HAL Id: hal-02094679
https://hal.archives-ouvertes.fr/hal-02094679
Submitted on 9 Apr 2019
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Glycolipid self-assembly: micellar structure
Marie-Christine Cecutti, Bonaventura Focher, Bruno Perly, Thomas Zemb
To cite this version:
Marie-Christine Cecutti, Bonaventura Focher, Bruno Perly, Thomas Zemb.
Glycolipid
self-assembly: micellar structure. Langmuir, American Chemical Society, 1991, 7 (11), pp.2580-2585.
�10.1021/la00059a031�. �hal-02094679�
OATAO is an open access repository that collects the work of Toulouse
researchers and makes it freely available over the web where possible
Any correspondence concerning this service should be sent
to the repository administrator:
tech-oatao@listes-diff.inp-toulouse.fr
This is an author’s version published in:
http://oatao.univ-toulouse.fr/23551
To cite this version:
Cecutti, Marie-Christine
and Focher, Bonaventura and Perly, Bruno and
Zemb, Thomas Glycolipid self-assembly: micellar structure. (1991)
Langmuir, 7 (11). 2580-2585. ISSN 0743-7463
1991, 7,
Glycolipid
Self-Assembly:
Micellar
Structure
Christine
Cecutti,*-*
Bonaventura
Focher,*
Bruno
Perly,8
and Thomas
Zemb§
Ecole
Nationale
Supérieure
deChimie
de Toulouse, 118 route deNarbonne,
F.31077Toulouse
cedex,
France,
C.N.R., P.zzaL.
daVinci,
1.20133Milano,
Italy,
and
CEA-CEN
de Saclay,Service de
Chimie Moléculaire,
F.91191Gif
surYvette
cedex,France
Small-angle scattering is used to investigate a
typical glycolipid
micelle structurein
conjunctionwith
NMR determination of
sugar cycle conformation.It
is shownthat
the ellipsoidal shapeof
the micelleoriginates
from
two constraints: sugar rings perpendicular to the interface induce alimited
areaat
thechain-head interface. Together
with
thebulky
hydrated heads,this
imposes an ellipsoidal shape.Introduction
Single-chain surfactants
areusually classified
into
two
families: ionic
andnonionic
molecules.In
the
phasediagram,
adiluted, optically
isotropic
fluid
micellar
phaseLi
existsfor
both types
of
surfactants.
The
micellar
structure,
i.e.,the number
of
water
moleculesbound per
headgroup,
the
radius
of
the
micellar hydrophobic
core,and
the
area persurfactant
head, isdetermined by
geometric
constraints1 andby the
balancebetweenhead-group
repulsion and
hydrophobic effects
betweenapolar
chains.
In
the
caseof
most
ionic single-chain
moleculeswithout
addedsalt, the
sizeandstructure
of
theaggregatesin
micellar
phaseisroughly independent
of
concentration
and temperature at
anypoint
of the
phasediagram
far
from the
phaselimits.
Nonionic
micellesexhibit
asphereto rod
transition
in binary solutions.
At
increasingtem-perature, the lower
consolutepoint
is dueto
attractive
interactions
appearing betweennonionic
headgroups whenthe
interfacial
radius
of
curvature
decreases.2Typical
values
of
thephysicalquantities
describing these two typesof
micelles,including the
microstructural
parameters,aregiven
in
Table
I for
two
classical examples: 2% SDSin
D2O
and
5% C12E5in
D2Oat
roomtemperature.
Weexamine here
the
caseof
abiologically
important
molecule, /3-dodecyl maltoside.
This
isanonionic
moleculewith
anextremely
largehydrophilic
headgroup. Wefirst
want to
assessthe following
questions: asthe
largehead-group
gives riseto
alargesterical repulsive term,
will this
besufficient
to
inducethe
characteristic structure
of
ionicmicelles,
with
someparticularities
suchasthe
absenceof
a
salt
effect
dueto the
absenceof
counterions?
Onthe
other
hand,will
glycolipid
micelles present thesame phasebehavior
andmicrostructure
asother nonionic
systems?Our aim
in
this work
isto
identify
thedominant
featuresof
glycolipid
self-assemblyin
the micellar
state using/3-dodecyl
maltoside
(/3C12M) as atypical
molecule./3C12M presentsalarge and
flexible headgroup
madeby
two
sugar rings.Therefore, after
identification of
the
phases
in
abinary
concentration
andtemperature
phasefEcoleNationaleSupérieuredeChimiedeToulouse,
lC.N.R.
