THE DIMENSIONAL BEHAVIOR OF INVAR By
BERNARD SAMUEL LEMENT
B.Sc. (Met), M.I.T., Cambridge, Massachusetts 1938
Submitted in Partial Fulfillment of the ReqOuirements for the Degree of
DOCTOR OF SCIENCE from the
Massachusetts Institute of Technology 1949 Signature of Author Department of Metallurgy Signature of Professor in Charge of Research Signature of Chairman Department Committee on Graduate Research
Signature redacted
Signature redacted
Signature redacted
1(
4,
TABLE OF CONTENTS
Chapter Page
Number Number
List of Tables ... vi
List of Figures . . . . . . . . . ... viii
Acknowledgments. ... x
I Introduction ...
1
II Summary. . . . . . . . . . . . . . . . . . . 2
III 'Previous Work ...
3
A. Prior Investigation ... ..
3
B. Possible Explanations of Invar . . . . . . 7
1. Magnetic Inversion of Cementite . . . 7
2. Precipitation of Cementite, Graphite, or Nickel Carbide. . .... . . . 8
3. Transformation of Austenite to Ferrite 12 4. Transformation of Austenite to Marten-site. .o ... 13
5. Residual Stress Formation and Stress Relief ... 14
6. Alteration of Magnetic State by Cold Work ... 16
IV Plan of Experimental Work and Materials Used . 17 V Experimental Equipment and Techniques . . . . 20
A. Heat Treatment . .. . . .. . 20
1. Quenchingo. . . . .. . . .. . . .. 20
2. Furnace Cooling . . . . . * . 21
Chapter Page
Number Number
V 4. Carburization and Homogenizatio4 . . . 22
5. Decarburiz4tion . . . 25
B. Precision Length Measurements . . . 25
C. Specific Volume Measurements . . . . . . . 27
D.
Drop Tests . . . . 27E. Heyn Analysis for Residual Stress . . . . 27
F. X-ray Diffraction Measurements . . . 29
1. Precision Lattice Parameter Measurements 29 2. Identification of Phases Present . . . 31
G. Electrolytic Extraction. . .
31
H. Hardness Tests ... 32
I. Magnetic Measurements . . . 32
J. Measurement of Thermal Expansion Coefficient 32 VI Experimental Results . . . . ... . 34
A. Dimensional Charges on Aging . . .
34
1. Invar (0.07 C, 0.44
Mu,
36.8 Ni) . . . 34a. Aging Following Quenching from 8300 C . . .
34
b. Aging at 700 C Following Quenching from Temperatures up to 540" C . 35 c. Re-Aging at 500 C Following Aging at 700 C . . .
35
d. Aging Following Quenching from
2050
C . ... . . . ...36
e. Hot Quenching from 205* C to 70* C 39 f. Aging Following Cold Working . . . 40
B. Drop Tests . . . - . . . C. Residual Stress . . . . . . . . .
1. Complete Stress Distribution . . . . 2. Surface Stress Values . . . . D. X-ray Measurements. . . . .
1. Determination of Solid Solubility of Carbon . . . .
51
52
52
53
55
55
Chapter Numbervi
-iii-Page Number g. Aging Following Furnace Coolingfrom 8300 C . .. . . .
40
h. Rate of Cooling from 8300 C and
3150 C . . . .- - - 41 i. Quenching in Water from 5400 C 42 2. Decarburized Invar (0.01 C, 0.44 Mn,
36.8 Ni).
. . .43
a. Aging Following Quenching from 8300C 43
b. Aging Following Furnace Cooling from
8300
C . . . .
.44
c. Summary . . .45
3.
Invars of Varying Carbon Content (0.02 to 0.58 C, 0.10 Mn, 36.0 Ni) . . .45
a. Aging Following Furnace Cooling from$300
C
. . . 45 b. Aging Following Quenching in Waterfrom 830
C...
... 48c. Aging Following Quenching from 8300 C andl2 0
C.
. . . .... ... 48-
iv
-Chapter Page
Number Number
VI 2. Changes in Lattice Parameter due to
Aging at 200 C to 2050 C. ... 57 3. Debye Patterns of Electrolytic
Ex-tractions ... 60
4. Existence of Martensite or Ferrite . 60 E. Metallographic Examination . . .
63
F.
Hardness Tests .. . .. . . .. . .. .65
1.
Rate of Cooling from 8300 C . . . 65-2. Temperature and Time of Aging . . . . 65
3. Aging Following Quenching from 8300 C
andl2 0
C.
. .. . . .69
G. Magnetic Tests .... . .. . .. ...70
1. Determination of Saturation Field . . 70 2. Variation of Magnetization withTemperature . . .. . .. . . . .. . 70
3.
Rate of Cooling from 8300 C .. .. . 724.
Aging Following Furnace Cooling from830*
0
. . . . . . . . 72 5. Aging Following Quenching from 8300 C 75 6. Aging Following Quenching from 8300 Candl2 0
C.
. . . 75 H. Coefficient of Thermal ExpansionMeasure-ments . . . . . . . . .* 77 1. Rate of Cooling from 8300 C . . . 77 2. Aging Following Furnace Cooling . . . 80 I. Summary of Experimental Results . . . . . 80 1. Aging of Quenched Invar . . . 80 2. Furnace Cooled Invar . . .. .. .. 82
Bibliography 9 . 0 . . 0 . 0 0 Chapter
Number VII
Appendix A - Formulae and Data Used in Calculating Changes in Length Due to Phase Transformations . . . . Appendix B - Calculation of Length Change due to Formation of Cementite in Invar . . . . Appendix C - Calculation of Length Change due Formation of Graphite in Invar. . . . Appendix D - Calculation of Length Change due to Formation of Nickel Carbide in Invar . . . Appendix E - Calculation of Length Change for Formation of Iron-Nickel Carbide in Invar . Appendix F - Calculation of Length Change due to Formation of Ferrite in Invar. . . . . Discussion of Results . . . . .
A. Formation of Residual Stress B. Relief of Residual Stress C. Formation of and Disappearance
Preston Zones . .
D. Precipitation and Re-solution Graphite . . . . E. Summary * . . * . * .* . Conclusions
Suggestions for Further Work VIII Ix Page Number
83
83
84
86
88
89
90
91 92.
1
3
to.
5
.
6
.
