Publisher’s version / Version de l'éditeur:
Polymer Degradation and Stability, 34, 1-3, pp. 309-323, 1991
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/0141-3910(91)90125-B
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Polyethylene hydroperoxide decomposition products
Lacoste, J.; Carlsson, D. J.; Falicki, S.; Wiles, D. M.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=854430b9-d412-433e-85ab-ec26a71bea96 https://publications-cnrc.canada.ca/fra/voir/objet/?id=854430b9-d412-433e-85ab-ec26a71bea96
Polymer Degradation and Stability 34 (1991) 309-323
Polyethylene Hydroperoxide D e c o m p o s i t i o n Products
J. Lacoste,* D. J. Carlsson, S. Falicki
& D. M. Wiles
Institute for Environmental Chemistry, National Research Council of Canada, Ottawa, Canada K1A 0R6
A B S T R A C T
The decomposition products from pre-oxidized, linear low-density polyethy- lene have been identified and quantified for films exposed in the absence of oxygen to ultra-violet irradiation, heat or y-irradiation. Films were pre- oxidized under controlled conditions by 7-irradiation in air. Vacuum decomposition products were then analyzed by infrared derivatization techniques to allow the quantification of carboxylic acid, ketone, ester, 7- lactone, alcohol, hydroperoxide and unsaturation products. From the relative proportion of these products it is possible to compare the mechanisms of the three initiation methods. These mechanisms are compared and contrasted with previously proposed mechanisms for polyethylene degradation.
I N T R O D U C T I O N
Polyethylene is the simplest m e m b e r o f the polyolefin family. However, its oxidative d e g r a d a t i o n is still only poorly u n d e r s t o o d with several apparently conflicting proposals for the oxidative mechanism.1 - 7 Scott and coworkers have proposed the involvement o f hydroperoxides in both the thermal and photo-oxidation o f polyethylene.1 Nevertheless, the nature and stability o f hydroperoxide products and the origin o f the backbone scission reaction(s) are controversial. Hydroperoxide species ( R O O H or their dimers) * Present address: Ecole Nationale Sup6rieure de Chimie de Clermont Ferrand et URA CNRS 433, F-63177 Aubi6re Cedex, France.
309
Polymer Degradation and Stability 0141- 3910/91/$03" 50 © 1991 Elsevier Science Publishers Ltd, England. Printed in Great Britain
310 J. Lacoste, D. J. Carlsson, S. Falicki, D. M. Wiles
have been suggested to be unstable to both near ultra-violet (UV) and heat exposure and to cleave to give radicals capable of initiating the oxidative breakdown of any hydrocarbon (RH) (reactions (1)--(3)). a'7 The secondary peroxyl radicals expected from PE should terminate rapidly to generate equimolar yields of a secondary alcohol and a backbone ketone (reaction (4)). 2
R O O H ~ R light O + "OH " R. ~ R" + H 2 0 / R O H (1)
R" + 0 2 ~ RO~ (2)
RO~ + R H • R O O H + R" (3)
2RO 2 ,. ROH + R'C(---~O)R" + 02 (4) Reactions (1)-(4) predict the accumulation of hydroperoxide, alcohol and ketone groups but do not explain backbone scission, the essential factor leading to embrittlement, or other suggested products such as esters and lactones, carboxylic acids and unsaturation. 2-7 The secondary alkoxyl radical (RO') from hydroperoxide decomposition can be expected to H- abstract to form alcohol (reaction (1)) or (less likely) to undergo some fl- scission (reaction (5)) which will cause backbone scission and generate an
H H I I - - - , C H 2 - - C - - C H 2 ~ , ----~H2--C + " C H 2 ~ I II O" O
(5)
aldehydic chain end which should oxidize to carboxylic acid. However, embrittlement and carboxylic acid formation also accompany the v-initiated oxidation of PE, which results predominantly from C - - H cleavage caused by the radiation (reaction (6) followed by reactions (2)-(4)): 6,7
PE ~ - - - C H 2 - - ( ~ - - C H 2 ~ 0~, RO 2 (6) H
Gugumus has pointed out that hydroperoxide groups do not accumulate in photo-oxidized PE and proposed that these groups are not apparently
H/CH2 ~ H C H 2 ~
I
d /
C... . _ . C ~ 2 0 \ o h, " ' ~ H 2 OH / \ I + H + .----.CH2--CH2~ H 2 0 + ...C==CH2 I H (7)Polyethylene hydroperoxide decomposition products 311
photo-initiators# 's In addition, the simple scheme (reactions (1)-(6)) does not explain the observed Vinyl and trans-vinylene products. As an alternative, Gugumus has suggested the novel reactions (7)-(9) to account for some of the products that he has observed (especially vinyl and trans-vinylene unsaturation). Aldehyde (reaction (9)) was not clearly identified (by, for example, observation of its unique ~ 2720cm-1 IR absorption).
