Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Organometallics, 38, 6, pp. 1186-1199, 2018-09-24
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=e04d0ab6-f6f0-4852-8fa9-a4f9e0b90021 https://publications-cnrc.canada.ca/fra/voir/objet/?id=e04d0ab6-f6f0-4852-8fa9-a4f9e0b90021
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/acs.organomet.8b00508
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Non-noble iron group (Fe, Co, Ni)-based oxide electrocatalysts for
aqueous zinc–air batteries: recent progress, challenges, and
perspectives
Yi, Jin; Liu, Xiaoyu; Liang, Pengcheng; Wu, Kai; Xu, Jie; Liu, Yuyu; Zhang,
Jiujun
Institute for Sustainable Energy/College of Sciences, Shanghai University, 99 Shangda Road, Shanghai 200444, China
‡School of Environment and Materials Engineering, Shanghai Polytechnic University, 2360 Jinhai Road, Shanghai 201209, China §Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University, Tianjin 300071, China
ABSTRACT: With the increasing energy demand, energy conversion and storage technology has garnered intense attention. As one of the competitive electrochemical energy technologies, the zinc−air battery (ZAB) has been considered to be a promising candidate due to its advantages, including low cost and environmental friendliness. To overcome its technical challenges of low activity and stability of ZAB air electrode electrocatalysts, substantial efforts have been made
to develop highly active and stable materials for improving the performance. In this paper, a comprehensive review of one type of the ZAB electrocatalyst, that is, iron group (Fe, Co, Ni)-based oxides, is provided. The discussion will be concentrated on the recent progress and challenges associated with this type of electrocatalyst as well as corresponding perspectives.
■
INTRODUCTIONWith increasing energy demand worldwide, the use of renewable energy sources has attracted tremendous attention. Subsequently, energy storage technologies, in particular, electrochemical batteries with low cost, environmental friend-liness, and high energy density, are urgently required.1−5 Because one of the reactants (O2) is obtained from the
operating environment, metal−air batteries, including lithium− air batteries and zinc−air batteries, display energy densities significantly higher than that of the state-of-the-art lithium-ion battery systems. As one typical metal−air battery, the lithium− air battery has drawn increasing attention due to its high energy density (5200 Wh kg−1 when the mass of O2 is included) and operating potential.6 However, the explosive reactivity of Li metal toward H2O leads to the requirement of employing non-aqueous electrolyte, hybrid electrolyte, or all-solid-state electrolyte, which require a strict moisture-controlled manufacturing environment and limit the further application of the lithium−air battery.7−12 Compared to the lithium−air battery, the components of the zinc−air battery (ZAB) are stable toward H2O, which indicates that the
manufacturing process of ZABs is easier and cheaper. Meanwhile, the zinc metal is abundant and environmentally benign with a stable discharge voltage.13−19 The above significant advantage enables the ZAB to be a very appealing candidate.
As illustrated inFigure 1, ZABs are mainly composed of four main components: a zinc metal as the anode, an alkaline electrolyte, an air electrode as the cathode, and a separator that employs to separate the anode and the cathode. During discharge, the Zn anode is oxidized to form ZnO, releasing
electrons to the cathode through the external circuit (eqs 1and
2); meanwhile, at the cathode, O2 from the air accepts
electrons, being reduced to oxygen-containing compounds through a four-electron route (eq 3) or a two-electron route
(eqs 4and5). The total reaction can be summarized ineq 6.
During charging, the processes reverse, whereby the metal is
Special Issue: Organometallic Electrochemistry: Redox Catalysis Going the Smart Way
Received: July 20, 2018
Figure 1.Schematic cell configurations for ZABs.
© XXXX American Chemical Society A DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX
recovered and O2is regenerated. Several reviews have provided
the basic insights on the mechanisms governing the perform-ance of ZAB.13−15,20Although many efforts have been made to improve the performance of ZABs, numerous challenges associated with the components of ZAB are required to be overcome, which strongly impede their large-scale application in energy storage devices. In terms of zinc metal anode, it usually exhibits poor cycling life, which is mainly caused by the uneven current distribution within the zinc anode, uneven reaction zones, ZnO precipitation, and hydrogen evolution reaction (HER).21−26 According to the previous studies, the challenges of zinc anodes can be concluded as dendrite, corrosion, shape change, and passivation.13,27,28In addition to the zinc anode, the separator also faces several challenges, including chemical (oxidation) resistance and penetration of the zincate ion (i.e., Zn(OH)42−). An ideal separator is expected to be porous, highly chemical (oxidation) resistant, and stable in the alkaline rechargeable battery.29,30 Although aqueous alkaline solutions (e.g., KOH, NaOH) with low viscosity and high ionic conductivity have been widely used in ZABs, the long-standing issues related to carbonate (i.e., ZnCO3) precipitation, dissolution of metallic zinc, and electrolyte evaporation are required to be addressed.20,22,31−34 Some issues are more concerning because the electrolyte used in ZABs with an open system is highly sensitive to CO2.
Furthermore, there is another urgent need, but it is still a significant challenge to rationally design and prepare high-efficiency electrocatalysts for ZABs with low cost and benign environmental impact.
+ −→ −+ e−
Zn 4OH Zn(OH)42 2 (1)
→ + +
− −
Zn(OH)42 ZnO H O2 2OH (2)
+ + e−→ − O2 H O2 4 4OH (3) or + + e−→ −+ − O2 H O2 2 HO2 OH (4) → + − − HO2 OH 1/2O2 (5) + → E=
overall: 2Zn O2 2ZnO, 1.66V vs SHE (6)
As indicated byeq 6, a theoretical voltage of 1.66 V can be calculated for a ZAB. During discharge/charge cycles, the sluggish kinetics of the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) lead to the high overpotential (η) and low-energy efficiency, which become the major limitation for ZAB performance.35,36 Thus, the development of highly efficient and cost-effective electro-catalysts for the ORR and OER of ZABs is highly desirable. Although many efforts have been made to develop bifunctional electrocatalysts to speed up the kinetics of both ORR and OER for improving the performance of ZABs, the results are still not satisfactory in terms of the catalysts’ activity and stability.35,37 In the past several decades, several kinds of electrocatalysts have been explored, such as precious metals,38,39 non-noble metal oxides,40,41carbonaceous materials,42,43and so on,44,45 whereas the non-noble metal oxides are considered as promising alternatives of precious metals because of their low cost and potential commercial applications. Among the various metal oxides, iron group (Fe, Co, Ni)-based oxide electrocatalysts have drawn intense attention due to their incompletely filled d-orbitals, providing numerous possible oxidation states, leading to the high intrinsic electrochemical
activity. In addition, the performance improvement can also be achieved through doping or combining with other materials such as carbon-based materials. In this review, the recent progress and challenges in the iron group (Fe, Co, Ni)-based oxide electrocatalysts for ZABs are presented, and the corresponding perspectives are provided for facilitating the continuing research and development in this area.
■
RECENT PROGRESS IN (FE, CO, NI)-BASED OXIDE ELECTROCATALYSTS FOR ZABSCompared to the bare metals, metal oxide electrocatalysts are considered to be more likely active toward OER in addition to the ORR. The electrocatalytic reactions can be triggered by the interaction between metal ions and oxygen intermediates, together with the formation of a M−O bond by altering the valence state (as demonstrated in eqs 7−9), which largely depend on the site geometry of metal cations.16,37Therefore, a study of metal oxide electrocatalysts employed in ZABs is of great importance, in particular, the iron group (Fe, Co, Ni)-based oxide electrocatalysts. As demonstrated in Figure 2,
three typical iron group (Fe, Co, Ni)-based oxides display similar crystal structures, which are presented as the representative space groups of Fd3̅m, Fd3̅m, and Fm3̅m for Fe2O3, Co3O4, and NiO, respectively. Subsequently, it is highly
significant to disclose the structure-dependent electrocatalytic properties of iron group (Fe, Co, Ni)-based oxides for ZABs.
