HAL Id: hal-03106430
https://hal.inrae.fr/hal-03106430
Submitted on 11 Jan 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
bacterial symbionts but has no effect on the form or
parameters of Taylor’s law
Robin Guilhot, Simon Fellous, Joel Cohen
To cite this version:
Robin Guilhot, Simon Fellous, Joel Cohen. Yeast facilitates the multiplication of Drosophila bacterial symbionts but has no effect on the form or parameters of Taylor’s law. PLoS ONE, Public Library of Science, 2020, 15 (11), pp.e0242692. �10.1371/journal.pone.0242692�. �hal-03106430�
RESEARCH ARTICLE
Yeast facilitates the multiplication of
Drosophila bacterial symbionts but has no
effect on the form or parameters of Taylor’s
law
Robin GuilhotID1*, Simon Fellous1, Joel E. CohenID2,3,4
1 CBGP, INRAE, CIRAD, IRD, Montpellier SupAgro, Univ Montpellier, Montpellier, France, 2 Laboratory of Populations, Rockefeller University, New York, New York, United States of America, 3 Earth Institute and Department of Statistics, Columbia University, New York, New York, United States of America, 4 Department of Statistics, University of Chicago, Chicago, Illinois, United States of America
*guilhoro@gmail.com
Abstract
Interactions between microbial symbionts influence their demography and that of their hosts. Taylor’s power law (TL)–a well-established relationship between population size mean and variance across space and time–may help to unveil the factors and processes that determine symbiont multiplications. Recent studies suggest pervasive interactions between symbionts in Drosophila melanogaster. We used this system to investigate theoret-ical predictions regarding the effects of interspecific interactions on TL parameters. We assayed twenty natural strains of bacteria in the presence and absence of a strain of yeast using an ecologically realistic set-up with D. melanogaster larvae reared in natural fruit. Yeast presence led to a small increase in bacterial cell numbers; bacterial strain identity largely affected yeast multiplication. The spatial version of TL held among bacterial and yeast populations with slopes of 2. However, contrary to theoretical prediction, the facilita-tion of bacterial symbionts by yeast had no detectable effect on TL’s parameters. These results shed new light on the nature of D. melanogaster’s symbiosis with yeast and bacteria. They further reveal the complexity of investigating TL with microorganisms.
Introduction
Animals and plants are often associated with several symbiotic microorganisms, the interac-tions of which affect the ecology and evolution of hosts and symbionts alike [1,2]. Encompass-ing the diversity of symbiotic communities is challengEncompass-ing. However, experimental systems of modest complexity enable the investigation of microbial interactions, their mechanisms and consequences for host phenotypes [3,4] and the dynamics of microbial symbionts [5]. Con-ceptual tools developed by ecologists for the study of population and community ecology may be used to unveil general processes at play in symbiotic communities [1,6].
Among these tools, Taylor’s law (TL) may be used to investigate the spatial and temporal distributions of symbiotic populations of microorganisms. TL asserts that the variance of
a1111111111 a1111111111 a1111111111 a1111111111 a1111111111 OPEN ACCESS
Citation: Guilhot R, Fellous S, Cohen JE (2020)
Yeast facilitates the multiplication of Drosophila bacterial symbionts but has no effect on the form or parameters of Taylor’s law. PLoS ONE 15(11): e0242692.https://doi.org/10.1371/journal. pone.0242692
Editor: Guido Favia, Universita degli Studi di
Camerino, ITALY
Received: June 23, 2020 Accepted: November 8, 2020 Published: November 23, 2020
Peer Review History: PLOS recognizes the
benefits of transparency in the peer review process; therefore, we enable the publication of all of the content of peer review and author responses alongside final, published articles. The editorial history of this article is available here:
https://doi.org/10.1371/journal.pone.0242692
Copyright:© 2020 Guilhot et al. This is an open access article distributed under the terms of the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability Statement: The dataset is
available in the open data repository Zenodo (DOI:
population density (or size) is a power function of the mean population density (or size) across a set of replicate populations [7]. This power-law relationship is equivalent to a linear relation-ship on log-log coordinates:log(variance of population density) = a + b.log(mean of population density). A spatial TL holds when the mean and the variance are calculated over populations that differ in spatial location and the variance is a power function of the mean. A temporal TL holds when the mean and the variance are calculated over different points in time for each population and the variance is a power function of the mean. Many other variants of TL exist. TL holds in populations of various organisms [8] including bacteria [9,10] and can be of inter-est to address quinter-estions and resolve issues in conservation biology [11], epidemiology [12,13], human demography [14,15], fisheries [16], forestry [17], and crop protection [18]. Whether the relationship between population means and variances follows TL, and TL’s parameters when TL holds, can shed limited light on the demographic processes in the populations stud-ied [8,19]. For example, a TL’s slopeb that falls in the range from 1 to 2 and differs from 2 may be due to interspecific interactions within ecological communities such as competition, predation or parasitism [8,20].
