Publisher’s version / Version de l'éditeur:
Propagation of Ornamental Plants, 11, 2, pp. 63-77, 2011
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Review of doubled Haploidy methodologies in ornamental species
Ferrie, Alison M.R.; Caswell, Karen L.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=94ea4b3a-386a-43fa-a111-72ef8d527794 https://publications-cnrc.canada.ca/fra/voir/objet/?id=94ea4b3a-386a-43fa-a111-72ef8d527794
REVIEW OF DOUBLED HAPLOIDY METHODOLOGIES IN ORNAMENTAL SPECIES 1 2 3 4 5 6 7 8 9 10 11 12
Alison M.R. Ferrie and Karen L. Caswell
Plant Biotechnology Institute – National Research Council
110 Gymnasium Place
Saskatoon, SK, Canada
Fax: (306) 975-4839
Abstract: 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
Ornamental species are an important group of plants produced mainly for their aesthetic value and to
enhance both our indoor and outdoor environments. Development of new germplasm with enhanced
colours, fragrances, or longevity is an important aspect of plant breeding programs. Tissue culture
methods have been utilized to propagate favourable genotypes. Haploidy methodology is commonly used
in many agronomically important crops to speed up the breeding program by developing uniform lines.
Haploids and doubled haploid protocols can be used for both practical application, as in breeding,
mutagenesis, and genetic transformation, as well as basic research (i.e. biochemical, physiological and
genomic studies). This review will focus on in vitro haploid production in several ornamental species.
Key words: anther culture, doubled haploid, embryogenesis, gynogenesis, microspore culture,
Introduction 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
There is a continual need for breeding and improving ornamental plants as well as generating novel
genetic variation for the production of new varieties that will be aesthetically appealing to consumers.
The term “ornamental species” covers a wide range of species and there are many breeding objectives,
but generally, these objectives would include new plant and leaf shapes, new flower colours, extended
flowering, pleasant fragrances and improved disease and weather tolerance. Doubled haploidy (DH) is a
tool used in plant breeding to enhance the development of new cultivars. This methodology has been
used in a number of agronomically important species to develop new breeding lines and cultivars.
A haploid plant is defined as a sporophytic plant having the gametophytic number of chromosomes. A
naturally occurring haploid angiosperm plant (Datura stramonium L.) was first described by Blakeslee in
1922 (Blakeslee et al. 1922). This led to many reports of other naturally occurring haploids (see review
by Dunwell 2010). Plant breeders and geneticists had long known the benefits of pure lines, and it
became apparent that haploid plants would be beneficial in generating these pure lines, as complete
homozygosity can be accomplished by doubling the chromosomes of a haploid plant. Over the last few
years there has been a revival of doubled haploidy research stimulating a number of review articles
(Dunwell 2010, Chen et al. 2011, Ferrie and Caswell 2011, Germanà 2011a, b). This review will focus
on in vitro doubled haploid methodology in ornamental species, although other methods will be
mentioned. Compared to some agronomic species [i.e. Brassica napus L. (canola), Hordeum vulgare L.
(barley)], there has been very little work on doubled haploidy in the ornamental species despite the
Haploid methods 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Haploid plants can occur spontaneously, through wide crosses which lead to chromosome elimination,
through pollination with irradiated pollen, or through the in vitro culture of the gametophytes
(microspores or ovules). Naturally occurring haploids have been observed in over 70 species (see reviews
by Horlow et al. 1996, Dunwell 2010). Cultivar development has utilized these naturally occurring
haploids. For example, ’Kleine Liebling‘ is a haploid cultivar of Pelargonium (Daker 1966), the tomato
(Lycopersicon esculentum Mill.) ’Marglobe‘ is a doubled haploid (DH) cultivar (Morrison 1932), as is
’Korall‘ rapeseed (as listed in Thomas et al. 2003), and there are examples of spontaneous haploids being
used in breeding commercial lines of corn (Zea mays L.) (Chase 1951). The frequency of these naturally
occurring haploids is generally low; consequently, this is not an efficient method for breeding.
Interspecific or intergeneric crosses in which the pollinator chromosomes are eliminated have been used
successfully to produce maternal haploids. Initial work with barley in which Hordeum vulgare L. was
crossed with Hordeum bulbosum L. resulted in the H. bulbosum chromosomes being eliminated and H.
vulgare haploid embryos being produced (Kasha and Kao 1970). A number of commercial barley
varieties have been produced using the bulbosum method (Devaux et al. 1996). A similar method is also
being used in oat (Avena sativa L.) (Rines 2003) and wheat (Triticum aestivum L.) (Laurie and Bennett
1986, O'Donoughue and Bennett 1994, Inagaki 2003) to produce doubled haploid plants and in the case of
wheat, new varieties (Thomas et al. 2003). Maize (Zea mays L.) is used as the pollinator plant in the
wheat and oat crosses. Embryo rescue is a necessary part of this technique as the endosperm does not
develop and therefore cannot provide nutrients for continued development of the embryo.
Another haploid production method is parthenogenesis in which the egg cell develops into an embryo
without fertilization by the sperm nucleus. This can be achieved by pollination with irradiated pollen or
with the addition of chemicals (Khush and Virmani 1996, Sestili and Ficcadenti 1996). There is generally
There are a few examples of ornamental species in which haploids have been produced by way of
irradiated pollen (Table 1). 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
In vitro haploidy methodology
As described previously, the frequency of spontaneous haploid production or induced haploid production
through parthenogenesis can be low or inconsistent, and therefore these methods are generally not used
for breeding purposes. This prompted researchers to focus on in vitro methods to develop haploids and
doubled haploid plants.
Androgenesis is defined as the process of embryo development from the culture of the male gametophytes
(i.e. microspores or anthers), with the subsequent regeneration of haploid and doubled haploid plants from
these cells. The in vitro culture of anthers was first described by Guha and Maheshwari (1964) in Datura.
Most of the early work focused on anther culture (AC) but more sophisticated protocols, such as isolated
microspore culture (IMC - the process of isolating the microspores from the anthers), are preferred. This
eliminates any possibility of regenerating somatic tissue (i.e. anther wall). Gynogenesis is the culture of
unfertilized female gametophytes (i.e. ovules, ovaries). This method is used when plants do not respond
to androgenetic methods, there is a problem with regenerating albino plants from anther culture, or the
donor plants are male sterile. Gynogenic methods were first described in 1976 in barley (San Noeum
1976) and now this method has been demonstrated for at least 24 species (Bohanec 2006). Gynogenesis
is usually less efficient than androgenesis and therefore fewer studies have been carried out in
gynogenesis.
As in most tissue culture systems, the frequency of embryogenic response is influenced by the genotype,
the environmental conditions in which the donor plants are grown, pretreatment of the floral organs,
media composition, developmental stage of the explant (i.e. microspore, ovule), and culture conditions
on embryogenesis, the exact factors that control the process have not been elucidated. Protocols are
different for all species and embryogenic response can vary among species, genotypes, and plants within
the same genotype. DH protocols are available in over 250 species. A book compiled by Maluszynski et
al. (2003) provided detailed protocols for 33 species and listed another 226 species and references relating
to them. There are no detailed protocols for what would be classified as ornamental species; most
protocols were for agronomically important crops. Table 2 gives a listing of ornamental species in which
in vitro doubled haploidy methods have resulted in callus, embryos, or plants (haploids or doubled
haploids). This review will highlight a few plant families and recent progress in haploidy methodology
within these families. Other current general review articles on androgenesis or gynogenesis include those
by Chen et al. (2011), Ferrie and Caswell (2011), and Germanà (2011a, b). 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Compositae (sunflower) family
Compositae or Asteraceae is the largest family of dicotyledonous plants, encompassing approximately
25,000 species found world-wide (Heywood 2007). The family includes plants that are used for food, oil
production, insecticides, medicinal remedies, industrial applications, and ornamentals [e.g. pot marigold
(Calendula officinalis L.), chrysanthemums (Chrysanthemum spp.), gaillardia (Gaillardia pulchella
Foug.), and asters (Aster spp.)]. Members of the Compositae have a head-like capitulum, which is
composed of many small individual flowers called florets (Heywood 2007). The florets are surrounded
by bracts, which may be covered with somatic hairs. Most members of the Compositae have five anthers,
which form a tube around the style and dehisce their pollen into the tube. Some members of the
Compositae, such as calendula and current hybrid sunflower (Helianthus spp.) are self-compatible, while
others such as rudbeckia (Rudbeckia hirta L.) and gaillardia, are obligate out-crossers.