− + → − − + + − − − + − − e Mm O2 OH M(m 1) O OH (7) − − + → − − + + − + − − + − − e M O OH OH M O O H O m m ( 1) 2 2 (8) − − → − + + − + − 2Mm O O2 2Mm O2 O2 (9)
Iron Oxides. Generally, noble metal/metal oxide electro-catalysts, such as platinum (Pt)-based materials and ruthenium (Ru)/iridium (Ir) oxides, are considered to be the most active catalysts for both OER or ORR. Unfortunately, the high cost of the ORR/OER electrocatalysts mentioned above have limited their large-scale application in ZABs. Recently, iron group (Fe, Co, Ni)-based oxides have been developed as appealing candidates for rechargeable ZABs because of their inherently high electrocatalytic activities toward ORR or OER. Compared to (Co, Ni)-based oxides, iron oxides (FeOx), such as Fe2O3
and Fe3O4, are more earth-abundant, cheaper, and less toxic, attracting numerous research interest for their application in fields of electrochemical research. For example, as the typical polymorph of Fe2O3, γ-Fe2O3shows a cubic crystal structure
and contains cations in the non-equivalent tetrahedral (A) and octahedral (B) positions, which can also be described as FeA(Fe5/3O1/3)BO4.46In order to enhance the electrocatalytic
Figure 2.Crystal structure of typical iron group (Fe, Co, Ni)-based
oxides (Fe2O3, Co3O4, NiO).
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX B
activity of Fe2O3, a synergistic effect between Fe2O3and other
electrocatalysts would play an important role in the improved performance. Previous works have reported that the Fe-active species in combination with N-doping carbon materials could give rise to more catalytic active sites and result in high
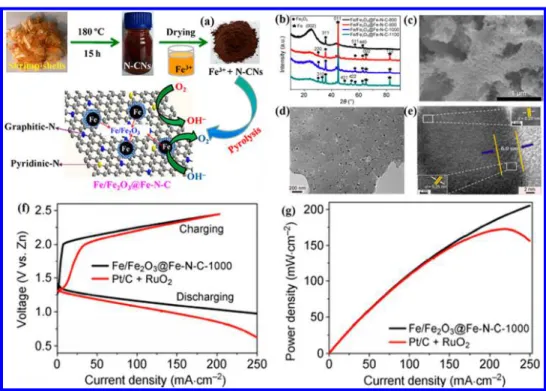
electrocatalytic activities for both ORR and OER.47−50 Accordingly, Zhao et al.51developed Fe/Fe2O3 nanoparticle-anchored Fe−N-doped carbon nanosheets (Fe/Fe2O3@Fe−
N−C) via a facile pyrolysis treatment employing shrimp-shell-derived N-doped carbon nanodots and FeCl3 as carbon,
Figure 3.(a) Schematic illustration of the preparation process for the Fe/Fe2O3@Fe−N−C composites; (b−e) XRD patterns, SEM and TEM of
Fe/Fe2O3@Fe−N−C-1000; (f,g) discharge/charge polarization curves and power density plots of rechargeable Zn−air batteries using various
electrocatalysts. Reproduced with permission from ref51. Copyright 2016 Springer.
Figure 4.(a) Schematic of the preparation process for the 3D Fe3O4/N-GAs catalyst. (b) Cyclic voltammetry curves of Fe3O4/N-GAs in N2- and
O2-staturated 0.1 M aqueous KOH electrolyte solutions. (c) Linear sweep voltammetries of Fe3O4/N-GAs in O2-saturated 0.1 M KOH at different
rotating disk electrode rotation rates. Reproduced from ref53. Copyright 2012 American Chemical Society.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX C
nitrogen, and iron sources. As illustrated in Figure 3, Fe/ Fe2O3@Fe−N−C have shown bifunctional electrocatalytic
activities toward ORR and OER in alkaline media, which can compare to the performances of commercial Pt/C for ORR and RuO2for OER. Through a facile and scalable method, the
Fe3O4 nanoparticle enchased in the wall structures of N/S-doped carbon nanotubes was prepared and displayed a largely reinforced synergistic effect, as evidenced by the achievement of high ORR performance with a four-electron selectivity. When it was used as a ZAB cathode material, the high performance can be comparable to that of commercial Pt/C, demonstrating a promising potential for its applications on future ZABs.52Compared to that of precious metals, the lower electronic conductivity of (Fe, Co, Ni)-based oxides is one reason for the depressed electrocatalytic activities, which would
be enhanced by cation substitution or combination with a conductive material, such as the 3D nitrogen-doped graphene aerogel (N-GA)-supported Fe3O4 nanoparticles (Fe3O4
/N-GAs) (Figure 4)53 and N-doped carbon-coated Fe3O4
nanorods.54,55For example, the Mn and Co codoping Fe3O4
on nitrogen-doped reduced graphene oxide (N-rGO) was used to simultaneously enhance the ORR and OER performance via a four-electron ORR process (eqs 10−12) and Bockris’s electrochemical oxide pathway (eqs 13−15). When it was used as electrocatalyst for a ZAB, the obtained performance is comparable to that of the commercial Pt/C air electrode, and the ZAB could operate steadily for 40 h at a high current density (15 mA cm−2).56
ORR process:
+ ↔ ···
2M O2 2M O2 (10)
Figure 5. (Left) Schematic image, HR-TEM of 3DOM Co3O4. (Right) Electrochemical performance of rechargeable ZABs: (a) schematic
illustration, (b) prototype in operation, (c,d) charge/discharge polarization curves and corresponding power density plots of air electrode using
3DOM Co3O4, bulk Co3O4, and Pt/C+Ir/C as bifunctional catalysts. Reproduced with permission from ref59. Copyright 2016 Wiley-VCH Verlag.
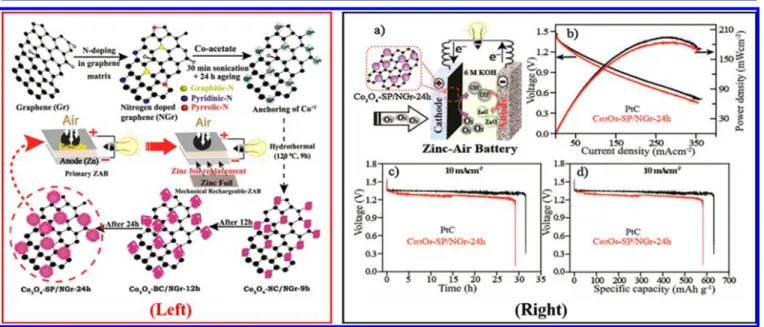
Figure 6.(Left) Schematic of synthetic routes for Co3O4−SP/NGr-24h catalyst and its application in primary and rechargeable ZABs. (Right) ZAB
performance based of Co3O4−SP/NGr-24h and Pt/C catalyst: (a) schematic representation, (b) polarization plots, (c) galvanostatic discharge
curves, and (d) specific capacity. Reproduced from ref60. Copyright 2015 American Chemical Society.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX D
··· + + e−→ + − 2M O2 2H O2 2 2MOH 2OH (11) +e−↔ + − MOH M OH (12) OER process: + −↔ +e− M OH MOH (13) + −→ + + e− MOH OH MO H O2 (14) ↔ + 2MO 2M O2 (15)
Cobalt Oxides. As the representative structure of cobalt oxides (CoOx), spinel-type CoOx are widely employed as ORR/OER electrocatalysts for ZABs.57,58 Typically, Co3O4
shows a unique cation distribution, in which the Co2+ions are
located in 1/8 of the tetrahedral sites, and the Co3+ions lie in 1/2 of the octahedral sites.37 The electrocatalytic activity of Co3O4 can be controllable by varying the composition,
structure, and morphology. Until now, a variety of preparation methods have been developed to prepare nanostructured Co3O4 with various morphologies in order to improve its electrocatalytic properties. For instance, a 3D ordered mesoporous cobalt oxide (3DOM Co3O4) synthesized by a template-derived process demonstrated improved electro-chemical durability together with highly active bifunctional performances, as shown in Figure 5.59 The various morphologies of Co3O4(nanocubic (NC), blunt cubic (BC), and spherical) were obtained through a simple hydrothermal method; subsequently, it was found that the spherical Co3O4 nanoparticle supported by the nitrogen-doped graphene (NGr) catalyst (Co3O4−SP/NGr-24h) for 24 h could display superior ORR activities. Furthermore, the ZABs based on Co3O4−SP/NGr-24h displayed a peak power density of ≈190 mW cm−2and high specific capacity of ≈590 mAh g−1, which could be comparable to that of Pt/C-based ZABs (200 mW cm−2 and 620 mAh g−1) (as displayed in Figure 6).60 In addition, a N-doping strategy is considered to be a promising solution to enhance the catalytic activity of Co3O4. For
instance, the N-doped Co3O4nanowires were prepared via a simple hydrothermal method by Yang et al.61The valence and conduction bands of Co3O4can be shifted negatively after N doping based on the results of DFT calculations, which is beneficial for improved electronic conductivity. Meanwhile, the decreased absorption energy of O2 and lengthened OO
double bond facilitate the adsorption and cleavage of O2, which are favorable for enhancing ORR activity. An onset potential of 0.94 V (vs reversible hydrogen electrode (RHE)) for ORR was achieved, and a high volumetric capacity of 98.1 mAh cm−3was obtained at 2.5 mA cm−3for the all-solid-state
ZABs on the basis of nitrogen-doped Co3O4 mesoporous
nanowire arrays. An open circuit voltage of ≈1.40 V and a stable discharge voltage over 40 h at a high current density (20 mA cm−2) were obtained for ZAB based on the composite electrocatalyst of Co@Co3O4@pyrolyzed polydopamine,
which was prepared through a hydrothermal reaction followed by a high-temperature pyrolysis and a mild heat treatment.62In addition to major advances in electronic conductivity, carbon-based materials can offer active sites and display high catalytic activity for the ORR in alkaline electrolytes. Thus, efforts have been made to combine carbon-based material with Co3O4to
form composite electrocatalysts with the expectation that they would deliver enhanced performance for ZABs through a synergistic effect between the two components. In 2011, an unexpected high ORR activity was reported by Liang et al. for a Co3O4/N-doped graphene hybrid, which exhibited not only
similar catalytic activity but also admirable stability in alkaline electrolytes. The high catalytic activity was considered to be caused from synergetic chemical coupling effects between Co3O4 and graphene.