«CEA-CENdeSaclay.
(1)Israelachvili,J.N.;Mitchell,D. J.;Ninham, B. W. J.Chem. Soc.,
Faraday Trans.2 1976, 72, 1525.
(2)Mitchell,D. J.;Tiddy,G. J.;Waring, L.; Bostock, T.; McDonald, . P.J.Chem. Soc.,Faraday Trans1 1983, 79, 975.
Table I.
Typical
Molecular Parametersfor
theIonicSurfactant
Sodium Dodecyl Sulfate(SDS),theNonionicPolyethylene(C12E5), and0-Dodecyl Maltoside(¡9C12M)
SDS C12E5 /SC12M
Vmoi,nm3 0.380 0.620 0.691
headgroupareas a, nm2 0.70 0.35 0.50
headgroupareas
',
nm2 0.60 0.35chain lengthl,nm 1.8 1.8 1.8
packingparameter,Vmoi/ 0.3 1.0 0.75
volumeofheadgroup,nm3 0.060 0.300 0.376
diagram,
westudied the
sugarring
conformation by
NMR
andthe
micellar
structure by X-ray
andneutron
scattering.The
samemethods
have been usedfor
other
surfac-tants
with
abulky
nonionic
headgroup.3·4With
amixture
of
lipopolysaccharides isolatedfrom Escherichia
coli,with
an average
of
four sugar rings per molecule,36longcylinders,
similar
to
thoseobtained
with
diheptanoylphosphatidyl-choline®
with
apH-dependent length
havebeenevidenced.Nonspherical
micelles have beenobtained
with
the
gan-glioside
GM1.4Materials
and
Methods
(1)
Scattering
Techniques. X-raysmall-anglescatteringofisotropicphases has beenperformedontheD24double-crystal
diffractometerin Lure(Orsay),usingawavelengthof0.122nm
andasampletodetector distanceof59.3cm. Theresultswere
identicalusing deuterated andprotonatedoctaneassolvent.The
obtainedqrangewas qmin= 0.03 nm"1andq,m= 6 nm"1; i.e.,the
resolutionwas 2v/qmMX= l nm andnolong-rangeorderinghigher
than2ir/qmill = 200 nm was considered. The absolute scaling
was made by comparison
with
the isotropiccoherentscattering®ofasampleof1.5-mmthicknessofwater between25-#tm
thick
Mylarsheets. Dueto watercompressibility,eachwater molecule
givesthesame uniformscatteringas6.35independentelectrons.
Thistype of normalization,versus averyweakbutconstantsignal,
ensures
that
the setupisperfectlyaligned andthat
background correctionissuccessfullyachieved.7 Neutronsmall-anglescat-teringwasperformedon thePACEsetup at
LLB
Orphée, using0.5-1-nm wavelengthswithtwodifferentwavelengths,whichgives
aqrangeofqm¡„= 0.08 nm"1to = 3nm"1. Absolute scaling
(3)Hayter,J. B.;Rivera,M.; McGroarty,E. J.J. Biol.Chem. 1987,
262, 5100.
(4)Cantu,L.; Corti, M.; Degiorgio,V.; Piazza, R.;Rennie,A. Prog.
Colloid Polym.Sci. 1988,76, 216.
(5)Lin,T. L.;Chen,S.H.; Gabriel, N.E.;Roberts, M. F.J.Phys.
Chem. 1987,91,406.
(6)Zemb,T.; Charpin,P.J.Phys. 1985,46,249.
(7)Levelut,A.M.Science Phys. Thesis, Orsay, 1968;p334.
was made using the incoherent scattering
of
water.8 Lyotropicliquid-crystal identification was done using a Guinier camera
equipped with a linear detector, giving a qm¡„ = 0.2 nm"1 to q—
= 8 nm"1. The lyotropic
liquid
crystals were identified by peakspacing. After identification of the symmetry, the area per molecule was derived from measurement of the molecular volumes
by densitometry.