~ C /
\ /
0 / \ H h, C I * II +H20
+ H (8) H / O ~ C / / \ O ---C--H H HH~C/
H /
O / \CH2
h, ~C
CH2
I J IE 11 C - - H 0 + CH/ \
I
H H H 2 0 (9)By the use of pre-oxidized PE samples, some of the authors have recently shown that hydroperoxide groups do photo-cleave to initiate oxidation but reach a (photostationary) plateau level. 7 In this paper, the authors examine the products generated by UV, heat or y-exposure of sec--hydroperoxide in pre-oxidized PE films. In order to simplify the identification of breakdown products, samples were exposed in the absence of 02, when hydroperoxides can only decompose. The observed hydroperoxide breakdown products are then compared with those from the proposed free-radical and molecular decomposition routes.
EXPERIMENTAL
Linear, low-density polyethylene (LLDPE) film (DuPont Sclair, 105 #m thickness) was exhaustively extracted to remove additives (48 h acetone Soxhlet extraction) and dried under vacuum. It has previously been shown that this film has 0.53 mol kg-1 of tert-C--H sites and that the branch groups are predominantly --C2H5. 6 Some of this extracted LLDPE film was pre-oxidized to 5, 10 or 15 Mrad total dose by exposure in an AECL Gammacell 220 (0.7 Mrad h-1 dose rate) in air. Pre-oxidized and non- oxidized films were exposed, after being sealed under vacuum in 1 cm ID borosilicate tubes, to either ),-radiation from the Gammacell, or to the radiation from a xenon arc WeatherOmeter (Atlas, 2500W, borosilicate inner and outer filters, silver panel temperature 55°C) or in a forced air oven at 85°C.
312 J. Lacoste, D. J. Carlsson, S. Falicki, D. M. Wiles
The products in the pre-oxidized films and in the films subsequently exposed under vacuum to heat, light or ),-radiation were identified and quantified by Fourier transform infrared (FTIR) spectroscopy (Perkin Elmer 1500, DTGS detector, or Nicolet 7199, MCT detector, 200 scans signal averaged in each case) or by FTIR after derivatization. Derivatization was used to improve the IR resolution and sensitivity and involved film exposure to nitric oxide or sulfur tetrafluoride, s'9 The NO treatment quantitatively converts hydroperoxide groups into nitrates which can be identified by FTIR as primary, secondary or tertiary nitrates from the corresponding hydroperoxides. Similarly, NO quantitatively converts alcohols to nitrites which can be identified by FTIR as primary, secondary or tertiary nitrites from the corresponding alcohols. The SF 4 treatment fluorinates all-OH species, converting hydroperoxide and alcohol groups to fluorides and carboxylic acid to the corresponding acid fluoride. This treatment greatly simplifies the carbonyl region by eliminating the H - - bonding - - O H and - - O O H species and also the widely spread carboxylic acid absorptions to give the sharp, intense acid fluoride, at ,-~ 1848 cm -1, well separated from the absorptions of the residual carbonyl species. The NO and SF4 treatments and peak identification have been described previously.S'9 To eliminate the interference ripples, which are often found in the IR spectra of films and which complicate the accurate measurement of IR absorbances, films were tilted at the Brewster angle and a polarizer placed in the sample beam as proposed by Harrick. 1°
Total hydroperoxide species were quantified by iodometry. 11 Vinyl (909 c m - 1), vinylidene (887 c m - 1) and
trans-vinylene
(965 c m - 1) unsatur- ation was also quantified by FTIR. Radicals generated by y-irradiation of pre-oxidized films under vacuum at - 196°C were identified and quantified by electron spin resonance (esr) spectroscopy (Varian, E4, Q-band coupled to a microcomputer for data storage and signal integration). Spectra were measured at 0.5 mW power (when neither alkyl or peroxyl radicals were found to power saturate, see below) and at 10mW when alkyl signals saturate whereas peroxyl signals do not. Radical concentrations were quantified by double integration and comparison with the signal from cupric acetyl acetonate powder dispersed in KC1. This standard was used because it obeyed the Curie Law from ambient down to - 196°C.RESULTS