63,64
Furthermore, owing to the high electrical conductivity of the carbon nanotube (CNT) hybrids, the CoO/CNT hybrid showed better performance than the graphene hybrid counterpart in charge transport as well as active catalytic sites.65 Therefore, several CNT-based CoOx
composites have been developed and used as electrocatalysts for ZABs, such as Co@CoOx-embedded 1D N-doped CNT (Co@CoOx/N-CNT)66 and CoO/N-CNT.67 A ZAB as-sembled with CoO/N-CNT displayed a performance com-parable to that of Pt/C. The voltages remain stable when galvanostatically discharged at 5 and 50 mA cm−2for 22 and 12 h, respectively. Specific capacity of 570 mAh g−1and energy density of 470 Wh kg−1were obtained.67Recently, the cobalt/ carbon-based electrocatalyst was shown to have bifunctional electrocatalytic activity for ZABs. For instance, a rechargeable ZAB was constructed using the Co/CoO nanoparticles immobilized on Co−N-doped carbon (Co/CoO@Co−N− C) as the electrocatalyst, which delivered comparable current densities to the ZAB using Pt/C-RuO2 as catalyst.68Doping with other metallic elements with CoOxwould also promote
the four-electron ORR pathway and have high ORR activity, such as Mn2Pb2CoOx, leading to the ZAB delivering the high
current density of 125 mA cm−2and power density of 75 mW cm−2.69 In addition, compared with monometallic oxide catalyst (i.e., Co3O4), the spinel bimetallic structures MCo2O4 (M = Zn, Ni, Mn, or Cu) have been widely
investigated as ORR/OER catalysts for ZABs owing to their abundant electrochemically active surface sites as well as enhanced conductivity.70−72As a typical reaction mechanism
Figure 7.Schematic the OER/ORR processes under the cooperation of ZnCo2O4/N-CNT. Reproduced with permission from ref70. Copyright
2016 Wiley-VCH Verlag.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX E
of the OER/ORR processes illustrated in Figure 7, it can be found that, during the OER process, more active centers of ZnCo2O4/N-CNT for OER are obtained owing to the
inherent electron cloud migration around C and Co after N doping, which offer Co with a high valence state and the reduced energy consumption from Co3+ to CoO2. The OER
process has been accelerated under the cooperation of ZnCo2O4/N-CNT. For the ORR process, the O2absorption is facilitated because of the created edges and/or defects after N doping; subsequently, a four-electron path for the ORR is provided under the synergistic effect between ZnCo2O4and N-CNT coupled with the high electron transportation rate, whereby the ORR process has been improved. Therefore, several spinel bimetallic oxide catalysts have been reported for
their intrinsic high catalytic reaction. For example, CuCo2O4 shows a better electrocatalytic activity than Co3O4, which
favors a total of 4e−for the ORR process with a higher current density.71 When the N-rGO was used as a supporter for CuCo2O4, the hybrid CuCo2O4/N-rGO composite offers higher ORR catalytic activity than CuCo2O4 or N-rGO
alone; furthermore, the hybrid composite affords more favorable durability than the commercial Pt/C electro-catalyst.73 A similar phenomenon was also observed for N-CNTs decorated by CuCo2O4quantum dots. From the results
obtained from spectroscopic characterization and electro-chemical investigations, a remarkable synergetic effect between CuCo2O4 and N-CNTs derived from a large active surface
area, high electronic conductivity, and increased number of
Figure 8.(a) Schematic illustration of formation mechanism. (b) SEM. (c,d) TEM images of NiO/Ni(OH)2mesoporous spheres. (e,f) Schematic
illustration of high-power usage of a hybrid battery for electrical vehicle applications during regenerative breaking and acceleration, respectively. (g)
Galvanodynamic charge/discharge profiles and (h) power density profile of NiO/Ni(OH)2 mesoporous spheres. Reproduced from ref 82.
Copyright 2016 American Chemical Society.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX F
catalytic sites evoked by Cu doping can be responsible for the overall electrocatalytic activities. When it was used for ZABs, an prominent discharging/charging performance, large energy density, and long lifetime were obtained for ZAB using liquid alkaline or solid-state electrolytes.74 Although these mixed metal oxides with spinel structure exhibit high catalytic capability as the bifunctional electrocatalysts, the complicated spinel structures make the investigations more complicated. The nonspinel CoxMn1−xO demonstrates high catalytic performance for its application in ZABs. Generally, the manganese and cobalt with spinel structure have multiple oxidation states (Co2+, Co3+, Mn2+, Mn3+, and Mn4+), whereas only a single oxidation state (Co2+and Mn2+) is detected in the
case of the nonspinel structure. When it was used in ZABs, a low charge/discharge voltage gap (0.57 V) and stable cycling performance were achieved at the current density of 7 mA cm−2. The much improved performance metrics of N-CNT/ CoxMn1−xO can be comparable to state-of-the-art Pt/C or Pt/
C+IrO2noble metal electrocatalysts.75
Nickel Oxides. As a resourceful and indispensable semiconductor oxide, nickel oxides (NiOx) have been extensively used in various fields, such as batteries,76 supercapacitors,77 electrocatalysis,78 and so on. For example, NiO shows electrocatalytic activity for the formation of the OH−ion.79,80The porous NiO/CoN interface nanowire arrays were developed by Yin et al., which offered not only oxygen vacancies but also a solidly interconnected interface between NiO and CoN regions, and improved electrocatalytic perform-ance and stability were observed for both OER and ORR. The ZABs using the NiO/CoN nanowire arrays showed an open circuit potential of 1.335 V and good cycling performance, which powered a timer to operate sustainably for more than 12 h.40 A N-CNT supported CoO−NiO−NiCo nanocomposite (N-CNT/CoO-NiO-NiCo) bifunctional electrocatalyst was prepared by Liu et al., which displayed both prominent activity and stability for ORR/OER in alkaline electrolyte. Moreover, the primary and rechargeable ZABs based on the N-CNT/ CoO-NiO-NiCo bifunctional electrocatalyst exhibited remark-able performance, which can compare to state-of-the-art Pt/C or Pt/C+IrO2electrocatalysts.81In addition, a hybrid battery
combining Ni−Zn and Zn−air reactions employing NiO/ Ni(OH)2mesoporous spheres as the active material illustrated the high power density (volumetric = 14 000 W L−1; gravimetric = 2700 W kg−1) and energy density (980 Wh kg−1), as illustrated inFigure 8.82
Hybrid Iron Group (Fe, Co, Ni)-Based Oxides. In order to tune the electronic and/or surface structures of the (Fe, Co, Ni)-based oxides, the deliberate introduction of other metal ions in the oxides lattice would improve their electrocatalytic activities. Therefore, hybrid metal oxides have attracted increasing attention when they are explored as bifunctional electrocatalysts for the ORR and OER due to the combination of the advantages of each active site.83 Mixed-valence spinel-type oxides with a general formula AB2O4(A, B = metal) and a
space group of Fd3m have attracted extensive interest because of their potential as bifunctional electrocatalysts.84,85Zn-, Ni-, Cu- and Mn-substituted Co3O4display the advantages of high activity and stability as electrocatalysts for the ORR.86−89 Further research has demonstrated that NiCo2O4 shows
superior electrocatalytic activity toward the ORR among the spinel oxides NiCo2O4, NiMn2O4, ZnCo2O4, and ZnMn2O4, which can be attributed to the differences in the structure and composition of spinel oxides listed above.86−88 As a typical mixed-valence oxide with spinel structure, the nickel in NiCo2O4 occupies the octahedral sites, whereas cobalt occupies both the octahedral and tetrahedral sites. The solid-state redox couples (Co3+/Co2+, Ni3+/Ni2+) in NiCo2O4
enable its high electrocatalytic activity.90 With increasing attention, NiCo2O4 with various morphologies was designed and prepared. For example, the hierarchical nanostructured 1D-spinel NiCo2O4 displayed an excellent electrocatalytic