8
.9
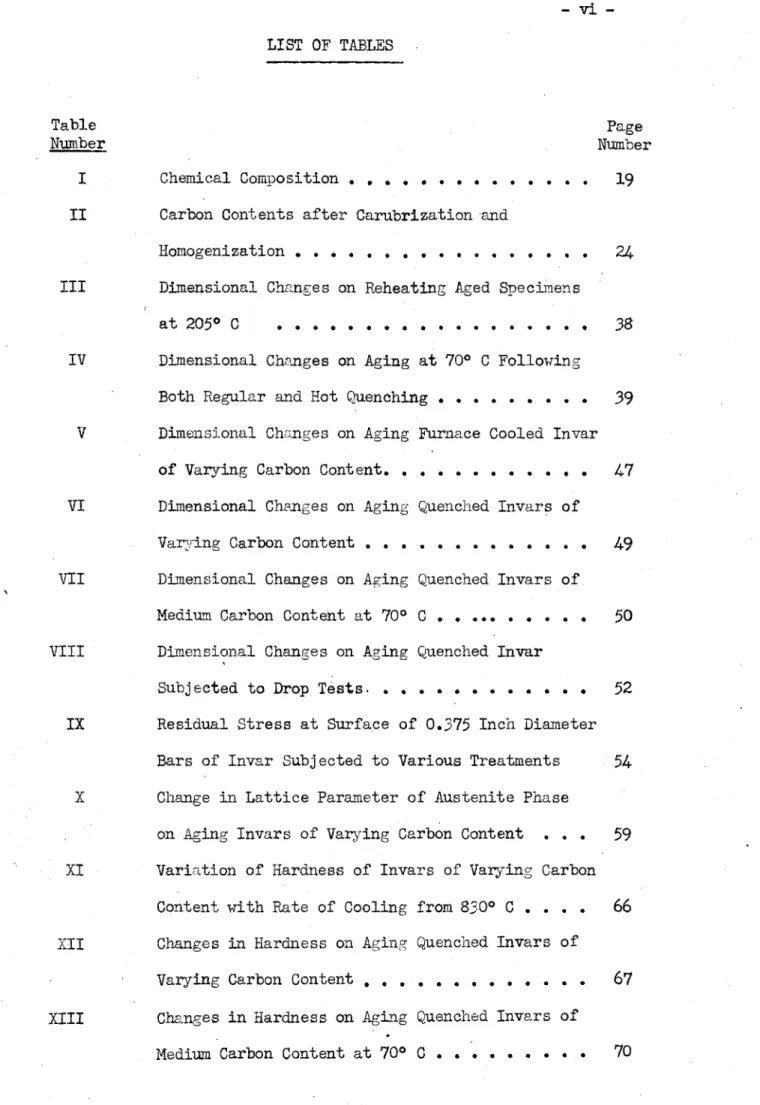
of Guinier-of Carbide and . 0 0 .P . . . . 0 . .* . . . 0 . . .0 . . . .0 . 0 . . . . .-vi-LIST OF TABLES
Table Page
Number Number
I Chemical Composition.... ... . .
19
II Carbon Contents after Carubrization and
Homogenization.... ... . . . 24 III Dimensional Changes on Reheating Aged Specimens
at 205* C ...
38
IV Dimensional Changes on Aging at 700 C Following
Both Regular and Hot Quenching. . .. . .. 39
V Dimensional Changes on Aging Furnace Cooled Invar of Varying Carbon Content. . . . .. . . . 47 VI Dimensional Changes on Aging Quenched Invars of
Varying Carbon Content. . . . .. . . . . ...
49
VII Dimensional Changes on Aging Quenched Invars of
Medium Carbon Content at 700 C . . ... . . .
50
VIII Dimensional Changes on Aging Quenched Invar
Subjected to Drop Tests, . . . 52 IX Residual Stress at Surface of 0.375 Inch Diameter
Bars of Invar Subjected to Various Treatments 54 X Change in Lattice Parameter of Austenite Phase
on Aging Invars of Varying Carbon Content . . .
59
XI Variation of Hardness of Invars of Varying CarbonContent with Rate of Cooling from 8300 C . . . . 66 XII Changes in Hardness on Aging Quenched Invars of
Varying Carbon Content... ... . . .
67
XIII Changes in Hardness on Aging Quenched Invars of- vii
-Table Page
Number Number
XIV Variation of Both Magnetization and Curie Point
of Low Carbon Invar with Treatment . . . . . . . 71 XV Variation of Magnetization of Invars of Varying
Carbon Content with Rate of Cooling from 8300 C 73 XVI Changes in Magnetization on Aging Furnace Cooled
Invars of Varying Carbon Content . . . 74 YVII Changes in Magnetization on Aging Quenched Invars
of Varying Carbon Content . . .. . . . 76 XVIII Changes in Magnetization on Aging Quenched Invars
of Medium Carbon Content at 700 C . . . . . 77 XIX Variation of coefficient of Thermal Expansion of
Invars of Varying Carbon Content with Rate of
Cooling from 8309 C. . . . 78 XX Changes in Coefficient of Thermal Expansion on
Aging Furnace Cooled Invars of Varying Carbon
- viii
-LIST OF FIGURES
Figure Page
Number Number
1 Atmosphere Controlled Furnace . . .
95
2 Apparatus for Measuring Coefficient of ThermalExpansion... . . . ... . . .
96
3
Dimensional Changes on Aging Quenched Invar(0.07 C, 0.44 Mn, 36.8 Ni)
. . ...97
4 Dimensional Changes on Aging Invar (0.07 C,
0.44
t,
36.8 Ni)
at 700
.
. ... .98
5 Dimensional Changes on Re-Aging at 500 C
Following Aging at 700 C ... . . 99 6 Dimensional Changes on Aging quenched Invar (0.07 C,
0.44
Mn,
36.8 Ni) .. . . . 100 7 Dimensional Changes on Aging Quenched Invar(0.07 C, 0.44
Mn,
36.8 Ni) at 70 C . ... 101 8 Dimensional Changes oh Aging Cold Worked Invar(0.07
C,
0.44 Mn, 36.8 Ni)
. .
. . . .
. .
102
9 Dimensional Changes on Aging Annealed'Invar
(0.07 C, 0.44
Mn,
36.8 Ni)
.. *...103
10 Dimensional Changes on Aging Invar (0.07 C, 0.44 Mn, 36.8 Ni) Cooled at Different Rates from Both 830 C and 3150 C ... .. . .. .. . 104 11 Dimensional and Volume Changes on Aging Annealed
Invar (0.07 C, 0.44 Mn, 36.8 Ni) at 5400 C and
Figure Number 12 13 14
15
16
17 18 19 20Dimensional Changes on Aging Decarburized and Quenched Invar (0.01 C, 0.44 Mn, 36.8 Ni). . 106 Dimensional Changes on Aging Decarburized and Annealed Invar (0.01 C, 0.44 Mn, 36.8 Ni). . 107 Dimensional Changes Remilting from Drop
Tests of Quenched and of Annealed Invar (0,7 C, 0.44
Mn,
36.8.Ni) . . ..108
Distribution of Residual Stress in Quenched Invar (0.07 C, 0.44 Mn, 36.8 Ni) . . . . Plot of Lattice Parameter vs. Carbon Content of Invar (0.44 Mn, 36.8 Ni) Heated 500 Hours at Various Temperatures. . . . .. . . . 110 Solubility of Carbon in Austenitic Phase of Invar (0.44 Mn, 36.8 Ni) . . . 111 Plot of J vs. H for Quenched Invar (0.07 C, 0.44
un,
36.8 Ni) at 130 C . . . 112 Variation of Saturation J with Temperaturefor Annealed Invar (0.07 C, 0.44 Mn, 36.8 Ni 113 Variation of Saturation J with Temperature
for Quenched Invars (0.10 Mn, 36.0 Ni) of
Varying Carbon Content . . . 114 Microstructures. . . . 115 21
- ix
-Page Number
ACIaOWLEDGMENTS
The writer wishes to express his appreciation to:
Professors Morris Cohen and Benjamin L. Averbach under whose joint direction this research was carried out, for their advice and deep interest;
Professor John T. Norton, for use of x-ray equipment and advice regarding x-ray technique;
John M. Fitzpatrick, Walter Fitzgerald, Leonard Sudenfield, and Harold Ludwig for assistance in the various tests and
techniques used;
My wife, Annette Lement, for measurements of the x-ray dif-fraction films and for taking care of more than her share of our domestic and social tasks.