The small-angle scattering of micellar solutions was
first
checked by the measurement of the invariant Q*, which is given
in a system
with
two scattering length densities bi and b%, withthe volumes
and <fo:9
Q* =
2 ( 1
-b2)% 2= dq
This expression is valid whatever the microstructure of the
solution, isolatedspheres, connected cylinders,or random
bi-layers;10theonlyunderlying assumptionisthat itisatwo-medium
structure. Then, theshapeofthemicelleshastobedetermined
by comparing thescatteringI(q)obtainedwiththe scatteringof
modelstructures. Since thepresenceorabsenceof intermicellar
attractionor repulsionisnot knownapriori, determination of
the radiusof gyrationhasno physical meaning. The
determi-nationofthemicellarshapecan only relyon acomplete calculation on an absolutescaleofthe scatteringcurve for differentideal
modelshapesofthe micelle. Fortunately, in binarysolutions,
micellarradiiand scattering lengthsareknown; theyareimposed
by chemicalcomposition and molecular volumes. There isno
free parameter adjustmentexcept aggregation number, itself
relatedtothearea per headgroup,once the generalshapeofthe
aggregate has been chosen,as explained below.
Our aimisto compare theshapewiththe surfactant parameter
p,thelaterbeing deducedfromsterical considerations.
Usually, the volume of the polar headgroups Vp is small
comparedto
that
ofthehydrophobicchains Vc- Thepackingparameterp isthen definedas10
P= Vool/6.1=
(Vp+Vc)/6.1
= Vc/6.1In
the presentcase,the packingofthe molecule requirestaking intoaccountthe whole molecular volume to evaluate tosurfac-tant parameter. The length ofthe molecule is now the total
length(TableI). Using the
first
definition,(=
18A and p= 0.33whileusing the totallength (l = 24A) yieldsalsop = 0.33.
Model
of Spherical Micelles.
Wesupposeherethat
theglycolipid molecules packinto sphericaldroplets
with
hydro-phobic chains inside: one singlequantity,the interfacialarea
per moleculea,determinesthe whole scattering spectrum:
I(q)= P(q)S(q)
The structure factor S(q) is
first
taken forindependent hardspheres, neglecting other types of interaction. S(q) can be
calculated analyticallyat anyq withgood precision when the radius R
of
the hard core ofmicelles, thedensity n (cm-3) of micelles, and thetotalvolumefraction ofmicellesare known.11 Thesethreequantitiescan beevaluated by molecular parametersonce thearea per molecule isfixed.
(a)Thesurfaceofmicelles imposesarelationbetweenNand R:
4 R2=
where
N
isthe aggregation number and the single adjustableparameter, which hasto bebetween 2.5and 7 nm forsterical reasons. Usually, isdefined atthemicelle-solventinterface.
For typical surfactantssuchasSDS, aisca. 70A2/molecule.
It
doesnotmake any differencein thiscasetodefine atthe
chain-headgroup interfacesincethe volumeofthe headgroupisonly
10% ofthetotal surfactantmolecular volume. For glycolipids,
however, the sugarrings represent themajor part ofthe
mo-lecular volume. Wethereforedifferentiatebetweenthe
micelle-solventinterface andthehydrophobiccore-sugar headgroup
interface '.
(8)Jacrot,B.; Zaccai,G.Biopolymers1981,20, 2413.
(9)PorodG.InSmall AngleX-rayScattering·,Clatter, Krakty,Eds.;
Springer: Berlin,1982.
(10)Duplessix,R.; Cabane,B.;Zemb,T.J. Phys. 1985, 46, 2161.
(11)Hansen, J. P.;Hayter,J.B.Mol.Phys.1982,46, 651.
(b) The micellar volume imposes another relation between
N
andR:
*/3*R3= NVaol
where Vmoiisthe known molecular volume, measured by
den-sitometryusing theAntonPaar high-precision densitometer.
Aggregation number
N
andmicellarradiusRare now fixed:N
= 4*R2/a R= 3Vmol/Thearea per moleculeatthe core-headgroupinterfaceisnow
fixed.
If
theshaperemains spherical, the radiusof
the sphereincludingthehydrophobicchainsR'isdefined as
4*R'2=
NS
i/3*R'3
= NVdu¡nTherecan bea conflictbetween the values of and '. The
aggregate shape, whennonspherical,isanenergy-effective packing
solution tosolvethis conflict.
Theexcluded-volume fraction ofthehydrated micelle is
alsodeducedfromsterical considerations. Thevolumefraction ofthe micelleisgiven by
N
molecular volumesincludingh-10water molecules bysurfactantinsidethe hard-sphere volume.