The Compositae species are regarded as recalcitrant in terms of microspore embryogenesis. Several
reports appear in the literature of attempts to produce doubled haploid lines of sunflower (Gurel et al
Microspore and anther culture have also been attempted with Dendranthema grandiflorum (Ramat.)
Kitam. (Yang et al. 2005), but there was no response (i.e. divisions of microspores) from the isolated
microspores. Callus was initiated from the anther culture experiments, however, all 76 resulting plants
showed a chromosome number and a zymogram banding pattern similar to the donor plants. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Several Composite species were evaluated for microspore and anther culture response in the author’s lab
(Table 2). A wide variety of media and conditions (i.e. pretreatment of the floral organs, microspore
density, culture conditions) were evaluated in sunflower, calendula, chamomile (Matricaria recutita
Linn.), gaillardia, and rudbeckia. One of the most serious problems encountered with isolated microspore
culture in the Compositae is the presence of somatic hairs (Figure 1), which often divide and produce
callus. Somatic hairs are difficult to eliminate, as they are similar in diameter to the microspores and
therefore pass through screens used to separate the microspores from debris. In order to reduce or
eliminate the presence of somatic hairs, we have used multiple filtrations and removal of the florets from
among the bracts (which are hair covered) using a microscope prior to grinding and filtration (Caswell
and Ferrie, unpublished). Both techniques have been successful in reducing, but not eliminating, somatic
hairs in culture. Anther culture methods can be used as an alternative method of eliminating the presence
of somatic hairs in culture, but this method is very labour intensive.
Sunflowers are a valuable species for use as ornamentals, for oil production, and for use as a confection or
bird seed. In sunflower, no embryos were produced from any of the isolated microspore culture
treatments, however, calli were produced in several cases. No shoots were produced from any of the
calli. In anther culture studies of sunflower, utilizing many types of medium, callus was produced around
the filament of each anther, and in cases where the filament was removed at culture initiation, a smaller
amount of callus was produced at the same end of the anthers (Figure 2). A few anthers also produced
callus along the anther walls. In addition to the callus, organized or leafy structures (Figure 3) were
M2, M3 and BR1 (Gurel et al. 1991b)]. Flow cytometry tests have been conducted on five shoots of
which two have been determined to be haploid, proving that they originated from microspores, not from
diploid anther wall or filament tissue (Caswell and Ferrie, unpublished). 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Calendula is a common pot or garden flower. Two cultivars were evaluated for androgenic response:
‘Erfurter Orangefarbigen’, which is commercially grown in Europe, and ‘Pacific Beauty’ from Butchart
Gardens, Victoria, Canada. Many types of medium, microspore density, bud sizes, and culture conditions
were evaluated. Callus was produced with many treatments, but generally, the most callus was produced
from both varieties with R92.01 medium (Theiler-Hedtrich and Hunter 1996), with an incubation
temperature of 24°C (Caswell, Allen, and Ferrie, unpublished). In some experiments, hundreds of calli
per plate were produced with these conditions. Microspores from donor buds larger than 15 mm in
diameter did not produce callus. Using cold liquids (4°C) during microspore extraction was beneficial,
while the effects of cold pretreatments of buds were difficult to assess. Microspores isolated from donor
plants grown at 10 / 5°C produced few calli as compared to donor plants grown in the standard 22/ 12°C
conditions. Callus produced from microspore cultures was placed on regeneration medium and in some
cases, shoots were produced (Figure 4). One shoot produced buds while on rooting medium in vitro and
once in soil flowered while small in stature (approximately 12 cm tall). Flow cytometry was conducted
on 10 of the plants from microspore culture. Two of the plants were determined to be haploid, while the
other eight were diploid. Anther culture experiments were conducted with Calendula anthers, but these
anthers are smaller than those of sunflower, making anther culture more difficult. Callus has been
produced around the filament end of anthers on several types of medium, most notably M1, M2, M3 and
BR1 (Gurel et al. 1991b). Callus has been transferred to regeneration medium and to date four shoots
have been produced. Flow cytometry was conducted on the one shoot that survived; this shoot proved to
Several different types of medium have been studied with microspore culture of chamomile. Callus was
produced only with R92.01 (Theiler-Hedtrich and Hunter 1996), where sucrose was replaced with
maltose (4 – 12%). One plant was produced, displayed a normal phenotype (Figure 5), and flow
cytometry analysis determined it to be a tetraploid (Caswell, Allen, and Ferrie, unpublished). 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Although many experiments were conducted with isolated microspores of gaillardia, very little callus was
produced. From one experiment using N6 medium (Chu 1978) [5% sucrose, 0.5 mg l-1 BA
(benzylaminopurine) and 0.01 mg l-1 NAA (naphthalene acetic acid)], one callus was produced which
gave rise to 13 plants. The plants were determined to be diploid. In another experiment using FHG
medium (Kasha et al. 1990), one embryo-like structure was produced which later gave rise to five shoots.
Gaillardia plants from microspore culture showed two distinct phenotypes (Figure 6), with some being
extremely short and others taller and similar to the donor plants (Caswell, Allen, and Ferrie, unpublished).
A very reliable and efficient system for producing plants from microspore culture of Rudbeckia hirta has
been developed (Caswell, Allen, and Ferrie, unpublished) using FHG medium developed by Kasha et al.
(1990). From callus produced in the microspore cultures, 189 plants have been produced from three
varieties (‘Richter’s S1437’, ‘Chocolate Orange’, and ‘Chim Chiminee’). Of the 16 plants evaluated with
flow cytometry, 15 were diploid and 1 was haploid. This system presents two challenges. Firstly, as with
other Compositae microspore culture experiments, eliminating somatic hairs is difficult, and somatic hairs
swell, divide and produce at least some of the callus. Plants described as diploid may have arisen from
such tissue, or may have arisen from microspores and spontaneously doubled. Secondly, Rudbeckia is an
obligate out-crosser, so potential doubled haploid lines will not set seed when selfed.
Purple coneflower (Echinacea purpurea L.) is both a popular medicinal herb and an ornamental species.
