HAL Id: hal-02371209
https://hal.archives-ouvertes.fr/hal-02371209
Submitted on 17 Nov 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Males’ calls carry information about individual identity
and morphological characteristics of the caller in
burrowing petrels
Charlène Gémard, Thierry Aubin, Francesco Bonadonna
To cite this version:
Charlène Gémard, Thierry Aubin, Francesco Bonadonna. Males’ calls carry information about indi-vidual identity and morphological characteristics of the caller in burrowing petrels. Journal of Avian Biology, Wiley, 2019, 50 (12), pp.e02270, doi:10.1111/jav.02270. �10.1111/jav.02270�. �hal-02371209�
Call rate, fundamental frequency and syntax determine
1male-call attractiveness in blue petrels Halobaena caerulea
2Charlène Gémard
1,2, Thierry Aubin
2, Eliette L. Reboud
1, Francesco Bonadonna
13
1 CEFE, Univ Montpellier, CNRS, Univ Paul Valéry Montpellier 3, EPHE, IRD, Montpellier, France
4
2 Equipe Communications Acoustiques, UMR 9197, Neuro-PSI-CNRS, Université Paris-Sud, Bat.446, 91405
5
Orsay, France.
Acknowledgments
7
This work was financially and logistically supported by the French polar institute Paul Emile Victor (IPEV,
8
program no. 354 ETHOTAAF). We are very grateful to Jessica Graham who improved the English of this
9
manuscript, and to Guilhem Battistella and Jean-Yves Barnagaud for their precious help on the field.
10 11
Abstract
12
In blue petrels (Halobaena caerulea), females are supposed to be particularly choosy and mate choice 13
can take a couple of years. In these lifelong monogamous seabirds, choosing a good mate is crucial and 14
has a strong influence on their fitness. Due to their nocturnal habits, the absence of sexual dimorphism 15
and the physical barrier between males calling from their burrow and females flying above the colony, 16
vocal signals seem to be one of the main channels for males to communicate with potential mates. 17
However vocal communication is also costly as it attracts predators. In a previous study, we investigated 18
whether acoustic traits of male calls carry information about morphological characteristics that might 19
be indicators of males’ qualities. Here, we test whether these acoustic traits linked to male characteristics 20
are actually attractive to females. To do so, we played-back modified calls of males to females in a 21
colony of blue petrels of the Kerguelen archipelago. We found that flying females were more attracted 22
by high-pitched calls and by calls broadcasted at a high call rate. Previous studies showed a relationship 23
between pitch and bill depth and length. In filter-feeding birds, such as blue petrels, bill morphology 24
influences feeding efficiency. A high call rate is an indicator of sexual motivation and makes the caller 25
easier to locate by potential mates and predators in the hubbub of the colony. We thus hypothesized that 26
producing frequent high-pitched calls may be the result of a trade-off between predation avoidance and 27
conspicuous sexual signalling. 28
Keywords
29
Vocal communication, sexual signal, attractiveness, mate choice, seabird, blue petrel 30
Introduction
1
Knowing how animal communication systems work is crucial to understanding social 2
behaviours. Indeed, social interactions are mediated by signals in one or several modalities, such as 3
acoustic, olfactory, or visual (Bradbury and Vehrencamp 2011). Among these, vocal signals provide 4
information mainly to conspecifics, but not exclusively (see Magrath et al. 2015 for a review). Signals 5
carry different kinds of information, stable information on long-term (e.g. species, sex, social status, 6
group membership, phenotypic characteristics: Searcy and Nowicki 2005) and temporary information 7
through acoustic modulations (e.g. motivation: Morton 1977, emotion: Briefer 2018). Vocalizations may 8
also bear stable individual signatures to allow efficient discrimination among individuals (Beecher 1989, 9
Tibbets and Dale 2007), particularly in social species. For instance, it has been shown in rodents that 10
alarm calls contain more individual information in species living in large groups than species living in 11
small ones (Pollard and Blumstein 2011). 12
Informative content of vocal signals has been particularly well-studied in birds in the framework 13
of sexual selection likely because the “dual function hypothesis” states that birdsongs serve two main 14
purposes: attract mates and deter rivals (Kroodsma and Byers 1991, Catchpole and Slater 2008). Due to 15
high costs (predation, social aggression, etc) and physiological constraints associated with their 16
production, vocalizations are considered as honest and reliable signals of male phenotypic qualities, 17
such as body size and body condition (Gil and Gahr 2002). Individual differences in phenotypic qualities 18
among males are translated by differences in structure of vocal signals (Rowe and Houlde 1996). 19
Several acoustic parameters have been shown to reflect a singer’s characteristics linked to 20
overall male quality, and there is evidence that females are attracted by specific acoustic parameters 21
related to male qualities (see Nowicki and Searcy 2004 for a review). A well-known example is the 22
fundamental frequency (or pitch) which negatively correlates with body size (e.g. Galeotti et al. 1997, 23
Mager et al. 2007, Favaro et al. 2017, Kriesell et al. 2018). In many species, larger males have a higher 24
breeding success because body mass is an indicator of physical strength and/or foraging success (Chastel 25
et al. 1995, Salton et al. 2015) and females rely on fundamental frequency as a sexual signal (review in 26
Cardoso 2012). Besides frequency parameters, females rely on song output structure, complexity, and 27
vocal performance (Nowicki and Searcy 2004). For instance, they are attracted by songs constituted of 28
many and/or complex elements (Martín-Vivaldi et al. 2000, Martín-Vivaldi 2004) which may indicate 29
a male in good body condition (Martín-Vivaldi et al. 1998). Females are also attracted by males that 30
perform challenging songs (i.e. with a high rate and/or a large frequency bandwidth), that indicates body 31
size, age, and endurance (Ballentine et al. 2004, Ballentine 2009, Byers et al. 2016). 32
In many bird species, among the functions that calls and songs fulfil, there is the transmission 33
of individual signature information (Falls 1982, Lambrechts and Dhont 1995). Vocal individual 34
signature is a crucial piece of information as it provides the basis for individual recognition required for 35
almost all aspects of social life (Tibbetts and Dale 2007). Individual recognition is possible when an 36
individual identifies another according to the easily-distinctive characteristics of its signal, for example 37
acoustic parameters of a call (Falls 1982, Dale et al. 2001). Vocal signature has been assessed in many 38
non-songbird species, especially seabirds (e.g. Robisson et al. 1993, Charrier et al. 2001, Aubin and 39
Jouventin 2002, Aubin et al. 2007, Favaro et al. 2017). In many seabirds, adults usually breed in dense 40
colonies (Croxall and Prince 1980, Wittenberg and Hunt 1985), form lifelong monogamous bonds 41
(Warham 1996), and forage far from the colony. Individual recognition would be advantageous when 42
returning to the nest/mate/chick (Warham 1990, Mathevon et al. 2003, Jouventin et al. 1999, Curé et al. 43
2011), especially for species that have no nest site to meet at (Robisson et al. 1993, Aubin and Jouventin 44
2002). 45
Among Procellariidae, burrowing petrels occupy deep burrows and are active, at the colony, at 46
night during the breeding period (Warham 1990). In this context, visual communication seems strongly 47