Thevolumefraction ofthedispersedphase istherefore known
a
priori
when isfixed:= (Vmol+
hx 30)Mi
Sincethe volumeofonesingle water moleculeis 30A3, atwo-step modelofthemicelleissufficientto calculate the form factor P(q)
in thisqrange:10 wesupposeahydrophobiccore ofradiusR3and
ahydratedheadgroup concentric shellofradiusR3. Theinternal
sphereofradiusRicontainsonlythe
N
hydrophobic chains.Theconcentric shell betweenRiandR3contains
N
headgroups andhN
watermolecules. Sincethe molecular volumes and scatteringlengthdensitiesare known, the valuesofRiandR3aswellasthe
contrastisknownonce
N
and hare fixed, hisimposedby thesimulation at high volume fraction. As usual, we make the
assumption
that
hisnotconcentrationdependent. Wefoundh= 10an acceptable valueforthiswhole study. The scattering
lengthdensities (electronic densities) are therefore calculated
numericallyforboth X-rayand neutron-scattering densitieswith
thesame parmaters
N
andh:eP(q)=
(£(*>,
+ 1-b^/^fiqR,))2
where
f(x)
= 3(sinx- xcosx)/x3If
thestructureisspherical,both X-rayandneutron-scatteringspectracan bereproducedon an absolutescale
with
thissingle parameter . When the calculated scattering cannotbefitted
tothe observedone byvarying , at leastone ofthetwounderlying
assumptions, i.e., (I) the only interaction between droplets is hard-sphererepulsion, and
(II)
micellesare spherical,hasto bemodified. Fordouble-chain surfactants,wehave recently shown
ina similarcase
that
assumptionII
is wrong.12In
thecaseof
glycolipids, theabsenceof critical points inthephasediagram andno effect of temperature ofscattering data
shows
that
it
is also merely assumptionII
which has to bemodified: theshape isnotspherical. We therefore turn now to
thecalculationofindependentcylindricalmicelles. An
infinite
flexiblecylinderis easilydetected byaq-1decayofthescattering.
In thiscase,the scatteringisvery intense at lowqand
it
isgivenby®
P(q)= (C-
C^r/qN^B
-bKlnJ2
exp(-W/2)
Where
M
isthe aggregationnumberperunit
micellarlength,Cthe concentration,
Cj*
thecritical micellar concentration,and5the averagescatteringlength density intheaggregate. The
relevant plotlogIq2versus logqdoesnot showalinearbehavior
inthe presentcase.
(12)Barnes,I.S.;Hyde,S.T.;Ninham, B. W.; Derian,P. J.;Drifford,
2582
Langmuir,
Vol. 7, No. 11, 1991Cecutti
et al.Wedonotseesuch signalsinour measurements,so wecalculate
thecase offinitecylinders.13 Othershapes(bilayers,
flat
disks,etc.)have beenconsidered,butthe scatteringofthemdoesnot correspondeither on an absolutescaleor qualitatively to the
observedshape.14
Model
of
Finite Cylinders.
Weapproximate finitesphero-cylinders madeof hydrophobiccore coatedwithaheadgroup
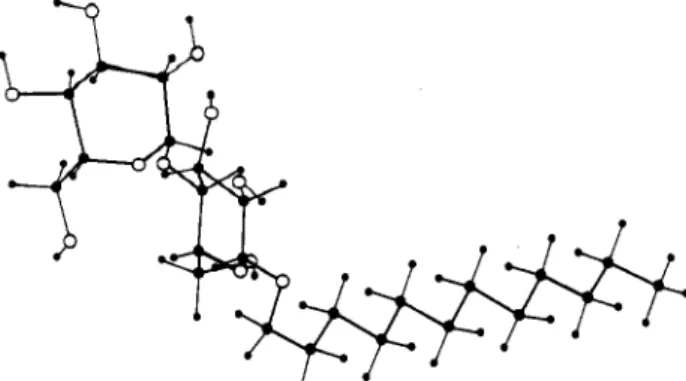
shell byanelongatedellipsoid(Figure4). Now, two parameters
are required tocalculatethe whole scatteringcurve for X-ray
and neutron scattering:thearea persurfactantheadgroup and the
ellipiticity
e. The S(q) termissetto1becauseno theoreticalcalculationofS(q)exists forthiscaseandonedoesnotseesensible
decreaseofthe scattering at lowq,whichisthe usualindication
ofsterical hindrance. P(q)iscalculatedforaprolateellipsoid of
ellipticity
e;theform factorisgiven byP(9)=
n(£(6i+1
-b;)2“/a*!?,·3/2X
_
(qRfVcos2 + e2sin2
)
cos d<?)Thepresenceofthe
ellipticity
parametereallowssolution oftheconflictbetweenthe surface
',
which includesall hydrophobictails,and , includingthe wholesurfactantmoleculeaswellas
hydrationwater.
Polydispersity.