The ~-initiated oxidation of polyolefins gives the simplest and most controllable mix of oxidation products. 6'7 Oxidation product con- centrations increase linearly with radiation dose (0-20 Mrad) so that a
Polyethylene hydroperoxide decomposition products 313
Q
40'00 35'00 30'dt) 1B~O0 16'00 141)0 1200 10'00 m 800
Wavenumbers (crn -1)
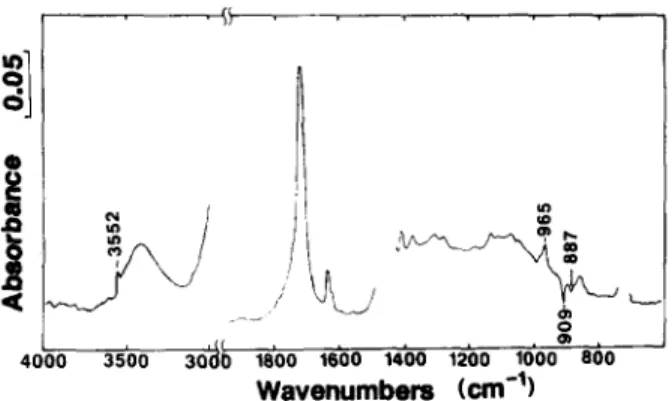
Fig. l. FTIR spectrum of oxidation products in 10Mrad irradiated LLDPE. Spectrum obtained by subtracting that of a non-oxidized from that of the ~,-irradiated sample. constant ratio of products can be achieved at progressively increasing total product levels. LLDPE films were studied after pre-oxidation at the 5-20 Mrad dose levels. All samples gave self-consistent data and only the 10 Mrad samples are discussed here. The FTIR spectrum of this stock, pre- oxidized film is shown in Fig. 1. The broad 3400 cm-1 and ~ 1715 c m - absorptions indicate the presence of various hydrogen bonded - - O H species (--OH and - - O O H ) and various carbonyl species, respectively. Sharp unsaturation peaks are visible, the negative 909 cm-~ peak resulting from vinyl loss during irradiation. The 3552cm -~ peak is unique for non- hydrogen bonded hydroperoxide groups. Oxidation products were determined in detail using the derivatization methods, as described previously, with FTIR determination of the products. 8'9 The observed product composition in this stock pre-oxidized LLDPE sample is given in Table 1. Also shown are the levels of unsaturation. Vinylidene (> C~---CH2) and vinyl ( - - C H ~ C H 2 ) and trans-vinylene ( - CH~---CH ,-~ ) levels were 7, 31 and 8mmol kg-1 respectively in the initial, non-oxidized film. The two former species decrease during 7-initiated oxidation, whereas trans-vinylene
( - - C H = C H - - ) increases. The experimental IR extinction coefficients are also shown in Table 1. 7 Iodometric analysis of total hydroperoxide agreed closely with the value from IR, quantified after conversion of all s e c - O O H
(free and hydrogen bonded) to the corresponding sec-nitrate by reaction with NO.
In order to compare initiation mechanisms, samples of pre-oxidized LLDPE film were subjected to thermal, 7- and UV-exposure in the absence of oxygen. The directly observed FTIR changes for these samples are shown in Fig. 2. Only broad details of the product changes can be identified from these data. Negative peaks, indicate loss of oxidation products. The broad 3400 cm-1 absorption from hydrogen bonded - - O H species (alcohol and hydroperoxide) and the free (non-hydrogen bonded) hydroperoxide
314 J. Lacoste, D. J. Carlsson, S. Falicki, D. M. Wiles
TABLE 1
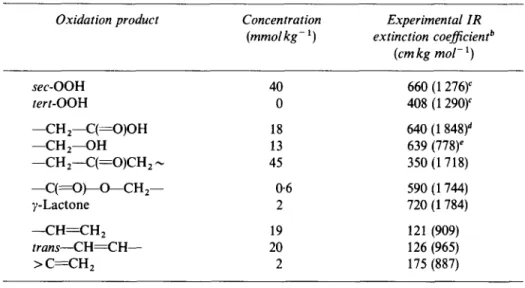
Oxidation Products and Unsaturation in Pre-y-oxidized LLDPE Stock Film"
Oxidation product Concentration Experimental IR (mmol kg- 1) extinction coefficient b
(cmkg mol- 1) sec-OOH 40 660 (1 276) c tert-OOH 0 408 (1 290) c - - C H 2 - - C ( = O ) O H 18 640 (1 848) d - - C H 2 - - O H 13 639 (778) e --CH2---C(~---O)CH 2 ~ 45 350 (1 718) - - C ( ~ O ) ~ O ~ C H : - - 0.6 590 (1 744) y-Lactone 2 720 (1 784) --CH--~-CH 2 19 121 (909) trans--CH~-CH-- 20 126 (965) > C-~-CH 2 2 175 (887)
"10 Mrad irradiation in air at ~40°C.
bAbsorption maxima (cm -1) shown in parenthesis. Values determined from model compounds.
c As nitrate after NO derivatization. dAs acid fluoride after SF4 derivatization. e As nitrite after NO derivatization.
