HAL Id: hal-02921646
https://hal.archives-ouvertes.fr/hal-02921646
Submitted on 26 Aug 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Distributed under a Creative Commons Attribution| 4.0 International License
Antimicrobial properties against Aeromonas hydrophila
and immunostimulant effect on Clarias gariepinus of
Piper betle, Psidium guajava, and Tithonia diversifolia
plants
Nunak Nafiqoh, S. Sukenda, Muhammad Zairin Junior, A. Alimuddin, Angela
Lusiastuti, Samira Sarter, Domenico Caruso, Jean-Christophe Avarre
To cite this version:
Nunak Nafiqoh, S. Sukenda, Muhammad Zairin Junior, A. Alimuddin, Angela Lusiastuti, et al.. An-timicrobial properties against Aeromonas hydrophila and immunostimulant effect on Clarias gariepinus of Piper betle, Psidium guajava, and Tithonia diversifolia plants. Aquaculture International, Springer Verlag, 2020, 28 (1), pp.1-13. �10.1007/s10499-019-00439-6�. �hal-02921646�
1
Antimicrobial properties against Aeromonas hydrophila and immunostimulant effect on
Clarias gariepinus of Piper betle, Psidium guajava and Tithonia diversifolia plants
Nunak Nafiqoh1, Sukenda2, M. Zairin Jr2, Alimudin2, Angela Lusiastuti1, Samira Sarter3, Domenico Caruso3, Jean-Christophe Avarre3*
1
Research Institute for Freshwater Aquaculture and Fisheries Extension, Bogor, Indonesia
2
Bogor Agriculture University, Bogor, Indonesia
3
ISEM, IRD, CIRAD, CNRS, EPHE, University of Montpellier, Montpellier, France
*Corresponding author
Jean-Christophe Avarre
Institut des Sciences de l’Evolution de Montpellier University of Montpellier, Place Eugène Bataillon, cc065 34095 Montpellier cedex 5, France
+33467143748
jean-christophe.avarre@ird.fr ORCID 0000-0001-6899-7052
2
Abstract
Aquaculture of the African catfish Clarias gariepinus has rapidly increased in South-East Asia over recent years, which has now become one of the most cultivated species. This culture intensification has resulted in the development of various diseases, among which the Motile Aeromonas Septicaemia (MAS) caused by Aeromonas hydrophila. The present study aimed at investigating the potential of three plants, Piper betle, Psidium guajava and Tithonia
diversifolia, to prevent this disease using an enriched fish diet during four weeks. Though the
minimum inhibitory and bactericidal concentrations of acetone–extracts were relatively high (2-32 mg mL-1 and 8-64 mg mL-1, respectively), all 3 plant powders imbedded in feed significantly reduced fish mortalities following an experimental infection with A. hydrophila, as indicated by relative percent survivals (RPS) ranging from 77% to 79%. When used in combination, the 3 plant extracts showed no interaction in vitro. Even though they improved RPS in comparison with controls, they were, however, less efficient in reducing mortalities than single plants. None of the plant-enriched diet had a clear effect on blood cell count or on reactive oxygen species (ROS) production (except for P. betle, which significantly increased ROS production after infection); however, both individual plants and their combinations significantly limited the drop of haematocrit following infection. Altogether, these results indicate that selection of plants for herbal therapy is complex and might depend on several in
vivo criteria, and as such should not solely rely upon measurement of their antimicrobial
activity.
3
Introduction
1 2
Aquaculture provides nearly half of the fish consumed in the world, and developing countries 3
account for 61 % of the world’s traded sea food (Fao 2018). Diseases of aquatic animals are 4
considered as a severe threat to the sustainability of aquaculture, as they jeopardize the efforts 5
and outcomes of millions of small fish farmers throughout the world. Global outbreaks in 6
aquaculture contribute to the loss of several billion US dollars per year (Assefa and Abunna 7
2018). 8
Aquaculture intensification over the world has often been associated with a considerable use 9
of antibiotics, widely recognized for their adverse effects on the environment microbial 10
ecosystems, as well as on public and animal health. Antimicrobial resistance has been 11
reported in both European and tropical aquaculture, not only in pathogenic bacteria, but also 12
in commensal and environmental isolates (Cabello et al. 2013). Moreover, resistant bacteria 13
or their resistance genes may spread among the aquatic communities, which in turn may 14
enter human food chain (Chen et al. 2015). 15
Eco-friendly alternatives for therapeutic and prophylactic purposes in health management of 16
aquatic animals are therefore of high priority, as they meet both environmental and societal 17
demands (a high sanitary quality of seafood products). In this regard, the use of plants may 18
provide an eco-friendly solution for the development of a sustainable health management of 19
fish farms. There is growing scientific evidence about the beneficial effects of diets enriched 20
with plant extracts or natural compounds on fish health and prevention of disease outbreaks 21
(Reverter et al. 2014). The active molecules contained in plants (such as alkaloids, 22
terpenoids, saponins and flavonoids) may provide inhibitory activity against pathogenic 23
microorganisms, without eliciting bacterial resistance as is often the case with antibiotics 24