Using small-angle scattering, there is nodistinctionon a setofscattering data betweensize andmass
polydispersity oftheaggregates.16
But
thereisadirectwayofdeterminationofthe variance ofthemass distributionusing
mass action law and the averagemass variation with
concen-tration:
2=
6 /(
In(*T-*!))
where is the total surfactant molar fraction, X\ the free
monomer molefraction,and
N
the aggregation number.Measurement ofmean aggregation number
N
for differentmolarfractionsallows thereforethedetermination ofthemass
polydispersity ofthe micelles.
(2)
NMR
Techniques.NMR
experimentswere performedinthe micellar andliquid-crystallinestatestogetinsight intothe local organizationwithspecialattention toward the local
con-formation ofthe polarhead.
NMR in
theMicellar
State.All
experimentswere performed at310Kusinga24mM solution in deuteriumoxide andaBrokerWM500spectrometeroperatingat500.13
MHz
forprotons.In
afirst
stage,allsignalsarisingfrom nonlabileprotonshavetobeassignedusing two-dimensionalCOSYandmultistepRelay
experiments.16 This is facilitated by the fact
that
anomericprotonsfromthe twoglucoseunitsare clearlyidentifiedon the
spectrum owingtotheirspecific chemicalshiftsandtocoupling constants relatedtolocal anomeric characters.
NOE experiments were then carried out to derive spatial
proximitybetweenprotonsandtomodelthe corresponding overall
average conformation oftheglycolipid molecule.
NMR in
theLiquid-Crystalline
State. Whenliquidcrystalsare considered, deuterium
NMR
offers the most powerfulapproach to local molecular order and conformation. Forthis purpose,adeuterium-labeled glycolipidwas preparedasfollows.
Laurieacidwas reducedto the corresponding alcohol using
Li-A1D<andthe obtained
, '-deuterated
dodecanolwas graftedtoactivated maltose using theclassicalprocedures. A95%labeling
ofthe
first
carbonofthealiphaticchainwas thus achieved. ForNMR
experiments, all samples were prepared indeuterium-depleted water (CEA)afterfreeze-dryingofthesolidglycolipid
from thissolvent. Thisensures that isotropiclines observedin
theforthcoming deuteriumspectraarisefromthe labels and not
fromresidualdeuteriumfromthe water.
All
experimentswereperformed at 310
K
using a Broker MSL300 spectrometer(13)Hjelm,R. P.,Jr. J.Appl.Crystallogr.1985, 18, 452.
(14)Porte,G.J.Phys. Chem. 1983,87, 3541.
(15)Hayter,J.B.InProceedings of theXCCorsoInternationalSchool
ofPhysics·,Degiorgio, V., Corti, M.,Eds.;NorthHolland: Amsterdam,
1985.
(16)Berthault,P.; Bossenec,V.;Perly, B. Analusis, 1990,18,184.
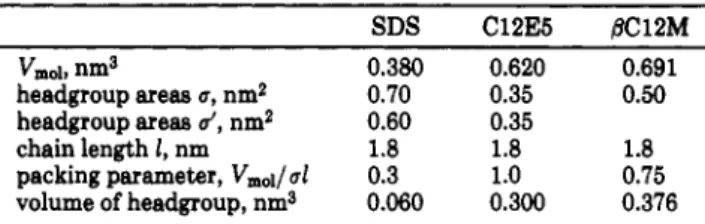
Figure
1. 2HNMRspectrum of/3-dodecylmaltoside in theliquid-crystallinestate.
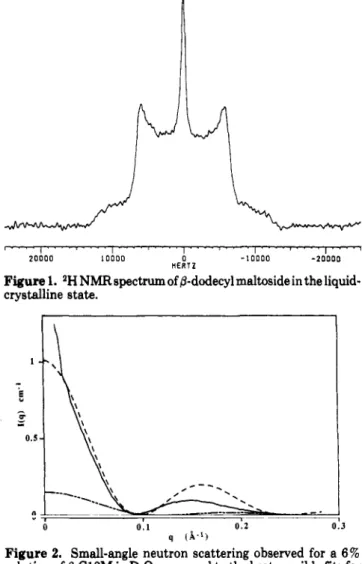
Figure
2. Small-angle neutron scattering observed for a6%solution of d-Cl2M inDzOcompared to the best possiblefits for
spherical micelles
(---)
withafixedarea per molecule (</=0.50 nm2) andshort ellipsoids (e = 1.2;
—).
operating at 46 MHz for deuterium. The quadrupolar-echo
sequence17 was usedto avoidphasedistorsions and signallosses.