I. INTRODUCTION
The problem of making metal parts that will maintain constant dimensions has become increasingly important to industrial develop-ment. Metals changpdimensions when subjected to variations in
temperature or to aging at a constant temperature. These dimensional changes are due to normal thermal expansion or contraction, phase transformations, and stress phenomena. To minimize such dimensional changes requires proper selection of alloy composition, method of processing, and heat treatment.
Because of its very low coefficient of thermal expansion, the 36:64 nickel-iron alloy "Invar" has an inherent advantage over other alloys for applications requiring constant dimensions. However, even with this alloy there is still the problem of achieving dimensional stability with respect to aging in the ambient range. Furthermore, the coefficient of thermal expansion of Invar has been found to de-pend on prior thermal and mechanical treatment.
Although Invar has been used successfully in the past for such applications as length standards, geodetic tape, clock pendulums and wheels, and thermostatic strips, even more exacting dimensional
characteristics are being required by modern high precision equipment. To meet this challenge, a more basi: understanding of the
metal-lurgical phenomena responsible for the dimensional behavior of Invar is necessary. It is for this reason that the present thesis investi-gation was undertaken.
-2-II. SUMMARY
The dimensional behavior of Invar was studied by means of pre-cision length, specific volume, x-ray diffraction, hardness, magnetic, and thermal expansion measurements. SpWg;al techniques involving decarburization, carburization, stress analysis, x-ray diffraction,
electrolytic extraction, and metallograplhic examination were employed.
This investigation showed that there are several expansion and contraction effects that play a significant role in the
dimensional behavior of Invar. The metallurgical phenomena believed responsible for these effects are as follows:
(1) Formation of residual stress by rapid cooling, which results in an expansion.
(2) Relief of residual stress by aging or by mechanical "shock", which results in a contraction.
(3) Formation and disappearance of Guinier-Preston zones within the austenitic solid solution, which result in an
expansion and contraction respectively at low aging temperatures.
(/) Precipitation and re-solution of both carbide and graphite, which result in a contraction and expansion respectively at moderately high aging temperatures.
-3-III. PREVIOUS WORK
A. Prior Investigations
The discovery of the alloy vInvar" was made in the year 1896 by C. E. Guillaume of the International Bureau of Weights and
Measures in France. Guillaume was looking for a cheaper alloy as a substitute for the platinum-iridium alloy used in length standards. He found that iron-nickel alloys in the range of about 30 to 60 percent nickel possess lower coefficients of thermal expansion at room temperature than either pure iron or pure nickel and that the alloy containing 36 percent nickel possesses the minimum coefficient. This alloy was named "Invar" since it is practically invariable with respect to temperature changes in the vicinity of room temperature.
Several theories(1) have been advanced to explain the low co-efficient of Invar. These theories may be summarized as follows:
1. Existence of a compound or superlattice corresponding to Fe2Ni.
2. Reversible transformation of austenite to ferrite in the neighborhood of room temperature.
3. Reversible transformation of a ferromagnetic austenite phase to a paramagnetic phase coexisting below the Curie point.
4. Effect of ferromagnetism on lattice parameter of austenite. No experimental evidence has been presented to support the existence of a compound or superlattice, reversible ferrite-austenite trans-formation, or coexistence of ferromagnetic and paramagnetic auste-nites as claimed in theories 1, 2 and 3. On the contrary, evidence
(-4-obtained by Owen, Yates, and Sully(2) indicates that the overall change in length of a bar of Invar can be correlated with the change in lattice parameter of the austenite phase. Although Owen and SuLLy( have shown that below about 4700 C (8800 F) the equilibrium structure of Invar consists of both austenite and ferrite, the
austenite to ferrite reaction does not occur at an appreciable rate at room temperature and the amount of ferrite resulting from an annealing treatment is probably small. Therefore, the anomalous
expansion of Invar must be due to the effect of ferromagnetism as claimed by theory
4.
(4)
According to the band theory , ferromagnetism may occur in elements or alloys with unfilled d-bands provided that the magne-tized state has a lower energy than the unmagnemagne-tized state. The relative magnitudes of the Fermi and exchange energy associated with the d-band determines which state has the lower energy. The Fermi or kinetic energy depends on the height to which the band is
filled with electrons as well as on the shape of the band. The exchange energy which is the potential energy of electrostatic interaction between electrons is dependent on the relative numbers of electrons of opposite spin. If there are more electrons spinning in one direction, than in the opposite direction, the exchange
energy is lower than for equal numbers of opposite spin. In order for ferromagnetism to occur, there must be more electrons in the d-band spinning in one direction than in the opposite direction so that a net magnetic moment results. This condition can be stable provided that the associated decrease in exchange energy is larger than the increase in Fermi energy.
-5-The difference between the Fermi and exchange energies is called the energy of magnetization and is equal to the difference in
energy between the unmagnetized and magnetized states. Knowing both the energy of magnetization and energy corresponding to the
magnetized state as a function of atomic separation, it is possible to determine the variation of the energy corresponding to the Un-magnetized state as a function of atomic separation.
In the case of Invar the minimum in the energy curve corres-ponding to the unmagnetized state is believed to have a higher value and to occur at a smaller separation than the minimum in energy curve corresponding to the magnetized state. This means that below its Curie point Invar in the ferromagnetic state is more stable than in the paramagnetic state and has a higher lattice parameter than would be the case if it were paramagnetic. If the ferromagnetism decreases, this is equivalent to a sliift of the ferromagnetic energy curve toward that of the paramagnetic energy curve. Thus on heating up from absolute zero there is- found a de-crease in lattice parameter of the austenite as loss of ferro-magnetism occurs due to the effect of increased temperature which
causes the orientation of the magnetic domains to become more and more random. This contraction effect superimposed on the normal
expansion due to temperature increase results in a coefficient of almost zero in the vicinity of room temperature. Above room tempera-ture, the coefficient increases with temperature until the Curie point, about 2600 C (5000 F),is reached and the alloy is in the para-magnetic condition. On heating above this temperature the coefficient is fairly constant and has a value even higher than that of pure iron.