activity toward ORR/OER in the aqueous alkaline electrolyte. The extraordinary bifunctional catalytic activity was obtained from the low overpotential (0.84 V), which was superior to that of noble metal catalysts (Pt/C (1.16 V), Ru/C (1.01 V), and Ir/C (0.92 V)) and demonstrated a great advantage as a promising cathode candidate for metal−air batteries.91 Addi-tionally, although the spinel oxides display poor electrical conductivity, the number of charge carriers in the conduction band can be increased with a concomitant reduction in the
Figure 9.(Left) SEM and TEM images of NiCo2O4-G. (Right) electrochemical performance of NiCo2O4-G: (a) ORR polarization curves in O2
-saturated 0.1 M KOH at various rotation rates, (b−d) ORR polarization curves of NiCo2O4-G, Co3O4-G, 20 wt % Pt/C, NiCo2O4, NiCo2O4-G-2 at
a rotation rate of 900 rpm. Reproduced with permission from ref92. Copyright 2013 Royal Society of Chemistry.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX G
resistivity on the conditions of the presence of defects and phase separation. The overall conductivity of the catalyst can be improved by employing the conducting supports, as well. A hybrid NiCo2O4 and graphene (NiCo2O4-G) were prepared
through a one-pot synthesis process, which could be used as an active bifunctional electrocatalyst for both ORR and OER.92 As evidenced in Figure 9, the homogeneously distributed meso-sized pores in the NiCo2O4 nanoplatelets are obtained,
providing more active sites and faster transport of reactant species during electrocatalysis owing to the increased surface area. On the other hand, the introduction of a Ni atom into the spinel lattice could enhance the electrical conductivity of metal oxide and increase the number of catalytically active sites, which significantly improved both ORR and OER perform-ances of Co3O4. Moreover, as displayed in Figure 10, by
employing a NiCo2O4−CNT hybrid as a cathode catalyst, the obtained practical primary and electrochemically rechargeable ZABs could deliver an appealing discharge peak power density (320 mW cm−2) and a high current density of 210 mA cm−2at 1.0 V.93The impressive performance contributed to the hybrid effect (coupling effect) between NiCo2O4 and CNTs, which
could produce a synergistic enhancement for both catalytic ORR and OER. Furthermore, MnO2/Co3O494,95 and NiO/
Co3O4
80
hybrid nanomaterials could also show good electro-catalytic activity for ORR/OER as well as cyclic stability during cycling.
In addition to spinel-type oxides, the metal oxides with a perovskite structure have also received tremendous attention as an efficient electrocatalyst for ZABs owing to their low cost and high ORR and OER activity, such as lanthanum-based perovskites (LaFeO3,96 LaCoO3,97 LaNiO397,98). The ORR and OER activity of perovskites can be affected by their crystal structure. For instance, LaNiO3−δ perovskite with cubic
structure shows bifunctional electrocatalytic activity for ORR/OER higher than that with rhombohedral structure, which can be prepared at different calcination temperatures followed by quenching treatment. The elongated Ni−O bond
length in cubic perovskite is considered to enhance the ORR and OER activity, which would attract new research interest for disclosing some fundamental challenges and improving the properties of perovskites.99Moreover, the large-scale applica-tion of the perovskite oxides in ZABs still faces considerable challenges. For example, the low electronic conductivity of some of the perovskite oxides has limited their further development. As a widely used method, the employment of carbon materials as a support is used to prepare perovskite oxide-based composites with enhanced electronic conductive as efficient electrocatalyst for ZABs, such as LaNiO3/CNTs,100
LaCo1−xMnxO3/CNT,
101
and LaMn0.9Co0.1O3-graphene.
102
On the other hand, the control of ionic and electronic conductivities through the doping method is considered as a promising strategy. For example, the composite with diameters of ∼200 ± 20 nm was constituted by perovskite LaTi0.65Fe0.35O3−δ(LTFO) nanoparticles (∼10 nm) entangling
both at the surface and within the nitrogen-doped carbon nanorods (NCNR), which was prepared by the electrospinning technique and carbonization under argon. Due to the excellent properties, including high surface area and highly active LTFO centers on NCNR with high nitrogen functional groups, the LTFO/carbon (LTFO-C) as a bifunctional catalyst delivers the ORR onset potential at about 0.92 V, which is∼60 and 80 mV more positive than that of NCNR and the bare LTFO (LTFO-B). A direct four-electron process has been confirmed during ORR for LTFO-C. When it was used for ZABs, significantly reduced overpotentials over 60 cycles compared to those for LTFO-B and NCNR at 5 A g−1 in 10 min interval cycles were obtained, where the potential gap increased slightly from 0.57 to 0.83 V after 60 cycles. Furthermore, the stability of different electrocatalysts was compared to the catalytic activity of prepared and 50th cycled electrodes by the galvanodynamic method at different current rates. The obtained results demonstrate the excellent rechargeability for LTFO-C, whereas after 50 cycles, both NCNR and Pt/C display obvious potential degradation, as shown in Figure
Figure 10.(Left) TEM images and FFT pattern of NiCo2O4−CNTs. (Right) Electrochemical performance of Zn−air batteries using NiCo2O4−
CNTs as the bifunctional catalyst: (a) charge/discharge polarization curves, (b,c) cycling data at 10 and 50 mA cm−2in short cycle periods, (d)
cycling data at 30 mA cm−2in 8 h per cycle. Reproduced with permission from ref93. Copyright 2016 Elsevier.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX H
11.103 On the other hand, a series of LaCo0.97O3−δ with
systematic Ni in place of Co octahedral sites were prepared by Vignesh et al., through which the electrocatalytic activity has been greatly altered. The optimized La(Co0.71Ni0.25)0.96O3−δ
composition delivered the lowest OER overpotential of 324 and 265 mV at 10 mA cm−2in 0.1 and 1 M KOH, respectively, which was lower than that of the noble-metal-based electro-catalysts, such as IrO2, Ru/C, and Pt/C. The La-(Co0.71Ni0.25)0.96O3−δ with intrinsic structure, interconnected
particle arrangement, and unique redox characteristics would be responsible for its superior oxygen evolution activity. When it was used in ZABs, an overpotential of 0.529 and 0.878 V during thefirst cycle was obtained for La(Co0.71Ni0.25)0.96O3−δ
and LaCo0.97O3−δ, respectively. What is more, a small potential gap of 0.792 V for La(Co0.71Ni0.25)0.96O3−δcan compare with
20 wt % Pt/C catalyst.104 Generally, perovskite has a general formula of ABO3. Except for partially substituting a cation B′ for B, the ionic defects or changes in the catalytically active metal A can be induced by partial substitution. For example, the substitution of Ca2+ for La3+ has been achieved for
La1−xCaxCoO3, which increases the oxygen vacancy
concen-tration and oxygen ion conductivity. As a promising bifunc-tional catalyst, the 0.89 V of potential difference between charge and discharge was obtained.105
■
CHALLENGES AND PERSPECTIVESAs competitive candidates for future energy storage systems, ZABs have attracted intense attention. As one of the indispensable components, the performance of ZABs is highly dependent on the activities of air electrode electrocatalysts. Although noble metal/metal oxide electrocatalysts can display high electrocatalytic activity for OER or ORR, their promising application in ZABs is restricted by their high expense and rarity. In order to reduce cost and increase the possibility of application for rechargeable ZABs, much work has been done to develop bifunctional metal oxide catalysts for their abundance of oxidation states and structures as well as favorable cost, as displayed in Table 1. Despite this, development of iron group (Fe, Co, Ni)-based oxide catalysts for ZABs still faces numerous challenges, as summarized in
Figure 11.(a) SEM and (b) TEM of LTFO-C. (c) Charge/discharge profiles of NCNR, LTFO-B, and LTFO-C at 5A g−1in 10 min interval cycles.