8
i
"~ i I I 4000 35'00 30~I) 1800 (DO1 ( D o 16()0 14'00 12'00 10'00 800 Wavenumbers (crn -1)Fig. 2. FTIR changes produced by photo-, thermal- and y-induced reactions, LDDPE films pre-oxidized by 10Mrad irradiation in air. Decomposition reactions all under vacuum. Spectra obtained by subtracting of the spectrum of the initial, pre-oxidized film. (a) After 96 h thermal decomposition at 85°C; (b) after 10Mrad y-exposure; (c) after 69h xenon arc
Polyethylene hydroperoxide decomposition products 315
) o r l v a t l m ~ , Derlvatlsat _ign: NO
2000 1800 16()0 1800 16()0 1400 12()0 1000 860 600 Wavenumbers (cm -1)
Fig. 3. FTIR changes after derivatization reactions. LLDPE films pre-oxidized by 10 Mrad irradiation in air. Decomposition reactions all under vacuum. Each spectrum obtained by subtraction of the spectrum of the initial, pre-oxidized film which had been subjected to the
equivalent derivatization reaction (either NO or SF 4 exposure). Note differing absorbance
scales. (a) After 96 h thermal decomposition at 85°C; (b) after 10 Mrad v-exposure; (c) after 69 h xenon arc exposure.
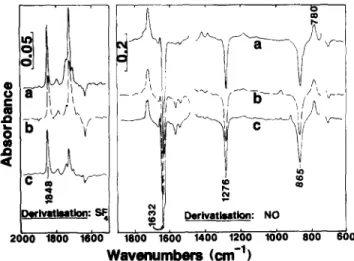
absorption at 3550cm-1 decrease in concert although the alcohol level is actually increasing (see below). Carbonyl species (,-~ 1715cm-1) and free alcohol groups (3625cm-1) increase, although in varying levels. Spectral subtraction after derivatization is much more informative and shows dramatically the loss and formation of species (Fig. 3). For NO treated samples, nitrate peaks (at 1276 and 865 c m - 1) are negative and give the loss of secondary hydroperoxide, whereas the nitrite peak (at 778cm-1) is positive and indicates sec-alcohol formation. The negative peaks at ,-~1630cm -1 are a mixture of sec-nitrate loss bands and sec-nitrite
formation bands. For SF4-treated samples, increases in acid fluoride (at 1848cm -1) from carboxylic acid end groups are distinctly visible and quantifiable together with ketone, ester and v-lactone in the (now simplified) carbonyl region. Changes in the oxidation products and unsaturation groups derived from FTIR data such as that shown in Figs 2 and 3 are collected in Figs 4-6.
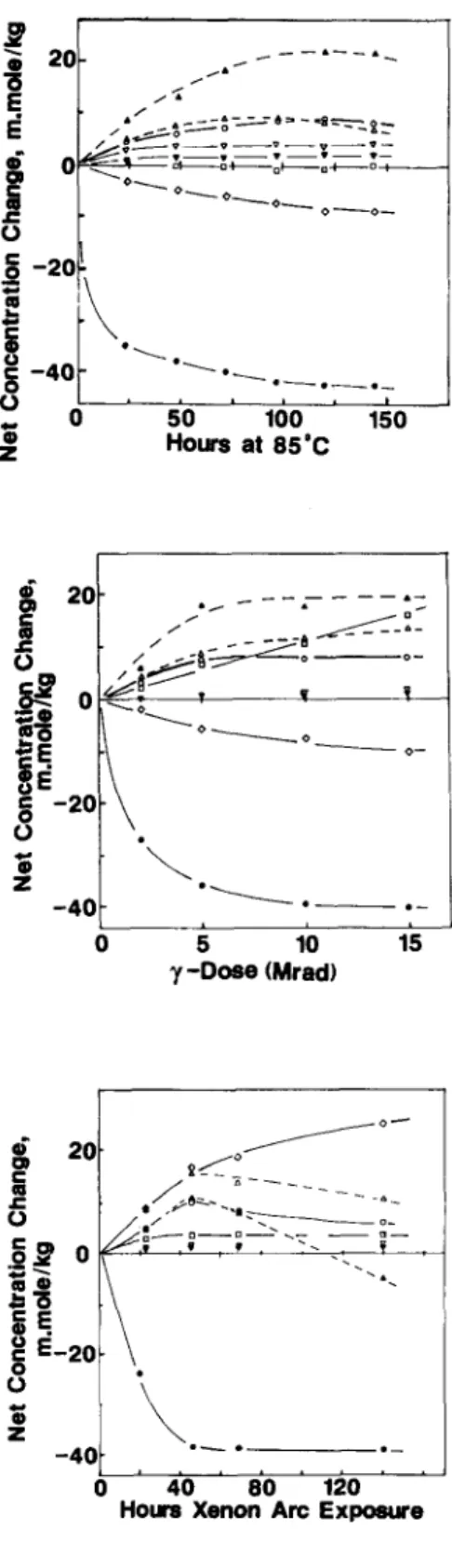
Thermal decomposition under vacuum gave the simplest changes in species (Fig. 4). Hydroperoxide was destroyed completely after --~ 100 h with the formation of backbone ketone, chain end carboxylic acid and sec
alcohol. Small increases as ester and v-lactone were also observed. Neither
trans-vinylene nor vinylidene changed in concentration whereas vinyl groups decreased to a plateau value (essentially zero concentration). All product changes appear to correlate with hydroperoxide decomposition,
316 J'. Lacoste, D. J. Carlsson, S. Falicki, D. M. Wiles
Fig. 4. Concentration changes during the thermal exposure of pre-oxidized LLDPE. Film pre-oxidized by 10 Mrad of),-irradiation in air. Initial concentrations of species shown in Table 1. Thermal exposure under vacuum at
85°C. O, sec-hydroperoxide; A, ketone; A,
sec-alcohoi; O, - - C H 2 - - C ( ~ O ) O H ; ~ , ~ H 2 - - C ( ~ O ) ~ H 2 ~ ; V, )'-lactone; ~ ,
--CH~---CH2; I--1, trans-CH-~CH. Vinylidene
concentration was essentially constant.