The coefficient of exoansion of Invar was found by Guillaume(5)P Scott
(6),
Russell (7) and Hunter to be affected by thermal and mechanical treatments. Quenching from about 8000 C (14700 F) resultsin a lower coefficient than furnace cooling from this temperature. By cold working it is possible to decrease the coefficient below the
as-quenched value and even obtain negative coefficients. However, low coefficients obtained by cold working are found to increase with time. Similarly, low temperature aging treatments increase the value of the coefficient of either cold worked or as-quenched Invar.
(9)
On reheating cold worked Invar, Hood found that the coefficient increases to a maximum value at about 4700 C (8750 F) and then decreases at higher temperatures. He suggested that the increase in coefficient at 4700 C (8750 F) might be due to transformation from
(9)
austenite to ferrite. Sachs and Spretnak disputed this theory on the ground that the alloy is in the single phase austenite field of the equilibrium Iron-Nickel diagram at the temperature considered. In the same investigation Sachs and Spretnak found that cold working is not effective in causing transformation of austenite to ferrite in Invar.
Although Invar possesses a low coefficient of thermal expansion at room temperature, Guillaume(10) found that this alloy is subject to dimensional changes which occur on aging at and above room
temperature. Guillaume spent over thirty years of research in at-tempting to determine the cause of this dimensional instability and how to overcome it. In brief, he found that two types of dimensional change occur: (1) an expansion or progressive change, and (2) a
contraction or transitorr change. Guillaume attributed these changes to the presence of carbon in commercial heats of Invar. He reasoned that carbon was combined in the form of cementite (FeC) which under-goes a volume change in transforming either from the non-magnetic to the magnetic state or vice versa. In support of this theory, he showed that dimensional instability increases with increasing carbon content and decreases with increasing content of carbide forming elements.which he assumed act to prevent the formation of cementite. In order to stabilize Invar without use of carbide forming elements, Guillaume recommended aging for several days at 1000 C (2120 F) and then cooling very slowly to room temperature over a period of several months.
B. Possible Eplanations of Invar
On the basis of the previous work done on the dimensional be-havior of Invar, it appears that a basic understanding of the
various phenomena observed is lacking. In order to form a basis upon which to plan the experimental program, possible explanations of these ohenomena were given consideration. The explanations that were considered involve several metallurgical phenomena known to occur in steel. A discussion of these phenomena and their possible
connection with the dimensional behavior of Invar follows.
1. Magnetic Inversion of Cementite. Guillaume's theory that the low temperature expansion is associated with the magnetic in-version of cementite has been mentioned previously. Le Chatlier and Chevenard( have shown that on cooling annealed steel an ex-pansion superimposed on the normal contraction due to temoerature change sets in at the cementite Curie point,2100 C (4100 F). This
effect increases with carbon content and must be associated with the magnetic inversion of cementite since it always occurs at the Curie point. In order to relate this effect to Invar, it is necessary to assume that cementite is present and that its Curie point is lowered to at least 1000 C (2120 F) where the low temperature expansion has been observed. It is plausible that this might occur due to the presence of some nickel in solid solution in the cementite. However, it is also necessary to assume that cementite can be retained in the non-magnetic state below its Curie point and that a change to the magnetic state occurs on aging. In view of the fact that the Curie
point of other ferromagnetic materials has been found to be virtually independent of heat treatment, it remains to be proven whether
cementite if it exists in Invar is an exception.
2. Precipitation of Cementite, Graphite or Nickel Carbide. Ac-(8)
cording to Hunter , the interpretation of Guillaumes' explanation of the dimensional instability of Invar is changing solubility of carbon in austenite during heat treatment. In order to explain either the expansion or contraction that occurs during the low temperature aging of Invar it is necessary to show that the solid
solubility of carbon in 36:64 nickel-iron austenite decreases with temperature. The pioneering work done on the iron-nickel-carbon
(12)
system by T. Kase indicates that the solid solubility of carbon in 36:64 nickel-iron austenite may decrease with decreasing tempera-ture. Unfortunately, Kases' work was based on chemical analysis of combined carbon content which could be the sum of carbon in the form of a carbide and carbon in solid solution. Therefore, the
-9
-location of the solid solubility line for carbon is still in doubt. There' are three main possibilities as to the form of the
precipitated carbon: cementite, graphite, and nickel carbide. Cementite and graphite are definitely known to exist in steel
whereas the evidence for the existence of nickel carbide is sketchy. According to Jacobson and Westgren , nickel carbide (Ni3C) has a hexagonal close-packed strouture and is stable at temperatures below about 3000 C (5700 F). Whether precipitation of carbon
either in the form of cementite, graphite, or nickel carbide results in an overall expansion or contraction depends on the relative
specific volume and amount of the austenite phase and carbon con-taining phase present after precipitation. All three
carbon-containing phases have a higher specific volume than the austenite phase; but this tendency for expansion is opposed by the decrease in specific volume of the matrix solution due to depletion of
carbon. An additional factor is the effect of nickel content on the specific volume of the austenite phase. Starting with pure iron the addition of nickel increases the specific volume of nickel-iron austenite to a maximUm value at 40 percent nickel after which the specific volume decreases to the value for pure nickel.
In the event carbon precipitates in the form of cementite (Fe3C) there should be a decrease in specific volume of the austenite phase due to the loss of carbon and an increase in specific volume due to
lowered iron content. If the resultant change is an increase in specific volume, then precipitation of cementite, which is slightly more voluminous than the austenite phase, should result in an overall
- 10
-expansion. If, however, the resultant change is a decrease in the specific volume of the austenite phase, then either an overall ex-pansion or contraction will occur depending on whether the total volume of the precipitated cementite phase is larger or smaller than the decrease in volume of the original austenite phase.
In the event carbon precipitates in the form of graphite, then the only significant change in the specific volume of the austenite phase would be a decrease due to loss of carbon. This change in volume of the austenite phase is opposed by the formation of the voluminous graphite phase which is approximately 3 1/2 times greater in specific volume.
Thus an overall expansion or contraction will be observed depending on whether the total volume of the precipitated graphite is larger or smaller
than the decrease in volume of the original austenite phase.
If carbon comes out of solid solution in the form of nickel car-bide (Ni3C), then there should be a decrease in the specific volume of the austenite phase due to loss of both carbon and nickel. Since the specific volume of nickel carbide is larger than that of the austenite phase, an overall expansion or contraction will be observed depending on whether the total volume of the precipitated nickel carbide is larger or smaller than the decrease in volume of the original austenite phase.