In this study, we investigated whether the spatial TL describes well the relation of the vari-ance to the mean of population density in experimental populations of bacterial symbionts of Drosophila; and tested whether the addition of another Drosophila symbiont–a yeast–in the system affects TL.Drosophila melanogaster larvae rely on both bacteria and yeast for larval development [21,22]. However, although interactions between microorganisms associated withDrosophila flies have been reported [4], the pervasiveness and nature of these relation-ships in the wild remains unclear [22,23]. So far, no study has investigated the nature of yeast-bacteria interactions when associated toDrosophila larvae in ecologically realistic conditions using numerous strains freshly isolated from the wild. Our study hence aimed at describing and understanding the numerical effects of symbionts on each other in a context relevant to naturalDrosophila biology. We assayed twenty bacterial strains in the absence and in the pres-ence of a wildHanseniaspora uvarum yeast strain. The growth of these microorganisms was studied in association with the larvae of a wildD. melanogaster population reared in natural fruit.
Material and methods
Biological material
We used twenty bacterial strains that were isolated from wild adultDrosophila and fruit homogenates collected in Montpellier (SF’s garden and Montpellier SupAgro campus) and in Montferrier-sur-Lez (private property), southern France, except for threeAcetobacter and Lac-tobacillus strains (Table 1). The yeast strainHanseniaspora uvarum Dm6y (MN684824) was isolated from a wildD. melanogaster fly collected in Montpellier (SF’s garden). Most of these microbial taxa had previously been identified as associated withDrosophila [24–26]. All field-isolated microorganisms were cultured a single time in the laboratory and stored in sterile Phosphate-Buffered Saline (PBS) solution (20% glycerol) at -80˚C until they were used in experiments. This ensured minimal adaptation to the laboratory of the tested microorganisms. TheDrosophila melanogaster population was established from a few dozen wild individuals collected in Montferrier-sur-Lez (private property) about a year before the experiment. Con-ventionally reared flies had been maintained on a carrot-based laboratory medium (11.25 g.L-1 agar, 37.5 g.L-1sugar, 15 g.L-1corn meal, 37.5 g.L-1dried carrot powder (Colin Ingredients SAS), 22.5 g.L-1inactive dry yeast, 5 ml.L-1propionic acid, 3.3 g.L-1nipagin, 25 ethanol ml.L
-1
). All biological samples were collected with the permission of the private owners and the Montpellier SupAgro administration.
Funding: This project was supported by French
National Research Agency through the ‘SWING’ project (ANR-16-CE02-0015).
