Publisher’s version / Version de l'éditeur:
Journal of Thermal Spray Technology, 5, 1, pp. 49-52, 1996-03-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Long-term factors influencing the adhesion of metallized zinc to
concrete
Brousseau, R. J.; Arnott, M. R.; Baldock, B.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=38c46b62-4ccb-4237-a8e7-01f95fe0e84d
https://publications-cnrc.canada.ca/fra/voir/objet/?id=38c46b62-4ccb-4237-a8e7-01f95fe0e84d
http://www.nrc-cnrc.gc.ca/irc
Long-t e rm fa c t ors influe nc ing t he a dhe sion of m e t a llize d zinc t o
c onc re t e
N R C C - 3 8 7 8 5
B r o u s s e a u , R . J . ; A r n o t t , M . R . ; B a l d o c k , B .
M a r c h 1 9 9 6
A version of this document is published in / Une version de ce document se trouve dans:
Journal of Thermal Spray Technology, 5, (1), pp. 49-52, March 01, 1996
The material in this document is covered by the provisions of the Copyright Act, by Canadian laws, policies, regulations and international agreements. Such provisions serve to identify the information source and, in specific instances, to prohibit reproduction of materials without written permission. For more information visit http://laws.justice.gc.ca/en/showtdm/cs/C-42
Les renseignements dans ce document sont protégés par la Loi sur le droit d'auteur, par les lois, les politiques et les règlements du Canada et des accords internationaux. Ces dispositions permettent d'identifier la source de l'information et, dans certains cas, d'interdire la copie de documents sans permission écrite. Pour obtenir de plus amples renseignements : http://lois.justice.gc.ca/fr/showtdm/cs/C-42
jTTEE55:49-52
©ASM Inlernational
Long-Term Factors Influencing the Adhesion
of Metallized Zinc to Concrete
R. Brousseau,M.Arnott, and B. Baldock
Metallized zinc cathodic protection often is used to mitigate steel reinfort::ement corrosion in concrete. The zinc is flame or8rc sprayed to the surface of the concrete structure to be protected. The metallized coating should be applied such that its adhesion to concrete is maximized. FaclOl·s believed to affect。、セ
hesion were investigated for pure zinc and aXszョセQUaャ alloy. On reinforced concrete samples polarized for more than 800 days at three different current densities, adhesion decreased. The reduction incrcuscd as afunction of time and of the current density applied. When no current was applied, there was a small
initial Increase of adhesion with time. For the metallized samples that were freeze-thaw cycled, no loss of
adhesion was fonnd afler 70 cycles.
1.
Introduction
CORROSION of reinforcing steel in concrete due to chloride
attack is a serious problem in northern climate regions and on coastal structures. Cathodic protection often is llsed toュゥエゥセgatesteel reinforcement corrosion. It involves negatively po-larizing the steel reinforcement in order to promote chloride ion migration away from the reinforcement and to reduce the rate of anodic oxidation ofiron. This technique is usually
ap-plied
10older and chloride-contaminated structures
experi-encing corrosion-related damage.
Itrequires the application
of an anode, such as metallized zinc, on the surface of Ihe
concrete structure to deliver the protective current to there-bars.
Zinc coatings have been successfully implemented as
im-pressed-current anodes in Califomia (Ref 1, 2). Oregon (ReO).
and Ontario (Ref 4, 5). The Florida Departmenl of
Transporta-tion (Ref 6) has also begun to use metallized zinc as a sacrificial
anode onmarine structures.Itis. however. still unknown howIhis type of anode will perform in the long term.
In order for the metallized zinc
toperform adequately as an
anode, it must have good adhesion to the surface concrete of thc
cathodically protected structure. Steps should be taken to
opti-mize the adhesion of the zinc coating when it is thermally
sprayed on the concrele surface. Earlier work (Ref 7-10) has
shown that a number of parameters can influence the adhesion ofarc-sprayed zinc to concrete.This paper discusses phenomena that may affect adhesion
af-ter zinc has been metallized onto concrete. The effects of anodic
polarization, time from application, and freeze-thaw cycling are
reported. A85Zn-15Alalloy was also thermally sprayed in these
experiments. However,
itshould be noted Ihal zinc-aluminum
coatings are not recommended for impressed-current cathodicprotection (Ref 11, (2).