Results
(1) Phase
Diagram.
The
phasediagram
presentsonly
two
regions: anisotropic
fluid
micellar
phase existsup to
50%
(w/w)
in
water, andat higher concentration,
aviscousbirefringent
lamellar
phase isobtained.
The nature
of
thesephases isnot
affected by temperature up to
353K.
The
periodicity
D*
andthe thickness 2t
of
thebilayer
aremeasured by
small-angle
X-ray
scattering at 50%:
2t= 2.9nm
D*
= 5.3nmThe
areaper surfactant headgroup calculated
with
thesevaluesis
therefore
= 0.57nm2/molecule.
In
the
caseof
lamellar packing,
a ='.
A typical NMR
spectrum
in
the
liquid-crystalline
stateis
displayed in Figure
1.The corresponding 11.4-KHz
quadrupolar
splitting
indicates high molecular ordering
at
the corresponding
carbon, andthe overall
shapeof
thespectrum
istypical of axially
symmetric bidimensional
structures. The sharper central line
correspondsto
iso-tropically tumbling
micelles
in
thermodynamical
equi-librium with
the
liquid
crystals.(2)
Structure of Micelles.
Westudy
now an aqueoussolution
of
j8-dodecylmaltoside
(6%w/v)
at 310K:
Neutron
andX-ray
scattering
curves are compared on(17)Davis,J.H.; Jeffrey, K.R.;Bloom, M.; Valic, . I.;Higgs,T.P.
Glycolipid
Self-Assembly:Micellar
Structure
Langmuir,
Vol. 7,No. 11, 1991 2583TableII.
Structural
Parameters Describing the 0-DodecylMaltosideMicelleat 6%
(w/w) in
Waterat 310K
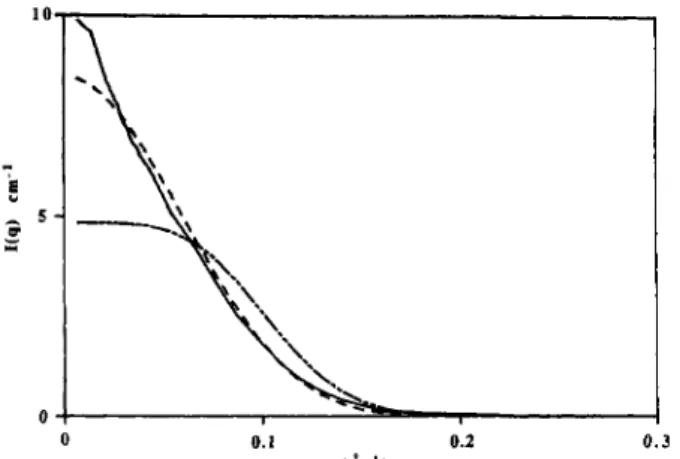
Figure
3. Small-angleX-rayscatteringofthesample describedin Figure2comparedtothe scatteringofperfectspheres
(---)
andshortellipsoids
(—).
Thesame numericalvalues (areas,molecular volumes, aggregation numbers)were usedtosimulate
X-ray
andneutron-scatteringspectra.Figure
4. Schematicviewofthemicellaraggregate.and absolute
scale (cm-1)to calculated model
curvesfor
spheres
and cylinders (Figures
2and
3).The
bestresult
is
obtained
with
the
short cylinder model (Figure
4),for
both neutron and
X-ray
techniques,
taking
thefollowing
parameter
values:v - 86 A2 = 51A2
which
gives an aggregationnumber
N
= 82.Micellar
structural
parameters
arereported
in
Table
II.
A
goodagreement
isnot
obtained
from pure spherical
micelles. The short ellipsoid model
fits
better
onthe
absolute
intensity
scale.The
best agreement isobtained
with
anellipticity
e= 1.2.The resulting scattering length
density
isgiven
in
Figure
4.(3)
Polar
Head
Conformation.