(d) Corresponding potential gap vs cycle number. The typical charge/discharge profiles of air electrodes before cycling and after the 50th cycling:
(e) NCNR, (f) LTFO-C, and (g) Pt/C. Reproduced with permission from ref103. Copyright 2015 Elsevier.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX I
Table 1. Summary of the Performance of (Fe, Co, Ni)-Based oxide Electrocatalysts for ZABs a catalysts ORR performance OER performance Δ E (V) current density cycling condition ref iron oxides Fe/Fe 2 O3 @Fe − N − C Ej=3 = − 0.18 V vs Ag/AgCl Ej=10 = 0.69 V vs Ag/AgCl 0.87 51 Fe 3 O4 /N-GAs Eonset = − 0.19 V vs Ag/AgCl 53 NCC@Fe 3 O4 Eonset = 0.024 V vs Hg/HgO 106 cobalt oxides 3DOM Co 3 O4 Eonset = − 0.197 V vs SCE Eonset = 0.7 V vs SCE 0.897 10 mA cm − 2 2 h per cycle for >400 h 59 Co 3 O4 nanowire Eonset = 0.94 V vs RHE 12.5 mA cm − 2 80 min per cycle for >28 h 61 Co 3 O4 @N-CNT/SS Eonset = 0.95 V vs RHE Eonset = 1.49 V vs RHE 0.54 25 mA cm − 2 20 min per cycle for >500 h 107 Co 3 O4 − SP/NGr-24h Eonset = 0.03 V vs Hg/HgO 60 nickel oxides NiO/CoN Ej=3 = 0.67 V vs RHE Ej=10 = 1.55 V vs RHE 0.88 3 mA cm − 2 10 min per cycle for >500 min 40 N-CNT/NiO-Ni Eonset = 0.75 V vs RHE 20 mA cm − 2 10 min per cycle for >100 cycles 81 hybrid (Fe, Co, Ni)-based oxides 1D NiCo 2 O4 Ej=3 = 0.78 V vs RHE Ej=10 = 1.62 V vs RHE 0.84 20 mA cm − 2 40 min per cycle for >2000 min 91 NiCo 2 O4 -G Eonset = − 0.12 V vs SCE Eonset = 0.55 V vs SCE 0.67 92 MnO 2 − Co 3 O4 /NiF Eonset = 0.95 V vs RHE Eonset = 1.59 V vs RHE 0.64 1 mA cm − 2 4 h per cycle for >400 h 95 MnO 2 /Co 3 O4 Eonset = 1.05 V vs RHE 15 mA cm − 2 14 min per cycle for >60 cycles 94 Co 3 O4 /MnO 2 − CNTs Eonset = 0.958 V vs RHE Eonset = 1.454 V vs RHE 0.496 10 mA cm − 2 10 min per cycle for >198 h 108 NiCo 2 O4 − CNTs Eonset = 0.934 V vs RHE Eonset = 1.43 V vs RHE 0.496 10 mA cm − 2 10 min per cycle for >40 h 93 LaCoO 3 fiber Eonset = − 0.145 V vs SCE Eonset = 0.693 V vs SCE 0.838 97 LaNiO 3 /graphene Eonset = − 0.185 V vs Hg/HgO 25 mA cm − 2 1 h per cycle for >97 cycles 98 LaTi 0.65 Fe0.35 O3‑ δ Eonset = 0.92 V vs RHE 5000 mA g − 1 1 h per cycle for >6 cycles 103 LaCo 1− x Mn x O3 /CNT Eonset = − 0.10 V vs Ag/AgCl Eonset = 0.58 V vs Ag/AgCl 0.68 2.5/1.25 mA cm − 2for discharge/charge 12 h per cycle for >34 cycles 101 a Stainless steel, SS; N-doped carbon-coated, NCC; EOER − EORR ,Δ E . DOI:10.1021/acs.organomet.8b00508 Organometallics XXXX, XXX, XXX−XXX J
conductive materials as support, such as carbonaceous materials, with iron group (Fe, Co, Ni)-based oxide electro-catalysts, allows the improved electrochemical properties. However, the unavoidable carbon corrosions often take place during the ORR with the formation of HO2−through the two-electron route (eq 4) and the OER under high oxidation potentials, which further leads to the aggregation of the catalysts and blocks the active sites by the formed carbonates. The stability of composite electrocatalysts cannot be non-negligible, which is expected to withstand long cycling time before requiring substitution. Furthermore, more investigations are expected to clarify the possible reasons that result in the failure of composite electrocatalysts in durability. Meanwhile, with the aim to avoid the use of carbon materials, the employment of other carbon-free conductive materials would enhance the stability of electrocatalysts during long operation life. The introduction of vacancies and the insertion of dopants can also be applied to tune the electronic structure of (Fe, Co, Ni)-based oxides and improve their stability and electro-catalytic active for both the ORR and OER. In addition, with the aim to facilitate a four-electron route reaction and the formation of the desired OH− species, how to achieve the selective catalytic reaction via a bifunctional catalyst with a high selectivity is urgent. Several aspects of the catalyst structure have also been considered to affect activity and selectivity of (Fe, Co, Ni)-based oxides for ORR and OER, including composition, lattice structure, active catalytic sites, particle size, porosity, and morphology. For example, the surface area to volume ratio of the catalyst would be a key point that determines the accessible catalytic active sites for reactions; therefore, the (Fe, Co, Ni)-based oxides with high surface areas and large porosity are suggested to be designed and prepared to provide more accessible active sites and offer large space to accumulate the products. Consequently, novel preparation methods are required to tunnel the morphology and structure of (Fe, Co, Ni)-based oxides. Moreover, the mechanism for ORR/OER at the (Fe, Co, Ni)-based oxide active sites is still not fully clear yet, and employment of technologies such as density functional theory and in situ characterization technique could provide a comprehensive understanding. Last but not least, the structure of the air electrode using (Fe, Co, Ni)-based oxides as electrocatalysts also plays a critical role in the performance improvement of
*E-mail:xyliu@sspu.edu.cn. *E-mail:liuyuyu@shu.edu.cn. *E-mail:jiujun.zhang@i.shu.edu.cn. ORCID Jin Yi: 0000-0001-6203-1281 Jiujun Zhang:0000-0002-6858-4060 Notes
The authors declare no competingfinancial interest.
■
ACKNOWLEDGMENTSWe are grateful for the partial financial support from the Shanghai Pujiang Program (18PJ1403800), Shanghai Sailing Program (17YF1406500), Shanghai University Young Teacher Training Program (ZZegd16005), Young Eastern Scholar Program of the Shanghai Municipal Education Commission, and 111 Project, B12015.
■
REFERENCES(1) Parker, J. F.; Chervin, C. N.; Pala, I. R.; Machler, M.; Burz, M. F.; Long, J. W.; Rolison, D. R. Rechargeable nickel-3D zinc batteries: An
energy-dense, safer alternative to lithium-ion. Science 2017, 356, 415−
418.
(2) Bruce, P. G.; Freunberger, S. A.; Hardwick, L. J.; Tarascon, J.-M.
Li-O2and Li-S batteries with high energy storage. Nat. Mater. 2012,
11, 19−29.
(3) Guo, S.; Yi, J.; Sun, Y.; Zhou, H. Recent advances in titanium-based electrode materials for stationary sodium-ion batteries. Energy
Environ. Sci. 2016, 9, 2978−3006.
(4) Yi, J.; Li, X.; Hu, S.; Li, W.; Zhou, L.; Xu, M.; Lei, J.; Hao, L. Preparation of hierarchical porous carbon and its rate performance as
anode of lithium ion battery. J. Power Sources 2011, 196, 6670−6675.
(5) Su, H.; Jaffer, S.; Yu, H. Transition metal oxides for sodium-ion
batteries. Energy Storage Mater. 2016, 5, 116−131.
(6) Lee, J.-S.; Tai Kim, S.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K. T.;
Cho, J. Metal−Air Batteries with High Energy Density: Li−Air versus
Zn−Air. Adv. Energy Mater. 2011, 1, 34−50.
(7) Li, H.; Wang, Y.; Na, H.; Liu, H.; Zhou, H. Rechargeable Ni-Li Battery Integrated Aqueous/Nonaqueous System. J. Am. Chem. Soc.
2009, 131, 15098−15099.
(8) Zhou, H.; Wang, Y.; Li, H.; He, P. The Development of a New Type of Rechargeable Batteries Based on Hybrid Electrolytes.
ChemSusChem 2010, 3, 1009−1019.
(9) Wang, Y.; He, P.; Zhou, H. A lithium-air capacitor-battery based
on a hybrid electrolyte. Energy Environ. Sci. 2011, 4, 4994−4999.