Keywords adhesion strength. cathodic protection. current density. freeze-thaw cycling. zinc on concrete
R.Brousseau, M. Arnou, and B.Baldock,NationalResearch
Coun-cil ofCanada. Ottawa. Canada.
Journal of Thennal Spray Technology
2.
Experimental Method
2.1 Thermal Spraying
Zinc and 85Zn-15AI were arc sprayed onto concrete hlocks
using a Thermion 500eleelrical arc gun. All thermal spmy
appli-cations were performed with 3 mm wires. using 620 kP:'1 airpressure, 26 V, 300 A, and a spray distance of 15.2 cm.
All "uto-mated application system was used to travel the electrical. arcgun inlhex-y planc during melallizing
10produce" uniform 0.4
mm thick coating.
2.2 Manufacture of Concrete Samples
The concrete samples were manufactured in thrce different
configurations and sizes to meet test requirements. The concrete mix design was the samefor each of thethree tests.Thecollcreteformulationusedacement/sand/aggregateratioof1:2:3, type
10 Portland cement, and a water
10cement ratio of 0.43.
Chlo-rides were also introduced into the concrete mix tobetter simu-late aggressive environments. The samples were cured in ahumid room at 23'C and 100% relative humidity for 28 day•.
Unless otherwise indicated. the concrete surfaceswere blastedwirh silica sand at 758 kPa air pressure prior to metallization
with
zinc.2.3 Adhesion Measurements
Bond strength measurements were obtained using a pneu-matic adhesion tester referred to as a HPatti." This equipment
uses 50 mm dollies. The pulling force is perpendicular to the
sur-face of the samples aud is applied at a constant rate that is not
op-erator dependent. A thick, high-strength, slow-setting, two-part
epoxy was used to glue the dollics.
2.4 Samples under Impressed Current
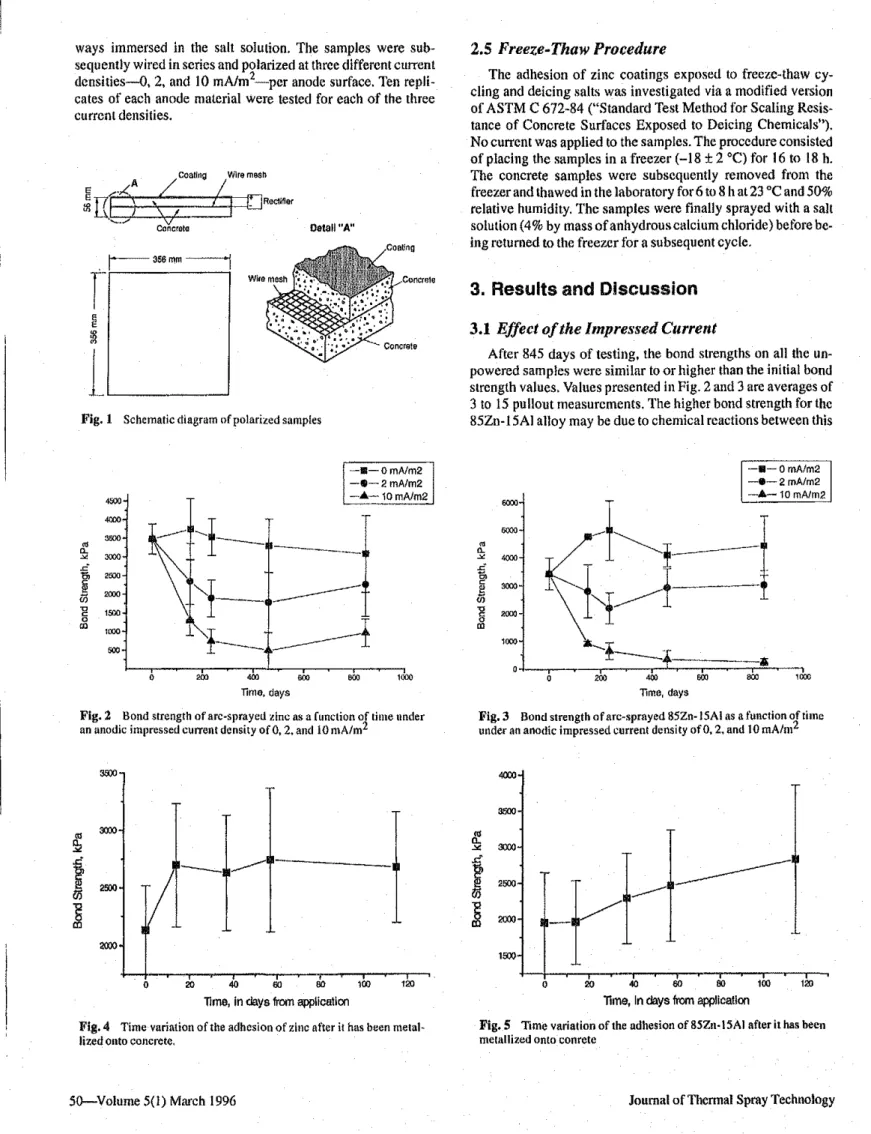
The effect ofimpressed currents on metallized zinc adhesion
was investigated using 60 concrete samples reinforced with a
mild steel mesh (Fig.