restricted whereas olfactory and vocal signals may be used for long-range communication. The role of 48
olfaction has been investigated in petrel social interactions. Studies suggest that the chemical signature 49
of nests is used by mates to find their own burrow (Bonadonna et al. 2001, Bonadonna et al. 2004), and 50
that individual body’s odour carries individuality signals and some genetic information that may 51
influence mate choice (Bonadonna and Nevitt 2004; Bonadonna and Sanz-Aguilar 2012; Leclaire et al 52
2017). Comparatively, acoustic communication has been poorly studied so far. For some burrowing-53
petrel species, vocal communication is extremely costly because it exposes callers to an increased risk 54
of predation (Mougeot and Bretagnolle 2000). However, during pair formation, bachelor males looking
for a mate intensely vocalize at night from their burrow, while females fly and call over the colony 56
(Bretagnolle 1990, Warham 1996). These costly calls are likely to be reliable sexual signals that attract 57
and/or stimulate suitable mates (Bretagnolle 1990), but we still ignore which one, between male or 58
female, stimulates the other. Petrels also vocalize after being vocally challenged by a conspecific close 59
to the entrance of their burrow. These vocalizations might be aggressive signals to defend the own 60
burrow (occupied year after year) from intruders (Warham 1990, Warham 1996). According to 61
Bretagnolle (1996), burrowing petrels have a small vocal repertoire. For instance, genera Halobaena 62
and Pachyptila produce a single major call produce in both sexual and agonistic interactions. 63
As previously mentioned, calling in the colony increases the predation risks (Mougeot and
64
Bretagnolle 2000) and should thus provide important benefits to balance these costs. Following the 65
suggestions of the wide literature on other bird species, we can hypothesize that calls play a role in mate 66
choice and/or male competition for mate and burrow. However, informative content of most burrowing-67
petrel species’ calls have been neglected so far. Nevertheless, previous studies have shown that heavy 68
males call with a higher rhythm than light males in blue petrels (Genevois and Bretagnolle 1994) and 69
that large heavy males produce low-pitched calls in snow petrels (Barbraud et al. 2000), suggesting 70
several ways of body size signalling. Here, we investigated the informative content of calls in two 71
burrowing petrels: the blue petrel (Halobaena caerulea) and the Antarctic prion (Pachyptila desolata) 72
(Procellariidae, Gmelin 1789). These two petrel species, with similar breeding and feeding ecology 73
(Warham 1990, Warham 1996), are highly vocal and suffer from a high predation pressure by the brown 74
skua (Mougeot and Bretagnolle 1998). We tested the hypotheses that acoustic parameters in both 75
temporal and spectral domains of a petrel calls (i) reflect morphological characteristics, and (ii) bear an 76
individual vocal signature. 77
Material and Methods
78
Studied species
79Blue petrels (H. caerulea) and Antarctic prions (P. desolata) are monogamous medium-sized 80
(about 190 and 160 g, respectively) seabirds belonging to the Procellariidae. They spend most of their 81
life at sea but they breed in dense colonies on coastal grass slops. This long-lived seabirds do not reach 82
sexual maturity for five years. A couple of years is needed for establishing stable pair-bonds. They show 83
a high partner fidelity, low extra-pair paternities, and divorces are rare. After pairing, pairs produce a 84
single egg per year. From the incubation (50 days in blue petrels, 45 in Antarctic prions) to the fledging 85
(50 and 53 days, respectively), they exhibit bi-parental cares. Partners regularly alternate fasting period 86
in the burrow and feeding trips at sea (Warham 1990, Warham 1996, Brooke 2004). 87
Callers are mainly non-breeder males and females looking for a mate but they are tricky to catch, 88
as they occupy inaccessible burrows and fly over the colony, respectively. On the contrary, breeders 89
usually have high mate and burrow fidelity over years (Warham 1996, Brooke 2004). Occupied burrows 90
can be equipped with an artificial access to the incubating chamber, by digging a tunnel and closing it 91
with a stone. From this artificial door, birds become easily accessible. For this reason, we worked only 92
on breeding birds. After some infructuous attempts to obtain females’ replies to playback, we 93
constrained our study to only on males’ calls. According to the literature, very few breeding females
94
react to the playback of bachelor males’ calls (Brooke 1978, Bretagnolle and Lequette 1990, Bretagnolle 95
1996). A possible explanation is that, in natural conditions, females call only in flight, whereas males 96
call from their burrow. The study was thus restricted on breeding males of the two petrel species: 31 97
blue petrels (16 in 2017 and 15 in 2018) and 24 Antarctic prions (16 in 2017 and 8 in 2018). Mates take 98
a shift every eight to ten days incubating the eggs (Warham 1990), and occasions when both mates are 99
present in the nest at the same time are quite rare. This enabled us to record the male alone. Petrels well 100
tolerate weak human disturbance (Bergès et al. 2019), allowing us to conduct several recording sessions 101
on the same individuals to assess the individual signature. All the birds were metal ringed and 102
individually identifiable. 103
Study location
104Fieldwork was performed in a small sub-Antarctic island (Ile Verte, 49°51′ S, 70°05′ E) of the Kerguelen 105
Archipelago, in the southern Indian Ocean, where blue petrels and the Antarctic prions gather in stable 106
colonies during the breeding season. We conducted the study during the 2017 and 2018 birds’ incubation 107
period (November 25th 2017 to December 12th 2017 and November 27th 2018 to December 20th 2018 for 108
blue petrels; December 23rd 2017 to January 16th 2018 and December 25th 2018 to January 13th 2019 for 109
Antarctic prions). 110
Playback procedure and recording of provoked calls
111During incubation, breeding petrels are silent in the colony, rarely calling spontaneously, 112
seemingly to avoid predation (Mougeot and Bretagnolle 2000). However, it is possible to elicit vocal 113
responses by broadcasting calls of same-gender conspecifics (Taoka et al. 1989, Bretagnolle & Lequette 114
1990, Curé et al. 2011). Petrel calls consist of a repetition of distinct sections called “phrases”, 115
themselves composed of indivisible elements called syllables (see Catchpole and Slater 2008 for 116
definition, see Fig. 2a and Bretagnolle 1996 for sonograms). Signals to broadcast were graphically 117
synthesized from spontaneous calls of nine bachelor male blue petrels and five bachelor male Antarctic 118
prions recorded in the same colonies in 2013 and 2017 (i.e. series of phrases, themselves constituted of 119
syllables). We used the signal processing software Avisoft–SASLab Pro v 5.2.11 (Specht 2017). Each 120
synthesized call consisted in a two-phrase call of a single blue petrel or Antarctic prion male, separated 121
by a silence of 200 ± 60 ms (5.5 ± 1.6 s and 3.43 ± 0.85 s, respectively; mean ± SD). To avoid 122
pseudoreplication (McGregor et al. 1992), nine blue petrel call series and five Antarctic prion call series 123
were played-back. 124
At the beginning of the 2017 and 2018 breeding seasons, we checked each burrow of the colony to 125
detect occupied burrows with the presence of an egg. We then choose distant burrows (i.e. separated by 126
a minimum of five meters) for each species. Several recording sessions were performed on each male, 127
with an interval of two to five day between sessions to avoid habituation. In total, we performed a mean 128
