ICTON-MW'09 FrP.15
978-1-4244-5747-2/10/$26.00 ©2009 IEEE 1
Structural and Optical Characterization of Hybrid
Nanocomposite ZnO/PMMA
M. Sebais1, A. Chaieb1,2, O. Halimi1, L. Bouhdjer1, B. Boudine1, B. Sahraoui2
1 Laboratory of Crystallography, Physics Department, Mentouri University Ain El-Bey Road 25000 Constantine Algeria
2 Laboratory POMA, FRE CNRS 2988, University of Angers 2, Boulevard Lavoisier, 49045 Angers, France Tel : 00 213 31 92 06 38, E-mail: msebais@yahoo.fr ABSTRACT
Thepresent study focuses on the structural and optical properties of thin filmsof ZnO/PMMA nanocomposite deposited on glass substrate using a colloidal solution and spin-coating technique. XRD study reveals that the ZnO particles in amorphous PMMA host matrix have hexagonal (wurtzite) structure and they have arbitrary orientation. Optical absorption spectrum shows three bands in the UV-Visible area at 377.70, 461.14 and 624.81 nm with a shift to high energies of the absorption edge because of quantum confinement induced by the very small size of ZnO crystallites which was estimated to 4.77 nm. Photoluminescence spectrum at low temperature (77°K) (excitation with 355 nm) shows a wide and intense emission band in the visible area (400 – 700 nm). Emission is due to excitons, trapped imperfection sites and surface electrons.
Keywords: nanocomposite ZnO/PMMA, X-ray diffraction, optical absorption, quantum confinement, photoluminescence.
1. INTRODUCTION
The ZnO semiconductor exhibits an intense optical activity in the ultraviolet- visible area and it’s used in the manufacture of many optical and optoelectronic components such as UV lasers, blue to UV light emitting diodes
and UV detectors [1-3]. The organic matrix of PMMA is optically transparent in the ultraviolet-visible area and
it allows the study of optical properties of ZnO nanocrystals in this field. The nanosized-material properties,
which are significantly different from these of the bulk material, have attracted much attention because of the
effect of quantum confinement mainly due to the size effect and high ratio of surface to volume. The ZnO nanoparticles can be synthesized by different methods like homogeneous precipitation [4], mechanical milling
[5] and spray pyrolysis [6]. Physical and particularly optical properties depend in part on the quality of material
and consequently depend on the conditions and method of preparation. So in this work we present the results of the structural and optical characterization of ZnO/PMMA nanocomposite thin films deposited on a glass substrate using a colloidal solution and spin-coating technique.
2. PREPARATION OF ZNO/PMMA NANOCOMPOSITE THIN FILM
The ZnO nanocrystals doped polymer PMMA (host matrix) was prepared by colloidal solution. Firstly a host solution was prepared by dissolving the polymer in tetrahydrofuran (THF) with a concentration of 0.02 g/ml. This solution was stirred at 50°C for 2 hours. Besides, a second solution was prepared by dispersing a submicrometric powder of ZnO crystallites in THF. Then we take just the part of solution near the surface which containing only very small crystallites of ZnO and inject it in host solution. The mixture was stirred for several minutes and then allowed to stabilize. Thin films of hybrid nanocomposite ZnO/PMMA were deposited on glass substrate by spin coating technique performed under various conditions depending on the viscosity of the mixture.
3-CHARACTERIZATION OF ZNO/PMMA NANOCOMPOSITE THIN FILM 3.1 X-ray diffraction
Structural properties of thin films were studied by using X-ray Bruker diffractometer D8 Advance model with Ni filtered Cu radiation generated at 30 kV and 30 mA (λCuKα = 1.542 Ả) in the range of diffraction angles 2θ = 30°
to 65° at a rate of 2° (in 2θ ) per minute.
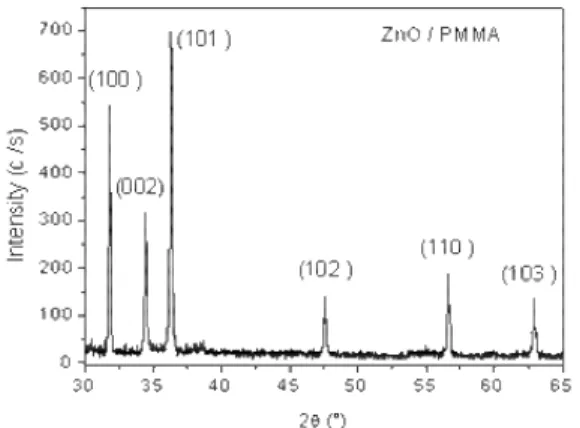
Figure 1 shows the diffraction spectrum of the ZnO/PMMA nanocomposite thin film obtained at room temperature. We can observe a series of diffraction peaks at 2θ = 31.75°, 34.41°, 36.24°, 47.52°, 56.58° and 62.85° which correspond to (100),(002), (101), (102), (110) and (103) plans of the hexagonal (wurtzite) ZnO structure. This result indicates the presence of ZnO nanoparticles in host amorphous matrix PMMA with an arbitrary orientation of crystallites. And peaks width provides information that ZnO particles have nanometric size. The small shift in positions of diffraction peaks, compared to the positions of the JCPDS card (36-1451) of ZnO, is due to the contraction of lattice parameters induced by reducing the size.