In order to calculate the overall length change resulting from the precipitation of graphite or carbide, it is necessary to know the
specific volumes of the precipitated phases as well as the specific volume of austenite as a function of both nickel and carbon. With the exception of the effect of carbon, sufficient data were found in the literature from which to make these calculations. It was
there-fore decided to determine the effect of carbon on the lattice parameter and consequently specific volume of 36:64 nickel-iron austenite. As
- 11
-will be discussed later, such information enabled calculations to be made of the overall dimensional change resulting from the precipitatiin of carbide or graphite from Invar containing 0.05 percent carbon. This
carbon content was selected since it is the lowest for which theax-pansion effect at low aging temperatures was observed.
Guillaume's recommendation for the elimination of the expansior effect by a very slow cool after prior aging at 1000 C (2120 F) could-be understood on the basis of precipitation of carbon in the form of a
carbide or graphite. Assuming that precipitation occurs at a maxi-mum rate at 1000 C (2120 F), then aging at this temperature would
be effective in removing most of the carbon from solid solution and reducing the tendency for dimensional change. However, there should still be some carbon in solution corresponding to the solid solu-bility at 1000 C (2120 F). If the alloy is simply air cooled to room
temperature, the carbon may remain dissolved but eventually must precipitate out of solution. In order to minimize this effect, it
is necessary to precipitate all the carbon in excess of the
solubility limit at room temperature. Theoretically all one has to do to accomplish this is to age long enough at room temperature; however, due to the exceedingly low rate of diffusion, such a
pro-cess might take an infinite length of time. In order to minimize the time for complete precipitation, it would be logical to age at the temperature of maximum rate of precipitation until the process
stops, lower the temperature so that the solid solution becomes more supersaturated and age until the process again stops, and repeat until room temperature is reached. In effect, Guillaume's recom-mended very slow rate of cooling can be considered equivalent to a
- 12
-The opposite of precipitation, that is re-solution, could con-ceivably account for part of the dimensional change observed on
aging Invar. If during slow cooling, for example, some of the carbon is precipitated before room temperature is attained, subsequent aging at a high enough temperature could cause re-solution as manifested by an expansion or contraction depending on whether precipitation
results in a contraction or expansion. If the precipitated phase has a fine enouch particle size, it is possible for retrogression to occur. This phenomenon involves re-solution of particles below a certain critical size and can orecede further precipitation on aging.
3. Transformation of Austenite to Ferrite. The possibility that the low temperature expansion is due to a transformation from auste-nite to ferrite as suggested by Russell is supported by the equili-brium diagram of Owen and Sully(3) which shiows that at a temperature of 3000
c
(5700 F) a pure Invar should consist of about 55 percent austenite (58:42 nickel-iron) and 45 percent ferrite (5:95nickel-iron). However, in order to attain equilibrium conditions it was found necessar7 to severely cold work and age for long periods of time. After ordinary heat treatment it is generally claimed that Invar is entirely in the austenitic condition. It is conceivable that a small amount of transformation could occur on aging at 1000 C
(2120 F) although the rate of such transformation would be very slow at this temperature. The effect of impurities such as carbon, manganese, and silicon found in commercial Invar on this transformation is not known; however, based on the effect of these elements on ferritic
hardenability in ordinary steel, it is suspected that the trans-formation would be retarded by their presence.
It is also possible that austenite transforms to a carbide-ferrite or graphite-carbide-ferrite aggregate. Either an overall expansion or contraction will occur depending on the relative amounts and
-13-specific volumes of the constituents making up the aggregate and how the specific volume of the untransformed austenite phase is af-fected. It is obvious that there are numerous possibilities in con-nection with this type of transformation and therefore a detailed analysis of dimensional changes would be of great complexity due to the many assumptions required at this point.
Assuming that transformation of austenite to ferrite results in an overall expansion, if any ferrite were present in Invar at room temperature, on reheating there would be a reverse transformation of ferrite to austenite which should result in a contraction. However, it is doubtful whether such a contraction would occur at low aging temperatures where an approach to equilibrium in the opposite direction is more likely. It is believed, therefore, that the ferrite to auste-nite reaction could not account for the observed contraction at low aging temperatures as suggested by Russell .
In order to explain why the coefficient of thermal expansion at room temperature should be lower after fast cooling than after slow cooling, the possibility that annealing favors, whereas quench-ing suppresses, transformation of the austenite to ferrite was con-sidered. If ferrite formed, the resulting two-phase alloy should have a higher coefficient than the alloy in the single phase con-dition due to the much higher coefficient of ferrite containing about 5 percent nickel as compared with austenite containing about 36 percent nickel.
4. Transformation of Austenite to Martensite. The possibility of a martensite reaction occurring isothermally at a low temperature was also considered. Although the martensite reaction was in the
-14-on
past believed to take place only/cooling, recent work by Averbach
(14)
(15)
and Cohen and Kurdjumov has indicated that it can also take place isothermally. On the basis of the austenite-martensite volume
change occurring in steel, a length increase of about 140 microinches per inch for each percent of transformation would be expected.
If the austenite-martensite reaction were reversible, then the contraction on aging could be accounted for. There is also the possibility that decomposition of martensite by rejection of carbon on aging could at least partially account for the observed contraction.
The occurrence of an austenite to martensite transformation could account for the fact that a lower coefficient results after quenching than after furnace cooling in the same way as suggested
for an austenite to ferrite transformation provided that the martensite has a higher coefficient than the austenite from which it forms.
5. Residual Stress Formation and Stress Relief. Stress relief could account for dimensional changes on aging Invar. The occurrence of residual stress in a metal object which has been subjected to
rapid cooling from a relatively high temperature is well known. Where there is no phase change involved, the setting up of residual stress must be due to the difference in cooling rate between the center and surface portions of the metal object. The cooling stresses usually result in residual compression at the surface and residual tension in the interior. On aging relief of this residual stress occurs giving rise to dimensional changes. Wheth .r a contraction or ex-pension results depends on the exact stress distribution, the
- 15
-variation of elastic limit in both tension and compression with temperature, and the rate of heating to and cooling from the aging temperature.
Guillaume's recommendation for the achievement of dimensional stability can be partially understood on the basis of stress relief. Heating to 1000 C (2120 F) for several days might result in a suf-ficient degree of stress relief so that negligible dimensional changes would occur subsequently provided that Invar is not heated above this temperature in service. Slow cooling from 1000 C (2120 F) to 200 C (680 F) over a period of several months as recommended by Guillaume is not an efficient way of achieving stress relief. Holding longer at 1000 C (2120 F) will result in greater stress relief than slow cooling.
Residual stress can also be introduced by cold working. The distribution of residual stress and the resulting dimensional changes due to stress relief on aging will depend on how the cold working is applied.