Competing interests: The authors have declared
Experimental design
Each experimental unit consisted of a halved grape berry that we first surface-sterilized follow-ing the procedure of Behar et al. [28] and embedded in jellified purified water (6 g.L-1agar) in a small petri dish. This protocol ensures the removal of all microorganisms present at the sur-face of fruit, but does not eliminate possible microbial endophytes [29]. Our results hence reflect the biology of symbiotic bacteria and yeast in conditions comparable to those of the field, but not in sterile medium. We manually deposited fifteen fly eggs on each half grape berry. The eggs had been laid by groups ofD. melanogaster females offered jellified grape juice plates (300 ml.L-1grape juice, 6 g.L-1agar) supplemented with cycloheximide (1 mg.L-1) to inhibit yeast growth, and chloramphenicol (10 mg.L-1) to inhibit bacterial growth. Repeated assays showed eggs produced in this manner are free of culturable microorganisms. After egg deposition, 105cells of each bacterium were inoculated to fruit flesh either alone (seven repli-cates per bacterium, except for strain R6b that had six replirepli-cates) or together with 105cells of the yeast strain (seven replicates per bacterium). The experiment was spread into seven blocks: one replicate of each bacterium× yeast combination was set up each day over seven days. Experimental units were incubated at 25˚C.
To measure microbial growth, we sampled fruit flesh after three days of incubation in a non-destructive fashion (analyses ofD. melanogaster development will be described in a sepa-rate manuscript). Fruit flesh was sub-sampled by randomly inserting ten sterile pipette tips in the surface of each fruit, collecting approximately one twentieth of the flesh in total. Flesh sam-ples were pooled per replicate, homogenized in 100μl of sterile PBS solution and serially diluted. Cell counts were carried out by plating samples (serially diluted) on appropriate selec-tive agar media (Table 1). For bacterial detection, we used Trypto-Casein-Soy (TCS) agar, Mannitol (MAN) agar or De Man, Rogosa and Sharpe (MRS) agar, all supplemented with cycloheximide (1 mg.L-1). For yeast detection, we used Yeast Extract-Peptone-Dextrose (YPD)
Table 1. Bacterial strains used in this study.
Strain Species Origin GenBank accession number Type of agar plate Temperature of incubation
R3b Gluconobacter sp. Grape berry not referenced MRS 24˚C R6b Staphylococcus sp. Grape berry not referenced TCS 24˚C R8b Gluconobacter thailandicus Grape berry not referenced TCS 24˚C Dm2b Yersinia sp. D. melanogaster not referenced TCS 24˚C Dm5b Gluconobacter sp. D. melanogaster not referenced TCS 24˚C Dm6b Escherichia coli D. melanogaster not referenced TCS 24˚C Dm8b Enterobacter sp. D. melanogaster not referenced TCS 24˚C
Dm10b Erwinia sp. D. melanogaster not referenced MRS 24˚C
Dm11b Enterobacteriaceae D. melanogaster not referenced MRS 24˚C
Ds3b Serratia fonticola D. suzukii not referenced TCS 24˚C Ds4b Gluconobacter kondonii D. suzukii not referenced TCS 24˚C Ds6b Lelliottia jeotgali D. suzukii not referenced TCS 24˚C Ds9b Erwinia injecta D. suzukii not referenced TCS 24˚C
Ds10b Lelliottia jeotgali D. suzukii not referenced TCS 24˚C
Ds25b Lelliottia jeotgali D. suzukii not referenced MRS 24˚C
Ds27b Serratia liquefaciens D. suzukii not referenced MRS 24˚C
Ds28b Lelliottia sp. D. suzukii not referenced MRS 24˚C
LpWJL
Lactobacillus plantarum D. melanogaster EU096230 [27] MRS 35˚C LbWJL Lactobacillus brevis D. melanogaster EU096227 [27] MRS 35˚C ApWJL Acetobacter pomorum D. melanogaster EU096229 [27] MAN 35˚C
agar supplemented with chloramphenicol (10 mg.L-1). Microbial colonies were counted after four days of incubation at species-specific temperatures and growth media (Table 1). We are confident the bacteria counted at the end of the experiment were those inoculated at the begin-ning for two reasons. First, we observed no bacterial growth in bacteria-free controls, which rules out the presence of culturable endophytes or contaminants in the fruits used for the experiment. Second, lack of cross-contaminations between bacterial treatments was attested by the systematic match between the morphology and metabolic abilities of the bacterial strains inoculated and those of the counted cells. These comparisons were possible because the majority of the strains used could be discriminated on the basis of the temperature and medium that enabled colony growth, as well as colony color, shape and transparency.