!
201 ~ ~ --~ , .~ i -o----o . . . o o_g -2o
o o go ' 16o ' I ; o Hours at 85°C zFig. 5. Concentration changes during the ),- irradiation of pre-oxidized LLDPE. Sample details and symbols as in Fig. 4. ),-Irradiation
at ~ 40°C on film under vacuum.
L
/ / . . . ~ . ~ ~ o v " ~ " ' ° ~ ° ~ ' ° ~ o-- 0 - 4 0 "- . - 7 - D o s e (Mrad)Fig. 6. Concentration changes during the photolysis of pre-oxidized LLDPE. Sample details and symbols as in Fig. 4. Photolysis of films under vacuum in borosilicate tubes at
55°C.
~e o ~ , ; ~ - - . - - --.,-2--~-,
\ --.-! ~:-2o \ .
"
40
i~"
i i i , J , ,0 40 80 120
Polyethylene hydroperoxide decomposition products 317
ceasing when all of the hydroperoxide groups were destroyed. Prolonged heating at 85°C beyond ~ 125 h caused a small decrease in ketone, alcohol and carboxylic acid groups possibly as a result of the slow volatilisation of fragments resulting from multiple chain scissions.
The ~-irradiation of pre-oxidized LLDPE gave a more complex range of changes (Fig. 5). Hydroperoxide groups were completely destroyed by a 10Mrad dose. Ketone, alcohol and carboxylic acid again increased as hydroperoxide disappeared, and plateaued at about 10Mrad dose. Only traces of ester and 7-1actone formed. Unsaturation showed a more complex change than found under heat exposure. The
trans-vinylene
groups increased linearly with dose, vinylidene was largely unaffected whereas vinyl decreased to a plateau value (essentially zero concentration). In a separate series of experiments, the 7-irradiation of non-oxidized LLDPE film under vacuum indicated that these unsaturation changes were the direct result of 7- induced reactions in the polymer and independent of the degree of pre- oxidation.Several oxidation products passed through clear maxima during the photolysis ofpre-oxidized LLDPE (Fig. 6). All hydroperoxide was destroyed after --~ 50 h of xenon arc exposure whereas alcohol, carboxylic acid and (especially) ketone began to decrease at this point after an initial rapid increase.
Trans-vinylene
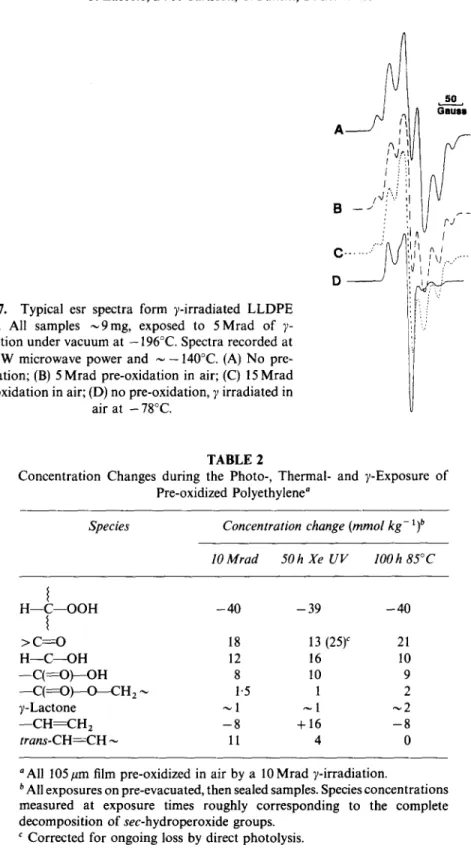
showed a small increase then plateaued whereas vinyl initially increased rapidly, then~progressively more slowly as all of the hydroperoxide was destroyed. Vinylidene was unchanged by UV exposure. For ease of comparison, the net changes in the various oxidation products and unsaturation are compared in Table 2. This comparison has been made at the point corresponding to close to complete loss of hydroperoxide for each of the decomposition mechanisms. The distinctive 2720cm -1 absorption of aldehyde was not detectable in any of the samples. In addition, peracid was not detected in any of our samples by the diazomethane derivatization reaction. 8In an attempt to establish the nature and quantities of free radical species produced by the 7-irradiation of LLDPE, esr spectra of non-oxidized and pre-oxidized films were recorded after a 5 Mrad 7-irradiation under vacuum at - 196°C. Pre-oxidized films were irradiated directly in evacuated, sealed esr tubes. Because of the strong signal in 7-irradiated borosilicate glass, esr tube ends were annealed with the (opposite) tube end containing the film samples immersed in liquid nitrogen. The films were then slid into the annealed end which had been pre-cooled in liquid nitrogen before insertion into the cold esr cavity. The esr spectra of vacuum irradiated films are shown in Fig. 7 together with the spectrum of a film (D) irradiated in air for comparison. (A spectrum identical to D resulted from admission of air to samples A, B or C at -78°C.) Signal intensities were estimated by double
318 J. Lacoste, D. J. Carlsson, S. Falicki, D. M. Wiles