(10) Li, F. J.; Kitaura, H.; Zhou, H. S. The pursuit of rechargeable
solid-state Li-air batteries. Energy Environ. Sci. 2013, 6, 2302−2311.
(11) Li, F.; Zhang, T.; Zhou, H. Challenges of non-aqueous Li-O2
batteries: electrolytes, catalysts, and anodes. Energy Environ. Sci. 2013,
6, 1125−1141.
(12) Yi, J.; Guo, S.; He, P.; Zhou, H. Status and prospects of
polymer electrolytes for solid-state Li-O2 (air) batteries. Energy
Environ. Sci. 2017, 10, 860−884.
Figure 12.Schematic of challenges for the development of iron group
(Fe, Co, Ni)-based oxide electrocatalysts.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX K
(13) Fu, J.; Cano, Z. P.; Park, M. G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685.
(14) Gu, P.; Zheng, M. B.; Zhao, Q. X.; Xiao, X.; Xue, H. G.; Pang, H. Rechargeable zinc-air batteries: a promising way to green energy. J.
Mater. Chem. A 2017, 5, 7651−7666.
(15) Tan, P.; Chen, B.; Xu, H.; Zhang, H.; Cai, W.; Ni, M.; Liu, M.; Shao, Z. Flexible Zn- and Li-air batteries: recent advances, challenges, and future perspectives. Energy Environ. Sci. 2017, 10, 2056−2080.
(16) Wang, Z. L.; Xu, D.; Xu, J. J.; Zhang, X. B. Oxygen electrocatalysts in metal-air batteries: from aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746−7786.
(17) Wu, S.; Yi, J.; Zhu, K.; Bai, S.; Liu, Y.; Qiao, Y.; Ishida, M.;
Zhou, H. A Super-Hydrophobic Quasi-Solid Electrolyte for Li-O2
Battery with Improved Safety and Cycle Life in Humid Atmosphere. Adv. Energy Mater. 2017, 7, 1601759.
(18) Yi, J.; Liu, Y.; Qiao, Y.; He, P.; Zhou, H. Boosting the Cycle
Life of Li−O2 Batteries at Elevated Temperature by Employing a
Hybrid Polymer−Ceramic Solid Electrolyte. ACS Energy Lett. 2017, 2,
1378−1384.
(19) Luo, W.-B.; Gao, X.-W.; Chou, S.-L.; Wang, J.-Z.; Liu, H.-K.
Porous AgPd−Pd Composite Nanotubes as Highly Efficient
Electro-catalysts for Lithium−Oxygen Batteries. Adv. Mater. 2015, 27, 6862− 6869.
(20) Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257−5275.
(21) McBreen, J. Zinc Electrode Shape Change in Secondary Cells. J.
Electrochem. Soc. 1972, 119, 1620−1628.
(22) McLarnon, F. R.; Cairns, E. J. The Secondary Alkaline Zinc Electrode. J. Electrochem. Soc. 1991, 138, 645−664.
(23) Sunu, W. G.; Bennion, D. N. Transient and Failure Analyses of
the Porous Zinc Electrode. J. Electrochem. Soc. 1980, 127, 2007−2016.
(24) Nichols, J. T.; McLarnon, F. R.; Cairns, E. J. Zinc Electrode Cycle-Life Performance In Alkaline Electrolytes Having Reduced Zinc
Species Solubility. Chem. Eng. Commun. 1985, 37, 355−379.
(25) Wang, K.; Pei, P.; Ma, Z.; Xu, H.; Li, P.; Wang, X. Morphology control of zinc regeneration for zinc−air fuel cell and battery. J. Power
Sources 2014, 271, 65−75.
(26) Stamm, J.; Varzi, A.; Latz, A.; Horstmann, B. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sources 2017, 360, 136−149.
(27) Chen, L. N.; Yan, M. Y.; Mei, Z. W.; Mai, L. Q. Research Progress and Prospect of Aqueous Zinc Ion Battery. Wuji Cailiao
Xuebao 2017, 32, 225−234.
(28) Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc−air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315−324.
(29) Saputra, H.; Othman, R.; Sutjipto, A. G. E.; Muhida, R. MCM-41 as a new separator material for electrochemical cell: Application in zinc−air system. J. Membr. Sci. 2011, 367, 152−157.
(30) Hwang, H. J.; Chi, W. S.; Kwon, O.; Lee, J. G.; Kim, J. H.; Shul, Y. G. Selective Ion Transporting Polymerized Ionic Liquid Membrane Separator for Enhancing Cycle Stability and Durability in Secondary Zinc-Air Battery Systems. ACS Appl. Mater. Interfaces 2016, 8,
26298−26308.
(31) Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc-air batteries: a promising way to green energy. J.
Mater. Chem. A 2017, 5, 7651−7666.
(32) Mainar, A. R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Alberto Blazquez, J. Alkaline aqueous electrolytes for secondary zinc-air batteries: an overview. Int. J. Energy Res. 2016, 40, 1032−1049.
(33) Wruck, W. J.; Reichman, B.; Bullock, K. R.; Kao, W. H.
Rechargeable Zn-MnO2Alkaline Batteries. J. Electrochem. Soc. 1991,
138, 3560−3567.
(34) Lee, S. H.; Park, D. J.; Yang, W. G.; Ryu, K. S. Comparison of electrochemical performance for zinc anode via various electrolytes and conducting agents in Zn-air secondary batteries. Ionics 2017, 23,
1801−1809.
(35) Cai, X.; Lai, L.; Lin, J.; Shen, Z. Recent advances in air electrodes for Zn−air batteries: electrocatalysis and structural design.
Mater. Horiz. 2017, 4, 945−976.
(36) Neburchilov, V.; Wang, H.; Martin, J. J.; Qu, W. A review on air cathodes for zinc−air fuel cells. J. Power Sources 2010, 195, 1271− 1291.
(37) Yang, D.; Zhang, L.; Yan, X.; Yao, X. Recent Progress in Oxygen Electrocatalysts for Zinc-Air Batteries. Small Methods 2017, 1, 1700209.
(38) Cui, Z.; Fu, G.; Li, Y.; Goodenough, J. B. Ni3FeN-Supported
Fe3Pt Intermetallic Nanoalloy as a High-Performance Bifunctional
Catalyst for Metal-Air Batteries. Angew. Chem., Int. Ed. 2017, 56, 9901−9905.
(39) Cui, Z.; Chen, H.; Zhao, M.; DiSalvo, F. J. High-Performance Pd3Pb Intermetallic Catalyst for Electrochemical Oxygen Reduction. Nano Lett. 2016, 16, 2560−2566.
(40) Yin, J.; Li, Y.; Lv, F.; Fan, Q.; Zhao, Y. Q.; Zhang, Q.; Wang, W.; Cheng, F.; Xi, P.; Guo, S. NiO/CoN Porous Nanowires as Efficient Bifunctional Catalysts for Zn-Air Batteries. ACS Nano 2017,
11, 2275−2283.
(41) Lee, J.-S.; Nam, G.; Sun, J.; Higashi, S.; Lee, H.-W.; Lee, S.; Chen, W.; Cui, Y.; Cho, J. Composites of a Prussian Blue Analogue and Gelatin-Derived Nitrogen-Doped Carbon-Supported Porous Spinel Oxides as Electrocatalysts for a Zn−Air Battery. Adv. Energy Mater. 2016, 6, 1601052.
(42) Xu, Y.; Zhang, Y.; Guo, Z.; Ren, J.; Wang, Y.; Peng, H. Flexible, Stretchable, and Rechargeable Fiber-Shaped Zinc-Air Battery Based on Cross-Stacked Carbon Nanotube Sheets. Angew. Chem., Int. Ed. 2015, 54, 15390−15394.
(43) Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N,P-Codoped Carbon Networks as Efficient Metal-free Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Angew. Chem., Int. Ed. 2016, 55, 2230−2234.
(44) Wang, H. F.; Tang, C.; Wang, B.; Li, B. Q.; Zhang, Q. Bifunctional Transition Metal Hydroxysulfides: Room-Temperature Sulfurization and Their Applications in Zn−Air Batteries. Adv. Mater. 2017, 29, 1702327.
(45) Wei, L.; Karahan, H. E.; Zhai, S.; Liu, H.; Chen, X.; Zhou, Z.; Lei, Y.; Liu, Z.; Chen, Y. Amorphous Bimetallic Oxide−Graphene Hybrids as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn−Air Batteries. Adv. Mater. 2017, 29, 1701410.