of 3 ± 1 recording sessions on 31 blue petrel males and we recorded 172 calls (6 ± 4 calls per male; 129
mean ± SD). We performed 4 ± 2 recording sessions on 24 Antarctic prion males and we recorded 224 130
calls (5 ± 3 calls per male; mean ± SD). Playbacks were carried out at night during the period of maximal 131
vocal activity of the colony (i.e. between 22:00 and 02:00, unpublished data) in dark nights (Mougeot 132
and Bretagnolle 2000), and in calm weather (i.e. wind speed < 4 km/h and no rain) to limit background 133
noises. 134
The recording equipment was composed of an omnidirectional Sennheiser K6-ME62 microphone 135
(frequency response: 20-20 000 Hz ± 2.5 dB) connected to a Marantz PMD 660 digital recorder 136
(sampling frequency: 44.1 kHz, dynamic: 16 bits). The microphone was positioned on the ground, at the 137
burrow entrance, and one randomly selected synthesized call was broadcast at a natural sound pressure 138
level (± 67 dB, measured on 111 calls from 54 males with a sound level meter) using a TASCAM DR-139
07MKII digital recorder (sampling frequency: 44.1 kHz, dynamic:16 bits). Calls emitted in reply by the 140
burrow owner were recorded during the playback and two minutes after the end of the playback stimulus. 141
If the focal individual did not reply, the playback was repeated every 30 seconds, up to three times. 142
Morphometric measurements
143Soon after recording, the identity of the bird was checked by capturing the birds and reading the 144
metal ring. The day after, birds were caught again in order to take a blood sample from the brachial vein 145
(0.2 mL in Queen lysis buffer, Seutin et al. 1991) and the following seven morphometricmeasurements 146
(some shown in Fig. 1, Cramp and Simmons 1977): bill length (CL), from edge of implantation of 147
feathers to the bill tip; bill depth (BD), from angle of gonys to dorsal surface of hook; head length (HL), 148
from supraoccital to front edge of bill; tarsus length (TL), from middle of mid-tarsal joint to distal end 149
of tarso-metatarsus; wing chord length (WL), maximum flattened chord, from carpal joint to tip of 150
longest primary; mass (MS); wing area (WA). 151
Head and tarsus measurements (CL, BD, HL, and TL) were taken using a calliper with an 152
accuracy of ± 0.1 mm. Wing length was measured with a stainless-steel rule with an accuracy of ± 1.0 153
mm. To limit measurement biases, a single person (CG) took each measurement three times and 154
calculated the mean. We also took standardized pictures of both sides of the right wing for each tested 155
individual, using a digital camera (Olympus TG-610). Wing area was then measured by a single person 156
(CG) by counting the number of calibrated pixels using ImageJ image analysis software version 1.52a 157
(National Institute of Health, USA). Correlation scores between morphometric measurements and 158
summary are given in Appendices I and II, respectively. 159
We chose morphometric measurements likely to be indicators of body size and/or to have an 160
effect on call production based on the literature, and/or may be important in the biology of the species. 161
Head size has been used as an indicator of body size in swallows (Winkler and Allen 1996, Patel et al. 162
2010) and nestling growth in blue tits (Plummer et al. 2013). Similarly, tarsus and wing lengths are good 163
indicators of body size in passerines (Senar and Pascual 1997, Gosler et al. 1998), although it has not 164
been documented if these indicators are suitable in seabirds. Bill is considered as the end of the vocal 165
tract and its morphology (length and depth) may influence sound production. Bill is also the small 166
petrels’ feeding means (Warham 1996), as they filter small crustaceans at the surface of the water. Wing 167
area seems a relevant morphological trait because petrels are a soaring species and travel great distances 168
at sea to feed (Cherel et al. 2002). 169
Body mass is highly variable in petrels due to their feeding habits. Every ten days or so, mates 170
alternate fasting incubation shifts in the burrow and foraging trips where they restore their energy 171
reserves (Warham 1990). The longer they stay in the burrow incubating, the lighter they get. For 172
instance, in blue petrels, breeders loose about 45 ± 6 g in 10 ± 2 days (mean ± SD) during the incubating 173
shift (Chaurand and Weimerskirch 1994a, 1994b). Therefore, we did not include mass in the following 174
statistical analyses. 175
Birds were genetically sexed following methods for non-ratite birds (Fridolfsson and Ellegren 176
1999). 177
Acoustic analysis
178Calls recorded were first down sampled at 11.025 kHz to increase the precision of frequency 179
measurements and high-pass filtered (Cutting frequency: 0.10 kHz, FFT filter) to remove the 180
background noise. We described calls at a phrase level by measuring 11 acoustic parameters in the 181
temporal and frequency domains (see Table 1 for abbreviations and description), on oscillograms and 182
spectra of the signal-processing software Avisoft–SASLab Pro v 5.2.11, respectively (Specht 2017). 183
Temporal variables were automatically extracted on the amplitude envelopes using the “Pulse Train 184
Analysis” function, with a resolution of 0.09 ms (hysteresis: +30 dB; start/end threshold: -30 dB). 185
Settings were not manipulated during measurements, except the threshold which was manually adjusted 186
to detect all syllables. Mean fundamental frequencies and variables describing energy spectral 187
distribution were automatically extracted on linear amplitude spectra with a resolution of 22 Hz (FFT 188
length: 512, Blackman window) (Fig. 2). Automatic extractions are based on clear criteria preliminarily 189
set, insuring objectivity in element demarcation and thus replicable measurements (Fischer et al. 2013). 190
Syllables and phrases were counted on sonograms (Fig. 2). Correlation scores between acoustic-191
parameter measures and summary are given in Appendices III and IV, respectively. 192
Statistical Analysis
193We implemented all analysis results under the R software environment version 3.4.4 (R Core 194
Team 2018) with the ade4 package (Dray and Dufour 2007). 195
Relating acoustic parameters and morphological traits
196
Body mass has been shown to be related to vocal performance in birds, i.e. heavy males have a 197
higher calling activity than light males (e.g. Berg et al. 2005, Barnett and Briskie 2011, Yamada and 198
Soma 2016). As previously mentioned, mass is highly variable in petrels and decreased during the 199
fasting periods in the burrow (Chaurand and Weimerskirch 1994a, 1994b), and consequently between 200
successive playback sessions. To avoid potential bias related to the high variation of mass between 201
playback sessions, we considered only the first session of each individual and we omitted mass in our 202
statistical analyses. 203
We investigated covariance among morphometric measurements and acoustic parameters with 204
a Co-Inertia Analysis (CIA), an ordination method designed to reveal the co-structures among two data 205
tables (Dolédec and Chessel 1994, Dray et al. 2003). A summary table of acoustic and morphometric 206
data are given in Appendices I and II, respectively. We first summarized the morphometric measurement 207
table with the two first axes of a normed Principal Component Analysis (PCA1), which explained 208
together 55.6 % for blue petrel (32.2 and 23.3 % for PC11 and PC12, respectively) and 60.6% of total 209
inertia for Antarctic prion (34.6 and 26.0 % for PC11 and PC12, respectively). We ordinated the acoustic 210
parameters table with a Between-Class Analysis (PCA2), a PCA which accounts for the clustering of 211
object (here phrases) by a grouping factor (here individuals), similarly as in a redundancy analysis 212