Anomeric protons
were hence used as
starting
points
for
the complete
assignment
of all
other
signals.A
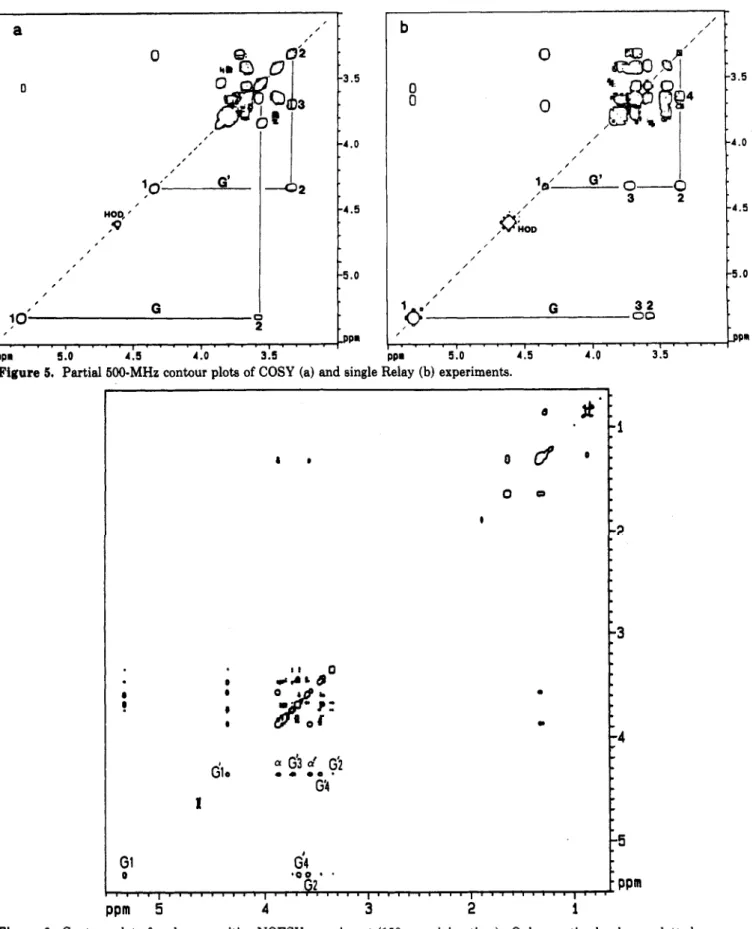
typical contour
plot
ofaCOSYexperiment
isdisplayedin Figure
5.The corresponding coupling constants
werederived
further from
computer-assisted spectral
simula-tion. The relevant parameters
arereported
in
Table
III.
Ri totalshort radiusofthe micelle 2.4nm
Ri short radiusofthe apolarhydrophobiccore 1.8 nm
N aggregationnumber 82
h watermolecules persurfactantmolecule 10
e ellipticityofthemicelle 1.2
a areapersurfactanthead atthe
water-micelle interface 0.87nm2
(ri area per surfactanthead atthe
chain-headgroup interface 0.50 nm2
61 electronic density ofthemicellarcore 285emn'3
6V electronic density ofthe sugar
headgroupregion 433enm"3
bi scattering length density ofthemicellarcore -0.4X1010cm"*
bi scattering length densityofthe sugar
headgroupregion 3.8 X1010cm'2
TableIII. Chemical
Shifts
andCoupling Constants ofAll
Protons of the d-Dodecyl Maltoside Polar Head
proton chemshift, ppm couplingconst,Hz
Hi
Ha H3 H4 h6 He-Hg'H'i
H'a H's H'4 H'6 H'g-H'g-TableIV. 5.30 3.60 3.65 3.40 3.65 3.80 4.35 3.35 3.70 3.65 3.45 3.80 Ji-2— 4.2 </a-3= 8.7 J3-4= 10.0 J*.g= 9.4 «/fr4= 4.0,Jis-g'= -12.4 J'1-2= 8.3J'
2-3 = 8.5 =/'3-4= 9.0 c/'4-5= 8.5 t/'g-g'= -12.4Structure of the Micelles
for
Different
Surfactant Concentrations* concn (w/w),% aggregationno. N Ri Ri e 1 120 0.014 21 36 1.2 4 115 0.056 21 36 1.2 6 82 0.084 18 24 1.2
0eistheellipticityfor which thebestfitwas achieved. R\and
Riare the hydrophobiccore radius (short axis) andthe external
radius, respectively. The excluded-volume fraction includes 10
watermolecules per headgroup.
A
two-dimensional
NOESY experiment
wasperformed
with
150-msmixing
time.The corresponding contour
plot
is
displayed
in
Figure
6and
showsanumber
of
structure-related
cross-peaks.More
accuratequantitative
datawerethen obtained by NOE experiments using the
buildup
technique. NOE
buildup
rateswereobtained at variable
transfer times, and
theseratescould
then
beconverted
into
effective
interproton
distances.A
complete
ratio-nalization
of all
data
was achieved using amolecular
modeling
program,18allowing proposal
of
amodel
for
asingle
glycolipid
molecule in
the micelle.