Fig. 7. Typical esr spectra form ?-irradiated L L D P E films. All samples ~ 9 m g , exposed to 5 M r a d o f y- radiation under vacuum at - 1 9 6 ° C . Spectra recorded at 0-5 m W microwave power and ~ - 1 4 0 ° C . (A) N o pre- oxidation; (B) 5 Mrad pre-oxidation in air; (C) 15 M r a d pre-oxidation in air; (D) no pre-oxidation, y irradiated in
air at - 78°C. 5 0
A
I~
Gaus~
I j:. t !'! i H i H / q f"v',1
; F j ~ - C . . . " ' I [ \) ,../"' ~. I I l ! : ',, ":./ TABLE 2Concentration Changes during the Photo-, Thermal- and y-Exposure o f Pre-oxidized Polyethylene a
Species Concentration change (retool k g - 1)b lO Mrad 50h Xe UV lOOh 85°C H - - C - - O O H - 40 - 39 - 40 > C - ~ O 18 13 (25) ~ 21 H - - C - - O H 12 16 10 - - C ( ~ O ) - - O H 8 10 9 - - C ( ~ - ~ O ) - - O - - C H 2 ~ 1.5 1 2 y-Lactone ~ 1 ~ 1 ~ 2 - - C H ~ C H 2 - 8 + 16 - 8 t r a n s - C H ~ C H ~ 1 ! 4 0
a All 105 pm film pre-oxidized in air by a 10 M r a d y-irradiation.
b All exposures on pre-evacuated, then sealed samples. Species concentrations measured at exposure times roughly corresponding to the complete decomposition o f sec-hydroperoxide groups.
Polyethylene hydroperoxide decomposition products 319
integration and radical concentrations calculated by comparison with the cupric standard. All four samples shown had virtually identical total radical yields [(12+_2) x 10-3mol kg-1]. The non-oxidized film gave an esr spectrum consistent with predominantly macro-alkyl species and possibly some allyl radicals. ~2 The 0 2 exposed samples gave a signal which is predominantly attributable to peroxyl radicals (D in Fig. 7). 13 Both the 5 Mrad and 15 Mrad pre-oxidized films appeared to contain predominantly alkyl and small amounts of peroxyl species from their esr spectra. From the intensity of the small peaks in the esr wings which are exclusively from alkyl species, the 5 Mrad pre-oxidized film contained ~ 10 x 10 -3 and ~ 2 x
10-3mol kg -~ of alkyl and peroxyl radicals whereas the 15Mrad pre- oxidized film contained ~ 9 x 10- 3 and -~ 3 x 10- 3 mol kg- ~ of alkyl and peroxyl radicals.
Dole et al. have suggested that the microwave power saturation of the alkyl signal in the esr spectra of irradiated polyolefins varies with structure. At 77 K, they have reported that the upper power limit for alkyl radicals is 0.35mW in irradiated polypropylene, but only 0-063mW for poly- ethylene. ~ 2.14. In the authors' studies, the spectra of irradiated polypropylene and polyethylene both follow a linear (microwave power) 1/z dependence up to 0"5 mW and this power limit has been used in the reported work.
DISCUSSION
The photolysis reactions proposed by Gugumus for pre-oxidized LDPE (reactions (7)-(9)) can together explain the loss of hydroperoxide and the formation of vinyl, trans-vinylene, ketone and secondary alcohol shown in Fig. 6. 4,5 Indeed, the similarity between the authors' data (degradation in vacuum) and those of Gugumus implies that oxygen depletion quickly established close to anaerobic conditions within his relatively thick samples (0.5 mm). In fact, although it is not explicitly stated in Gugumus' papers that the pre-oxidized samples were xenon arc exposed subsequently in air, the steady increase in carbonyl absorption in his data (as opposed to the authors' clear maximum Fig. 3), implies that some oxidation was occurring. 4,5
Although the rearrangement reactions (7)-(9) that have been proposed by Gugumus can explain some of the authors' observed products, these reactions are novel and not supported by prior studies. Rather than using these reactions, for all three sets of decomposition data here (~-, UV and thermal) it is possible to explain reasonably all of the products by well established free-radical reactions as follows.