Haploid plants have been regenerated from Echinacea (Zhao et al. 2006). Two basal media [N6 (Chu
(2,4-Dichlorophenoxyacetic acid), BA, NAA]. The N6 medium with 2.22 μM BA and 0.54 μM NAA was more conducive to callus production than the MS medium or the 2, 4-D treatments. A total of 30
plants were regenerated and 19 were determined to be haploid. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Gerbera (Barberton daisy) is used as a cut flower or potted plant and has been increasing in popularity
over the last few years. It is grown throughout the world but is native to South Africa and Asia. Gerbera
jamesonii Bolus ex Hooker f. is the only Gerbera species grown commercially. Gerbera can be
propagated sexually and asexually, but vegetative methods are predominant to maintain purity. These
methods are very time consuming, hence tissue culture techniques have been developed to accelerate
propagation. Genetic transformation methods have also been developed for Gerbera (Elooma et al. 1993,
Nagaraju et al. 1998). In addition, Gerbera is very responsive to gynogenic methods (Kanwar and Kumar
2008). Unfertilized mature ovules are plated on an MS based medium supplemented with IAA
(indoleacetic acid) and BA (Tosca et al. 1990). One of the major factors influencing embryogenesis is
genotype: 12 out of 21 G. jamesonii genotypes evaluated produced callus and six of those generated
shoots (Tosca et al. 1990). In another study, 13 out of 17 genotypes produced callus (Miyoshi and
Asakura 1996). Gynogenic response was also influenced by the season. One genotype was cultured in
spring (April – May) and fall (September – October) on 17 MS-based types of media, with different
growth regulators (Cappadocia et al. 1988). It was observed that callus production was higher in the fall,
but that callus produced in the spring had a higher morphogenetic capability. They also observed that
callus production occurred on a wide range of media and growth regulators so no conclusive results on
medium composition were reported except that activated charcoal was detrimental. In another study, two
of four genotypes evaluated produced more callus in the spring (April, May, June) than in the summer
(July and August) and responded poorly in autumn (September and October). The third genotype had the
worst response in the spring and summer and produced the most callus in the autumn, whereas the fourth
genotype gave a poor response regardless of the season (Tosca et al. 1999). There was no correlation
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Caryophyllaceae (Pink) family
The Caryophyllaceae which is commonly referred to as the pink or carnation family is a large family with
86 genera and about 2200 species (Heywood et al. 2007). They are found mostly in the temperate areas
of the world and include annuals, perennials, woody plants, and small trees. As ornamental species, they
are used as cut flowers or garden plants. Common ornamental species include carnation (Dianthus
caryophyllus L.), Sweet William (Dianthus barbatus L.), soapworts (Saponaria spp.), and baby’s breath
(Gypsophila paniculata L.). Doubled haploid protocols are available for some of these species.
Carnation is a popular cut flower that is vegetatively propagated. There are disadvantages in using
vegetative propagation as the costs are higher than seed production, shelf life of the cuttings is short, and
it is essential to have exceptionally high standards of disease control in a nursery. The production of F1
seed would be preferable to clonal propagation and for this, inbred lines are a necessity. Inbreeding
depression occurs in carnation, thus it is impossible to produce seeds by conventional means. Attempts
have been made to develop an anther culture or microspore culture protocol. Callus was produced from
anthers cultured on MS medium with 2,4-D and BA (Mosquera et al. 1999). From these experiments, 30
embryos and one plant were regenerated. Similar results were achieved with anther cultures of D.
chinensis L. and D. barbatus (Nontaswatsri et al. 2008). Callus was generated on MS medium containing
2,4-D and NAA or MS medium with TDZ (thidiazuron) and NAA, while shoots were produced only on
medium with TDZ. Unfortunately, all plants were diploid. Other studies have evaluated genotype,
pretreatments of the floral buds, and medium composition on callus production in D. chinensis. All
resulting plants were diploid or tetraploid and it was determined that the plants originated from the anther
wall (Fu et al. 2008).
Doubled haploid carnation has been produced by inducing ovules to develop haploid embryos by
and then applied to the ovule donor plants (Sato et al. 2000; Dolcet-Sanguan et al. 2001). The ovaries
started to swell after 1 week and aborted after 4 weeks, therefore, in later experiments, ovaries were
rescued after 2 – 3 weeks. Two plants were regenerated that the authors concluded to be doubled haploid
(Sato et al. 2000). In another study, pollen was irradiated at 1000Gy from a Co60 gamma ray source.
Pollination took place and after 3 – 4 weeks embryos were rescued. The origin of the embryos was
determined by isozyme markers, and out of 449 embryos evaluated 46 proved to be homozygous, proving
gametophytic origin. The ploidy of the regenerated plants proved to be haploid, diploid, triploid, or
tetraploid. Forty-one plants were established in soil and studied for Fusarium oxysporum resistance,
which was observed in 24 plants. These plants were incorporated into breeding programs. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Isolated microspore culture is the preferred androgenic method that avoids the potential of anther wall
cells regenerating into embryos and plants as has been demonstrated in a number of experiments. A
microspore culture protocol has been developed for Saponaria vaccaria L. (Kernan and Ferrie 2006), a
member of the Caryophyllaceae family. This species can be used as an ornamental but also has valuable
properties as a nutraceutical and a doubled haploid protocol would assist in breeding lines with specific
biochemical properties. Genotypic differences were observed among five lines evaluated. The most
embryogenic line (cv. White Beauty) produced more than 350 embryos/100 buds. Of several media
compositions investigated, full-strength NLN (Lichter 1982) with 15% sucrose resulted in the most
embryos. As with B. napus (Ferrie and Keller 1995), a heat shock of 32°C for 3 days was beneficial.
Over 800 DH plants were regenerated and produced seed (Kernan and Ferrie 2006). Some of these lines
were evaluated under field conditions and different chemotypes were selected (Kernan and Ferrie,
unpublished).
Polemoniaceae (Phlox) family
Phlox drummondii Hook. belongs to the Polemoniaceae family and is grown in North America and
drummondii is a heterozygous plant which suffers from inbreeding depression, this restricts the
development of inbred lines by conventional plant breeding methods. Development of a doubled haploid
protocol would be advantageous for continued improvement of this species. A number of factors were
evaluated using cv. Pink (Razdan et al. 2008). Microspores at the early to late uninucleate stage were the
most responsive in terms of callus production (Razdan et al. 2008). This developmental stage is optimal
for many species (Ferrie and Caswell 2011). No callus developed from binucleate microspores. Two
basal media [MS (Murashige and Skoog 1962) and B5 (Gamborg et al. 1968)], two growth regulators
(2,4-D and BA) as well as three sucrose concentrations (3%, 9%, and 12%) were evaluated. MS medium
with 9% sucrose, 10 μM 2,4-D and 5 μM BA for 8 weeks followed by a reduction in the sucrose concentration to 3% and 10 μM BA and 5 μM NAA was the best combination for continued callus production. The age of the callus was important for shoot differentiation with the maximum number of
shoots observed at week 13. Shoot initiation continued to be observed for up to 68 weeks in culture.
Rooting required IAA in the medium. From these experiments, 60 plants were produced of which 50%
were haploid, 30% were diploid, and 20% were aneuploid (Razdan et al. 2008). 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Ranunculaceae (buttercup) family
The buttercup family is found throughout the world, but mostly in the wet areas of the temperate and cold
regions. Most of the plants in this family are perennial while some are annual, but they are rarely shrubs
or trees. There is a great deal of variation in flower structure and methods of pollination. Many of the
Ranunculaceae species are ornamental plants (e.g. Delphinium, Clematis, Anemone, Nigella). Some
species are used in traditional medicine (e.g. Hydrastis canadensis L.), but other species are poisonous
(e.g. Aconitum).
Early work by Johansson and Eriksson (1977) established anther culture protocols for wild Anemone
species. Further experimentation focused on the economically important Anemone coronaria (Laura et al.
medium (Nitsch and Nitsch 1969) with activated charcoal, whereas the top layer was liquid NN medium
without charcoal. Embryos were observed after 12 – 14 weeks of culture. Genotypic differences were
observed in anther culture experiments (Laura et al. 2006). Of the 19 genotypes evaluated, seven did not
respond whereas 12 did show regeneration with up to 16.9 plants per 100 anthers. The authors felt that
these numbers were adequate for breeding purposes. Plants regenerated from anther culture flowered
after 15 months. The regenerated plants had various ploidy levels, including some plants which were
haploid. All plants regenerated differed genetically from their parental donor based on RAPD-based
DNA fingerprinting. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Hepatica nobilis Schreber var. japonica Nakai is a perennial native to Japan and is used as an ornamental.
Anther culture techniques were developed by Nomizu et al. (2004). Anthers containing uninucleate
microspores were precultured at 35°C for 4 days then cultured on NN basal medium supplemented with
activated charcoal. Microspore divisions were observed after six days and embryos developed after 20
weeks. Four media types were evaluated: liquid, solid, solid with 1% activated charcoal, and double layer
(comprised of solid media containing activated charcoal overlaid by liquid media and activated charcoal).