I).The metallized concrete samples were
placed in individual plastic containers partially filled with a
saturated salt solution. The lower 1.0 em of each sample was
..,.
I
ways immersed in the salt Solulion. The samples were sub-sequently wired in series and polarized at three differem current
densities-o, 2, and 10 mA/m2-per anode surface. Ten
repli-cates of each anode material were tested for each of the three
current densities.
E /.",;A セ
e,,,.,
I
w;"m,,'
セ
ITI ') '0
I FUR&ctlfler'...
Concrete Detail "A"
.r---
356m'"セr
セm
L,----Fig. 1 Schematic diagram of polarized samples
2.5 Freeze-Thaw Procedure
The adhesion of zinc coatings exposed to freeze-thaw cy-cling and deicing salts was investigated via a modified version of ASTM C 672-84 ("Standard Test Method for Scaling Resis-tance of Conerele Surfaces Exposed to Deicing Chemicals"). No eurrem was applied 10 the samples. The procedure consisted
of placing the samples in a freezer (-18 ±2 'C) for 16to 18 h.
The concrete samples were subsequently removed from the freezer and thawed in the laboratory for 6 to 8 h al23 'C and 50% relative humidity. The samples were finally sprayed with a salt solution (4% by mass of anhydrous calcium chloride) before be-ing returned to the freezer for a subsequent cycle.
3. Results and Discussion
3.1 Effect
ofthe Impressed Current
After 845 days of testing, the bond strengths on all the
un-powered samples were similar to or higher than the initial bond
strength values. Values presented in Fig. 2 and 3 are averages of 3 to 15 pullout measurements. The higher bond strength for the 85Zn-15AI alloy may be due to chemical reactions between this
6000 6000
•
a. セ 4000"'
'g>"""
セ
'0 2000 § OJ 1000Fig.3 Bond strength of。イ」セウーイ。ケ・、 85Zn-15Al as a function of time under an anodic impressedCllrrentdensity of 0, 2. and
to
mA/m2-.-OmA/m2 -e-2mA/m2
セiセ
-4-10 mAlm2I
!-*---!---t-·---a
1000 BOO'-1-'1'-,
-m-OmNm2 -.-2mA/m2 -A-10mA/m2"'"
4000"""
•
a. セ"""
"'
0> '500セ """
-g 1500 0'"
1000 500""
TIme. daysFig.2 Bond strength of arc-sprayed zinc as a function of time tinder
an anodic impressed current density ofO. 2. and lOmA/m2
"""
o ro セ 00 00 100
Time,Indaysfrom application
120
"""
35008'.
. " .'"
.c
MイKMセ
i
'500 /jjセ """
1500 0 20 40 SO 00 100 120Tim.,Indaysfrom application Fig.4 Time variation of theadhesion of zinc after it has been
metal-lized onto concrete.
Fig.S Time variation of the adhesion of 85Zn·15Al afterithas been metallized onto conrete
'000 セ Q. セ
"'"
.c '6>c セ Iii 20001!
0 tn 1000metallized alloy and the concrete. Aluminum is known to chemi-cally rcact in higher pH environment. as a result of its am-photeric oxides. The limiting factor for many of the bond strength tests on the unpowcred samples was the tensile strength of the concrete. Failure often occurred in the concrete (cohesi ve failure).