ICTON-MW'09 FrP.15
2
Fig. 1. XRD spectrum of ZnO/PMMA nanocomposite thin film deposited on glass substrate at room temperature.
3.2 Absorption measurement
Optical absorption was measured in the wavelength range 350 – 850 nm by using an UV-VIS-NIR spectrophotometer (perking Elmer, model Lambda 19). The absorption spectrum data of ZnO/PMMA nanocomposite were collected at room temperature. The spectrum (Fig. 2) exhibits absorption bands at 624.81, 461.14 and 377.70 nm with weak intensity which indicates a small concentration of ZnO nanoparticles in PMMA matrix. The optical gap of ZnO particles (Fig. 3) is determinated using the second derivative method [8-9] and was found equal to 3.43 eV. Also the absorption edge shifts to the shorter wavelengths compared to one of ZnO massive crystal. This shift is a consequence of quantum confinement induced by the small size of ZnO crystallites which was estimated by effective mass approximation model [18] to 4.77 nm. This size indicates that ZnO crystallites are in intermediate confinement because exciton Bohr radius in massive ZnO is about 2.4 nm.
Fig. 2. UV-Visible spectrum of ZnO/PMMA nanocomposite thin film.
Fig. 3. Optical gap of ZnO nanocrystallites in PMMA matrix.
ICTON-MW'09 FrP.15
3 3.3 Photoluminescence measurement
The photoluminescence (PL) spectrum was carried out by exciting the samples with continuous wave from a Q-switched Nd:YAG laser working at 355 nm. For the measurements at low temperature (77°K) samples were mounted in a variable temperature cryogenic Dewar.
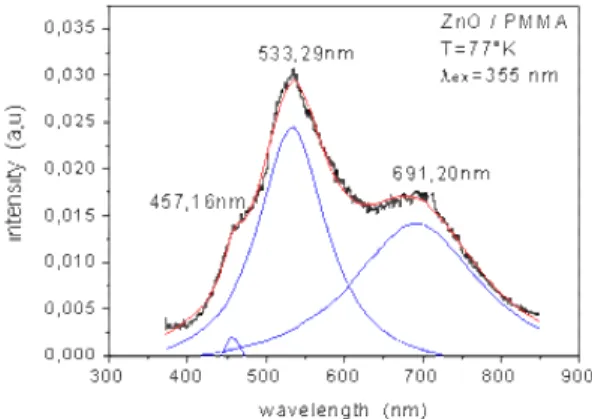
The photoluminescence spectrum (excitation with 355 nm) shows a wide and intense emission band covering the whole 400 – 700 nm visible area with remarkable peaks located at 457.16, 533.29 and 691.20 nm (Fig. 4). The blue emission (457.16 nm) is due to excitonic transitions. The green emission at 533.29 nm (2.32 eV) has an energy smaller than the band gap of ZnO (3.2 eV) and corresponds to an electronic transition via a defect. It is due to trapped deep holes assigned to oxygen vacancies in ZnO [10]. The red emission at 691.20 nm is due to surface electrons and others imperfections.
These results confirm the dispersion of ZnO nanocrystallites in the amorphous polymer PMMA and reveal a strong optical activity in the visible area for the fabricated ZnO/PMMA nanocomposite.
Fig. 4. Photoluminescence spectrum of ZnO/PMMA nanocomposite thin film at low temperature 77 °K and excitation with 355 nm.
4. CONCLUSION
The ZnO/PMMA nanocomposite thin films have been fabricated using a simple chemical technique. The results of structural characterization confirm the incorporation of ZnO nanocrystallites with hexagonal structure in the amorphous PMMA host matrix. The optical investigation reveals an intense optical activity in the visible area for the fabricated samples. The observed blue shift of absorption and emission bands is mainly due to the quantum confinement induced by the nanometric size (4.77nm) of ZnO crystallites.
ACKNOWLEDGEMENTS
The authors acknowledge the Service Commun d’Imageries et Analyses Microscopiques of the University of Angers for performing AFM measurements.
REFERENCES
[1] R.F. Service. Science 276 (1997) 895.
[2] D. P. Norton and all. Mater. Today 7 (2004)34.
[3] S.J. Pearton, D.P. Norton, K. Ip, Y.W. Heo, T. Steiner. Prog. Mater. Sci. 50 (2005) 293. [4] J.H. Kim, W.C. Choi, H.Y. Kim, Y. Kang, Y.-K. Park. Powder Technol. 153 (2005) 166-175. [5] L.C. Damonte and all. Powder Technol. 148 (2004) 15–19.
[6] X.Y. Zhao, B.C. Zheng, C.Z. Li, H.C. Gu. Powder Technol. 100 (1998) 20-23. [7] P. Nemecqnd and all. Mater. Sci. Eng. B69-70 (2000) 500-50.
[8] J. Joseph-Charles, M. Bertucat, P. Levillain. Bull. Soc. Pharm. Bordeaux, 1997, 136, 56-76 [9] Y. Kayanuma. Phys .Rev B 38 (1988), 9797-9805.
[10] D. Sridevi, K.V. Rajensran. Bull. Mater. Sci., Vol. 32, No. 2, April 2009, pp. 165-168.