The best embryogenic response was found with the solid media with activated charcoal and the double
layer. Germination of the embryos was higher at temperatures of 8°C or 15°C rather than 25°C. All
plants regenerated were haploid.
Gentianaceae (Gentian) family
Members of the Gentian family, found all over the world, are used in traditional medicines (e.g. Gentiana
lutea) or as ornamental plants (e.g. Exacum). This group covers annuals, perennials, herbs, shrubs, and
some tropical trees. Gentiana species are most commonly used in rock gardens and borders.
Inbreeding depression in perennial gentians makes it difficult to produce homozygous lines for hybrid
anther culture protocol for G. triflora Pall. They evaluated three genotypes and bud sizes in the range of
7 – 15 mm. Half-strength NLN medium supplemented with 10, 13, or 16% sucrose was evaluated along
with different growth regulators. Embryos were detected 2 – 4 months after the anthers were cultured.
Of the three genotypes, one responded with 19.8 embryos per 100 anthers, whereas the other two
genotypes had poor or no response (i.e. 0.3 embryos or 0 embryos/100 anthers). Buds 9 – 13 mm
produced the highest number of embryos which correlated to the late uninucleate to early binucleate stage
of microspore development. Experiments evaluating medium composition were inconclusive, however
results did show that solid medium was more effective than liquid medium in terms of the number of
embryos produced. A total of 138 plants were regenerated: 5% of these were haploid, 25% were diploid,
and the majority were triploid (70%). Confirmation by ISSR (Inter Simple Sequence Repeat) analysis
indicated that the one diploid analyzed was a doubled haploid. In another study, both anther and ovary
cultures were evaluated using a basal medium consisting of NN macro salts and MS micro salts with 3%
sucrose (Pathirana et al. 2011). Both naphthoxyacetic acid (NOA) and BA were necessary for callus
production from ovary and anther culture, with a greater plant regeneration response from the ovary
cultures. Globular structures similar to embryo development were observed with 2,4-D and TDZ
treatments but these did not develop any further. Haploid, diploid, triploid and tetraploid plants were
regenerated and all were confirmed by RAPD bands to be of gametophytic origin. It is interesting that
both anthers and ovaries, given similar conditions (i.e. bud sizes, stress treatments, growth regulators)
produced haploid and doubled haploid plants. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Anther culture methods were attempted with G. scabra (Japanese Gentian) but these were not successful
(Doi et al. 2011), therefore unfertilized ovule culture was evaluated with this species. A solid
half-strength NLN medium with 10% sucrose was used for ovule culture. Embryos were observed after 1
month of culture and these could be regenerated into plantlets. Genotypic variation was observed as in
most tissue culture studies. A total of 176 plants were regenerated, of which 76.1% were green and
also haploid (31.3%), triploid, tetraploid, hexaploid, and chimeric plants (Doi et al. 2011). DNA markers
identified 96.3% of the diploid plants as doubled haploid. This ovule culture method was also used for G.
triflora and the hybrid (G. triflora x G. scabra) with success. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Araceae (Arum) family
The Araceae family are a monocot group of plants mostly found in the tropical regions of South East
Asia, Africa and America, although a few species are found in the temperate and boreal zones. Most of
the species are perennial, many form tubers and some are epiphytes and climbers. The inflorescence is a
spadix with numerous flowers surrounded by a modified leaf called a spathe. Most species are
insect-pollinated. A number of the Araceae are economically important as edible tubers [e.g. taro (Colocasia
esculenta (L.) Schott)]. As for ornamentals, they are used as garden plants in tropical climates or popular
house-plants or cut flowers in North America (e.g. Arum, Dieffenbachia, Zamioculcas, Anthurium
andraeanum Linden ex André). Some of the species can be used as aquarium plants (e.g. Cryptocoryne
Fisch. ex Wydler).
Anthurium species
Anthurium andreanum Linden ex André (Flamingo lily) is a common house-plant in North America.
This plant is propagated by seed but the seeds do not store well and are not uniform. It also takes up to
three years of growth before plants can be used in a breeding program. Anther culture methods would be
valuable for this group of plants. A novel half-anther culture method has been developed wherein
anthers were isolated from the plant, the middle to top part of the anther was excised and cultured with the
adaxial side on solid media. Comparisons with whole anthers showed that the half anthers produced more
callus (Winarto et al. 2010). Callus regenerated from half anthers displayed a variety of colours with
differing regeneration frequencies. Morphological differences were observed in the resulting plants.
colour, and spadix length. The ploidy also ranged from haploid, diploid, and triploid to aneuploid. These
differences could be exploited in breeding this ornamental species. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Spathiphyllum wallisii a member of the Araceae family can be easily propagated by in vitro methods.
Although success with haploidy methodology has been limited, an ovule culture method has been
reported (Eeckhaut et al. 2001). Genotypic differences were observed among three genotypes evaluated.
’Alfa‘ gave the best embryogenic response, but the embryos from this genotype were all determined to be
somatic in origin. The genotype ’Stefanie‘, which gave a lower embryogenic response, did yield two
homozygous plants as confirmed by AFLP (Amplified Fragment Length Polymorphism) analysis. The
basal medium consisted of MS macroelements, NN microelements, as well as myo-inositol,
thiamine-HCl, sucrose, and agar. Different growth regulators were evaluated along with fungicide treatments. The
use of TDZ (0.25 – 1 μM) was important for ovary cultures but was not essential for culture of ovules. The addition of fungicide allowed the ovules to swell making isolation of the ovules easier (Eeckhaut et
al. 2001).
Amaryllidaceae (Daffodil) family
This family is mainly found in the tropics and subtropics but a few species grow in the temperate zones.
These are mainly bulbous plants with linear leaves and umbel-like inflorescences. The main economic
use of the Amaryllidaceae is ornamental. Daffodils (Narcissus pseudonarcissus L.), amaryllis
(Hippeastrum spp.), snowdrops (Galanthus spp.), and Crinum lilies (Crinum spp.) are just a few of the
plants used for cut flowers, potted plants, or nursery plants.
Narcissus is a popular plant for gardens and cut flowers. Vegetative propagation of this species is very
time consuming and there is a risk of disease infection. Narcissus tazetta L. var. chinenesis Roem, is a
triploid species important as a flower and for its fragrance. Several factors influencing microspore
produced the most callus. For ease of selection, the authors were able to correlate anther length and
anther colour with microspore stage. Growth regulators were essential for the callus response as there
was no response from media lacking growth regulators. For callus induction, the best response was with
a combination of 0.5 mg l-1 2,4-D and 0.5 – 2 mg l-1 BA. After 10 weeks on induction media, calli were
transferred to differentiation media for bulblet production. The best differentiation media used 3 mg l-1
BA as a growth regulator (Chen et al. 2005). Those media compositions with 2,4-D inhibited bulblet
regeneration. The plants produced were diploid and were found to be of anther wall origin. While not
useful for the production of DH plants, this method may be used for in vitro propagation. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Conclusions
Ornamental species are produced mainly for their aesthetic value and to enhance both our indoor and
outdoor environments. They may also be of value in the production of pharmaceutically important
substances, preventing soil erosion, and improving soil and air quality. Consumers create a continual
demand for new ornamental plant varieties displaying new colours, fragrances, plant architecture, and
resistance to stresses. Ornamental species are an extremely diverse group of plants from many families,
with different uses, morphologies, growth habits, and modes of reproduction, which makes it impossible
to form generalizations regarding the benefits of haploids and doubled haploids for all species.
Nevertheless, for most ornamental species there are very few genetic resources available for conventional
breeding, which could be enhanced and accelerated with the use of doubled haploid lines. Doubled
haploids have been successfully used in breeding programs with crop species to produce new breeding
lines and cultivars. Microspore-derived embryos and DH lines can also be used in mutagenesis breeding
as well as for genetic modification.