Statistical analyses
Analyses were split in two steps. First, we investigated the effects of each type of symbiont, and their interactions, on mean cell numbers. We hence tested whether yeast affected (i.e.
increased or decreased) bacterial densities using microbial counts from each replicate as data-points. We used a linear mixed model with the restricted maximum likelihood method. ‘Modality’ (i.e. presence / absence of yeast), ‘bacterial strain’ and their interaction were defined as fixed factors; ‘experimental block’ was defined as a random factor. A similar analysis was also carried out to test whether bacteria had variable effects on yeast growth. Finally, we tested whether numbers of yeast and bacterial cells correlated among bacterial treatments. To this end we used a symmetrical Major Axis regression with mean cell numbers per treatment as datapoints.
In a second stage, we investigated the variance of microbial cell numbers across twenty bac-terial strains. We hence tested whether the relationship between the means and variances of bacterial densities followed a mixed-species power law (i.e. log10(variance) =a + b.log10
(-mean)) [8] and whether its parametersa and b depended on yeast presence. We hence used a linear model with ‘modality’, ‘log10(mean)’ and their interaction as fixed factors. We further
included in early model formulations the quadratic term ‘[log10(mean)]2’ and its interaction
with ‘modality’ to investigate deviation from a power law. A similar analysis was carried out to test whether the relationship between the means and variances of densities of the yeast Hanse-niaspora uvarum in presence of the twenty bacterial strains followed a single-species power law (i.e. log10(variance) =a + b.log10(mean)). We used a linear model with ‘log10(mean)’ as a
fixed factor. We further included in early model formulations the quadratic term ‘[log10
(-mean)]2’ to investigate deviation from a power law. We found no significant evidence of non-linearity in the two relationships.
Analyzes were performed with JMP (SAS, 14.1) and R (3.6.2) (package lmodel2 [30]).
Results
Interactive effects of symbionts on microbial numbers
Bacterial density was significantly greater on average across treatments in the presence of yeast than in its absence (F-test ofmodality main effect: F1,229= 4.02, p = 0.0463). However, the
effect was modest–approximately 0.18 orders of magnitude (± 0.09 standard error of the mean)–compared to among-strain variation–approximately 3 orders of magnitude (F-test of bacterial treatment main effect: F19,229= 11.07, p < 0.0001) (Fig 1A). Even though some
bacte-rial strains seemed to benefit more from yeast presence than others, the interaction between the presence / absence of the yeast and the bacterial strain identity was not significant (F-test ofmodality�bacterial treatment interaction: F
Yeast density was significantly influenced by bacterial strain (F19,110= 4.12, p < 0.0001)
(Fig 2A). Unfortunately, we did not measure yeast growth in the absence of bacteria. It was
therefore not possible to assess whether bacteria had a generally beneficial or costly effect on the density ofH. uvarum yeast. Overall, yeast and bacterial mean densities did not correlate significantly. Using Major Axis regression, the correlation coefficient between bacterial mean density and yeast mean density was 0.2198; the slope was 2.7458 (95% CI [-2.1477, 0.4665])
5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 ApWJL Dm10 b Dm5b Dm6b Dm8b Ds3bLpWJL R6b R8bDs10b Ds25b Ds27b Ds28b Ds4b Ds6b Ds9bLbWJL R3b Dm 11b Dm2b log 10
bacterial density (CFU.ml
-1) 6.50 6.75 7.00 7.25 7.50 7.75
absence of yeast presence of yeast
log
10
bacterial density (CFU.ml
-1)
modality bacterial strain
A B
Absence of yeast Presence of yeast
Fig 1. Effect of yeast on bacterial numbers in fruit across all bacterial strains (A) and by bacterial strain (B). Bacterial densities are expressed in log10
of colony forming units (CFU) per milliliter of fruit homogenate. At the time of inoculation, log10bacterial density was 2.7 on the Y axis. Error bars
indicate standard errors around the mean.