(46) Machala, L.; Tuček, J.; Zbořil, R. Polymorphous Trans-formations of Nanometric Iron(III) Oxide: A Review. Chem. Mater. 2011, 23, 3255−3272.
(47) Wu, Z.-Y.; Xu, X.-X.; Hu, B.-C.; Liang, H.-W.; Lin, Y.; Chen, L.-F.; Yu, S.-H. Iron Carbide Nanoparticles Encapsulated in Mesoporous Fe-N-Doped Carbon Nanofibers for Efficient Electrocatalysis. Angew.
Chem., Int. Ed. 2015, 54, 8179−8183.
(48) Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Deng, J.; Sun, G.; Bao, X. Iron Encapsulated within Pod-like Carbon Nanotubes for Oxygen Reduction Reaction. Angew. Chem., Int. Ed. 2013, 52, 371−375.
(49) Wang, J.; Wu, H.; Gao, D.; Miao, S.; Wang, G.; Bao, X. High-density iron nanoparticles encapsulated within nitrogen-doped carbon nanoshell as efficient oxygen electrocatalyst for zinc−air battery. Nano Energy 2015, 13, 387−396.
(50) Jia, Q.; Ramaswamy, N.; Hafiz, H.; Tylus, U.; Strickland, K.; Wu, G.; Barbiellini, B.; Bansil, A.; Holby, E. F.; Zelenay, P.; Mukerjee, S. Experimental Observation of Redox-Induced Fe−N Switching Behavior as a Determinant Role for Oxygen Reduction Activity. ACS Nano 2015, 9, 12496−12505.
(51) Zang, Y.; Zhang, H.; Zhang, X.; Liu, R.; Liu, S.; Wang, G.;
Zhang, Y.; Zhao, H. Fe/Fe2O3nanoparticles anchored on Fe-N-doped
carbon nanosheets as bifunctional oxygen electrocatalysts for
rechargeable zinc-air batteries. Nano Res. 2016, 9, 2123−2137.
(52) Zeng, S.; Lyu, F.; Nie, H.; Zhan, Y.; Bian, H.; Tian, Y.; Li, Z.; Wang, A.; Lu, J.; Li, Y. Y. Facile fabrication of N/S-doped carbon
nanotubes with Fe3O4 nanocrystals enchased for lasting synergy as
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX L
efficient electrocatalyst for ORR. Nano Energy 2017, 40, 462−470. (56) Zhan, Y.; Xu, C.; Lu, M.; Liu, Z.; Lee, J. Y. Mn and Co
co-substituted Fe3O4nanoparticles on nitrogen-doped reduced graphene
oxide for oxygen electrocatalysis in alkaline solution. J. Mater. Chem. A 2014, 2, 16217−16223.
(57) Wang, X.; Wang, F.; Wang, L.; Li, M.; Wang, Y.; Chen, B.; Zhu, Y.; Fu, L.; Zha, L.; Zhang, L.; Wu, Y.; Huang, W. An Aqueous
Rechargeable Zn//Co3O4 Battery with High Energy Density and
Good Cycling Behavior. Adv. Mater. 2016, 28, 4904−4911.
(58) Lee, D. U.; Choi, J.-Y.; Feng, K.; Park, H. W.; Chen, Z. Advanced Extremely Durable 3D Bifunctional Air Electrodes for Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2014, 4, 1301389. (59) Park, M. G.; Lee, D. U.; Seo, M. H.; Cano, Z. P.; Chen, Z. 3D Ordered Mesoporous Bifunctional Oxygen Catalyst for Electrically
Rechargeable Zinc−Air Batteries. Small 2016, 12, 2707−2714.
(60) Singh, S. K.; Dhavale, V. M.; Kurungot, S. Surface-Tuned
Co3O4Nanoparticles Dispersed on Nitrogen-Doped Graphene as an
Efficient Cathode Electrocatalyst for Mechanical Rechargeable Zinc-Air Battery Application. ACS Appl. Mater. Interfaces 2015, 7, 21138− 21149.
(61) Yu, M.; Wang, Z.; Hou, C.; Wang, Z.; Liang, C.; Zhao, C.;
Tong, Y.; Lu, X.; Yang, S. Nitrogen-Doped Co3O4 Mesoporous
Nanowire Arrays as an Additive-Free Air-Cathode for Flexible Solid-State Zinc−Air Batteries. Adv. Mater. 2017, 29, 1602868.
(62) Wang, Z.; Li, B.; Ge, X.; Goh, F. W. T.; Zhang, X.; Du, G.;
Wuu, D.; Liu, Z.; Hor, T. S. A.; Zhang, H.; Zong, Y. Co@Co3O4@
PPD Core@bishell Nanoparticle-Based Composite as an Efficient Electrocatalyst for Oxygen Reduction Reaction. Small 2016, 12, 2580−2587.
(63) Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai,
H. Co3O4 nanocrystals on graphene as a synergistic catalyst for
oxygen reduction reaction. Nat. Mater. 2011, 10, 780−786.
(64) Li, Y.; Zhong, C.; Liu, J.; Zeng, X.; Qu, S.; Han, X.; Deng, Y.;
Hu, W.; Lu, J. Atomically Thin Mesoporous Co3O4Layers Strongly
Coupled with N-rGO Nanosheets as High-Performance Bifunctional Catalysts for 1D Knittable Zinc−Air Batteries. Adv. Mater. 2018, 30, 1703657.
(65) Liang, Y.; Wang, H.; Diao, P.; Chang, W.; Hong, G.; Li, Y.; Gong, M.; Xie, L.; Zhou, J.; Wang, J.; Regier, T. Z.; Wei, F.; Dai, H. Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes. J. Am. Chem. Soc. 2012,
134, 15849−15857.
(66) Lin, C.; Shinde, S. S.; Jiang, Z.; Song, X.; Sun, Y.; Guo, L.; Zhang, H.; Jung, J.-Y.; Li, X.; Lee, J.-H. In situ directional formation of Co@CoOx-embedded 1D carbon nanotubes as an efficient oxygen electrocatalyst for ultra-high rate Zn-air batteries. J. Mater. Chem. A 2017, 5, 13994−14002.
(67) Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J. E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805.
(68) Zhang, X.; Liu, R.; Zang, Y.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Co/CoO nanoparticles immobilized on Co-N-doped carbon as trifunctional electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Commun.
2016, 52, 5946−5949.
Dou, S. X.; Kim, J. H.; Lee, J. W. One-dimensional manganese-cobalt oxide nanofibres as bi-functional cathode catalysts for rechargeable metal-air batteries. Sci. Rep. 2015, 5, 7665.
(73) Ning, R.; Tian, J.; Asiri, A. M.; Qusti, A. H.; Al-Youbi, A. O.;
Sun, X. Spinel CuCo2O4 nanoparticles supported on N-doped
reduced graphene oxide: a highly active and stable hybrid electro-catalyst for the oxygen reduction reaction. Langmuir 2013, 29, 13146−13151.
(74) Cheng, H.; Li, M. L.; Su, C. Y.; Li, N.; Liu, Z. Q. Cu-Co Bimetallic Oxide Quantum Dot Decorated Nitrogen-Doped Carbon Nanotubes: A High-Efficiency Bifunctional Oxygen Electrode for Zn− Air Batteries. Adv. Funct. Mater. 2017, 27, 1701833.
(75) Liu, X.; Park, M.; Kim, M. G.; Gupta, S.; Wang, X.; Wu, G.; Cho, J. High-performance non-spinel cobalt−manganese mixed oxide-based bifunctional electrocatalysts for rechargeable zinc−air batteries.
Nano Energy 2016, 20, 315−325.
(76) Liu, H.; Wang, G.; Liu, J.; Qiao, S.; Ahn, H. Highly ordered mesoporous NiO anode material for lithium ion batteries with an excellent electrochemical performance. J. Mater. Chem. 2011, 21, 3046−3052.
(77) Lu, Q.; Lattanzi, M. W.; Chen, Y.; Kou, X.; Li, W.; Fan, X.; Unruh, K. M.; Chen, J. G.; Xiao, J. Q. Supercapacitor electrodes with high-energy and power densities prepared from monolithic NiO/Ni
nanocomposites. Angew. Chem., Int. Ed. 2011, 50, 6847−6850.
(78) Kuhlenbeck, H.; Shaikhutdinov, S.; Freund, H.-J. Well-Ordered
Transition Metal Oxide Layers in Model Catalysis− A Series of Case
Studies. Chem. Rev. 2013, 113, 3986−4034.
(79) Trunov, A. Analysis of oxygen reduction reaction pathways on
Co3O4, NiCo2O4, Co3O4−Li2O, NiO, NiO−Li2O, Pt, and Au
electrodes in alkaline medium. Electrochim. Acta 2013, 105, 506−513.