Haploids and doubled haploids have been produced with some ornamental species. Different
methodologies have been used to generate haploid and doubled haploid plants with each species, and
varying significantly among species. Within a species, there are genotypic differences in response to a
protocol as has been observed with doubled haploidy protocols in crop species. With some of the
ornamental species, callus is produced from isolated microspores or anthers and subsequently shoots or
embryos are derived from the callus. In these cases, ploidy and/or molecular marker analyses are critical
to verify gametophytic origin of the tissue. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
Further research is required to develop and optimize protocols for production of doubled haploids from
ornamental species. Efficient protocols are essential since doubled haploids need to be produced in large
numbers to be beneficial to breeding programs. Basic research to further elucidate the factors that control
embryogenesis would be of benefit.
Acknowledgements
The authors acknowledge Kara Allen and Maureen Carter for their technical expertise in the Compositae
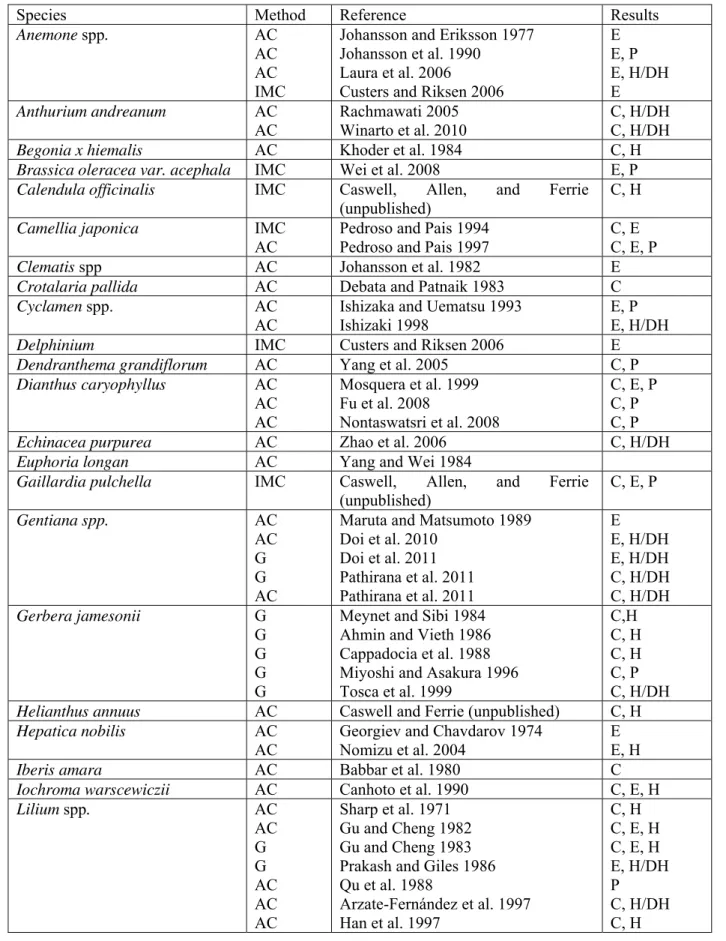
Table 1: Haploid or doubled haploid ornamental plants derived from irradiated pollen 1
Species Reference Results
Dianthus spp. Sato et al. 2000
Dolcet-Sanjuan et al. 2001
DH H/DH
Helianthus annuus L. Todorova et al. 1997 H/DH
Impatiens hawkeri W. Bull. Bastar et al. 2006 P
Lilium Vassileva-Dryanovska 1966a E
Mimulus aurantiacus Jelerčič et al. 2006 Murovec et al. 2007
P DH
Petunia Raquin 1985 H
Rosa spp Meynet et al. 1994 Lim et al. 2005 H H Tradescantia Vassileva-Dryanovska 1966b E 2 3 4
Table 2: In vitro generated haploids of ornamental species 1
Species Method Reference Results
Anemone spp. AC
AC AC IMC
Johansson and Eriksson 1977 Johansson et al. 1990 Laura et al. 2006
Custers and Riksen 2006
E E, P E, H/DH E Anthurium andreanum AC AC Rachmawati 2005 Winarto et al. 2010 C, H/DH C, H/DH
Begonia x hiemalis AC Khoder et al. 1984 C, H
Brassica oleracea var. acephala IMC Wei et al. 2008 E, P
Calendula officinalis IMC Caswell, Allen, and Ferrie (unpublished)
C, H
Camellia japonica IMC AC
Pedroso and Pais 1994 Pedroso and Pais 1997
C, E C, E, P
Clematis spp AC Johansson et al. 1982 E
Crotalaria pallida AC Debata and Patnaik 1983 C
Cyclamen spp. AC
AC
Ishizaka and Uematsu 1993 Ishizaki 1998
E, P E, H/DH
Delphinium IMC Custers and Riksen 2006 E
Dendranthema grandiflorum AC Yang et al. 2005 C, P
Dianthus caryophyllus AC AC AC Mosquera et al. 1999 Fu et al. 2008 Nontaswatsri et al. 2008 C, E, P C, P C, P
Echinacea purpurea AC Zhao et al. 2006 C, H/DH
Euphoria longan AC Yang and Wei 1984
Gaillardia pulchella IMC Caswell, Allen, and Ferrie (unpublished) C, E, P Gentiana spp. AC AC G G AC
Maruta and Matsumoto 1989 Doi et al. 2010 Doi et al. 2011 Pathirana et al. 2011 Pathirana et al. 2011 E E, H/DH E, H/DH C, H/DH C, H/DH Gerbera jamesonii G G G G G
Meynet and Sibi 1984 Ahmin and Vieth 1986 Cappadocia et al. 1988 Miyoshi and Asakura 1996 Tosca et al. 1999 C,H C, H C, H C, P C, H/DH
Helianthus annuus AC Caswell and Ferrie (unpublished) C, H
Hepatica nobilis AC AC
Georgiev and Chavdarov 1974 Nomizu et al. 2004
E E, H
Iberis amara AC Babbar et al. 1980 C
Iochroma warscewiczii AC Canhoto et al. 1990 C, E, H
Lilium spp. AC AC G G AC AC AC Sharp et al. 1971 Gu and Cheng 1982 Gu and Cheng 1983 Prakash and Giles 1986 Qu et al. 1988 Arzate-Fernández et al. 1997 Han et al. 1997 C, H C, E, H C, E, H E, H/DH P C, H/DH C, H
Matricaria recutita IMC Caswell, Allen, and Ferrie (unpublished)
C, P
Narcissus tazetta L. var. chinensis Roem
AC Chen et al. 2005 C, P
Oenothera hookeri AC Martinez and de Halac 1995 C, P
Paeonia spp. IMC
AC
Ono and Harashima 1981 Lee et al. 1992 C C, E, P Papaver spp. AC AC Johansson et al. 1982 Dieu and Dunwell 1988
E C, H
Pelargonium spp. AC AC AC
Abo El-Nil and Hildebrandt 1973 Tokumasu and Kato 1979
Kato et al. 1980
H C, H C, P
Peltophorum pterocarpum AC Rao and De 1987 C, E, H
Petunia spp. IMC
AC AC AC G
Sangwan and Norreel 1975 Malhotra and Maheshwari 1977 Gupta 1982
Raquin 1983
DeVerna and Collins 1984
C, H E E, P E H
Phlox drummondii AC Razdan et al. 2008 C, H/DH
Primula spp. AC Bajaj 1981 C, E, P
Ranunculus asiaticus AC Meynet and Duclos 1990 C, E, P
Rosa spp AC
AC
Tabaeizadeh and Khosh-Khui 1981 Wissermann et al. 1996
C C
Rudbeckia hirta IMC Caswell, Allen, and Ferrie (unpublished) C, H Saintpaulia ionantha AC AC Hughes et al. 1975 Weatherhead et al. 1982 C, H C, H
Saponaria vaccaria IMC Kernan and Ferrie 2006 E, H/DH
Spathiphyllum wallisii G Eeckhaut et al. 2001 E, DH
Streptocarpus hybridus AC Wolff et al. 1986 C, P
Tradescantia spp. AC Dietert et al. 1982 E, H
Tropaeolum majus AC Dorle and Kulkarni 1984 C, P
Tulipa spp. IMC
IMC
Van den Bulk and Van Tuyl 1997 Custers and Riksen 2006
C, E E
Viola odorata G Wijowska et al. 1999 C
Zantedeschia IMC Custers and Riksen 2006 E
Zantedeschia aethiopica AC Zhang et al. 2011 C, H/DH 1 2 3 4 5 6 7
AC = anther culture, G = gynogenesis, IMC = Isolated microspore culture
1 2 3 4 5 6 7 8
Figure 1: Freshly isolated microspores of Calendula with somatic hair.