(Chandra et al. 2017). For these reasons, plants may be considered as a promising alternative 25
to the use of chemo-therapeutants, particularly antibiotics (Wink 2015). However, the 26
effectiveness of plant extracts used as antimicrobial depends on many factors linked to their 27
chemical composition, the hydrophobicity of bacteria tested, the exposure time and bacterial 28
cell concentration (Patra and Mohanta 2014). On the other hand, the use of plant 29
combinations may increase their effectiveness through synergistic effects (Ji et al. 2007). 30
Finally, in addition to their potential antibacterial activity, plant extracts may improve the 31
non-specific immunity of several cultured fish, as well as the digestibility and availability of 32
nutrients, thereby resulting in an increase in feed conversion efficiency and protein synthesis 33
(Talpur and Ikhwanuddin 2013). 34
4 Although the use of plants in freshwater aquaculture is widespread in Indonesia, the reasons 35
for their choice by the farmers should be developed further. A comprehensive ethno-botanic 36
survey carried out in West Java revealed that improvement of water quality and disease 37
management were the major drivers (63 % of cases) of plant use (Caruso et al. 2013). This 38
study also highlighted that Clarias spp. farming was strongly associated with the use of local 39
plants. In addition to their frequent usage by fish farmers, the three plants investigated in the 40
present study (Piper betle, Psidium guajava and Tithonia diversifolia) display antibacterial 41
activity against Aeromonas hydrophila (Caruso et al. 2016; Muniruzzaman and Chowdhury 42
2004), and may therefore be useful for preventing fish infection by this major pathogen in 43
tropical regions. 44
Clarias gariepinus was introduced to Indonesia in the early 1980s and its culture quickly
45
expanded to the whole country. Like other members of this genus (e.g. C. batrachus), it is 46
easy to breed and can grow in most aquaculture systems; as such, it has become a popular 47
food source to fulfil protein needs in several developing countries, including Indonesia, 48
where it is now the second bred freshwater species with a production of more than 1 M tons 49
y-1 (Pouomogne 2019). Due to its low oxygen demand, C. gariepinus is often cultured in 50
high density, which favors the development of various diseases, among which the Motile 51
Aeromonas Septicaemia (MAS) caused by Aeromonas hydrophila and may cause around 70-52
90% mortality during an outbreak (Hanson et al. 2014). The most common symptoms 53
associated with MAS disease are loss of appetite, sores around the mouth, skin haemorrhages 54
and lesions. As in other countries, this bacterial disease is mostly treated in Indonesia with 55
antibiotics such as penicilline, oxytetracycline or ciprofloxacin (Sukarni et al. 2012). 56
In order to promote an ecological alternative to antibiotics against MAS in Clarias 57
aquaculture, the present study aimed at investigating the antibacterial activities, 58
hematological and immunostimulant effects of three plants widely used by fish farmers: 59
Piper betle, Psidium guajava and Tithonia diversifolia. Leaves of the 3 plants were tested
60
alone and in combination, both for their in vitro and in vivo activity. 61
62
Materials and methods
63 64
Plant extractions
65
The plants were all purchased as leaf powder from the Indonesian Spices and Medicinal 66
Crops Research Institute (ISMERI) and selected due to their wide availability in Indonesia 67
and frequent use as medicinal purpose both for humans and animals. Extracts were obtained 68
5 from these plants using a maceration method because of its simplicity and low cost. For 69
maceration, one part of plant leave powder was immersed in 10 parts of acetone (g/v) as the 70
best solvent described by Eloff (1998), incubated for 24 hours at room temperature in an 71
orbital shaker (50 rpm) and then filtered through Whatmann (N°41) paper filters. Filtered 72
solutions were then evaporated by rotary evaporation (Heidolph WB 200) at 40°C and 60 rpm 73
until complete solvent removal. The resulting plant extracts were stored in dark glass bottles 74
at 4°C until use. 75
76
MIC and MBC tests
77
Each of the three plant extracts was tested against the Gram negative Aeromonas hydrophila 78
fish pathogenic bacteria to determine its ability to inhibit bacterial growth in vitro. The 79
bacterial strain used in this study was isolated from a diseased catfish (Clarias sp.) by the fish 80
health management laboratory, Research Institute of Freshwater Aquaculture and Extension 81
Fisheries, West Java, Indonesia. To confirm its pathogenicity, it was reinjected into a healthy 82
C. gariepinus and collected after the appearance of clinical signs, twice consecutively. It was
83
then identified as Aeromonas hydrophila with the kit API 20 NE (BioMérieux) and archived 84
at -80°C in 20% glycerol. For the present assays, the strain was thawed, passaged 4 times on 85
liquid medium and tested again twice on catfish as described above. It was incubated 86
overnight in Mueller Hinton Broth (MHB) medium (Himedia) at 30°C, such that the optical 87
density at 630 nm was around 0.08 to 0.09, corresponding to 107 CFU mL-1. Serial 1:2 88
dilutions of plant extracts, from 64 mg mL-1 to 0.031 mg mL-1, were prepared in water 89
