is fully reversible and the phosphate ions can be re-exchanged for Fe(CN)64. In the cyclic voltammograms, the significantly
reduced E1/2values for divalent ions also indicate the high affinity of these ions to PAH (see figure 5B).
The positive charge of the ammonium groups is neutralized if they form contact-ion pairs with the
Table 1. Literature values1 for the hydration number of different anions compared to their measured swelling ratios (see figure 4A) and their calculated normalized swelling (see figure 6).
Counter anion Hydration number a % Swelling (frequency) Normalized swelling (frequency) Fluoride 2.7 9.3 1.65 Chloride 2 10.5 1 Bromide 1.9 14.5 0.61 Perchlorate 1.4 6.4 0.16
divalent anions. Therefore, the Donnan potential (~ E1/2value) of the multilayer is reduced. Our results
also agree with Itaya’s and Ochiai’s findings that the binding strengths of monovalent anions to PAH are significantly lower than the binding strength of divalent ions.75,76
Conclusions
We investigated ion exchange processes in Fe(CN)64- containing PGA/PAH multilayers using
EC-QCM-D and FTIR spectroscopy.
We successfully measured the electrochemical swelling behavior of this system and related it to interactions of the multilayer with different counter ions and their hydration shell. The films showed perm selective behavior; during the electrochemical swelling, they exchanged only anions and were indifferent towards changes in nature, valency or concentration of the cations. At low pH values, the PEM has a large amount of extrinsically compensated ionic groups rendering the multilayer hydrophilic. This results in high hydration numbers of the anions that are exchanged during the electrochemical swelling and therefore in large swelling amplitudes. The opposite effect was observed for high pH values. Increasing anionic ionic strength of the buffer solution resulted in higher permeability of the multilayers for water molecules. The swelling behavior was used to describe the hydration properties of different counter anions in the multilayer. We ordered the ions according to their ability to hydrate the multilayer, and compared our results to the Hofmeister series. Applied to monovalent ions, our ranking is in good agreement with the series; the kosmotropic ions indicate strong hydration of the multilayer while the chaotropic ones cause the multilayer to be less hydrated. The swelling amplitudes of the divalent anions used could not be rationalized in the same way, possibly because they showed strong binding affinities to PAH. We also showed that they can be used to reversibly shuttle the ferrocyanide out of the multilayer.
Getting insight into the electrochemical swelling behavior of PEM contributes to a better comprehension of the interplay between counter ions, their hydration shell and the ionic groups along
the polymers. Understanding the swelling process we could quantify the hydration properties of the multilayer in dependence of external factors like pH value, ionic strength or the kind of salts used in the buffer solution. Due to their small dimensions, the determination of ionic hydration number in PEMs is not possible or complicated with conventional methods (size exclusion chromatography, ultrasonic velocity, x-ray or neutron diffraction and nuclear magnetic resonance).77-80 Thus, characterizing the swelling amplitudes in the EC-QCM-D presents an easy and versatile method to determine the hydration properties of multilyers and could have an important impact on the design and characterization of thin film based devices like ion-selective membranes or smart coatings with tunable hydrophobicity.
Acknowledgements
The authors thank ETH Zurich (research grant ETH-17 08-1) and the Germaine de Staël project for funding.
Supporting Information Available: AFM image showing the topology of a PEI-(PGA-PAH)5
multilayer (Figure S-1); effect of counter cation species and molarity on swelling behavior and cyclic voltammograms in the EC-QCM-D (Figure S-2); EC-QCM-D measurement showing the effect of phosphate containing buffer on the swelling behavior of PEI-(PGA-PAH)5 (Figure S-3); Linear regression of the normalized swelling versus the hydration number for different counter anions (Figure S-4); Influence of the pH value on the static swelling of PEI-(PGA-PAH)5-Fe(CN)64- multilayers
(Figure S-5). This material is available free of charge via the Internet at http://pubs.acs.org.
References
(1) Volkov, A. G.; Deamer, D. W. Liquid-liquid interfaces: theory and methods; CRC Press, 1996.
(2) Decher, G.; Hong, J. D.; Schmitt, J. Thin solid films 1992, 210, 831. (3) Decher, G. Science 1997, 277, 1232.
(4) Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N. A. Advanced Materials 2007, 9, 906. (5) Yoo, D.; Shiratori, S. S.; Rubner, M. F. Macromolecules 1998, 31, 4309.
(7) Dubas, S. T.; Schlenoff, J. B. Macromolecules 1999, 32, 8153.
(8) Salomaki, M.; Vinokurov, I. A.; Kankare, J. Langmuir 2005, 21, 11232. (9) Manning, G. S. Journal of Chemical Physics 1969, 51, 924.