1 2 3 4
1 2 3 4
1 2 3 4 5
1 2 3 4 5 6 7 8 9
Figure 5: Chamomile shoot derived from isolated microspore culture on rooting medium.
Reference List 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51
Abo El-Nil M.M., Hildebrandt A.C. (1973). Origin of androgenetic callus and haploid geranium plants. Canadian Journal of Botany, 51: 2107-2109.
Ahmin M., Vieth J. (1986). Regeneration of haploid plants of Gerbera jamesonii by ovules cultivated in
vitro. Canadian Journal of Botany, 64: 2355-2357.
Arzate-Fernández A.-M., Nakazaki T., Yamagata H., Tanisaka T. (1997). Production of doubled-haploid plants from Lilium longiflorum Thunb. anther culture. Plant Science, 123: 179-187.
Babbar S.B., Mittal A., Gupta S.C. (1980). In vitro induction of androgenesis, callus formation and organogenesis in Iberis amara Linn. anthers. Zeitschrift Fur Pflanzenzuchtung, 100: 409-414.
Bajaj Y.P.S. (1981). Regeneration of plants from ultra-low frozen anthers of Primula obconica. Scientia Horticulturae, 14: 93-95.
Bastar M.T., Hunold R., Bohanec B. (2006). Attempts of gynogenic haploid induction in New Guinea Impatiens. In: Touraev A., Forster B. (Eds.) The International Conference “Haploids in higher plants III”, Vienna, Austria, February 12-15, 2006: 50.
Blakeslee A.F., Belling J., Farnham M.E., Bergner A.D. (1922). A haploid mutant in the Jimson weed,
Datura stramonium. Science, 55: 646-647.
Bohanec B. (2006). Mechanisms of gynogenesis. In: Touraev A., Forster B. (Eds.) The International Conference “Haploids in higher plants III” , Vienna, Austria, February 12 – 15, 2006: 18.
Canhoto J.M., Ludovina M., Guimaraes S., Cruz G.S. (1990). In vitro induction of haploid, diploid and triploid plantlets by anther culture of Iochroma warscewiczii Regel. Plant Cell, Tissue and Organ Culture, 21: 171-177.
Cappadocia M., Chretien L., Laublin G. (1988). Production of haploids in Gerbera jamesonii via ovule culture: influence of fall versus spring sampling on callus formation and shoot regeneration. Canadian Journal of Botany, 66: 1107-1110.
Chase S.S. (1951). Efficient methods of developing and improving inbred lines. The monoploid method of developing inbred lines. Report of 6th hybrid corn industry research conference: 29-34.
Chen J.F., Cui L., Malik A.A., Mbira K.G. (2011). In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell, Tissue and Organ Culture, 104: 311-319.
Chen L.J., Zhu X.Y., Gu L., Wu J. (2005). Efficient callus induction and plant regeneration from anther of Chinese narcissus (Narcissus tazetta L. var. chinensis Roem). Plant Cell Reports, 24: 401-407.
Chu C. C. (1978). The N6 medium and its applications to anther culture of cereal crops. Proc. Symp. on Plant Tissue Culture, Beijing, China, Science Press: 43-50.
Coumans M., Zhong D. (1995). Doubled haploid sunflower (Helianthus annuus) plant production by androgenesis: fact or artifact? Part 2. In vitro isolated microspore culture. Plant Cell, Tissue and Organ Culture, 41: 203-209.
Custers J., Riksen T. (2006). Microspore embryogenesis successfully established in ornamentals;
Anemone, Tulipa, Zantedeschia and Delphinium. In: Touraev A., Forster B. (Eds.) The International Conference “Haploids in higher plants III” , Vienna, Austria, February 12 – 15, 2006: 66.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50
Daker M.G. (1966). ‘Kleine Liebling’ a haploid cultivar of Pelargonium. Nature, 211: 549-550.
Debata B.K., Patnaik S.N. (1983). In vitro culture of anther of Crotalaria pallida Ait. for induction of haploid. Indian Journal of Experimental Biology, 21: 44-46.
Devaux P., Zivy M., Kilian A., Kleinhofs A. (1996). Doubled haploids in barley. In: Scoles G., Rossnagel B. (Eds.) Proc. V International Oat Conference and VII International Barley Genetics Symposium. University of Saskatchewan, Saskatoon, Canada: 213-222.
DeVerna J.W., Collins G.B. (1984). Maternal haploids of Petunia axillaris (Lam.) B.S.P. via culture of placenta attached ovules. Theoretical and Applied Genetics, 69: 187-192.
Dietert M.F., Barron S.A., Yoder O.C. (1982). Production and culture of microspore-derived plants of
Tradescantia paludosa. Environmental and Experimental Botany, 22: 211-220.
Dieu P., Dunwell J.M. (1988). Anther culture with different genotypes of opium poppy (Papaver
somniferum L.): effect of cold treatment. Plant Cell, Tissue and Organ Culture, 12: 263-271.
Doi H., Takahashi R., Hikage T., Takahata Y. (2010). Embryogenesis and doubled haploid production from anther culture in gentian (Gentiana triflora). Plant Cell, Tissue and Organ Culture, 102: 27-33.
Doi H., Yokoi S., Hikage T., Nishihara M., Tsutsumi K., Takahata Y. (2011). Gynogenesis in gentians (Gentiana triflora, G. scabra): production of haploids and doubled haploids. Plant Cell Reports, DOI 10.1007/s00299-011-1017-y.
Dolcet-Sanjuan R., Clavería E., Llauradó M., Ortigosa A., Arús P. (2001). Carnation (Dianthus
caryophyllus L.) dihaploid lines resistant to Fusarium oxysporum F. sp. Dianthi. Acta Horticulturae, 560: 141-144.
Dorle U.P., Kulkarni A.R. (1984). Anther culture for production of pollen haploids in Tropaeolum majus Linn. Current Science, 53: 867-868.
Dunwell J.M. (2010). Haploids in flowering plants: origins and exploitation. Plant Biotechnology Journal, 8: 377-424.
Eeckhaut T., Werbrouck S., Dendauw J., van Bockstaele E., Debergh P. (2001). Induction of homozygous
Spathiphyllum wallisii genotypes through gynogenesis. Plant Cell, Tissue and Organ Culture, 67: 181-189.
Elooma P., Honkaanen J., Puska R., Seppanen P., Helariutta Y., Mehto M., Nevalainen L., Teeri T.H. (1993). Agrobacterium-mediated transfer of antisense chalcone synthase cDNA to Gerbera hybrida inhibits flower pigmentation. Bio/Technology, 111: 505-511.
Ferrie A.M.R., Caswell K.L. (2011). Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell and Tissue Culture, 104: 301-309.