320 J. Lacoste, D. J. Carlsson, S. Falicki, D. M. Wiles
weak O---O link in hydroperoxides (reaction (1)). The free-radical products can then attack adjacent
sec-hydroperoxide
sites to generatesec-peroxyl
radicals (reaction (10)).
'on l
I
"O C - - H + H---C---4)OHH2OI
I
,
HO---~--H +
H---({--O 2.
[----,CH2~(10)
The self-reaction of nearby
sec-peroxyl
radicals is normally assumed to lead to equimolar quantities of alcohol and backbone ketone (reaction (4)).2 Additional alcohol groups may also result from alkoxyl radical attack on the polymer matrix. In fact, the alcohol yield from ~- and thermal exposure is only about one half of the ketone yield (Figs 4 and 5). This is consistent with "OH, alkoxy or peroxyl attack on the activatedtert-hydrogen
of thesec-alcohol,
as reported previously for C2HJvinyl alcohol copolymers(reaction (11)). 15 Some of the evolved 02 may further react to give new peroxyl radicals which cross-terminate to give ketone (reaction (12)).
H---C---OH r~di~ "C---OH o2, .O2__C___OH (11)
I
a.aok
I
I
"O2--C--OH + "O2--C--H , HO C - - O H + / C = : O + 02
H20 +/C----O
(12)
Carboxylic acid might result from reactions analogous to reaction (11) but involving abstraction of the activated
tert-hydrogen
of thesec-
hydroperoxide groups rather than reaction (10). The unstable intermediate could then fl-scission to the acid and an alkyl radical (reaction (13)). The total yield of oxygen-containing products (Table 2) accounts for only ~ 70% of the oxygen lost by hydroperoxide thermal decomposition. The balance may be evolved as 02 into the vacuum, or may be present as dialkyl peroxides or ethers for which we know of no reliable analytical method.
During the ~-induced degradation of pre-oxidized LLDPE, the ~- radiolysis of the polymer itself will provide a constant source of macroalkyl and hydrogen radicals. 12 Some of these radicals will attack oxidation products. In particular, attack on the hydroperoxide groups will give peroxyl radicals (reaction (10)) or carboxylic acid (reaction (13)). The linear generation of
trans-vinylene
groups (Fig. 5) is a consequence of the directPolyethylene hydroperoxide decomposition products 3 2 1
#
H - - C - - O O H
radical)
"C--OOH - - : - . ---.-,C\oH+"CH2~
(13)radiolysis of the polymer and/or reactions of the initial radicals (reaction (14)). 12 The loss of the initial vinyl content of the polymer also results from radical attack (reaction (14)). 12 The authors' rate of trans-vinylene formation (Fig. 5) is consistent with the reported net G-value of its formation. 12
R / ~ H = = C ), \ LLDPE ~ PE" + H" H C = C + H 2 / I H unsaturation loss (14)
The small increase in trans-vinylene groups from the photolysis of LDPE (authors' vacuum data, Fig. 3 and the thick film data of Gugumus) may also result from multiple radical attack on the polymer matrix. The rapid vinyl formation and the ketone maximum (Fig. 6) can be simply explained by sequential Norrish type II photo-cleavages of ketones (reactions (15) and (16)) following an initial burst of ketone formation associated with the w.42H2__C(=O)__CH2 - h~ -M-CH2--C(=O)CH 3 + H 2 C = C H ~ (15)
CH3--C(=O)CH 3 + H2C---CH~ (16) hydroperoxide scission/radical attack cascade (reactions (10) to (13)). Guillet
et al. have reported type II quantum yields from model ketones and product data from
CEHJCO
copolymer photolysis which are consistent with the ketone loss and vinyl formation data. ~6'17 It must be remembered that the ketone loss occurs continuously throughout the photolysis (Fig. 6) but this is initially overwhelmed by its rapid formation from hydroperoxide decomposition reactions. Ketone photolysis will also accelerate as the terminal methyl ketone (from reaction (15)) begins to dominate because of its higher quantum yield for cleavage. 17 Norrish type I cleavage will also destroy the ketone groups but is much slower than the type II process for backbone ketones. 16'17 In the early stages of photolysis when both hydroperoxide and ketone groups are present, energy transfer to hydroperoxide from the more strongly absorbing ketones may also be occurring. ~ 8322 J. Lacoste, D. J. Carlsson, S. Falicki, D. M. Wiles
Although y-interaction with matter is normally statistical, some selective decomposition of polar groups has been suggested, x9'2° However, the authors' esr data indicate that the total radical yield is independent of the degree of polymer oxidation and that there is only a small contribution from peroxyl radicals. The increasing proportion ofperoxyl to alkyl radicals with increasing pre-oxidation (Fig. 7) could imply some attack by H" on hydroperoxide sites even at liquid nitrogen temperature. Radiolytic cleavage of oxidation products appears to be very small in the authors' samples, although Guillet et al. have reported increased reactivity of C2HJCO