(10) Manning, G. S. Journal of Chemical Physics 1969, 51, 934. (11) Manning, G. S. Journal of Chemical Physics 1969, 51, 3249. (12) Steitz, R.; Jaeger, W.; von Klitzing, R. Langmuir 2001, 17, 4471. (13) Dubas, S. T.; Schlenoff, J. B. Langmuir 2001, 17, 7725.
(14) Salomaki, M.; Laiho, T.; Kankare, J. Macromolecules 2004, 37, 9585. (15) Salomaki, M.; Kankare, J. Macromolecules 2008, 41, 4423.
(16) El Haitami, A. E.; Martel, D.; Ball, V.; Nguyen, H. C.; Gonthier, E.; Labbe, P.; Voegel, J.-C.; Schaaf, P.; Senger, B.; Boulmedais, F. Langmuir 2009, 25, 2282.
(17) Zhang, Y.; Cremer, P. S. Current Opinion in Chemical Biology 2006, 10, 658.
(18) Klitzing, R.; Wong, J. E.; Jaeger, W.; Steitz, R. Curr. Opin. Colloid Interface Sci. 2004,
9, 158.
(19) Salomaki, M.; Tervasmaki, P.; Areva, S.; Kankare, J. Langmuir 2004, 20, 3679. (20) Schlenoff, J. B.; Rmaile, A. H.; Bucur, C. B. J Am Chem Soc 2008, 130, 13589. (21) Hofmeister, F. Arch. Exp. Pathol. Pharmakol 1888, 24, 247.
(22) Jaber, J. A.; Schlenoff, J. B. Langmuir 2007, 23, 896.
(23) Zhang, Y.; Furyk, S.; Bergbreiter, D. E.; Cremer, P. S. J. Am. Chem. Soc 2005, 127, 14505.
(24) Schönhoff, M.; Ball, V.; Bausch, A. R.; Dejugnat, C.; Delorme, N.; Glinel, K.; Klitzing, R.; Steitz, R. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2007, 303, 14.
(25) Crouzier, T.; Picart, C. Biomacromolecules 2009, 10, 433.
(26) Richert, L.; Lavalle, P.; Payan, E.; Shu, X. Z.; Prestwich, G. D.; Stoltz, J.-F.; Schaaf, P.; Voegel, J.-C.; Picart, C. Langmuir 2004, 20, 448.
(27) Thompson, M. T.; Berg, M. C.; Tobias, I. S.; Rubner, M. F.; Van Vliet, K. J.
Biomaterials 2005, 26, 6836.
(28) Picart, C. Curr. Med. Chem. 2008, 15, 685.
(29) Dai, J.; Jensen, A. W.; Mohanty, D. K.; Erndt, J.; Bruening, M. L. Langmuir 2001, 17, 931.
(30) Tokarev, I.; Minko, S. Soft Matter 2009, 5, 511.
(31) Harris, J. J.; Bruening, M. L. Langmuir 2000, 16, 2006. (32) Wang, L. M.; Lin, Y.; Su, Z. H. Soft Matter 2009, 5, 2072.
(33) Bruening, M. L.; Sullivan, D. M. Chemistry- A European Journal 2002, 8, 3832. (34) Harris, J. J.; Stair, J. L.; Bruening, M. L. Chemistry of materials 2000, 12, 1941.
(35) Ouyang, L.; Malaisamy, R.; Bruening, M. L. Journal of Membrane Science 2008, 310, 76.
(36) Chen, J.; Koehler, R.; Gutberlet, T.; Moehwald, H.; Krastev, R. Soft Matter 2009, 5, 228. (37) Farhat, T. R.; Schlenoff, J. B. Journal of the American Chemical Society 2003, 125, 4627.
(38) Farhat, T. R.; Schlenoff, J. B. Langmuir 2001, 17, 1184. (39) Hiller, J.; Rubner, M. F. Macromolecules 2003, 36, 4078.
(40) Tanchak, O. M.; Barrett, C. J. Chmistry of materials 2004, 16, 2734. (41) Burke, S. E.; Barrett, C. J. Biomacromolecules 2005, 6, 1419.
(42) Schmidt, D. J.; Cebeci, F. C.; Kalcioglu, Z. I.; Wyman, S. G.; Ortiz, C.; Van Vliet, K. J.; Hammond, P. T. ACS Nano 2009, 3, 2207.
(43) Calvo, E. J.; Wolosiuk, A. Journal of the American Chemical Society 2002, 124, 8490. (44) Forzani, E. S.; Perez, M. A.; Teijelo, M. L.; Calvo, E. J. Langmuir 2002, 18, 9867. (45) Tagliazucchi, M.; Grumelli, D.; Calvo, E. J. Physical Chemistry Chemical Physics 2006,
(46) Calvo, E. J.; Forzani, E.; Otero, M. Journal of Electroanalytical Chemistry 2002, 538, 231.