Ferrie A.M.R., Keller W.A. (1995). Microspore culture for haploid plant production. In: Gamborg O.L., Phillips G.C. (Eds.) Plant Cell, Tissue and Organ Culture. Fundamental methods, Springer-Verlag, Berlin: 155-164. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51
Fu X., Yang S., Bao M. (2008). Factors affecting somatic embryogenesis in anther cultures of Chinese pink (Dianthus chinensis L.). In Vitro Cellular and Developmental Biology - Plant, 44: 194-202.
Gamborg O.L., Miller R.A., Ojima K. (1968). Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research, 50: 151-158.
Georgiev G., Chavdarov I. (1974). The anther culture – a new method of producing haploid plants. Genetetika i Selektsiia, 7: 404-415.
Germanà M.A. (2011a). Anther culture for haploid and doubled haploid production. Plant Cell and Tissue Culture, 104: 283-300.
Germanà M.A. (2011b). Gametic embryogenesis and haploid technology as valuable support to plant breeding. Plant Cell Reports, DOI 10.1007/s00299-011-1061-7.
Gu Z.P., Cheng K.C. (1982). Studies on induction of pollen plantlets from the anther cultures of lily. Acta Botanica Sinica, 24: 28-32.
Gu Z.P., Cheng K.C. (1983). In vitro induction of haploid plantlets from unpollinated young ovaries of lily and its embryological observations (Lilium davidii). Acta Botanica Sinica, 25: 24-28.
Guha S., Maheshwari S.C. (1964). In vitro production of embryos from anthers of Datura. Nature, 204: 497.
Gupta P.P. (1982). Genesis of microspore-derived triploid petunias. Theoretical and Applied Genetics, 61: 327-331.
Gurel A., Kontowski S., Nichterlein K., Friedt W. (1991a). Embryogenesis in microspore culture of sunflower (Helianthus annuus L.) Helia, 14: 123-128.
Gurel A., Nichterlein K., Friedt W. (1991b). Shoot regeneration from anther culture of sunflower (Helianthus annuus) and some interspecific hybrids as affected by genotype and culture procedure. Plant Breeding, 106: 68-76.
Han D.-S., Niimi Y., Nakano M. (1997). Regeneration of haploid plants from anther cultures of the Asiatic hybrid lily ‘Connecticut King’. Plant Cell, Tissue and Organ Culture, 47: 153-158.
Heywood V.H., Brummitt R.K., Culham A., Seberg O. (2007). Flowering plant families of the world. Firefly Books, Richmond Hill, Ontario, Canada, 424 pp.
Horlow C., Hamza S., Chupeau Y., Pelletier G. (1996). Conditional lethal markers: spontaneous haploid selection in plants. In: Jain S.M., Sopory S.K., Veilleux R.E. (Eds.) In vitro haploid production in higher plants, vol. 1, Kluwer Academic Publishers, Dordrecht, The Netherlands: 297-315.
Hughes K.W., Bell S.L., Caponetti J.D. (1975). Anther-derived haploids of the African violet. Canadian Journal of Botany, 53:1442-1444.
Inagaki M.N. (2003). Doubled haploid production in wheat through wide hybridization. In: Maluszynski M., Kasha K.J., Forster B.P., Szarejko I. (Eds.) Doubled Haploid Production in Crop Plants: A Manual. Kluwer Academic Publishers, Dordrecht: 53-58.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50
Ishizaki H. (1998). Production of microspore-derived plants by anther culture of an interspecific F1 hybrid between Cyclamen persicum and C. purpurascens. Plant Cell, Tissue and Organ Culture, 54: 21-28.
Ishizaki H., Uematsu J. (1993). Production of plants from pollen in Cyclamen persicum Mill. through anther culture. Japanese Journal of Breeding, 43: 207-218.
Jelerčič J., Bastar M.T., Hunold R., Bohanec B. (2006). Studies of gynogenic haploid induction in
Mimulus aurantiacus – Progress report. In: Touraev A., Forster B. (Eds.) The International Conference “Haploids in higher plants III” , Vienna, Austria, February 12-15, 2006: 51.
Johansson L., Andersson B., Eriksson T. (1982). Improvement of anther culture technique: Activated charcoal bound in agar medium and elevated CO2 concentration. Physiologia Plantarum, 54: 24-30.
Johansson L., Eriksson T. (1977). Induced embryo formation in anther cultures of several Anemone species. Physiologia Plantarum, 40: 172-174.
Johansson L.B., Calleberg E., Gedin A. (1990). Correlations between activated charcoal, Fe-EDTA and other organic media ingredients in cultured anthers of Anemone canadensis. Physiologia Plantarum, 80: 243-249.
Kanwar J.K., Kumar S. (2008). In vitro propagation of Gerbera – A review. Horticultural Sciencs, 35: 35-44.
Kasha K.J., Kao K.N. (1970). High frequency haploid production in barley (Hordeum vulgare L.). Nature, 225: 874-876.
Kasha K.J., Ziauddin A., Cho U.-H. (1990). Haploids in cereal improvement: anther and microspore culture. In: Gustafson J.P. (Eds.) Gene manipulation in plant improvement II. Plenum Press, New York: 213-236.
Kato M., Suga T., Tokumasu S. (1980). Effects of 2,4-D and NAA on callus formation and haploid production in anther culture of Pelargonium roseum. Memoirs of the College of Agriculture Ehime University, 24: 85-93.
Kernan Z., Ferrie A.M.R. (2006). Microspore embryogenesis and the development of a double haploidy protocol for cow cockle (Saponaria vaccaria). Plant Cell Reports, 25: 274-280.
Khoder M., Villemur P., Jonard R. (1984). Obtention de plantes monoploides et triploides par androgenese in vitro chez le Begonia X hiemalis Fotsch cv. (A). Bulletin de la Société Botanique de France, Paris, 131: 43-48.
Khush G.S., Virmani S. (1996). Haploids in plant breeding. In: Jain S.M., Sopory S.K., Veilleux R.E. (Eds.) In vitro haploid production in higher plants, vol. 1, Kluwer Academic Publishers, Dordrecht, The Netherlands: 11-33.
Laura M., Safaverdi G., Allavena A. (2006). Androgenetic plants of Anemone coronaria derived through anther culture. Plant Breeding, 125: 629-634.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50
Laurie D.A., Bennett M.D. (1986). Wheat x maize hybridization. Canadian Journal of Genetics and Cytology, 28: 313-316.
Lee B.K., Ko J.A., Kim Y.S. (1992). Studies on the thidiazuron treatment of anther culture of Paeonia
albiflora. Journal of the Korean Society for Horticultural Science, 33: 384-395.
Lichter R. (1982). Induction of haploid plants from isolated pollen of Brassica napus. Zeitschrift Fur Pflanzenzuchtung, 105: 427-434.
Lim K.Y., Werlemark G., Matyasek R., Bringloe J.B., Sieber V., El Mokadem H., Meynet J., Hemming J., Leitch A.R., Roberts A.V. (2005). Evolutionary implications of permanent odd polyploidy in the stable sexual, pentaploid of Rosa canina L. Heredity, 94: 501-506.
Malhotra K., Maheshwari S.C. (1977). Enhancement by cold treatment of pollen embryoid development in Petunia hybrida. Zeitschrift Fur Pflanzenzuchtung, 85: 177-180.
Maluszynski M., Kasha K.J., Forster B.P., Szarejko I. (2003). Doubled Haploid Production in Crop Plants: A Manual. Kluwer Academic Publishers, The Netherlands, 428 pp.
Martinez L.D., de Halac I.N. (1995). Organogenesis of anther-derived calluses in long-term cultures of
Oenothera hookeri de Vries. Plant Cell, Tissue and Organ Culture, 42: 91-96.
Maruta I., Matsumoto E. (1989). Induction of haploid plants from anther culture in Gentiana sp. Bulletin of the Nagano Vegetable and Ornamental Crops Experimental Station Japan, 5: 51-55 (in Japanese).