supplemented with 1% DMSO. Using 96-microwell plate, 20 µL of each diluted plant extract 90
were mixed with 20 µL of the bacterial suspension and 160 µL of MHB, and incubated 91
overnight at 30°C. Controls consisted of either 20 µL of bacterial suspension and 180 µl of 92
MHB (positive control), 200 µL of MHB alone (negative control) or 20 µL of plant extract 93
and 180 µL MHB (extract control as a blank). Each control was tested in triplicate and each 94
dilution was tested in 4 replicates. Optical density was measured after 24h of incubation, and 95
the lowest concentration of plant extract that inhibit the bacterial growth was determined as 96
the minimum inhibitory concentration (MIC). To measure the minimum bactericidal 97
concentration (MBC), full loops of the culture were striked onto MHA plates (Mueller Hinton 98
Agar) and incubated overnight at 30°C. The MBC was determined as the lowest concentration 99
that did not show any colony growth after sub-culturing (Fankam et al. 2015). 100
101
Bacterial growth interaction tests
6 To test a potential synergistic effect between the three plant extracts, two combinations with 103
varying proportions were tested: combination 1 (C1) contained an equal amount of each plant 104
extract (33%), whereas combination 2 (C2) was based on the individual MIC values that were 105
measured for each plant, and contained 19% of P. guajava, 5% of P. betle and 76% of T. 106
diversifolia. MIC and MBC tests for plant extract combinations were determined as describe
107
above. The fractional inhibitory concentration (FIC), defined as the ratio between the MIC of 108
the test sample in combination and the MIC of the sample used alone, were calculated for 109
each plant combination. FIC index, which corresponds to the sum of fractional inhibitory 110
concentrations, was calculated following Shafiei et al. (2016), and determined the interactions 111
among the plant extracts: 112
∑𝐹𝐼𝐶 = (𝐹𝐼𝐶 𝐼 + 𝐹𝐼𝐶 𝐼𝐼 + 𝐹𝐼𝐶 𝐼𝐼𝐼) 113
It was defined as synergistic when ∑FIC ≤ 1, antagonistic when ∑FIC ≥ 4, or non-interactive 114
when 1 < ∑FIC < 4 (van Vuuren and Viljoen 2011). 115
116
Experimental fish, feeding trials and bacterial challenge
117
Juvenile fish Clarias gariepinus (7±0.6 cm and 3.2±0.8 g) were purchased from local fish 118
farmer, placed in 30 L aquaria (30 fish / aquarium) filled with room temperature water (28-119
30°C) and treated with NaCl (10 ppm) for 3 days after arrival. They were acclimated to such 120
laboratory conditions for two weeks during which they were fed ad libitum with commercial 121
diet (Chaoroen Phokpand Prima Indonesia), and during which their health status was 122
frequently checked by visual inspection. Fish were treated according to Indonesian 123
regulations, and all experiments were carried out under the accreditation SNI 7583:2010. Fish 124
were split into six experimental groups: one group for each individual plant (P. guajava, P. 125
betle and T. diversifolia groups), two groups corresponding to the 2 combinations (C1 and C2
126
groups) and 1 control group. Each group comprised three replicates consisting of 20 fish 127
reared in a single 30L aquarium. 128
For the feeding trials, the same commercial feed as above was ground and mixed with plant 129
powder. For single plant treatments, 80 g of leave powder were mixed to 1 kg of grinded 130
pellets supplemented with 1% of carboxymethylcellulose as binder. Three hundred mL of 131
water were added to this mix, which were then repelleted. For C1 and C2 treatments, the 132
same proportions of dry leaves as used in in vitro combination tests were applied. Fish of the 133
control group were fed with dry pellets without plant supplementation. Fish from all groups 134
7 were fed with the experimental diet for four weeks, twice daily, representing ~5% of 135
biomass. Every 3 days, 40-50% of water was renewed. 136
137
In vivo challenge test
138
For the challenge test, A. hydrophila was grown in Tryptic Soy Broth (TSB) medium 139
overnight at 30C. The actual bacterial concentration was determined by measuring the 140
optical density at 630 nm (as described above) and confirmed by colony counting. The 141
culture was diluted in Phosphate Buffer Saline (PBS) to obtain a concentration of 107 CFU 142
mL-1. One hundred µL of diluted culture were intramuscularly injected into the dorsal part of 143
the fish at the end of the 4-week feeding trial. During the challenge, fish continued to receive 144
the same experimental diet, and mortality was recorded two times per day for 10 days. 145
146
Blood sampling procedure
147
Five fish from each treatment group were sampled at the beginning (day 1) and at the end of 148
the feeding period (day 28) as well as 24 hours post-challenge. Approximately 200 µL of 149
blood were withdrawn from the caudal vein of each individual fish with a 1mL syringe. The 150
withdrawn blood was treated as follow: one drop was smeared onto a microscope slide for 151
leucocyte differentiation, 10µL were injected into a heparinized microhaematocrit capillary 152
tube, whereas the remainder was mixed with Na-citrate (3.8%) as anticoagulant for reactive 153
oxygen species measurement. 154
155
Measurement of haematological and innate immunity parameters
156
Reactive oxygen species (ROS) production was measured by quantifying the reduction of 157