(80) Li, G.; Mezaal, M. A.; Zhang, K.; Lei, L. Synthesis and
Electrocatalytic Performance of NiO Modified Co3O4Composites for
Zinc-Air Batteries. Int. J. Electrochem. Sci. 2015, 10, 5395−5404.
(81) Liu, X.; Park, M.; Kim, M. G.; Gupta, S.; Wu, G.; Cho, J. Integrating NiCo Alloys with Their Oxides as Efficient Bifunctional
Cathode Catalysts for Rechargeable Zinc−Air Batteries. Angew.
Chem., Int. Ed. 2015, 54, 9654−9658.
(82) Lee, D. U.; Fu, J.; Park, M. G.; Liu, H.; Ghorbani Kashkooli, A.;
Chen, Z. Self-Assembled NiO/Ni(OH)2 Nanoflakes as Active
Material for High-Power and High-Energy Hybrid Rechargeable
Battery. Nano Lett. 2016, 16, 1794−1802.
(83) Malavasi, L.; Fisher, C. A. J.; Islam, M. S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: structural and mechanistic features. Chem. Soc. Rev. 2010, 39, 4370−4387.
(84) Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345.
(85) Kashyap, V.; Singh, S. K.; Kurungot, S. Cobalt Ferrite Bearing Nitrogen-Doped Reduced Graphene Oxide Layers Spatially Separated with Microporous Carbon as Efficient Oxygen Reduction
Electro-catalyst. ACS Appl. ACS Appl. Mater. Interfaces 2016, 8, 20730−
20740.
(86) Toh, R. J.; Eng, A. Y. S.; Sofer, Z.; Sedmidubsky, D.; Pumera, M. Ternary Transition Metal Oxide Nanoparticles with Spinel
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX M
Structure for the Oxygen Reduction Reaction. ChemElectroChem 2015, 2, 982−987.
(87) Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent Hybrid of Spinel Manganese−Cobalt Oxide and Graphene as Advanced Oxygen Reduction Electrocatalysts. J. Am.
Chem. Soc. 2012, 134, 3517−3523.
(88) De Koninck, M.; Marsan, B. MnxCu1−xCo2O4 used as
bifunctional electrocatalyst in alkaline medium. Electrochim. Acta 2008, 53, 7012−7021.
(89) Prabu, M.; Ramakrishnan, P.; Nara, H.; Momma, T.; Osaka, T.; Shanmugam, S. Zinc−Air Battery: Understanding the Structure and
Morphology Changes of Graphene-Supported CoMn2O4Bifunctional
Catalysts Under Practical Rechargeable Conditions. ACS Appl. Mater.
Interfaces 2014, 6, 16545−16555.
(90) Pletcher, D.; Li, X.; Price, S. W. T.; Russell, A. E.; Soenmez, T.;
Thompson, S. J. Comparison of the Spinels Co3O4and NiCo2O4as
Bifunctional Oxygen Catalysts in Alkaline Media. Electrochim. Acta 2016, 188, 286−293.
(91) Prabu, M.; Ketpang, K.; Shanmugam, S. Hierarchical
nanostructured NiCo2O4 as an efficient bifunctional non-precious
metal catalyst for rechargeable zinc-air batteries. Nanoscale 2014, 6,
3173−3181.
(92) Lee, D. U.; Kim, B. J.; Chen, Z. One-pot synthesis of a
mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its
oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A 2013, 1, 4754−4762.
(93) Ma, C.; Xu, N.; Qiao, J.; Jian, S.; Zhang, J. Facile synthesis of
NiCo2O4 nanosphere-carbon nanotubes hybrid as an efficient
bifunctional electrocatalyst for rechargeable Zn-air batteries. Int. J.
Hydrogen Energy 2016, 41, 9211−9218.
(94) Du, G. J.; Liu, X. G.; Zong, Y.; Hor, T. S. A.; Yu, A. S.; Liu, Z.
L. Co3O4nanoparticle-modified MnO2nanotube bifunctional oxygen
cathode catalysts for rechargeable zinc-air batteries. Nanoscale 2013, 5, 4657−4661.
(95) Kim, G.-P.; Sun, H.-H.; Manthiram, A. Design of a
sectionalized MnO2-Co3O4 electrode via selective electrodeposition
of metal ions in hydrogel for enhanced electrocatalytic activity in
metal-air batteries. Nano Energy 2016, 30, 130−137.
(96) Zhu, C.; Nobuta, A.; Nakatsugawa, I.; Akiyama, T. Solution
combustion synthesis of LaMO3 (M = Fe, Co, Mn) perovskite
nanoparticles and the measurement of their electrocatalytic properties for air cathode. Int. J. Hydrogen Energy 2013, 38, 13238−13248.
(97) Shim, J.; Lopez, K. J.; Sun, H. J.; Park, G.; An, J. C.; Eom, S.; Shimpalee, S.; Weidner, J. W. Preparation and characterization of
electrospun LaCoO3 fibers for oxygen reduction and evolution in
rechargeable Zn-air batteries. J. Appl. Electrochem. 2015, 45, 1005− 1012.
(98) Hu, J.; Liu, Q. N.; Shi, Z. W.; Zhang, L.; Huang, H. LaNiO3
-nanorod/graphene composite as an efficient bi-functional catalyst for zinc-air batteries. RSC Adv. 2016, 6, 86386−86394.
(99) Zhou, W.; Sunarso, J. Enhancing Bi-functional Electrocatalytic Activity of Perovskite by Temperature Shock: A Case Study of
LaNiO3−δ. J. Phys. Chem. Lett. 2013, 4, 2982−2988.
(100) Ma, H.; Wang, B. A bifunctional electrocatalyst α-MnO2
-LaNiO3/carbon nanotube composite for rechargeable zinc-air
batteries. RSC Adv. 2014, 4, 46084−46092.
(101) Ge, X.; Li, B.; Wuu, D.; Sumboja, A.; An, T.; Hor, T. S. A.;
Zong, Y.; Liu, Z. Nanostructured Perovskite LaCo1‑xMnxO3 as
Bifunctional Catalysts for Rechargeable Metal-Air Batteries. J. Mol. Eng. Mater. 2015, 3, 1540006.
(102) Hu, J.; Wang, L. N.; Shi, L. N.; Huang, H. Oxygen reduction
reaction activity of LaMn1‑xCoxO3-graphene nanocomposite for
zinc-air battery. Electrochim. Acta 2015, 161, 115−123.
(103) Prabu, M.; Ramakrishnan, P.; Ganesan, P.; Manthiram, A.;
Shanmugam, S. LaTi0.65Fe0.35O3−δ nanoparticle-decorated
nitrogen-doped carbon nanorods as an advanced hierarchical air electrode for
rechargeable metal-air batteries. Nano Energy 2015, 15, 92−103.
(104) Vignesh, A.; Prabu, M.; Shanmugam, S. Porous
La-Co1‑xNixO3−δ Nanostructures as an Efficient Electrocatalyst for
Water Oxidation and for a Zinc-Air Battery. ACS Appl. Mater.
Interfaces 2016, 8, 6019−6031.
(105) Eom, S. W.; Ahn, S. Y.; Kim, I. J.; Sun, Y. K.; Kim, H. S.
Electrochemical evaluation of La1‑xCaxCoO3cathode material for zinc
air batteries application. J. Electroceram. 2009, 23, 382−386.
(106) Hadidi, L.; Mahmoud, A. Y. F.; Purkait, T. K.; McDermott, M. T.; Veinot, J. G. C. Cellulose nanocrystal-derived hollow mesoporous carbon spheres and their application as a metal-free catalyst. Nanotechnology 2017, 28, 505606.
(107) Fu, J.; Hassan, F. M.; Li, J.; Lee, D. U.; Ghannoum, A. R.; Lui, G.; Hoque, Md. A.; Chen, Z. Flexible Rechargeable Zinc-Air Batteries through Morphological Emulation of Human Hair Array. Adv. Mater.
2016, 28, 6421−6428.
(108) Xu, N.; Liu, Y.; Zhang, X.; Li, X.; Li, A.; Qiao, J.; Zhang, J.
Self-assembly formation of Bi-functional Co3O4/MnO2-CNTs hybrid
catalysts for achieving both high energy/power density and cyclic ability of rechargeable zinc-air battery. Sci. Rep. 2016, 6, 33590.
DOI:10.1021/acs.organomet.8b00508
Organometallics XXXX, XXX, XXX−XXX N