(47) Grumelli, D. E.; Garay, F.; Barbero, C. A.; Calvo, E. J. J. Phys. Chem. B 2006, 110, 15345.
(48) Tagliazucchi, M.; Calvo, E. J.; Szleifer, I. Journal of Physical Chemistry C 2008, 112, 458.
(49) Tagliazucchi, M.; Williams, F. J.; Calvo, E. J. J. Phys. Chem. B 2007, 111, 8105. (50) Tagliazucchi, M.; Calvo, E. J.; Szleifer, I. Langmuir 2008, 24, 2869.
(51) Hodak, J.; Etchenique, R.; Calvo, E. J.; Singhal, K.; Bartlett, P. N. Langmuir 1997, 13, 2708.
(52) Laurent, D.; Schlenoff, J. B. Langmuir 1997, 13, 1552.
(53) Liu, A.; Anzai, J. Analytical and Bioanalytical Chemistry 2004, 380, 98.
(54) Zhang, S.; Yang, W.; Niu, Y.; Sun, C. Sensors & Actuators B: Chemical 2004, 101, 387. (55) Zheng, H.; Zhou, J.; Zhang, J.; Huang, R.; Jia, H.; Suye, S.-i. Microchimica Acta 2009,
165, 109.
(56) Hübsch, E.; Fleith, G.; Fatisson, J.; Labbe, P.; Voegel, J. C.; Schaaf, P.; Ball, V.
Langmuir 2005, 21, 3664.
(57) Laugel, N.; Boulmedais, F.; El Haitami, A. E.; Rabu, P.; Rogez, G.; Voegel, J. C.; Schaaf, P.; Ball, V. Langmuir 2009, 25, 14030.
(58) Takita, R.; Yoshida, K.; Anzai, J. Sensors & Actuators: B. Chemical 2007, 121, 54. (59) Grieshaber, D.; Vörös, J.; Zambelli, T.; Ball, V.; Schaaf, P.; Voegel, J.-C.; Boulmedais, F. Langmuir 2008, 24, 13668.
(60) Venyaminov, S.; Kalnin, N. N. Biopolymers 1990, 30, 1259.
(61) Diéguez, L.; Darwish, N.; Graf, N.; Vörös, J.; Zambelli, T. Soft Matter 2009, 5, 2415 (62) Boulmedais, F.; Ball, V.; Schwinte, P.; Frisch, B.; Schaaf, P.; Voegel, J. C. Langmuir
2003, 19, 440.
(63) Johannsmann, D.; Reviakine, I.; Rojas, E.; Gallego, M. Analytical Chemistry 2008, 80, 8891.
(64) Tellechea, E.; Johannsmann, D.; Steinmetz, N. F.; Richter, R. P.; Reviakine, I. Langmuir
2009, 25, 5177.
(65) Boulmedais, F.; Bozonnet, M.; Schwinte, P.; Voegel, J. C.; Schaaf, P. Langmuir 2003,
19, 9873.
(66) von Klitzing, R. Physical Chemistry Chemical Physics 2006, 8, 5012.
(67) Cheng, Y.; Corn, R. M. The Journal of Physical Chemistry B 1999, 103, 8726. (68) Choi, J.; Rubner, M. F. Macromolecules 2005, 38, 116.
(69) Riegler, H.; Essler, F. Langmuir 2002, 18, 6694.
(70) Tanchak, O. M.; Yager, K. G.; Fritzsche, H.; Harroun, T.; Katsaras, J.; Barrett, C. J.
Journal of Chemical Physics 2008, 129.
(71) Sauerbrey, G. Z. Phys 1959, 155, 206.
(72) Vogt, B. D.; Lin, E. K.; Wu, W. L.; White, C. C. Journal of Physical Chemistry B 2004,
108, 12685.
(73) Szwarc, M. Accounts Chem. Res. 1969, 2, 87. (74) Wang, B.; Anzai, J. Langmuir 2007, 23, 7378.
(75) Ochiai, H.; Anabuki, Y.; Kojima, O.; Tominaga, K.; Murakami, I. Journal of Polymer
Science Part B Polymer Physics 1990, 28, 233.
(76) Itaya, T.; Ochiai, H. Journal of polymer science. Part B. Polymer physics 1992, 30, 587. (77) Kiriukhin, M. Y.; Collins, K. D. Biophysical Chemistry 2002, 99, 155.
(78) Bergstrom, P. A.; Lindgren, J.; Read, M.; Sandstrom, M. J. Phys. Chem. 1991, 95, 7650. (79) Bleuzen, A.; Foglia, F.; Furet, E.; Helm, L.; Merbach, A. E.; Weber, J. Journal of the
American Chemical Society 1996, 118, 12777.