nitroblue tetrazolium into formazan, following the protocol described by Biller-Takahashi et 158
al. (2013), except that blood was incubated at 27°C instead of 25°C. Haematocrit percentage 159
was measured according to Sirimanapong et al. (2014). For leukocyte differentiation, the 160
smeared blood was air dried, stained with Giemsa and observed under microscope with 161
1000x magnification assisted with immersion oil. One hundred leukocytes were counted and 162
percentage of neutrophils, monocytes and lymphocytes was then calculated according to Adel 163 et al. (2015). 164 165 Statistical analyses 166
8 Normality and equal variance of data where checked using Shapiro-Wilk test and Brown-167
Forsythe test, respectively. When both criteria were respected, a one-way Anova followed by 168
a post hoc test (Holm-Sidak method) was applied to evaluate the effect of the different herbal 169
treatments; otherwise, a Kruskal-Wallis one-way Anova on Ranks was used. These analyses 170
were used to study the effect of the different herbal treatments on blood parameters between 171
the last day of feeding trial (day 28, before infection) and 1 day after A. hydrophila infection. 172
Comparison before infection and after infection within each treatment group was carried out 173
using a t-test. Mortalities resulting from the experimental infection were compared with Chi 174
square followed by a Kaplan-Meier survival analysis and post hoc analysis using Holm– 175
Sidak method. Differences between treatments were considered significant when P<0.05. 176
Relative Percent Survival (RPS) was calculated according to Amend (1981) as follows: 177
Relative percent survival = 1 −(% of treated group)(% of control group)x 100. 178 179 Results 180 181 Antimicrobial activity 182
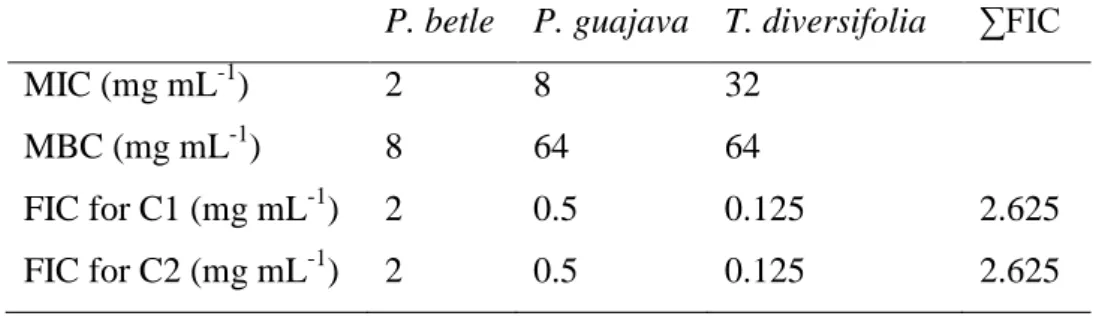
The minimum inhibitory concentrations and minimum bactericidal concentrations were 183
measured for the three plant extracts against the pathogenic bacteria A. hydrophila. Overall, 184
P. betle extracts showed the highest ability to inhibit bacterial growth, with MIC and MBC of
185
2 and 8 mg mL-1, respectively. P. guajava and T. diversifolia displayed higher MIC (8 and 32 186
mg mL-1, respectively) and MBC (64 mg mL-1 each) values (Table 1). The FIC index of 187
tested combinations among the 3 plant extracts indicated the absence of antagonistic or 188
synergistic effect between them. 189
190
Fish growth during feeding trials
191
After replacement of the regular feed by the experimental diet, fish from all groups stopped 192
eating. Whereas control fish recovered appetite after 2 days, fish from the other treatment 193
groups started to eat again after 3 days only. Body weight of each fish was measured every 7 194
days during the feeding trial. After one week of experimental diet, the average weight was 195
comparable in all groups, ranging from 2.90 ± 0.21 g (P. betle group) to 3.49 ± 0.17 g (C2 196
group). After 4 weeks, however, fish from the control group displayed a significantly higher 197
(P<0.05) body weight (7.00 ± 0.57 g) than those from all the other treatment groups (3.56 ± 198
9 0.27 g for P. betle group to 5.14 ± 0.39 g for P. guajava group). Moreover, no visible change 199
in vigour/health was observed in any group following administration of the experimental diet. 200
201
Haematological and immune parameters
202
Since almost no significant difference was found between day 1 and day 28, figures will only 203
illustrate the differences before and after experimental infection. 204
After 4 weeks of feeding, there were no differences in haematocrit values, except for fish fed 205
with P. betle, which had a significantly lower haematocrit than control animals (Anova on 206
Rank P=0.009). After infection, haematocrit was significantly lower in survivor fish of 207
control group than in fish fed with T. diversifolia leaves (One way Anova, P=0.03) (Fig.1). 208
Most notably, infection had no significant effect on the haematocrit of the treated groups, 209
whereas it significantly decreased that of the control group (t-test, P <0.001) (Fig 1). 210
Likewise, there was no variation in blood cell count after 4 weeks of herbal administration, 211
except in fish fed with T. divesifolia, which showed the highest percentage of monocytes; 212
however this difference was significant (one way Anova, P=0.021) only compared to group 213
C1 (not shown). Following experimental infection, significant variations in blood cell count 214
were observed in survivors of all groups; notably with a marked neutrophilia, monocytosis 215
and lymphocytopenia (Fig. 2). However, none of these modifications could be ascribed to 216
herbal treatment and no difference was observed with the control group in all three categories 217
of blood cells. 218
Reactive oxygen production, reflected by NBT test, showed no significant difference after 4 219
weeks between the different herbal treatments and the control (Anova on Rank, P=0.161). 220
Following infection, NBT increased for all treatments, including the control; however, this 221
increase was significantly higher in fish fed with P. betle than in the control animals (Fig. 3). 222
223
Experimental infection
224
Clinical signs of all moribund and dying fish consisted of red sores, skin ulcers, internal 225