It is possible that the reported differences in coefficient of expansion resulting from furnace cooling, cuenching, and cold working Invar may be due to the relief of residual stress. The reported dilatometer curves which were made for the purpose of determining coefficients of expansion usually show that heating was carried out well above room temperature. This could result in stress relief as manifested by a dimensional change superimposed on the normal heating curve and lead to a false measure of the coefficient, particularly if an average value over a range of temperatures is
-16
-being determined. Without knowledge of the magnitude of irreversible changes due to stress relief the reported values of the coefficient are open to question.
6. Alteration of Manetic State by Cold Work. The lowering of the coefficient by cold work could be attribut'ed to alteration of the ferromagnetic condition of the austenite. It is possible that the intensity of magnetization could be affected .by cold work in such a way that the temperature dependence of the contraction due to the gradual reversion from the ferromagnetic to the paramagnetic state on heating results in a lowered overall coefficient of ex-pansion at room temperature. The observed effect of reheating after cold working on the coefficient could also be explained on this basis. Reheating tends to remove the effects of cold working and consequently the coefficient would be increased.
- 17
-IV. PLAN OF EXPERIMENTAL WOEK AND MATERIALS USED
Based on consideration of the possibilities discusged in the previous section, a plan of the experimental work to be followed in
this investigation was decided upon. The main outline of this plan is as follows:
1. Determination of the effect of quenching, annealing, and
cold working on length changes during subsequent aging of Invars of varying carbon content.
2. Determination of the magnitude and distribution of residual stress in quenched Invar.
3. Determination of the solid solubility of carbon in 36:64 nickel-iron austenite.
4. Determination of the possible existence of cementite,
graphite, nickel carbide, ferrite, and martensite in Invar and the conditions under which they form.
5. Determination of the effect of heat treatment on the hardness of Invar.
6. Determination of the effect of thermal and mechanical treatment on the temperature dependence of intensity of magnetization of Invar.
7. Determination of the coefficient of thermal expansion at room temperature of Invar subjected to thermal and mechanical
treatments, taking into account irreversible length changes. The materials used in this investigation were iron-nickel-carbon alloys obtained in the form of 0.250 and 0.375 inch diameter rod mostly in the cold drawn condition. The chemical composition of
- 18
-these materials is given in Table I. Two series of Invars were investigated: (1) 0.44 Mn, 36.8 Ni; and (2) 0.10 Mn, 36.0 Ni.
-
19
-TABLE I CHEMICAL CMTPOSITION Invar Series C Mn Si 8 P Ni 0.44 Mn, 36.8 Ni* 0.07 0.44 0.24 0.011 0.007 36.82 0.10 Mn, 36.0 Ni 0.02 0.09, 0.01 0.012 0.008 36.02 0.10 0.12 0.15 0.080.08
0.010
0.009
36.10
0.17
0.021
0.009
36.03
0.25
0.05
0.20
0.022
0.010
36.60
0.40
0.10
0.07
0.013
0.009
35.89
0.58 0.07 0.22 0.022 0.01236.16
0.74
0.07
0.17
0.020
0.009
35.89
0.99 0.14 0.24 0.011 0.012 36.10* By decarburization and carburization techniques, Invars
varying in carbon content from 0.01 to 0.84 percent were made
- 20
-V. EXPERIMENTAL EQUIPNENT AiD TECNIQUES
A. Heat Treatment
1. Quenching. Heating prior to quenching of Invar specimens was carried out either in a lead pot furnace or a tube furnace. The
lead pot, 10 inches in diameter by 10 inches deep inside a chromel wound muffle, was maintained at 830 + 50 C (1525 + 100 F) for this
treatment. It was found that distortion resulting from water quench-ing specimens 0.250 inch in diameter by 4 inches in length could be minimized by use of a jig consisting of two parallel rods 0.25 inch
square and 8 inehes long welded 6 inches apart across a rod 12 inches long. Two specimens could be tied on the jig with iron wire in a position parallel to and on either side of the 12 inch rod. The loaded jig was placcd in the lead pot to completely suknerge the specimens about 4 inches from the surface. After 30 minutes of heating at 8300 C (15250 F) the loaded jirg was removed from the
lead pot and quenched in a tank of water using an up and down motion. With this procedure, distortion as measured by "bowing" of the speci-mens was usually found to be less than 0.005 inch.
When it was desired to quench specimens from as high as 1205* C (22000 F) a vertical tube furnL.ce shown in Figure 1 was used because the lead pot could not be heated above 8700 C (16000 F). The tube
furnace contained a muffle 2 inches in diameter and 24 inches long, wound with Kanthal A resistance wire. A quartz tube with
- 21
-of the muffle. The furnace provided a four inch long zone at the center of the tube throughout which the temperature was constant within 50 C (100 F). Either dry nitrogen, hydrogen, or argon gas was used as a protective atmosphere and entered the quartz tube through the bottom of the furnace. In practice, specimens were tied with iron wires and lowered into the
4
inch constant temperature zone. Because of the lack of space for a quenching jig and the higher temperatures employed, appreciable distortion often occurred. This necessitated heat treating a greater number of duplicatespecimens until enough specimens with an acceptable degree of bowing, less than about 0.01 inch, became available.
2.. Firnace Cooling. Heating of Invar specimens prior to furnace cooling was carried out in a small retort furnace heated by resistance elements. The retort contained inlet and outlet connections for a protective atmosphere. Hydrogen gas was found to result in the brightest surface after furnace cooling of Invar. The procedure was to place the specimens in the retort, turn on the hydrogen gas, heat to 830 + 80 C (1525 + 15*0 F) in about 3 hours, hold at tempera-ture for 1 hour, and furnace cool to room temoeratempera-ture in about 10 hours.
3. Aging. Aging at room temperature was carried out in a constant temperature room maintained at 20 + 10 C (68 + 20 F). Aging between 200 C (680 F) and 1500 C (3000 F) was carried out in oil pots maintained within 30 C (50 F) of the desired temperature. Aging above 1500 C (3000 F) and up to 5400 C (10000 F) was carried
- 22
-temperature. The following times were found necessary for the center of specimen 0.250 inch in diameter by 4 inches in length to attain within 50 C (100 F) of the temperature of the aging bath:
Tepperature Medium Time for Center to Reach Temperature
500 C Oil 1 minute
1500 C Oil 2 minutes
2050 C Salt 1 minute
4250 C Salt 0.5 minute
The time of cooling from a 3150 C (6000 F) salt bath to room tempera-ture for specimens 0.250 inch in diameter by 4 inches in length was found to be as follows:
Medium Time for Center to Reach Room Temoerature
Water 1 second
Air 20 minutes
Silocel 45 minutes
4. Carburization and Homogenization. Carburization was carried out in the tube furnace shown in Figure l A gas train similar to that used by Low and Gensamer (16) was constructed for the purpose of carrying out carburization, homogenization, and decarburization experiments. Carburization was carried out by bubbling dry purified hydrogen through liquid heptane. The steps involved in purifying and drying ordinary tank hydrogen consisted of passing this gas through platinized asbestos at 4000 C (7500 F), anhydrous calcium sulfate, anhydrous magnesium perchlorate, and a trap cooled by liquid nitrogen. The purpose of the platinized asbestos was to get rid of any oxygen by catalyzing the reaction 2H2 + 02 -- +. 2H20-The water formed by this reaction as well as any water initially
-23-present in the tank hydrogen is removed by the two chemicals and liquid nitrogen trap, and dry oxygen-free hydrogen gas is thus pre-pared. This gas when bubbled through liquid heptane maintained at 0' C (320 F) in an ice bath acts as a carrier of hydrocarbon vapor.