The bond strength values for all the powered samples de-creased over the course of the experiment. Samples powered at the higher current density experienced larger decreases in bond strength. The loss of adhesion of the metallized coating under anodic polarization is believed to he related to oxidation of the "anchors" that usually provide the mechanical bonding. Molten metal that penetrated into the pores of the concrete during
met-allizing is most susceptible to oxidation during the flow of
an-odic current because these "anchors" have a more intimate
contact with the concrete pore solution through which electro-lytic current flow is forced. This also suggests that excessively large current densities at the anode will be detrimental to the ad-hesinn of the zinc coating. The 85Zn-15AI alloy should not be
used as an impressed-current anode for reinforced concrete due
to its severe loss of adhesion at 10mA/m2. An unusual
forma-tion of oil' pockets alsoOCCUI'Sunderneath the disbanding
zine-aluminum coatings. with unusual darkening. Such severe
- a - Grit blasted before aging - e -GrIt blasted aftera Ing
"""
"'"
•
"'"
Q. セヲセ
t
2"" 2400 Iii 2200 '0c 0 2000 OJ/ '
'""
'""
-20 0 20 40 60 60 '00''''
'40 '60lime in days fromapplication
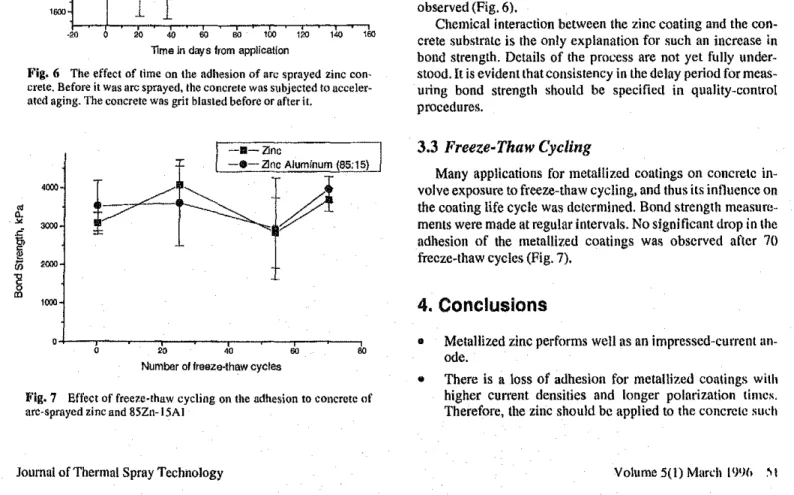
Fig. 6 The effect of lime on the adhesion of arc sprayed zinc con-crete. Before itWas llrcsprayed. theCOncretewas sllbjectcd to acceler-ated aging. The concrete was grit blasted before or afterit.
-a-Zinc
セッセLNB
physical changes are not observed for pure zinc. Photographs
il-lustrating this point are presented in Ref 11.
3.2 Fffect ofRest Time after Metallization
It has been speculated, based on field observations in Oregon and Florida, that a delay in testing the adhesion of metallized zinc after application frequently resnlts in higher hond strength values. This was also noticed in the present result'. More de-tailed experiments were undertaken to study this phenomenon. The changes in bond strength overtime were monitored for both zinc and 85Zn-15AI. The surfaces of 30 concrete blocks were pretreated in three ways prior to metallizing. After metallizing,
bond strengths were monitored on all the samples that were
maintained in a 50% relative humidity atlllosphere at 22 'C.
In the first test, concrete samples manufactured as described
in the experimental procedure were metallized shortly after
completion of theircuring period, The same experiment was
re-peated twice to ensure reproducibility. Each time, no polarizing current was applied to the samples. The individual values in Fig. 4 and 5 represent averages of nine pullout tests. The results
indi-cate an initial increase in bond strengthfOfat least a few weeks
after the metallizing. The magnitude of the increase in bond strengths varies, but in some cases can be as high as 25% of the original value.
Experiments
were also conducted on aged samples todeter-mine whether the apparent increase in bond strength was related to concrete strengthening. Concrete samples (half of which were already grit blasted) were stored for 5 months in tanks kept at 70% relative humidity and 22 'C. There was also a slight
posi-tive
overpressure
of CO2to promote carbonation of thecon-crete. Thnse samples that had previously been grit blasted were metallized without additional grit blasting. All other samples
wefegrit blasted and metallized according to the usual proce-dure. Increases in zinc bond strength over time were once again
observed (Fig. 6).
Chemical interaction between the zinc coating and the con-crete substrate is the only explanation for such an increase in
bond strength. Details of the process are not yet flilly under-stood.lt is evident that consistency in the delay period for meas-uring bond strength should be specifietl in quality-control procedures.