hemorrhages and hemorrhagic ascites, compliant with an infection by A. hydrophila. Data of 226
cumulative mortality and RPS are summarized in Table 2. Chi-square analysis revealed a 227
significant difference between treatments (78.631 with 5 degrees of freedom, P = <0.001). 228
The highest cumulative mortality was observed in the control group (83.6%). All mortalities 229
occurred during the first 7 days post-infection (Fig. 4). Except for fish fed with C1 230
combination, mortalities were significantly lower in all other herbal treatments. Survival 231
analysis (Fig. 4) showed that best survival rates were obtained in fish fed with individual 232
10 plants, and in a lesser extent with C2 combination. No synergistic effect on survival was 233
observed when the three plants were administrated together, compared to single plant 234 treatments. 235 236 Discussion 237
The aim of the present work was to evaluate the effect of three plants against MAS, caused 238
by Aeromonas hydrophila, by testing both in vitro and in vivo approaches in Clarias 239
gariepinus. MIC values of the 3 plants were all above 1 mg mL-1. Even though a compound
240
that displays a MIC value higher than 100 µg mL-1 has been reported to have a low 241
antibacterial activity (Kuete 2010), it is worth noticing that this threshold concerns isolated 242
compounds of plants and might vary depending on the methods used, which are not 243
standardized in the literature for plant extracts. For crude extracts, this threshold might be 244
too high, as attested by our study and other published work (Bussmann et al. 2010). The 245
ability of plant extracts to inhibit bacterial growth indicates that some of their natural 246
compounds have antimicrobial properties. P. betle main active compounds belong to 247
phenylpropene class, which includes eugenol, chavicol or chavibetol (Bajpai et al. 2010). In 248
contrast, Tithonia diversifolia and Psidium guajava share a number of common compounds, 249
such as α-pinene, β-pinene, germacrene D and β-caryophyllene, though at different 250
percentages (Rajan and Thenmozhi 2015; Wanzala et al. 2016). These natural compounds 251
may have various effects against different bacteria, depending on their biochemical 252
characteristics. The common modes of action of plant extracts against bacteria are not 253
specific, and include for instance increased cell permeability, cytotoxicity, gene silencing, 254
cell filamentation, membrane disruption, genomic DNA binding etc. (He et al. 2018; Wink 255
2015). The high survival rates obtained on treated fish after the bacterial challenge tend to 256
indicate that the in vitro properties of a plant are not directly correlated with its in vivo 257
effects, as was already reported (Anusha et al. 2014). Under these conditions, selection of 258
active plants should not solely rely upon measurement of their MIC. A recent review 259
highlighted that all antimicrobial tests carried out in vitro are difficult to reproduce in clinical 260
trials, and may have a limited predictive value (Mundy et al. 2016). In spite of this, in vitro 261
antimicrobial tests remain essential in the evaluation of potential antimicrobial candidates, 262
mainly because they do not require the sacrifice of animals. However, additional information, 263
such as ethnobotanical data or comparative usage in human or veterinary pharmacopeia, is 264
likely to help the discovery of efficacious plants for fish. 265
11 Application of combined plants may be considered as an alternative way for preventing 266
diseases. It is indeed acknowledged that the combination of two or more plants will provide a 267
better performance of plants as medicine (Che et al. 2013). For illustration, the use of four 268
herbs in combination significantly increased the survival of red seabream juveniles compared 269
to single herb application (Ji et al. 2007). In the present study, the three individual plant 270
extracts significantly reduced the mortality caused by A. hydrophila. However, when used in 271
combination, their efficiency was lower, as indicated by higher RPS values. Interestingly, the 272
two combinations used in vivo led to significantly different survival rates, suggesting that the 273
proportion of each active compound in the diet is an important factor to consider. A previous 274
study on the combination of P. betle with P. guajava and Andrographis paniculata showed 275
an ability to prevent MAS infection caused by A. hydrophila on Clarias sp. when 276
supplemented to fish diet (Wahjuningrum et al. 2007). In this study, the combination 277
0.25:0.75:1 elicited the best survival rate compared to other combinations. In herbal therapy, 278
complex interactions may appear when plants are formulated together with varying 279
proportions. An example of unwanted interaction between herbs is the presence of tannins. 280
These substances may hinder the absorption of proteins and alkaloids, or induce enzymes 281
such as cytochrome P450, which may in turn accelerate drug catabolism and result in blood 282
levels of active substances that are too low to elicit a therapeutic effect (Williamson 2001). 283
Surprisingly, growth of herbal-treated fish was lower than that of control animals. As already 284
reported (Bulfon et al. 2015), the impact of plants on fish growth may greatly vary. The 285