(Dolédec and Chessel 1987). We retained the two first axes of the PCA2 in both species (28.2% and 213
24.7% of variance explained by PC21 and PC22, respectively, in blue petrel; 34.6% and 24.9% in 214
Antarctic prion). We built the CIA as an ordination of PCA1 and PCA2. Overall similarity between the 215
two ordination tables was assessed by the RV coefficient, a correlation coefficient between two sets of 216
variables recorded from the same sample (0: no similarity, 1: equal tables). 217
Individual signature
218
We assessed whether males can be discriminated based on their calls using a Between-Class 219
Analysis (BCA, Dolédec and Chessel 1987). Then, we compared the intra and inter-individual variations 220
for each acoustic parameter to highlight the most discriminant parameters. As a single recording session 221
is not sufficient to highlight a vocal signature (Průchová et al. 2017), we considered only males that 222
have been recorded at least three times to run the BCA. In total, we had 127 blue petrel calls from 21 223
males (6 ± 4 calls per male), and 224 Antarctic prion calls from 16 males (5 ± 3 calls per male). 224
We calculated the Potential of Individuality Coding (PIC) of each call acoustic parameter. PIC 225
is defined as the ratio between intra-individual variation (CVw) and inter-individual variation (CVb) of 226
a given acoustic parameter. For each parameter and each individual, we calculated the coefficient of 227
intra-individual variation CVw corrected for small samples (Scherrer 1984): CV 100 1 228
with X the mean of the parameter measured, and n is the number of measures. We calculated the inter-229
individual variation coefficient as CV 100 where std and X arethe standard deviation and the 230
mean calculated with all measures of a given parameters, respectively, from all individuals. PIC values 231
have been calculated as PIC . If PIC>1, intra-individual variability is smaller than inter-individual 232
variability for a given parameter, suggesting that this parameter may potentially carry individual 233
information. PIC values are considered high (i.e. likely to carry individual information), when they are 234
superior to 2 (Robisson et al. 1993, Lengagne et al. 1997). 235
Results
236
Relation between acoustic parameters and morphological traits
237In blue petrels, the RV coefficient of the CIA between morphometric measurements and acoustic 238
parameters is 0.34. The first two axes of the CIA explained 51.0% and 22.8% of total variance, 239
respectively. The first CIA axis shows the duration and the syllable number of the phrase are mainly 240
related to head and bill length (HL, CL). More precisely, males with a small head (HL) and a short bill 241
(CL) produce short calls with few syllables. The second CIA axis shows rhythm and syllable rate are 242
related to both wing measurements (WL, WA). More precisely, males with short, large wings produce 243
slow (i.e. low phrase rate) calls with long syllables (i.e. high rhythm) and short interphrase silences. 244
Frequency parameters are weakly related to the chosen morphometric measurements (Fig. 3). 245
In Antarctic prions, the RV coefficient of the CIA between morphometric measurements and 246
acoustic parameters is 0.40. The first two axes of the CIA explained 52.9% and 25.6% of total variance, 247
respectively. The first CIA axis of shows head-bill length (HL) and wing area (WA) are related to energy 248
quartiles. More precisely, males with a short head (HL) and slim wings (WA) produce high-pitched 249
calls. The second CIA axis shows temporal parameters (duration, interphrase silences and phrase rate) 250
are mainly related to wing length (WL) and bill length (CL). In other words, males with long wings and 251
a short bill produce short, fast (i.e. high phrase rate, and short interphrase silences) calls (Fig. 4). 252
Tables of CIA scores for both species are given in Appendix V. 253
Individual signature
254
We focused here on the individual information carried in males’ calls. We did not investigate 255
the sexual signature or species signature, both already described in the literature (Bretagnolle 1996, 256
Bretagnolle and Genevois 1997). 257
The total inertia explained by the differences between males in blue petrels and Antarctic prions 258
was 74% and 65%, respectively, showing that the chosen acoustic parameters allow a significant vocal 259
discrimination of individuals (p <10-5 in both species). Composite plots of BCA analyses are given in 260
Appendix VI. 261
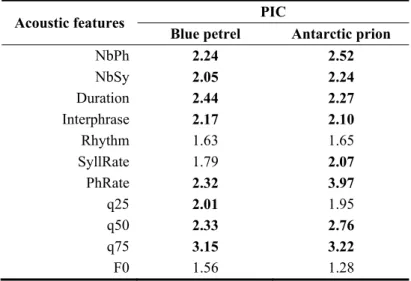
The PIC values of each of the 11 acoustic parameters used to describe blue petrel and Antarctic 262
prion calls are presented in Fig. 4. All values in both species are higher than one, suggesting that all 263
acoustic parameters considered here may bear the vocal signature (Robisson et al. 1993). Eight out of 264
11 parameters are higher than two, suggesting they potentially code more than others for individual 265
information (Robisson et al. 1993). In both species, acoustic parameters with highest PIC values are 266
upper energy quartiles in spectral domain, duration, and phrase rate in time domain (Table 2). BCA and 267
PIC methods gave similar results. 268
Discussion
269
We aimed to investigate the informative content of calls in two species of burrowing petrels, the 270
blue petrel and the Antarctic prion. Results show acoustic parameters carry information about the caller 271
individuality and morphological traits, for males of both species. 272
Relationship between acoustic parameters and morphological traits
273In both species, results obtained by CIA show a clear relationship between acoustic parameters 274
of males’ calls and some morphological traits which may constraint call productions (bill morphology, 275
body size indicators), and/or may be relevant considering the feeding ecology (bill morphology, wing 276
morphology) of these species. 277
Specifically, frequency parameters (i.e. fundamental frequency and energy quartiles) are related 278
to bill morphology, especially head-bill length, in both species even though the relationship is weak in 279
blue petrels. Males with a short head and bill produce low-pitched calls in blue petrels whereas they 280
produce high-pitched calls in Antarctic prions. Relationship between bill morphology and frequency is 281
unclear in birds and both opposite patterns have been observed in many species (e.g. Palacios and Tubaro 282
2000, Laiolo and Rolando 2003, Christensen et al. 2006). Other studies have found no relationships 283
between frequencies and bill morphology (e.g. Garcia and Tubaro 2018). 284
Head-bill length is also a body size indicators, suggesting frequencies are related to body size. 285
This frequency-body size relationship has been illustrated in many species at the light of the source-286
filter theory (e.g. Taylor & Reby 2010). This theory states that vocal signals result from a two-stage 287
production: the source signal is generated by membrane vibrations in the vocal organ (larynx in 288
mammals or syrinx in birds), and subsequently filtered by the vocal tract and the mouth/nostrils (Fant 289
1960). Consequently, the anatomy of the “source”, particularly the length and mass of vibrating 290
membranes, shapes the fundamental frequency. Muscular interactions changes the airflow in the 291
“source” and thus influence temporal parameters such as rhythm, duration and amplitude. The “filter” 292
influences the formants, i.e. the resonant frequencies (Titze 1994, Taylor and Reby 2010). Although this 293
theory is valid in mammals, the relationships between frequencies and body size remains unclear in 294
birds (Galeotti et al. 1997, Mager et al. 2007, Favaro et al. 2017, Kriesell et al. 2018 but see Patel et al.