A low flow rate adjusted to result in barely perceptible bubbling through
the liquid heptane was maintained during carburization. Appropriate temperature-time combinations were selected to result in the desired degree of carbrization.
In order to obtain powder of a given carbon content for the x-ray diffraction experiments, it was decided to carburize Invar specimens 0.250 inch in diameter by 2 inches in length weighing about 9 grams. This size specimen also allowed enough material for chemical analysis of carbon content at both the center and surface of the specimen. It was expected that after carburization a carbon
gradient would exist from surface to center and a homogenization treat-ment carried out in dry nitrogen would be required to equalize the
carbon content throughout the 0.250 inch diameter section. An initial experiment gave the following results:
Carburization Treatment Homogenization
%
C at % C at Treatment Surface Center12050 C - 6 hours none 0.824 0.770
12050 C - 6 hours 12050 C - 3 hours 0.783 0.775 Specimens were water quenched to room temperature after carburization and after homogenization. This experiment gave an idea of how much carbon could be introduced by carburization and showed that equali-zation throughout the 1/4 inch section to within 0.01 percent of
24
-carbon was attainable. Homogenization treatments at lower tempera-tures were tried but the times required became excessive below 10950 C (20000 F). A homogenization treatment consisting of 24
hours at 1095* C (20000 F) was finally selected for this work. The carburizing treatments and resulting carbon contents of the series of specinens used in the x-ray diffraction work are given in Table II.
TABLE II
CARBE0 CONTENTb -FTER CiJRBURIZbTION AND 'TOMOGENIZATION
Carbon Content zAfter Homogenizing 24, Hours at 10950 C
Carburizing Treatment Surface Center Average
None 0.070 0.070 0.070 9800 C - 2 hrs. 0.130 0.124 0.127 10400 C -
4
hrs. 0.270 0.266 0.268 10950 C - 2 hrs. 0.376 0.365 0.369 1095* C - 4 hrs. 0.421 0.411 0.416 10950 C 8 hrs. 0.505 0.485 0.495 11500 C - 8 hrs. 0.616 0.638 0.627 12300 C - 8 hrs. 0.832 0.844 0.838 Melting occurred wehen an attempt was made to carburize at 12600 C (23000 F); therefore a carbon content of about 0.84 percent wasconsidered the limit attainable by this technique using an 8 hour carburizing period.
7
- 25
-5.
Decarburization. Decarburization was carried out by the use
of wet hydrogen. To produce the wet hydrogen, tank hydrogen was first
passed through platinized asbestos at 40
00 C
(7500
F) to remove
oxygen and then through a water saturator. After some preliminary
experimentation,
it
was found that with a flow rate of 2 cubic feet
per hour and the water saturator maintained at
7
00
C
(1580
F)
effective decarburization of a 0.250 inch diameter Invar specimen
could be accomplished in
24 hours at 10950 C (20000 F). Chemical
analysis before and after this treatment gave the following results:
Treatment
Carbon
Pyen
Nitrogen
As received
0.070
0.0027
0.0058
24 hours at 10950 C in wet H
20.011
0.0036
0.0077
These results show a significant decrease in
carbon content along
with but a slight increase in oxygen and nitrogen contents.
B. Precision Length Meagurements
Precision length measurements were carried out by the use of a
Sheffield Comparator of 5000X magnification. Specimens 0.250 inch
in diameter and 4 inches long were spherically ground to a 2 inch
radius at their both ends in order to provide a reproducible high
spot for precise measurement and also to minimize the error due to
small deviations in positioning when held vertically in a jig. The
anvil to gaging point distance of the comparator was maintained at
4.120 inches
so that in making a
measurement
it was necessary for the
specimen to rest with its bottom end on a gage block of the proper
size which was wrung to the anvil. By moving the jig and
conse-quently the specimen back
and
forth
in
contact with the gaging
-26
-a 4.120 inch st-and-ard block c-alibr-ated to the ne-arest microinch. From the difference in readings between the specimen and standard block and a measurement of the temperature at which the measure-ments were taken, the length of the specimen prior to or after a given treatment could be determined. The sensitivity of the com-parator used is about 5 microinches so that changes in length of a
4 inch specimen could be determined with an accuracy of within 2 microinches per inch. The reproducibility of length change for
duplicate specimens varies with the magnitude of the change. In general it is believed that the precision of measurement of the average length change of duplicate specimens is + 2 microinches per inch or 5 percent, whichever is the larger.
In order to make a correction for the difference in coefficient of thernial expansion between the specimen and standard block, ac-curate measurement of temperature is required. Although length measurements were made in a "constant" temperature room maintained
at 20 + 10 C (68 + 20 F), the variation of even 10 C (20 F) results in a large error due to the great difference between the coefficient of expansion of the tool steel standard block and the Invar speci-mens (12 compared with less than 2 microinches per inch per OC). To reduce this error, a large copper block, 2 inches thick by 12 inches square was used for the purpoce of keeping both standard
block and specimens at constant temperature. This block was machined with grooves in order to attain intimate contact with cylindrical
K
-27-material and dimensions were kept side by side on a flat surface of
the block. A copper-constantan thermocouple was attached to the
dummy block and temperatures were measured by a potentiometer to
within + 005*
C
(0.100 F).C.
Specific Volume Measurements
The weigh-in-water, weigh-in-air method was used for measurement
of specific volume. This method has been described in detail by
(17)
Cohen
and Kohl.
The accuracy of measurement is about 0.00002
cubic ems. per gram.
D.
Drop Tests
It was noted that the accidental dropping of a quenched Invar
specimen resulted in a relatively large contraction.
It was
sus-pected that this change of length might be associated with a
redistri-bution of residual stress. In order to check this
hypothesis, drop
tests were carried out. These tests consisted of dropping specimens
from a height of 5 feet above a concrete floor and measuring the
resultant change in length. In order not to injure the spherical
ends of the specimen, a small electromagnet having two wound cores
was used to hold the specimen in a horizontal position. When the
current in the electromagnet was turned off, the specimen dropped and
maintained its
horizontal position on striking the floor. The
re-bound was as high as 2 feet above the concrete floor
and
specimens were
usually caught on the first bounce.
E.