In
thesecalcu-lations,
anormal
4C1chair
conformation
was usedfor
both
sugar
units
in
agreementwith
coupling
constants.Only
the interglycosidic and the glycosidic
bondswereconsid-ered
for local rotation. The relevant molecular structure
is
depicted
in
Figure
7.Discussion
The extended chain length for the
/3-dodecylmaltoside
isLc= 1.8 nm;10
The apolar volume
of
this chain
is Vc=0.315nm3.
Therefore, the maximum
aggregationnumber
of
aspherical apolar
corewithout
a holeat
the center
corresponds
to
N
= 82,whenRi
= Lcand = 0.50 nm2.(18)Langlet,G. 44émeRéunionInternationaledemodélisationdee
structures et propriétés moléculairesenchimie physique et biophysique,
2584
Langmuir,
Vol. 7,No. 11, 1991Cecutti
et al.Figure
5. Partial500-MHzcontour plots ofCOSY (a)and single Relay (b) experiments.Figure
6. Contour plot ofaphase-sensitiveNOESY experiment(150-msmixing time). Onlynegative levelsare plotted.The
sizeof
theextended
maltoside
headgroupis Ry= 1.2nm, and
the maximum radius
R2isR2 = Lc+ Rh= ca. 3nm.
In
the present
case,the external radius
of
themicelleis 24
A
alongthe small
axisand28A
alongthe long
axis,allowing
enoughroomfor
the
sugarheadgroups and alsosatisfying the conditions
of
occupancyof
thecenterof
themicelle;
i.e.,(a) Le>
Ri
and (b)eR%= Lc +Ly¡.When
the
long axis reaches30
A, linear growth
of
themicelle
stops.When there
is nostopping mechanism
for
miceller
growth,
infinite cylindrical
micellesare formed.14In
ourcase,
the
cylindrical
structure isforbidden by the volume
of
the headgroup,asit
should require dehydration
of
thesugar
polar
head.The micelle
hence growstoward
anellipsoid
but
cannot reachthe
cylindrical
state.Exper-imentally,
there
is no wayto
fit
the scattering
with
the
expressions
of
the scattering
of cylindrical
micelles.The ellipsoid length
islimited
tothe length
of
the
extended
molecule(3nm). The two constraints inducing
Glycolipid
Self-Assembly:Micellar
Structure
Langmuir,
Vol. 7, No. 11, 1991 2585Figure
7. Schematic lateral view of the polar headgroupconformation.
the ellipsoidal
shape are asfollows:
(a)the
areaat the
chain-headgroup interface
= 0.5 nm2;this
fixes
the
maximum value
of
R\. (b)The
total
headgroup volume
between
R\ and
Ri
hasto include
the
2N
hydrated
sugarcycles;
this
induces anellipsoidal
shape. Thesetwo
geometrical conditions
aresatisfied by the prolate
ellip-soidal
shapeof
the
glycolipid
micelle.
From the
observedscattering
alone, onecould
alsoinvoke
sizepolydispersity
of
micellesinstead
of
adistri-bution
of
monodisperseellipsoids,
asthis
will
yield similar
scattering
curves.An
independent evaluation
of
poly-dispersity
ishowever provided by the
variation
of
the
averageaggregation
number
with
concentration.6
Table
IV
givesthe
aggregationnumbers
at three concentrations.
The lack
ofvariation
of
thesenumberswith
concentration
implies
that
polydispersity should
belower
than
a fewpercent.
Asthis
valueistoo small toexplain
the scatteringcurves,ashape
deformation
hasto
beinvoked instead
of
a
contribution
of
polydispersity.
Conclusion
The two sterical constraints responsible
for
the
shapeof
the
micellar
aggregatearestrongly dependent
onthe
sugar
ring
conformation and
orientation
relative to the
chain-headgroup interface
plane.Therefore,
interaction
of
the sugarheadwith
anyother
molecule suchasaprotein
can
dramatically
changethe
valueof
'
whenthe
sugarconformation
ororientation
changes.The
valueof '
caneasily increase
by
alargefactor (up to
3)when
the ring
liesparallel to the interface.
This
should strongly
decreasethe
sizeof
the micelle. Along the
same idea, whenthe
glycolipid
ismixed
with another surfactant,
asin biological
membranes,
the
spontaneouscurvature
toward oil should
decrease
drastically.
This
is alsoa possiblemechanism
for inhibiting
the
decreaseof
the radius
of
gyration
if
binding of
aprotein
caninduce headgroup conformation
changes.