copolymers as compared to polyethylene and the authors have found increased peroxyl radical formation from C2HJvinyl alcohol copolymers as compared to polyethylene. 15,20
The authors' product data for the 7, photo- and thermal induced decomposition ofpre-oxidized LLDPE samples are in good agreement with the more limited IR results of Gugumus for xenon arc exposure of pre- thermally oxidized LDPE films. The main difference comes in the interpretation of the IR data, aided by the comparison of all three methods of initiation. Gugumus did not have access to derivatization reactions, and his data can be re-interpreted in several ways. First, his assumption that the 3400 cm-1 absorption is solely due to hydrogen-bonded hydroperoxide is not correct. It has previously been shown that both s e c - a l c o h o l s and sec-
hydroperoxides are produced in roughly equivalent quantities during photo-oxidation and both species contribute to the 3400cm-1 peak. 7 Secondly, vinyl unsaturation must be expected from the known photo- chemistry of ketone-containing polymers and is not a product of hydroperoxide decomposition. ~ 6,17 Thirdly, assignment of the ~ 1715 cm - absorption to ketone alone ignores the presence of carboxylic acid absorptions in this region, which are dominant products from photo- oxidation. 7 Finally, hydroperoxide scission to give radicals, hydroperoxide decomposition by radical attack and radical-radical reactions should occur. The reported absence of photo-initiation by LDPE hydroperoxide is an artefact?- 5 This resulted from a short kinetic chain length of the oxidation for the level of pre-oxidation and UV exposure conditions used. 7
From the product curves (Figs 4-6) and the data at complete sec-
hydroperoxide loss (Table 2), all hydroperoxide decomposition mechanisms lead to fairly similar product mixes. Thermal and 7-induced products are particularly alike. The product mix from photolysis is complicated by the concurrent ketone photo-cleavage. This can be compensated for approxi- mately based upon a linear extrapolation of the ketone loss after complete hydroperoxide decomposition (Fig. 6). The formation of similar quantities of carboxylic acid from all three methods implies a common mechanism based upon a radical-induced reaction. Reaction (13) is consistent with these
Polyethylene hydroperoxide decomposition products 323
data as is the cessation o f acid formation when all sec-hydroperoxide is destroyed. In addition, the authors have found that little carboxylic acid is formed from the photo- thermal- or y-induced decomposition o f pre- oxidized polypropylene where tert-hydroperoxide sites dominate.
A C K N O W L E D G M E N T S
The authors are indebted to N A T O and ICI Americas Inc. for financial support for J. L. and S. F., respectively.
This paper has been issued as N R C C # 32542.
R E F E R E N C E S
1. Amin, M. V., Scott, G. & Tillikeraine, L. M. K., Euro. Polym. J., 11 (1975) 85. 2. Decker, C., Mayo, F. R. & Richardson, H., J. Polym. Sci., Polym. Chem. Ed., 11
(1973) 2879.
3. Ginhac, J.-M., Gardette, J.-L., Arnaud, R. & Lemaire, J., Makromol. Chem., 182 (1981) 1017.
4. Gugumus, F., Die Angewandte Makromol. Chemie, 158/159 (1988) 151. 5. Gugumus, F., J. Polym. Deg. Stab., 27 (1990) 19.
6. Carlsson, D. J., Bazan, G., Chmela, S., Wiles, D. M. & Russell, K. E., J. Polym. Deg. Stab., 19 (1987) 195.
7. Lacoste, J. & Carlsson, D. J., J. Polymer Sci., Polym. Chem. Ed. (in press). 8. Carlsson, D. J., Brousseau, R., Zhang, C. & Wiles, D. M., Amer. Chem. Soc.
Syrup. Ser., 364 (1988) 376.
9. Carlsson, D. J., Brousseau, R., Zhang, C. & Wiles, D. M., J. Polym. Deg. Stab., 17 (1987) 303.
10. Harrick, N. J., Appl. Spectrosc., 31 (1977) 548.
11. Carlsson, D. J. & Wiles, D. M., Macromolecules, 2 (1969) 597. 12. Dole, D., Adv. Rad. Chem., 4 (1974) 307.
13. Hori, Y., Shimada, S. & Kashiwabara, H., Polymer, 18 (1977) 567.
14. Gvozdic, N., Basheer, R., Mehta, M. & Dole, M., J. Phys. Chem., 85 (1989) 1563. 15. Carlsson, D. J., Chmela, S. & Wiles, D. M., J. Polym. Deg. Stab., 31 (1991) 255. 16. Sitek, F., Guillet, J. E. & Heskins, M., J. Polym. Sci. Symp. Ser., 57 (1976) 343. 17. Guillet, J. E., Polymer Photophysics and Photochemistry. Cambridge University
Press, Cambridge, 1985.
18. Ng, H. C. & Guillet, J. E., Macromolecules, 18 (1985) 2299. 19. Whelan, D. J., Chem. Rev., 69 (1969) 179.