https://doi.org/10.1371/journal.pone.0242692.g001 A B 3 4 5 6 7 8 6 6.5 7 7.5 8 bacterial strain 3 4 5 6 7 8 log 10
yeast density (CFU.ml
-1)
log
10
(mean of yeast density)
log10(mean of bacterial density)
ApWJL Dm10b Dm5b Dm6b Dm8b Ds3bLpWJL R6b R8bDs10b Ds25 b Ds27b Ds28b Ds4b Ds 6b Ds9bLbWJL R3b Dm1 1b Dm2b
Fig 2. Effect of bacterial identity on yeast numbers in fruit (A) and Major Axis regression of log10(mean of yeast densities) on log10(mean of
bacterial densities) (B). Yeast densities are expressed in log10of colony forming units (CFU) per milliliter of fruit homogenate. In Fig 2A, at the
time of inoculation, log10yeast density was 2.7 on the Y axis. Error bars indicate standard errors around the mean. In Fig 2B, each point represents
the mean yeast density and the bacterial density for a given bacterial strain.
(Fig 2B). Using OLS regression, the slope of yeast density as a function of bacterial density was 0.2847 (95% CI [-0.3411, 0.9104]) and the slope of bacterial density as a function of yeast den-sity was 0.1697 (95% CI [-0.2033, 0.5426]), neither of which was significantly different from zero. Thus Major Axis regression and OLS regression consistently reported no correlation sig-nificantly different from zero.
Relationships between cell numbers mean and variances
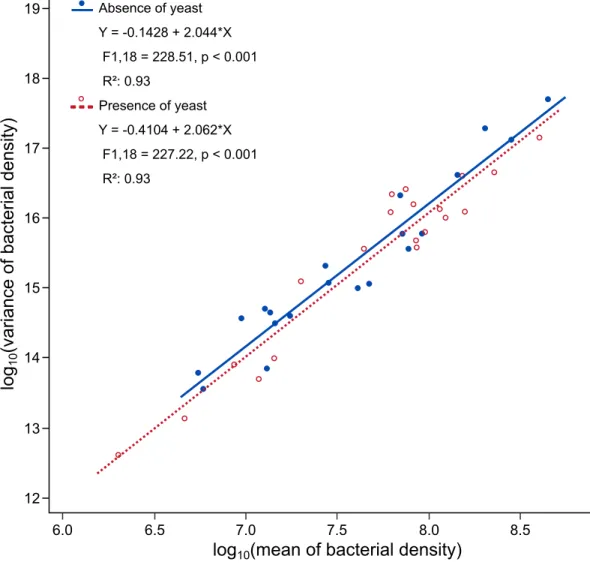
Overall, bacterial cell density across strains followed a spatial mixed-species TL. The slopes of the regression of log(variance bacterial density) on log(mean bacterial density) was 2.044 (95% CI [1.7601, 2.3284]) in the absence of yeast, and 2.062 (95% CI [1.7742, 2.3489]) in its presence. The intercepts and the slopes of the two linear regressions were not significantly different (F-test ofmodality main effect: F1,36= 1.56, p = 0.2199; F-test ofmodality�log10(mean) interaction:
F1,36= 0.01, p = 0.9290) (Fig 3). The coefficient of the quadratic term ‘[log10(mean)]2’ and the
interaction of the quadratic term with yeast presence or absence were not significant (F-test of
Fig 3. Taylor’s law holds in bacterial symbionts ofDrosophila, and TL parameters are not significantly affected by yeast
presence. Linear regressions of log10(variance) on log10(mean) for the densities of each bacterial strain in the absence (solid blue
line) and the presence (dashed red line) of yeast. Each point corresponds to one bacterial strain in the absence (blue filled circle •) or presence (red open circle o) of yeast.