Heyn Analysis for Residual Stress
The Heyn analysis
was used to determine the residual stress in
Invar specimens subjected to various heat treatments. This analysis
assumes the existence of longitudinal stress but no
radial
or
- 28
-circumferential stress. In carrying out the Heyn analysis it is necessary to determine the change of length resulting from machining off layers from the surface. If after reducing the area of a
cylindrical specimen to a value A, a change in length e measured on a unit length basis results, then the force F that the machined off surface layer exerted on the section of area A is given by the following:
(1) F
=
EAe Where E = Young's modulusThe stress S that must have existed at the radius corresponding to A prior to machining is given by:
(2) S = = -E A + e
Thus the residual stress distribution can be calculated knowing the value of E (21 X 106 p.s.i. for Invar), the rate of change of e with A at a given A, and the value of e at a given A. In order to deter-mine the variation of e with A, specimens 0.250 and 0.375 inch in diameter and 4 inches in length possessing spherical ends were centerless ground taking off 0.001 inch from the diameter at each pass. The change of length corresponding to decreased sectional area was determined by precision length measurements.
For the most part only the residual stress at the surface was desired and this as determined by machining 0.020 inch in steps of 0.001 inch off the diameter. The value of surfaoe stress was calculated using equation (2) substituting the value of d at thedA surface and zero for e. The accuracy of surface residual stess as determined by the Heyn method is estimated to be about 10 percent.
- 29
-F. X-rar Diffraction Measurements
1. Precision Lattice Parameter Measurements. Precision lattice parameter measurements of the austenite phase in Invar were made using a symmetrical back reflection camera 10 cms. in diameter. Specimens in the form of 325 mesh powder were used. A cobalt target was sel-cted in order to obtain enough high angle lines to facilitate determination of lattice parameters by the extrapolation method. The accuracy of this method is about 0.01 percent, which
0
amounts to + 0.0003 A for the austenite lattice parameter.
Three lines were obtained in the back reflection diffraction pattern resulting from the face-centered cubic austenitic phase. These lines are as follows:
Line hkl Radiation sin 2e Relative Intensity 1 400 Cobalt K alpha 1 0.99 Medium (diffuse) 2 400 Cobalt K alpha 2 0.99 Strong (sharp) 3 331 Cobalt K beta 1 0.97 Weak (sharp)
In order to calculate the lattice parameter corresponding to each line the following equations were used:
(1) = 90 - Where 8 = Bragg angle in degrees. or 6 = 90 - 0.14129 S S
=
Separation on film in mm.D = diameter of camera (101.38 mm). (2) a - 2 +
I
Where a=
lattice parameter= wavelength of radiation in angstroms
hkl = Miller indicies of atomic plane. The value of the lattice parameter corresponding to each line
was plotted against sin 26. The value obtained by extrapolating to sin2
e
= 1 was taken to be the lattice parameter of the specimen.30
-The parametric method of determining the solubility of carbon in 36:64 nickel-iron -austenite was employed. In the determination of the phase boundary of a terminal solid solution in a two component system by this method, there are two main steps. First, it is necessary to determine the relationship between lattice parameter and composition of the solid solution phase by quenching a series of specimens of
varying composition from the temperature of maximum solubility. Second, specimens of an alloy exceeding the maximum solubility of the solid solution are brought to equilibrium and quenched from temperatures up to that corresponding to maximum solubility. From the lattice para-meters of the solid solutions in equilibrium with the second phase at each temperature and the relationship between lattice parameter and composition of the terminal solid solution, the composition-tem-perature or phase boundary of the terminal solid solution can be con-structed.
The procedure uied for binary systems depends on the fact that the composition of two phases in equilibrium at a given temperature is independent of the amounts of the phases present. In a ternary system, the compositions of two phases in equilibrium at a given temperature are not necessarily fixed. Therefore, instead of determining the relation between the lattice parameter and carbon content at only the temperature corresponding to maximum solubility, it was planned to determine t-nis relation at all temperatures. It was expected that a plot of lattice parameter vs. carbon content would
exhibit a discontinuous change in slope (but not necessarily a horizontal break) at the carbon content corresponding to the limit of solubility for a given temperature. The solid solubility curve of carbon in 36:64 nickel-iron austenite could then be determined by plotting the carbon content at the discontinuity vs. temperature.
- 31
-2. Identification of Phases Present. In order to determine whether or not Invar contains phases other than austenite two x-ray diffraction techniques were used. One technique described by Averbach
(is)
and Cohen consists of exposing the polished and etched surface of a solid specimen 0.375 inch in diameter to monochromatic radiation in a modified Debye camera. Iron K-alpha radiation monochromatized by a bent rock salt crystal and exposure times of 12 hours were employed. The other technique involves use of the regular Debye camera and a powdered specimen made in the shape of a wire about 0.5 mi. in diameter with cellulose acetate as binder. The specimens
were prepared by an electrolytic extraction technique used to separate carbides from steel. The details of this technique are given in the following section.
G. Electrolytic Extraction
Electrolytic extractions for possible graphite or carbides were carried out in an acid cell designed by Blickwede (19). In this cell a specimen 0.250 inch in diameter by 4 inches long serves as anoLe and is surrounded by a cylindrical copper cathode. Both anode and cathode are immersed in an electrolyte, 5 percent hydrochloric acid. A current of 0.5 amps is used which results in a current density of 0.15 amp. per square inch. The duration of a run is usually about
48
hours. Every 8 hours specimens are scraped and washed with 2 percent hydrochloric acid to obtain the insolubleresidue. This residue is kept at 00 C (-32* F) until vacuum filtered. The filtered residue is washed with 200 cubic cms. of distilled water and dried in a vacuum oven for 8 hours at 650 C (1500 F) after which
slow cooling in vacuum is carried out. The filter and residue are placed in a dessiccator for 4 hours and finally weighed. From the
- 32
-weight of the residue and the dissolved specimen, the -weight percent of the residue can be calculated.
H. Hardness Tests
Measurements of Rockwell B hardness were made in a standard Rockwell machine. The values reported are accurate to + 1 Rockwell B unit.
I. Maanetic Measurements
Measurements of variation of intensity of magnetization with temperature were made using the apparatus described by Zmeskal and Cohen(20) for specimens 0.250 inch in diameter by
4
inches long. A rate of heating of 50 C (100 F) per minute was maintained in making a run. By calibration, the following relation was determined from which to calculate intensity of magnetization (J):J = 6.37 (D - D ) gauss. Ds
=
deflection of galvanometer for complete reversal of field with specimen in secondary cell. DA = deflection of galvanometer forcomplete reversal of field with-out specimen in secondary coil. A magnetic field intensity of H = 1100 oersteds which is well above saturation was used in these measurements.
J. Measurement of Thermal Expansion Coefficient
The determination of the coefficient of thermal expansion of Invar at room temperature represents a more difficult problem than for other metals because of the low magnitude involved. In order to measure the coefficient of Invar an apparatus shown in Figure 2 was