reduced growth that was observed after herbal treatment could be linked to the presence of 286
antinutritional compounds, or more simply to a loss of feed palatability that resulted in a 287
reduced feed intake. Though additional studies are needed to address this loss of growth, the 288
lower weight of animals fed with plants did not prevent their beneficial effect against A. 289
hydrophila infection. These results suggest that such herbal treatments should not be used as
290
long-term diets. 291
Nitroblue tetrazolium assay, which measures the reactive oxygen species (ROS) produced by 292
phagocytic cells, is a good indicator of phagocytic activity in fish (Biller-Takahashi et al. 293
2013). An increase in ROS production was observed in all fish groups only after the bacterial 294
challenge, and this increase was significantly higher in animals treated with P. betle 295
compared to controls. Concomitantly, the proportion of neutrophils and leucocytes also 296
increased after infection, yet in the same manner for all treatments. However, none of the 297
plant extracts tested here had a direct effect on ROS production before infection, as was 298
observed with date palm extract applied to European seabass (Guardiola et al. 2016) or with 299
12 different herbs and spices administered to the tilapia Oreochromis mossambicus (Gültepe et 300
al. 2014), and in contrast with the oral administration of Eclipta alca to O. mossambicus, 301
where ROS production increased after one week post-feeding, although fish were not 302
challenged with any pathogen (Christybapita et al. 2007). 303
Haemolysis is retained as a key factor of A. hydrophila virulence (Gonzáles-Serrano et al. 304
2002). As a consequence of infection, a drop of red blood cell counts was observed in the 305
control fish, and in a lesser extent in the C1 group. However, the fact that haematocrit of all 306
treated fish did not significantly change after bacterial infection suggests that the addition of 307
plants in the diet contributed to prevent blood cell lysis during infection. These results are in 308
good agreement with the survival rates measured after infection, and indicate that leave 309
powder of these plants may inhibit, at least partially, the haemolytic activity of A. hydrophila 310
or prevent red blood cell from haemolysis. 311
To conclude, in spite of contrasted MIC values, the 3 species of plants tested in this study 312
showed a high and nearly identical efficiency in reducing the mortality due to an infection 313
with A. hydrophila. Although the two tested combinations had no antagonistic interaction in 314
vitro and elicited higher survival rates than controls, they were less efficient in reducing
315
mortalities than the individual plants. In order to get better insights into the beneficial use of 316
these plants, additional combinations leading to synergistic interactions should be searched 317
and tested for their ability to increase survival rates. 318
319
Acknowledgements
320
Nunak Nafiqoh benefited from an IRD fellowship under the BEST program. We are thankful 321
to the Indonesian Ministry of Fisheries and Marine Affairs for providing PhD scholarship to 322
Mrs. Nafiqoh as well as experimental facilities. This is publication ISEM-DIVA-XXX. 323
324
Compliance with Ethical Standards
325
The authors declare that they have no conflict of interest. ). All experiments involving fish 326
complied with animal welfare and were carried out under the Indonesian accreditation SNI 327 7583:2010. 328 329 References 330
Adel M, Abedian Amiri A, Zorriehzahra J, Nematolahi A, Esteban Mn (2015) Effects of 331
dietary peppermint (Mentha piperita) on growth performance, chemical body 332
composition and hematological and immune parameters of fry Caspian white fish 333
13 (Rutilus frisii kutum) Fish Shellfish Immunol 45:841-847. 334
https://doi.org/10.1016/j.fsi.2015.06.010 335
Amend DF (1981) Potency testing of fish vaccines. International Symposium in Fish 336
Biologics: Serodiagnostics and Vaccines. Dev Biol Stand 49:447-454 337
Anusha P, Thangaviji V, Velmurugan S, Michaelbabu M, Citarasu T (2014) Protection of 338
ornamental goldfish Carassius auratus against Aeromonas hydrophila by treating 339
Ixora coccinea active principles. Fish Shellfish Immunol 36:485-493. 340
https://doi.org/10.1016/j.fsi.2013.12.006 341
Assefa A, Abunna F (2018) Maintenance of fish health in aquaculture: Review of 342
epidemiological approaches for prevention and control of infectious disease of fish. 343
Vet Med International 2018:1-10. https://doi.org/10.1155/2018/5432497 344
Bajpai V, Sharma D, Kumar B, Madhusudanan KP (2010) Profiling of Piper betle Linn. 345
cultivars by direct analysis in real time mass spectrometric technique. Biomed 346
Chromatogr 24:1283-1286. https://doi.org/10.1002/bmc.1437 347
Biller-Takahashi JD, Takahashi LS, Saita MV, Gimbo RY, Urbinati EC (2013) Leukocytes 348
respiratory burst activity as indicator of innate immunity of pacu Piaractus 349
mesopotamicus. Braz J Biol 73:425-429.
https://doi.org/10.1590/S1519-350
69842013000200026 351
Bulfon C, Volpatti D, Galeotti M (2015) Current research on the use of plant-derived 352
products in farmed fish. Aquac Res 46:513-551. https://doi.org/10.1111/are.12238 353
Bussmann RW et al. (2010) Minimum Inhibitory concentration of medicinal plants used in 354