295
2010). 296
Our results show some temporal parameters are related to body size in both species, suggesting 297
big males produce more challenging songs than small males. More precisely, in blue petrels, males with 298
a long head, wings and tarsus produce long, fast calls with many syllables. In Antarctic prions, males 299
with long wings produce short, fast calls. In many species, females are more attracted by males 300
performing physically challenging songs/calls (e.g. high rate, long duration), likely because they reflect 301
male characteristics linked to qualities such as endurance (Ballentine et al. 2004), survival (Byers et al. 302
2016), age, and size (Ballentine 2009). 303
Results show a strong relationship between acoustic parameters and wing morphology, 304
especially wing length in both species, and wing area even though this relationship is weaker in blue 305
petrels. We thus suggest that for soaring seabirds, which travel great distances at sea to feed, wing 306
morphology is a relevant parameter to be considered (Cherel et al. 2002). We can hypothesize that cues 307
that may inform females on this male characteristic might be important as wing morphology is correlated 308
with foraging behaviour (Hertel and Ballance 1999) and reproductive costs (Mauck and Grubb 1995). 309
In other Procellariidae, a reduction of parents’ flying abilities (e.g. clipping feather tips, removing flight 310
feathers, adding extra weight) affects the incubation routine and results in a decrease of their body 311
condition and/or a deterioration of chick condition (Saether et al. 1993, Weimerskirch et al. 1995, 312
Navarro and Gonzáles-Solís 2007). In many species, females are more attracted by males performing 313
physically challenging songs/calls (e.g. high rate, long duration), likely because they reflect male 314
characteristics linked to qualities such as endurance (Ballentine et al. 2004), survival (Byers et al. 2016), 315
age, and size (Ballentine 2009). 316
Blue petrels and Antarctic prions form lifelong monogamous bonds. Pair bonding takes up to 317
two years and partners equally share parental care during the incubation period (Chaurand and 318
Weimerskirch 1994a, Warham 1990, Warham 1996). Sexual maturity is reached tardily (around seven 319
years), they show low fecundity (one single egg per year without a replacement clutch), and extra-pair 320
paternity is extremely rare (Jouventin and Mougin 1981, Warham 1996). Consequently, choosing the 321
“wrong” mate affects fitness more than in species that change mates at each breeding. Females should 322
rely on attractive and reliable cues to evaluate the quality of a potential partner (Darwin 1871, Smith 323
1991). As in many bird species, males’ calls are likely to be sexual signals that attract and/or stimulate 324
a potential mate (Bretagnolle 1990). Especially as they are highly vocal, despite the predation cost, and 325
as living in burrows prevents visual communication on long-range. We hypothesize, thus, that the 326
information contained in male calls about the caller body size may have a significant role in sexual 327
selection.In blue petrels, bigger males at the beginning of the breeding season have a higher breeding 328
success (Chastel et al. 1995). Moreover, calls contain information about wing morphology, which may 329
be linked to reproductive success in these soaring birds (Saether et al. 1993, Weimerskirch et al. 1995, 330
Navarro and Gonzáles-Solís 2007). 331
Individual signature
332Our results suggest that individuality in calls of males of both species is coded by several 333
parameters in spectral (energy quartiles) and temporal domain (phrase rate and duration). Individual 334
identity coded by frequency parameters has been shown in numerous species (e.g. Robisson et al. 1993, 335
Jouventin et al. 1999, Charrier et al. 2001, Charrier et al. 2004, Favaro et al. 2017). Among these 336
parameters, the fundamental frequency often codes for individual signature in many birds, likely because 337
it is linked to anatomical structure of the vocal tract (Fletcher and Tarnopolsky 1999). Nevertheless, our 338
results show that fundamental frequency has the lowest PIC value and that its correlation with 339
morphological traits is weak. This is consistent with previous studies on blue petrels (Genevois and 340
Bretagnolle 1994), although a negative correlation between mass and fundamental frequency has been 341
highlighted in the snow petrel (Guillotin and Jouventin 1980). 342
In many seabirds, vocal identity is crucial in social interactions, such as mate reunion or kin 343
recognition (e.g. Spheniscidae: Robisson et al. 1993, Jouventin et al. 1999, Aubin and Jouventin 2002, 344
Procellariidae: Barbraud et al. 2000, Curé et al. 2011, Stercorariidae: Charrier et al. 2001, Laridae 345
Mathevon et al. 2003). However, in burrowing petrels, finding relatives and nest sites seems not based 346
on vocal signals. As they return from their foraging trip at sea, petrels rely on olfactory signals to find 347
their burrow (Bonadonna et al. 2001, Bonadonna et al. 2004) and take the place of their mate incubating 348
in the burrow, without emitting any call (FB and CG personal observation). Therefore, vocal signature 349
does not seem to be involved in mate reunion. Vocal signatures do not systematically imply recognition 350
(Townsend et al. 2010). So far, there is no clear evidence that burrowing petrels vocally recognize 351
conspecifics as shown in other seabird families (e.g. Spheniscidae: Robisson et al. 1993, Jouventin et al. 352
1999, Aubin and Jouventin 2002, Procellariidae: Barbraud et al. 2000, Curé et al. 2011, Stercorariidae: 353
Charrier et al. 2001, Laridae: Mathevon et al. 2003). Nonetheless, vocal signature could be used in 354
neighbour/stranger discrimination processes. Indeed, blue petrel and Antarctic prion males call from 355
their burrow in crowded colonies (respectively up to 0.7 and 1.4 burrows/m² according to Croxall and 356
Prince 1980). The significant costs of calling behaviour (Mougeot and Bretagnolle 2000) suggests that 357
males should call only when necessary, for instance in the presence of a stranger male not belonging to 358
the neighbouring community, to avoid higher predation risks and useless energy expenditures. This 359
“dear-enemy phenomenon” (Fisher 1954) has been documented in many territorial songbirds where 360
males are less aggressive with neighbours than with strangers when defending their breeding territory 361
(Temeles 1994). One hypothesis is that territory owners adjust their aggressive behaviour according to 362
the familiarity and/or threat degrees and thus minimizes fighting costs (Temeles 1994). 363
Knowing which information is passed through vocal signals is crucial to understanding many 364
social behaviours, such as female mate choice or male-male competition. Here, we investigated the 365
informative content of males’ calls in blue petrels and Antarctic prions, two under-studied species that 366
use vocal signals in their social interactions. Results highlight similarities on informative content of 367
males’ calls between the two study species, such as the relationship between body size - element rates, 368
and individual-identity coding strategy. However, results also highlight dissimilarities. Although these 369
species are phylogenetically and ecologically close (Warham 1990, Warham 1996), they exhibit calls 370
that differ by their structure, with two different strategies of body-size vocal signalling. In Antarctic 371
prions, frequencies bear information on the caller body size whereas this information is only coded by 372
temporal parameters in blue petrels. These results are consistent with previous studies on blue petrels 373
(Genevois and Bretagnolle 1994) and snow petrels (Barbraud et al. 2000), suggesting the existence of 374
different coding strategies in Procellariidae. Syntactic differences between blue petrel and Antarctic 375
prions calls (Bretagnolle 1996) or differences of the intensity of predation pressure (Mougeot et al. 1998) 376
might be possible explanations for these different coding strategies. 377
Globally, our results showing that several call parameters correlate with male body size, suggest 378
that they might be potential indicators of male quality for females. However, so far, there is no evidence 379
showing that females are more attracted to males with particular morphological traits. Several call 380
parameters also bear an individual signature. Nevertheless, individual recognition has not been explored 381
in these species. Our study is the first step to understand the importance of vocal signals in burrowing 382
petrels’ social lives. The direction for future studies will be to assess how the informative content of 383
males' calls influence conspecific behaviours, for instance, by using playback experiments to test the 384
existence of individual vocal recognition and whether call characteristics could be good predictors of 385
female preference. 386
387
Acknowledgments – We thank all people who kindly helped us in the field, especially Jean-Yves Barnagaud,
388
Guilhem Battistella, Matthieu Bergès, Samuel Perret and the French polar institute IPEV which provided logistic
389
support in the field. We are very grateful to Jean-Yves Barnagaud and Pierre Legendre for their sound advices and
390
guidance on statistical analyses. We are also grateful to Jessica Graham for her comments and English
391
improvement of the paper.
392
Conflict of interest – The authors declare no conflicts of interest associated with this study.
393
Funding – This work was supported by the French Polar Institute Paul Emile Victor (IPEV, program no. 354).
394
Permits – This study was performed according to guidelines established by both IPEV and CNRS for the Ethical
395
Treatment of Animals. All experiments comply with the current laws of the country where they were performed.