3.3 Freeze- Thaw Cycling
Many applications for metallized coatings on concrete in-volve exposure to freeze-thaw cycling, and thus its influence on the coating life cycle was determined. Bond strength measurc-mellls were made at regular intervals. No significant drop in the adhesion of the metallized coatings was observed afler 70 freeze-thaw cycles (Fig. 7),
4. Conclusions
Fig. 7 Bffect of fteele-thaw cycling on the adhesion to concrete of arc-sprayed zinc and 85Zn-15AI
o 20
Number of freeze-thaw cycles
60 60
•
Metallized
zincperforms weB
as animpressed-current
nn-ode.• There is a loss of adhesion for metallized coalings with
higher current densities and longer polarization times.
Therefore, the zinc should be applied to the cOllerete sUth
that its adhesion is maximized and should not be used where excessively high current densities arc required. • Metallized 85Zn-15AI shoUld not be used as an
impressed-current anode due to poor bonding performance and an un-usual formation of largc air gaps underneath the disbanded coating.
• Zinc bond strength on concrete increases slightly during tlte tlrst few weeks after metallizing when no current is applied. • The adhesion of metaHized zinc on concrete does not
ap-pear to be influenced by severe freeze-thaw cycling.
Acknowledgments
The authors wish to thank the Intemational Lead Zinc Re-search Organization and the National ReRe-search Council of Can-ada for funding this research project. Special thanks to Dr. Serge Dallaire and Mr. Jean-Guy Allard from IMI(NRC) tor their as-sistance with the metallizing of the samples.
References
1. lA,Apostolos, D.M, Parks, and R,A, Carella, "Cathodic Protection Using Metallized Zinc: A 3.5 Years Progress Report," paper 131, pre· sented at Corrosion '87. National Association of Corrosion Engineers. 1987
2. I.A.Apostolos. D.M. Parks. and R.A. Carella. "Development, Testing
and Field Application of MetaIHzed Cathodic Protection Coatings on Reinforced Concrete Substructures," Stale ofCalifomia Department of Transport, Repon No.fhwaOcaitlセXYOPTL National Technicallnfor-mati on Service, Springfield. VA. 1989
52-Volume 5(1) March 1996
3. M,S. McGovern, CPOK with GnOT,Concrete Repair Dig.,OctINov t994.p29t·295
4. D.G. Manning and H.C. Schell, "Early Perfonnance of Eighl Experi-mental Cathodic Protection Systems at the Burlington Bay Skyway TestSite," Transportation ResearchRecord1041
5, E,W. Gulis and H. Jagasia, "Cathodic Protection 1993 Report:' Minis-try ofTransport of Ontario. StructuralOffice, ReportSO-94-0I 6. A.A, Sagues and R.O. Powers,"Low-Cost SprayedZinc Galvanic
An-ode for the Control of Corrosion of Reinforcing Steel in Marine Bridge Substructures," Strategic Highway Research Program. FinalReport, SHRP-88-tD024. 1994
7, R, Brousseau. M. Amott, S,Dallaire, andR.Feldman,Factors Af-fecting the Adhesion of Zinc on Concrete. Corrosion, Vol 48 (No.
Il),t992. P 947
8. R.Brousseau, M, Arnott,andS.Dallaire,"The Adhesion ofMetllllized Zinc Coatings on Concrete: Part1,"paper331,presented at Corrosion '93. NACE International, 1993
9. R Brousseau. M. Amott. and B. Baldock, "Improving the Adhesion of ZincCoatingsused to Metallize Concrete,"Maler. Perform.•lan1994,
p40
10, R.Brousseau,S.Dallaire, M. Arnott, B, Baldock. and lG, Allard. "The Adhesion of Thermally Sprayed Zinc on Reinforced Concrete," Final
ReportILZRO Z8-399, International Lead Zinc ResearchOrganization
11. R. Brousseau, M.Amott.andB.Baldock. "Arc Sprayed Zinc on Rein-forced Concrete," Final Report ILZRO zeセSYPL Intcma!ional Lead ZincResearchOrganization
12. R. Brousseau, M. Amott. and B. Baldock. ·Laboratory Performance of Zinc Anodes for Impressed CUlTcnt Cathodic Protection of Reinforced Concrete, Corrosion. accepted for publication