Northern Peru as antibacterial remedies. J Ethnopharmacol 132:101-108. 355
https://doi.org/10.1016/j.jep.2010.07.0 356
Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A, Buschmann AH 357
(2013) Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial 358
resistance and to animal and human health. Environ Microbiol 15:1917-1942. 359
https://doi.org/10.1111/1462-2920.12134 360
Caruso D, Lusiastuti AM, Slembrouck J, Komarudin O, Legendre M (2013) Traditional 361
pharmacopeia in small scale freshwater fish farms in West Java , Indonesia : An 362
ethnoveterinary approach. Aquaculture 416-417:334-345. 363
https://doi.org/10.1016/j.aquaculture.2013.09.048 364
Caruso D, Lusiastuti AM, Taukhid T, Avarre J-C, Yuhana M, Sarter S (2016) Ethnobotanical 365
uses and antimicrobial properties of plants in small-scale tropical fish farms: The case 366
14 of Indonesian fish farmers in Java (Indonesia). J World Aquacult Soc. 367
https://doi.org/10.1111/jwas.12345 368
Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal dAR (2017) Antimicrobial 369
resistance and the alternative resources with special emphasis on plant-based 370
antimicrobials—A Review. Plants 6:11. https://doi.org/10.3390/plants6020016 371
Che CT, Wang ZJ, Sing M, Chow S, Wai C, Lam K (2013) Herb-herb combination for 372
therapeutic enhancement and advancement: Theory, practice and future perspectives. 373
Molecules 18:5125-5141. https://doi.org/10.3390/molecules18055125 374
Chen H et al. (2015) Antibiotics in typical marine aquaculture farms surrounding Hailing 375
Island, South China: Occurrence, bioaccumulation and human dietary exposure. Mar 376
Pollut Bull 90:181-187. https://doi.org/10.1016/j.marpolbul.2014.10.053 377
Christybapita D, Divyagnaneswari M, Dinakaran Michael R (2007) Oral administration of 378
Eclipta alba leaf aqueous extract enhances the non-specific immune responses and
379
disease resistance of Oreochromis mossambicus. Fish Shellfish Immunol 23:840-852. 380
https://doi.org/10.1016/j.fsi.2007.03.010 381
Eloff JN (1998) Which extractant should be used for the screening and isolation of 382
antimicrobial components from plants ? J Ethnopharmacol 60:1-8. 383
https://doi.org/10.1016/S0378-8741(97)00123-2 384
Fankam AG, Kuiate JR, Kuete V (2015) Antibacterial and antibiotic resistance modifying 385
activity of the extracts from Allanblackia gabonensis, Combretum molle and 386
Gladiolus quartinianus against Gram-negative bacteria including multi-drug resistant
387
phenotypes. BMC Complement Altern Med 15:1-12. https://doi.org/10.1186/s12906-388
015-0726-0 389
Fao (2018) The state of world fisheries and aquaculture 2018 - Meeting the sustainable 390
development goals. FAO. http://www.fao.org/documents/card/en/c/I9540EN. 391
Accessed 1 July 2019 392
Gonzáles-Serrano CJ, Santos JA, Garcí-Lopéz M-L, Otero A (2002) Virulence markers in 393
Aeromonas hydrophila and Aeromonas veronii biovar sobria isolates from freshwater
394
fish and from a diarrhoea case. J Appl Microbiol 93:414-419. 395
https://doi.org/10.1046/j.1365-2672.2002.01705.x 396
Guardiola FA, Porcino C, Cerezuela R, Cuesta A, Faggio C, Esteban MA (2016) Impact of 397
date palm fruits extracts and probiotic enriched diet on antioxidant status, innate 398
immune response and immune-related gene expression of European seabass 399
15 (Dicentrarchus labrax). Fish Shellfish Immunol 52:298-308. 400
https://doi.org/10.1016/j.fsi.2016.03.152 401
Gültepe N, Bilen S, Yılmaz S, Güroy D, Aydın S (2014) Effects of herbs and spice on health 402
status of tilapia (Oreochromis mossambicus) challenged with Streptococcus iniae. 403
Acta Vet Brno 83:129-136. https://doi.org/10.2754/avb201483020000 404
Hanson LA, Liles MR, Hossain MJ, Griffin MJ, Hemstreet WG (2014) Motile Aeromonas 405
Septicemia. In: Fish Health Section Blue Book: suggested procedures for the 406
detection and identification of certain finfish and shellfish pathogens., vol 1.2.9. 2014 407
edn. AFS-FHS, Bethesda, Maryland, pp 1-11 408
He T-F, Zhang Z-H, Zeng X-A, Wang L-H, Brennan CS (2018) Determination of membrane 409
disruption and genomic DNA binding of cinnamaldehyde to Escherichia coli by use 410
of microbiological and spectroscopic techniques. J Photochem Photobiol B-Biol 411
178:623-630 412
Ji SC, Takaoka O, Jeong GS, Lee SW, Ishimaru K, Seoka M, Takii K (2007) Dietary 413
medicinal herbs improve growth and some non-specific immunity of red sea bream 414
Pagrus major. Fish Sci 73:63-69. https://doi.org/10.1111/j.1444-2906.2007.01302.x
415
Kuete V (2010) Potential of Cameroonian plants and derived products against microbial 416
infections : A review. Planta Med:1479-1491 417
Mundy L, Pendry B, Rahman M (2016) Antimicrobial resistance and synergy in herbal 418
medicine. J Herb Med 6:53-58. https://doi.org/10.1016/j.hermed.2016.03.001 419
Muniruzzaman M, Chowdhury MBR (2004) Sensitivity of fish pathogenic bacteria to various 420
medicinal herbs. Bangl J Vet Med 2:75-84 421
Patra JK, Mohanta YK (2014) Antimicrobial compounds from mangrove plants: A 422
pharmaceutical prospective. Chin J Integr Med 20:311-320. 423
https://doi.org/10.1007/s11655-014-1747-0 424
Pouomogne V (2019) Cultured Aquatic Species Information Programme. Clarias gariepinus. 425
FAO. http://www.fao.org/fishery/culturedspecies/Clarias_gariepinus/en. Accessed 1 426