396 397
References
398Aubin, T. and Jouventin, P. 2002. How to vocally identify kin in a crowd: the penguin model. - Adv. 399
Study Behav. 31: 243–277. 400
Aubin, T., Mathevon, N., Staszewski, V. and Boulinier, T. 2007. Acoustic communication in the 401
kittiwake Rissa tridactyla: potential cues for sexual and individual signatures in long calls. - 402
Polar Biol. 30: 1027–1033. 403
Ballentine, B. 2009. The ability to perform physically challenging songs predicts age and size in male 404
swamp sparrows, Melospiza georgiana. - Anim. Behav. 77: 973–978. 405
Ballentine, B., Hyman, J. and Nowicki, S. 2004. Vocal performance influences female response to 406
male bird song: an experimental test. - Behav. Ecol. 15: 163–168. 407
Barbraud, C., Mariani, A. and Jouventin, P. 2000. Variation in call properties of the snow petrel, 408
Pagodroma nivea, in relation to sex and body size. - Aust. J. Zool. 48: 421–430.
409
Barnett, C. A. and Briskie, J. V. 2011. Strategic regulation of body mass and singing behaviour in 410
New Zealand robins. - Ethology 117: 28–36. 411
Beecher, M. 1989. Signalling system for individual recognition: an information theory approach. - 412
Anim. Behav. 38: 248–261. 413
Berg, M. L., Beintema, N. H., Welbergen, J. A. and Komdeur, J. 2005. Singing as a handicap: the 414
effects of food availability and weather on song output in the Australian reed warbler 415
Acrocephalus australis. - J. Avian Biol. 36: 102–109.
416
Bergès, M., Choquet, R. and Bonadonna, F. 2019. Impact of long-term behavioural studies in the wild: 417
the blue petrel, Halobaena caerulea, case at Kerguelen. - Anim. Behav. 151: 53–65. 418
Bonadonna, F., and Nevitt, G. A. 2004. Partnerspecific odour recognition in an Antarctic seabird. -419
Science 306: 835. 420
Bonadonna, F., and Sanz-Aguilar, A. 2012. Kin recognition and inbreeding avoidance in wild birds: 421
the first evidence for individual kin-related odour recognition. - Animal Behav. 84: 509-513. 422
Bonadonna, F., Spaggiari, J. and Weimerskirch, H. 2001. Could osmotaxis explain the ability of blue 423
petrels to return to their burrows at night? - J. Exp. Biol. 204: 1485–1489. 424
Bonadonna, F., Villafane, M., Bajzak, C. and Jouventin, P. 2004. Recognition of burrow’s olfactory 425
signature in blue petrels, Halobaena caerulea: an efficient discrimination mechanism in the dark. 426
- Anim. Behav. 67: 893–898. 427
Bradbury, J. W. and Vehrencamp, S. L. 1998. Principles of animal communication. 428
Bretagnolle, V. 1990. Behavioural affinities of the blue petrel Halobaena caerulea. - Ibis (Lond. 429
1859). 132: 102–105. 430
Bretagnolle, V. 1996. Acoustic communication in a group of nonpasserine birds, the petrels. - In: 431
Kroodsma, D. E. and Miller, E. H. (eds), Ecology and Evolution of Acoustic Communication in 432
Birds. Cornell Un. pp. 160–177. 433
Bretagnolle, V. and Lequette, B. 1990. Structural Variation in the Call of the Cory’s Shearwater 434
(Calonectris diomedea, Aves, Procellariidae). - Ethology 85: 313–323. 435
Bretagnolle, V. and Genevois, F. 1997. Geographic variation in the call of the blue petrel: effects of 436
sex and geographical scale. - Condor 99: 985–989. 437
Bretagnolle, V., Genevois, F. and Mougeot, F. 1998. Intra- and intersexual functions in the call of a 438
non-passerine bird. - Behaviour 135: 1161–1184. 439
Briefer, E. F. 2018. Vocal contagion of emotions in non-human animals. - Proc. R. Soc. B Biol. Sci. 440
285: 20172783. 441
Brooke, M. L. 1978. Sexual differences in the voice and individual vocal recognition in the Manx 442
shearwater (Puffinus puffinus). - Anim. Behav. 26: 622–629. 443
Brooke, M. 2004. Albatrosses and petrels across the world. 444
Byers, B. E., Akresh, M. E. and King, D. I. 2016. Song and male quality in prairie warblers. - 445
Ethology 122: 660–670. 446
Candolin, U. and Voigt, H. R. 2001. Correlation between male size and territory quality: consequence 447
of male competition or predation susceptibility? - Oikos 95: 225–230. 448
Cardoso, G. C. 2012. Paradoxical calls: the opposite signaling role of sound frequency across bird 449
species. - Behav. Ecol. 23: 237–241. 450
Catchpole, C. K. and Slater, P. J. B. 2008. Bird song: biological themes and variations. 451
Charrier, I., Jouventin, P., Mathevon, N. and Aubin, T. 2001. Individual identity coding depends on 452
call type in the south polar skua Catharacta maccormicki. - Polar Biol. 24: 378–382. 453
Charrier, I., Bloomfield, L. L. and Sturdy, C. B. 2004. Note types and coding in parid vocalizations. I: 454
The chick-a-dee call of the black-capped chickadee (Poecile atricapillus). - Can. J. Zool. 82: 455
769–779. 456
Chastel, O., Weimerskirch, H. and Jouventin, P. 1995. Body condition and seabird reproductive 457
performance: a study of three petrel species. - Ecology 76: 2240–2246. 458
Chaurand, T. and Weimerskirch, H. 1994a. Incubation routine, body mass regulation and egg neglect 459
in the blue petrel Halobaena caerulea. - Ibis (Lond. 1859). 136: 285–290. 460
Chaurand, T. and Weimerskirch, H. 1994b. The regular alternation of short and long foraging trips in 461
the blue petrel Halobaena caerulea: a previously undescribed strategy of food provisioning in a 462
pelagic seabird. - J. Anim. Ecol. 63: 275. 463
Cherel, Y., Bocher, P., Trouvé, C. and Weimerskirch, H. 2002. Diet and feeding ecology of blue 464
petrels Halobaena caerulea at Iles Kerguelen, Southern Indian Ocean. - Mar. Ecol. Prog. Ser. 465
228: 283–299. 466
Christensen, R., Kleindorfer, S. and Robertson, J. 2006. Song is a reliable signal of bill morphology in 467
Darwin’s small tree finch Camarhynchus parvulus, and vocal performance predicts male pairing 468
success. - J. Avian Biol. 37: 617–624. 469
Cramp, S. and Simmons, K. E. L. 1977. The birds of the Western Palearctic. Vol. 1. Ostrich to ducks. - 470
In: Handbook of the Birds of Europe. Oxford University Press, pp. 722. 471
Croxall, J. P. and Prince, P. A. 1980. Food, feeding ecology and ecological segregation. - 472
Environment: 103–131. 473
Curé, C., Aubin, T. and Mathevon, N. 2011. Sex discrimination and mate recognition by voice in the 474
yelkouan shearwater Puffinus yelkouan. - Bioacoustics 20: 235–250. 475
Dale, Lank and Reeve 2001. Signaling individual identity versus quality: a model and case studies 476
with ruffs, queleas, and house finches. - Am. Nat. 158: 75. 477
Darwin, C. 1871. The descent of man, and selection in relation to sex (Murray, Ed.). 478
Dolédec, S. and Chessel, D. 1994. Co-inertia analysis: an alternative method for studying species-479
environment relationships. - Freshw. Biol. 31: 277–294. 480
Dolédec, S., and D. C. 1987. Rythmes saisonniers et composantes stationnelles en milieu aquatique I- 481
Description d’un plan d’observations complet par projection de variables. - Acta Oecologica. 482
Oecologia Gen. 8: 403–426. 483
Dray, S. and Dufour, A.-B. 2015. The ade4 package: implementing the duality diagram for ecologists. 484
- J. Stat. Softw 22: 1-20. 485
Dray, S., Chessel, D. and Thioulouse, J. 2003. Co-inertia analysis and the linking of ecological data 486
tables. - Ecology 84: 3078–3089. 487