Meynet J., Barrade R., Duclos A., Siadous R. (1994). Dihaploid plants of roses (Rosa x hybrid, cv ‘Sonia’) obtained by parthenogenesis induced using irradiated pollen and in vitro culture of immature seeds. Agronomie, 2: 169-175.
Meynet J., Duclos A. (1990). In vitro culture of Persian buttercup (Ranunculus asiaticus L.) II. Production of plants through in vitro anther culture. Agronomie, 10: 213-218.
Meynet J., Sibi M. (1984). Haploid plants from in vitro culture of unfertilized ovules in Gerbera
jamesonii. Zeitschrift Fur Pflanzenzuchtung, 93: 78-85.
Miyoshi K., Asakura N. (1996). Callus induction, regeneration of haploid plants and chromosome doubling in ovule cultures of pot gerbera (Gerbera jamesonii). Plant Cell Reports, 16: 1-5.
Morrison G. (1932). The occurrence and use of haploid plants in tomato with especial reference to the variety marglobe. In: Jones D.F. (Ed.) Proceeding of 6th International Congress on Genetics, 2: 137-139.
Mosquera T., Rodríguez L.E., Parra A., Rodríguez M. (1999). In vitro adventive regeneration from carnation (Dianthus caryophyllus) anthers. Acta Horticulturae, 482: 305-308.
Murashige T., Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15: 473-497.
Murovec J., Stajner N., Jakse J., Javornik B. (2007). Microsatellite marker for homozygosity testing of putative doubled haploids and characterization of Mimulus species derived by a cross-genera approach. Journal of the American Society of Horticultural Science, 132: 659-663.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51
Nagaraju V., Srinivas G.S.L., Lakshmi Sita G. (1998). Agrobacterium-mediated genetic transformation in
Gerbera hybrida. Current Science, 71: 630-634.
Nitsch J.P., Nitsch C. (1969). Haploid plants from pollen grains. Science, 163: 85-87.
Nomizu T., Niimi Y., Han D.-S. (2004). Haploid plant regeneration via embryogenesis from anther cultures of Hepatica nobilis. Plant Cell, Tissue and Organ Culture, 79: 307-313.
Nontaswatsri C., Ruamrungsri S., Fukai S. (2008). Callus induction and plant regeneration of Dianthus
chinensis L. and Dianthus barbatus L. via anther culture. Acta Horticulturae, 788: 109-114.
O'Donoughue L.S., Bennett M.D. (1994). Durum wheat haploid production using maize wide crossing. Theoretical and Applied Genetics, 89: 559-566.
Ono K., Harashima S. (1981). Induction of haploid callus from isolated microspores of Peony in vitro. Plant and Cell Physiology, 22: 337-341.
Pathirana R., Frew T., Hedderley D., Timmerman-Vaughan G., Morgan E. (2011). Haploid and doubled haploid plants from developing male and female gametes of Gentiana triflora. Plant Cell Reports, DOI 10.1007/s00299-011-01012-3.
Pedroso M.C., Pais M.S. (1994). Induction of microspore embryogenesis in Camellia japonica L., cv. Elegans. Plant Cell Tissue and Organ Culture, 37: 129-136.
Pedroso M.C., Pais M.S. (1997). Anther and microspore culture in Camellia japonica. In: Jain S.M., Sopory S.K., Veilleux R.E. (Eds.) In Vitro Haploid Production in Higher Plants. Kluwer Academic Publishers, Dordrecht, Netherlands: 89-107.
Prakash J., Giles K.L. (1986). Production of doubled haploids in oriental lily. In: Genetic manipulation in plant breeding. Horn W., Jensen C.J., Oldenbach W., Schieder O. (Eds.) Walter de Gruyter & Co. Berlin: 335-337.
Qu Y., Mok M.C., Mok D.W.S., Stang J.R. (1988). Phenotypic and cytological variation among plants derived from anther cultures of Lilium longiflorum. In Vitro Cellular and Developmental Biology - Plant, 24: 471-476.
Rachmawati F. (2005). Kultur Anther pada Anthurium (Anthurium andreanum Linden ex andre). MSc. Thesis. Departemen Agronomi dan Hortikultura Fakultas Pertanian Institut Pertanian Bogor, Halaman, Indonesia, 146pp.
Rao P.V.L., De D.N. (1987). Haploid plants from in vitro anther culture of the leguminous tree,
Peltophorum pterocarpum (DC) K. Hayne (copper pod). Plant Cell, Tissue and Organ Culture, 11: 167-177.
Raquin C. (1983). Utilization of different sugars as carbon source for in vitro anther culture of Petunia. Zeitschrift Fur Pflanzenzuchtung, 111: 453-457.
Raquin C. (1985). Induction of haploid plants by in vitro culture of Petunia ovaries pollinated with irradiated pollen. Zeitschrift Fur Pflanzenzuchtung, 94: 166-169.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50
Razdan A., Razdan M.K., Rajam V., Raina S.N. (2008). Efficient protocol for in vitro production of androgenic haploids of Phlox drummondii. Plant Cell, Tissue and Organ Culture, 95: 245-250.
Rines H.W. (2003) Oat haploids from wide hybridization. In: Maluszynski M., Kasha K.J., Forster B.P., Szarejko I. (Eds.) Doubled haploid production in crop plants. Kluwer Academic Publishers, Dordrecht, The Netherlands: 155-159.
Saji K.V., Sujatha M. (1998). Embryogenesis and plant regeneration in anther culture of sunflower (Helianthus annuus L.). Euphytica, 103: 1-7.
San Noeum L.H. (1976). Haploides d’Hordeum vulgare L. par culture in vitro d’ovaries non fecondes. Annales De L Amelioration Des Plantes, 26: 751-754.
Sangwan R.S., Norreel B.S. (1975). Induction of plants from pollen grain of Petunia cultured in vitro. Nature (London), 257: 222-224.
Sato S., Katoh N., Yoshida H., Iwai S., Hagimori M. (2000). Production of doubled haploid plants of carnation (Dianthus caryophyllus L.) by pseudofertilized ovule culture. Scientia Horticulturae, 83: 301-310.
Sestili S., Ficcadenti N. (1996). Irradiated pollen for haploid production. In: Jain S.M., Sopory S.K., Veilleux R.E. (Eds.) In Vitro Haploid Production in Higher Plants, vol. 1, Kluwer Academic Publishers, Dordrecht, Netherlands: 263-274.
Sharp W.R., Raskin R.S., Sommer H.E. (1971). Haploidy in Lilium. Phytomorphology, 21: 334-337.
Tabaeizadeh Z., Khosh-Khui M. (1981). Anther culture of Rosa. Scientia Horticulturae, 15: 61-66.
Theiler-Hedtrich R., Hunter C.S. (1996). Microspore culture in chicory (Cichorium intybus L.). In: Jain S.M., Sopory S.K., Veilleux R.E. (Eds.) In Vitro Haploid Production in Higher Plants, vol. 3, Kluwer Academic Publishers, Dordrecht, Netherlands: 99-113.
Thomas W.T.B., Forster B.P., Gertsson B. (2003). Doubled haploids in breeding. In: Maluszynski M., Kasha K.J., Forster B.P., Szarejko I. (Eds.) Doubled Haploid Production in Crop Plants: A Manual. Kluwer Academic Publishers, Dordrecht: 337-349.
Todorova M., Ivanov P., Shindrova P., Christov M., Ivanova I. (1997). Doubled haploid production of sunflower (Helianthus annuus L.) through irradiated pollen-induced parthenogenesis. Euphytica 97:249-254.
Tokumasu S., Kato M. (1979). Variation of chromosome numbers and essential oil components of plants derived from anther culture of the diploid and the tetraploid in Pelargonium roseum. Euphytica, 28: 329-338.
Tosca A., Arcara L., Frangi P. (1999). Effect of genotype and season on gynogenesis efficiency in