July 2019 427
Rajan S, Thenmozhi S (2015) GC-MS analysis of bioactive compounds in Psidium guajava 428
leaves. J Pharmacogn Phytochem 3:162-166 429
Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish 430
aquaculture as an alternative to chemotherapy: Current status and future perspectives. 431
Aquaculture 433:50-61. https://doi.org/10.1016/j.aquaculture.2014.05.048 432
16 Shafiei Z, Haji Abdul Rahim Z, Philip K, Thurairajah N (2016) Antibacterial and anti-433
adherence effects of a plant extract mixture (PEM) and its individual constituent 434
extracts (Psidium sp., Mangifera sp., and Mentha sp.) on single- and dual-species 435
biofilms. PeerJ 4:e2519-e2519. https://doi.org/10.7717/peerj.2519 436
Sirimanapong W, Thompson KD, Kledmanee K, Thaijongrak P, Collet B, Ooi EL, Adams A 437
(2014) Optimisation and standardisation of functional immune assays for striped 438
catfish (Pangasianodon hypophthalmus) to compare their immune response to live 439
and heat killed Aeromonas hydrophila as models of infection and vaccination. Fish 440
Shellfish Immunol 40:374-383. https://doi.org/10.1016/j.fsi.2014.07.021 441
Sukarni, Maftuch, Nursya H (2012) Kajian penggunaan ciprofloxacin terhadap histologi 442
insang dan hati ikan botia (Botia macracanthus, Bleeker) yang diinfeksi bakteri 443
Aeromonas hydrophila. J Exp Life Sci 2:6-12
444
Talpur AD, Ikhwanuddin M (2013) Azadirachta indica (neem) leaf dietary effects on the 445
immunity response and disease resistance of Asian seabass, Lates calcarifer 446
challenged with Vibrio harveyi. Fish Shellfish Immunol 34:254-264. 447
https://doi.org/10.1016/j.fsi.2012.11.003 448
van Vuuren S, Viljoen A (2011) Plant-based antimicrobial studies – Methods and approaches 449
to study the interaction between natural products. Planta Med 77:1168-1182. 450
https://doi.org/10.1055/s-0030-1250736 451
Wahjuningrum D, Tarono, Angka SL (2007) Efficacy of Andrographis paniculata, Psidium 452
guajava and Piper betle as prevention on Motile Aeromonad Septicaemia infection in
453
African catfish (Clarias sp.). J Akuakultur Indonesia 6:127-133 454
Wanzala W, Osundwa EM, Alwala J, Gakuubi MM (2016) Chemical composition of 455
essential oil of Tithonia diversifolia (Hemsl.) A. Gray from the Southern slopes of 456
Mount Elgon in Western Kenya. IJEPP 2:72-83 457
Williamson EM (2001) Synergy and other interactions in phytomedicines. Phytomedicine 458
8:401-409 459
Wink M (2015) Modes of action of herbal medicines and plant secondary metabolites. 460
Medicines 2:251-286. https://doi.org/10.3390/medicines2030251 461
17 Table 1. MIC, MBC and FIC index for the three plant extracts and their combination tested 463
against A. hydrophila 464
P. betle P. guajava T. diversifolia ∑FIC
MIC (mg mL-1) 2 8 32 MBC (mg mL-1) 8 64 64 FIC for C1 (mg mL-1) 2 0.5 0.125 2.625 FIC for C2 (mg mL-1) 2 0.5 0.125 2.625 465 466 467 468 469
Table 2. Cumulative mortality rate, expressed as % ± CI (α=0.05), and relative percent 470
survival (RPS) of Clarias gariepinus according to herbal treatments following an 471
experimental infection with A. hydrophila (N=60) 472
Treatment % mortality Confidence interval RPS
P. betle 17.2% ±9.7% 79% P. guajava 18.2% ±11.4% 78% T. diversifolia 19.5% ±12.1% 77% C2 27.9% ±13.4% 67% C1 52.1% ±14.1% 38% Control 83.6% ±9.8% 0% 473 474 475
18
Figure 1. Haematocrit, expressed as Mean % ± SE, before and after infection (N=5).
476
Asterisks indicate significant differences with the respective control group at the same 477
treatment time, whilst double daggers indicate significant differences before and after 478
experimental infection. 479
480
Figure 2. Differential leucocyte count (%) of Clarias gariepinus before and after infection.
481
The proportion of lymphocytes (A), neutrophils (B) and monocytes (C), expressed as a Mean 482
± SE, amounts 100% (N=5). Except for control animals, each group of fish was fed for 4 483
weeks prior to infection with a diet enriched with plant extracts. Different letters indicate a 484
statistically significant difference (P<0.05). 485
486
Figure 3. Reactive oxygen production (Mean ± SE) of Clarias gariepinus before and after
487
infection (N=5). Except for control animals, each group of fish was fed for 4 weeks prior to 488
infection with a diet enriched with plant extracts. Different letters indicate a statistically 489
significant difference (P<0.05). 490
491
Figure 4. Kaplan-Meier Survival Analysis (N=60). Except for control animals, each group of
492
fish was fed for 4 weeks prior to infection with a diet enriched with plant extracts. Different 493
letters indicate a statistically significant difference (P<0.05). 494
495 496
19 Figure 1 497 498 499
0
5
10
15
20
25
30
35
40
45
Hae
ma
to
crit (
%)
pre-infection
*
*
#
20 Figure 2 500 501 a a a a a a b b b b b b 0 10 20 30 40 50 60 70 80 90 100 P er cen tag e (%)
Lymphocyte
pre-infection post-infection a a a a a a b b a b b a 0 5 10 15 20 25 P er cen tag e (%)Neutrophil
pre-infection post-infection a a a a a a b b a b b b 0 2 4 6 8 10 12 14 16 P er cen tag e (%)Monocyte
pre-infection post-infection21 Figure 3 502 503 504 * 0 0,02 0,04 0,06 0,08 0,1 0,12 0,14 0,16
P. guajava P. betle T. diversifolia c1 c2 Control
Ni tr oblu e Te tr az ol ium ( ab sorb ance 5 4 0 n m) pre-infection post-infection a a a a a a b b b b b b
22 Figure 4 505 506 507