Falls, J. B. 1982. Individual recognition by sounds in birds. - In: Kroodsma, D. E. and Miller, E. H. 488
(eds), Acoustic Communication in Birds. Academic Press, pp. 237–278. 489
Fant, G. 1960. Acoustic theory of speech production. 490
Favaro, L., Gamba, M., Gili, C. and Pessani, D. 2017. Acoustic correlates of body size and individual 491
identity in banded penguins. - PLoS One 12: e0170001. 492
Fisher, J. 1954. Evolution and bird sociality. - Evol. as a Process: 71–83. 493
Fletcher, N. H. and Tarnopolsky, A. 1999. Acoustics of the avian vocal tract. - J. Acoust. Soc. Am. 494
105: 35–49. 495
Fridolfsson, A.-K. and Ellegren, H. 1999. A simple and universal method for molecular sexing of non-496
ratite birds. - J. Avian Biol. 30: 116–121. 497
Funghi, C., Leitão, A. V., Ferreira, A. C., Mota, P. G. and Cardoso, G. C. 2015. Social dominance in a 498
gregarious bird is related to body size but not to standard personality assays. - Ethology 121: 84– 499
93. 500
Galeotti, P., Saino, N., Sacchi, R. and Møller, A. P. 1997. Song correlates with social context, 501
testosterone and body condition in male barn swallows. - Anim. Behav. 53: 687–700. 502
García, N. C. and Tubaro, P. L. 2018. Dissecting the roles of body size and beak morphology in song 503
evolution in the “blue” cardinalids (Passeriformes: Cardinalidae). - Auk 135: 262–275. 504
Genevois, F. and Bretagnolle, V. 1994. Male blue petrels reveal their body mass when calling. - Ethol. 505
Ecol. Evol. 6: 377–383. 506
Gil, D. and Gahr, M. 2002. The honesty of bird song: multiple constraints for multiple traits. - Trends 507
Ecol. Evol. 17: 133–141. 508
Gosler, A. G., Greenwood, J. J. D., Baker, J. K. and Davidson, N. C. 1998. The field determination of 509
body size and condition in passerines: a report to the British Ringing Committee. - Bird Study 510
45: 92–103. 511
Guillotin, M. and Jouventin, P. 1980. Le pétrel des neiges à Pointe Géologie. - Gerfaut 70: 51–72. 512
Hertel, F. and Ballance, L. T. 1999. Wing ecomorphology of seabirds from Johnston Atoll. - Condor 513
101: 549–556. 514
Jouventin, P. and Mougin, J.-L. 1981. Les strategies adaptatives des oiseaux de mer. - Rev. Ecol. 515
(Terre vie) 35: 217–249. 516
Jouventin, P., Aubin, T. and Lengagne, T. 1999. Finding a parent in a king penguin colony: the 517
acoustic system of individual recognition. - Anim. Behav. 57: 1175–1183. 518
Kriesell, H. J., Aubin, T., Planas-Bielsa, V., Benoiste, M., Bonadonna, F., Gachot-Neveu, H., Le 519
Maho, Y., Schull, Q., Vallas, B., Zahn, S. and Le Bohec, C. 2018. Sex identification in king 520
penguins Aptenodytes patagonicus through morphological and acoustic cues. - Ibis (Lond. 1859). 521
160: 755–768. 522
Kroodsma, D. E. and Byers, B. E. 1991. The function(s) of bird song. - Am. Zool. 31: 318–328. 523
Laiolo, P. and Rolando, A. 2003. Comparative analysis of the rattle calls in Corvus and Nucifraga: the 524
effect of body size, bill size and phylogeny. - Condor 105: 139-144. 525
Lambrechts, M. M. and Dhondt, A. A. 1995. Individual voice discrimination in birds. - In: Powers, D. 526
M. (ed), Current Ornithology. Springer US, pp. 115–139. 527
Leclaire, S., Strandh, M., Mardon, J., Westerdahl, H. and Bonadonna, F. 2017. Odour-based 528
discrimination of MHC-similarity in birds. - Proc. R. Soc. B 284: 2016-2466. 529
Lengagne, T., Lauga, J. and Jouventin, P. 1997. A method of independent time and frequency 530
decomposition of bioacoustic signals: inter-individual recognition in four species of penguins. - 531
Comptes Rendus l’Académie des Sci. - Ser. III - Sci. la Vie 320: 885–891. 532
Mager, J. N., Walcott, C. and Piper, W. H. 2007. Male common loons, Gavia immer, communicate 533
body mass and condition through dominant frequencies of territorial yodels. - Anim. Behav. 73: 534
683–690. 535
Magrath, R. D., Haff, T. M., Fallow, P. M. and Radford, A. N. 2015. Eavesdropping on heterospecific 536
alarm calls: From mechanisms to consequences. - Biol. Rev. 90: 560–586. 537
Martín-Vivaldi, M., Palomino, J. J. and Soler, M. 1998. Song structure in the hoopoe (Upupa epops) - 538
strophe length reflects male condition. - J. fur Ornithol. 139: 287–296. 539
Martín-Vivaldi, M., Palomino, J. J. and Soler, M. 2000. Attraction of hoopoe Upupa epops females 540
and males by means of song playback in the field: influence of strophe length. - J. Avian Biol. 541
31: 351–359. 542
Martín-Vivaldi, M., Palomino, J. J. and Soler, M. 2004. Strophe length in spontaneous songs predicts 543
male response to playback in the hoopoe Upupa epops. - Ethology 110: 351–362. 544
Mathevon, N., Charrier, I. and Jouventin, P. 2003. Potential for individual recognition in acoustic 545
signals: A comparative study of two gulls with different nesting patterns. - Comptes Rendus - 546
Biol. 326: 329–337. 547
Mauck, R. A. and Grubb, T. C. 1995. Petrel parents shunt all experimentally increased reproductive 548
costs to their offspring. - Anim. Behav. 49: 999–1008. 549
McGregor, P. K. 1992. Quantifying responses to playback: one, many, or composite multivariate 550
measures? - In: Playback and Studies of Animal Communication. Springer US, pp. 79–96. 551
Morton, E. S. 1977. On the occurrence and significance of Motivation-Structural Rules in some bird 552
and mammal sounds. - Am. Nat. 111: 855–869. 553
Mougeot, F. and Bretagnolle, V. 2000. Predation as a cost of sexual communication in nocturnal 554
seabirds: an experimental approach using acoustic signals. - Anim. Behav. 60: 647–656. 555
Mougeot, F., Genevois, F. and Bretagnolle, V. 1998. Predation on burrowing petrels by the brown 556
skua (Catharacta skua lönnbergi) at Mayes Island, Kerguelen. - J. Zool. 244: 429–438. 557
Navarro, J. and González-Solís, J. 2007. Experimental increase of flying costs in a pelagic seabird: 558
effects on foraging strategies, nutritional state and chick condition. - Oecologia 151: 150–160. 559
Nowicki, S. and Searcy, W. A. 2004. Song function and the evolution of female preferences: why 560
birds sing, why brains matter. - Ann. N. Y. Acad. Sci. 1016: 704–723. 561
Palacios, M. G. and Tubaro, P. L. 2000. Does beak size affect acoustic frequencies in woodcreepers? - 562
Condor 102: 553-560. 563
Patel, R., Mulder, R. A. and Cardoso, G. C. 2010. What makes vocalisation frequency an unreliable 564
signal of body size in birds? A study on black swans. - Ethology 116: 554–563. 565
Plummer, K. E., Bearhop, S., Leech, D. I., Chamberlain, D. E. and Blount, J. D. 2013. Winter food 566
provisioning reduces future breeding performance in a wild bird. - Sci. Rep. in press. 567
Podos, J. 2001. Correlated evolution of morphology and vocal signal structure in Darwin’s finches. - 568
Nature 409: 185–188. 569
Pollard, K. A. and Blumstein, D. T. 2011. Social group size predicts the evolution of individuality. - 570
Curr. Biol. 21: 413–417. 571
Průchová, A., Jaška, P. and Linhart, P. 2017. Cues to individual identity in songs of songbirds: testing 572
general song characteristics in chiffchaffs Phylloscopus collybita. - J. Ornithol. 158: 911–924. 573
Robisson, P., Aubin, T. and Bremond, J. 1993. Individuality in the voice of the emperor penguin 574
Aptenodytes forsteri: adaptation to a noisy environment. - Ethology 94: 279–290.
575
Rowe, L. and Houlde, D. 1996. The lek paradox and the capture of genetic variance. - Proc. Biol. Sci. 576
263: 1415–1421. 577
Sæther, B. E., Andersen, R. and Pedersen, H. C. 1993. Regulation of parental effort in a long-lived 578
seabird an experimental manipulation of the cost of reproduction in the Antarctic petrel, 579
Thalassoica antarctica. - Behav. Ecol. Sociobiol. 33: 147–150.
580
Salton, M., Saraux, C., Dann, P. and Chiaradia, A. 2015. Carry-over body mass effect from winter to 581
breeding in a resident seabird, the little penguin. - R. Soc. Open Sci. 2: 140390–140390. 582
Scherrer, B. 1984. Biostatistique. - Gaetan Morin Editeur. 583
Searcy, W. A. and Nowicki, S. 2005. The evolution of animal communication: reliability and 584
deception in signaling systems. 585
Senar, J. C. and Pascual, J. 1997. Keel and tarsus length may provide a good predictor of avian body 586
size. - Ardea 85: 269–274. 587
Seutin, G., White, B. N. and Boag, P. T. 1991. Preservation of avian blood and tissue samples for 588
DNA analyses. - Can. J. Zool. 69: 82–90. 589
Smith, J. M. 1991. Theories of sexual selection. - Trends Ecol. Evol. 6: 146–151. 590
Taylor, A. M. and Reby, D. 2010. The contribution of source-filter theory to mammal vocal 591
communication research. - J. Zool. 280: 221–236. 592
Temeles, E. J. 1994. The role of neighbours in territorial systems: when are they “dear enemies”? - 593
Anim. Behav. 47: 339–350. 594
Tibbetts, E. A. and Dale, J. 2007. Individual recognition: it is good to be different. - Trends Ecol. 595
Evol. 22: 529–537. 596
Titze, I. R., & Martin, D. W. (1998). Principles of voice production. 597
Townsend, S. W., Hollén, L. I. and Manser, M. B. 2010. Meerkat close calls encode group-specific 598
signatures, but receivers fail to discriminate. - Anim. Behav. 80: 133–138. 599
Warham, J. 1990. The petrels: their ecology and breeding systems (A Press, Ed.). 600
Warham, J. 1996. The behaviour, population biology and physiology of the petrels. - In: Elsevier, in 601
press. 602
Weimerskirch, H., Chastel, O. and Ackermann, L. 1995. Adjustment of parental effort to manipulated 603
foraging ability in a pelagic seabird, the thin-billed prion Pachyptila belcheri. - Behav. Ecol. 604
Sociobiol. 36: 11–16. 605
Winkler, D. W. and Allen, P. E. 1996. The seasonal decline in tree swallow clutch size: physiological 606
constraint or strategic adjustment? - Ecology 77: 922–932. 607
Wittenberger, J. F. and Hunt, G. L. 1985. The adaptive significance of coloniality in birds. - In: Avian 608
Biology. Academic P. xx, pp. 1–78. 609
Yamada, K. and Soma, M. 2016. Diet and birdsong: short-term nutritional enrichment improves songs 610
of adult Bengalese finch males. - J. Avian Biol. 47: 865–870. 611
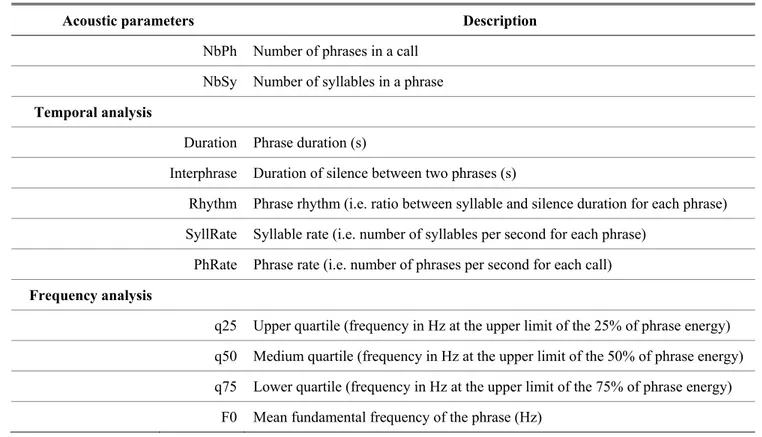
Table 1. Abbreviations and descriptions for the acoustic parameters measured on each phrase of blue petrel and 613
Antarctic prion provoked calls.
614
Acoustic parameters Description
NbPh Number of phrases in a call NbSy Number of syllables in a phrase
Temporal analysis
Duration Phrase duration (s)
Interphrase Duration of silence between two phrases (s)
Rhythm Phrase rhythm (i.e. ratio between syllable and silence duration for each phrase) SyllRate Syllable rate (i.e. number of syllables per second for each phrase)
PhRate Phrase rate (i.e. number of phrases per second for each call)
Frequency analysis
q25 Upper quartile (frequency in Hz at the upper limit of the 25% of phrase energy) q50 Medium quartile (frequency in Hz at the upper limit of the 50% of phrase energy) q75 Lower quartile (frequency in Hz at the upper limit of the 75% of phrase energy)
F0 Mean fundamental frequency of the phrase (Hz)
Table 2. Potential of Individual Coding (PIC) for the acoustic parameters measured on blue petrel (top) and 616
Antarctic prion (bottom) calls. Abbreviations of acoustic parameters as in Table 1. PIC values above 2.0 are in
617
bold.
618
Acoustic features PIC
Blue petrel Antarctic prion
NbPh 2.24 2.52 NbSy 2.05 2.24 Duration 2.44 2.27 Interphrase 2.17 2.10 Rhythm 1.63 1.65 SyllRate 1.79 2.07 PhRate 2.32 3.97 q25 2.01 1.95 q50 2.33 2.76 q75 3.15 3.22 F0 1.56 1.28 619
Figure captions
620Figure 1. Tarsus and head measurements from Cramp and Simmons (1977), taken on blue petrels and Antarctic prions: head
621
length (HL), bill depth (BD), bill length (CL) and tarsus length (TL).
622
Figure 2. (a) Sonogram (top) and oscillogram (bottom) of a male blue petrel call constituted of 2 phrases. (b) Linear amplitude
623
spectrum of a call phrase. Red lines indicate energy quartiles and blue line indicates the mean frequency of the phrase.
624
Figure 3. In blue petrels, correlation circles of CIA showing relationships between the two first components of the 625
CIA and (a) the morphometric measurements, and (b) the acoustic parameters of male calls.
626
Figure 4. In Antarctic prions, correlation circles of CIA showing relationships between the two first components 627
of the CIA and (a) the morphometric measurements, and (b) the acoustic parameters of male calls.