[log10(mean)]2main effect: F1,34= 0.06, p = 0.8152; F-test ofmodality�[log10(mean)]2
interac-tion: F1,34= 3.06, p = 0.0891). The variance of bacterial density throughout the different
micro-bial treatments therefore appeared to be consistent with a power function of mean bacterial density, as described by TL, and the parameters of TL were not affected by the presence of the yeast symbiont.
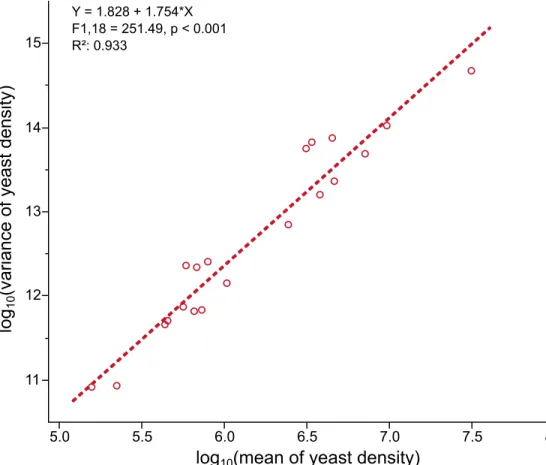
Taylor’s law also appeared to hold in yeast densities (Fig 4). We found a linear positive rela-tionship between log10(mean) and log10(variance) of yeast densities (F-test of log10(mean)
interaction: F1,18= 251.49, p < 0.001). The slope of the regression was 1.828 (95% CI [0.3867,
3.2686]). The quadratic term ‘[log10(mean)]2’ was not significant (F-test of [log10(mean)]2:
F1,17= 2.58, p = 0.1267).
Discussion
We demonstrated interactions betweenD. melanogaster’s bacterial and yeast symbionts in eco-logically realistic conditions. In our experiment, yeast presence slightly increased bacterial multiplication in fruit flesh infested withDrosophila larvae (Fig 1A). The twenty bacterial strains we tested also had variable effects on yeast multiplication (Fig 2). However, these inter-active effects did not change the spatial Taylor’s Law (TL): in the presence as in the absence of yeast, the variance of bacterial population density across the twenty bacterial strains was related to the mean of bacterial population density by a power law with a log-log slope indistin-guishable from 2 (Fig 3).
Fig 4. Taylor’s law holds in aHanseniaspora uvarum yeast strain in presence of different bacterial symbionts.
Linear regression of log10(variance of yeast density) on log10(mean of yeast density). Each point corresponds to a yeast
density for a given bacterial strain.
Interactions between microbial symbionts emerge as important factors affecting microbial dynamics [31,32]. However, the nature and prevalence of interactions between symbionts of Drosophila larvae in ecologically realistic conditions had never been investigated to our knowl-edge, in particular with wild microbial strains. Our study hence sheds light on novel aspects of Drosophila symbiosis in the field. We found that yeast apparently benefited bacteria, although modestly (Fig 1). Besides, we observed large variation inHanseniaspora uvarum yeast cell den-sities as a function of the strain of bacterium with which it shared the symbiotic environment
(Fig 2A). These interactive effects may be the result of cross-feeding, which is wide-spread in
symbiotic systems. It is well-established that yeast associated toDrosophila produce ethanol that is converted to acetic acid byAcetobacteraceae bacteria, a phenomenon referred to sour-rot in farming [33,34]. A similar cross-feeding interaction occurs betweenSaccharomyces yeast andLactobacillus bacteria [35], which are among the most important bacterial members of theD. melanogaster microbiota [24,26]. In our experimentH. uvarum yeast may have pro-vided nutrients to most of the bacterial strains, even though indirect effects through host phys-iology cannot be ruled out [5,36]. Independent of the mechanisms, interactive effects between symbionts may affect the dynamics of all partners at local and meta-population scales [37,38]. Interactions between organisms and their density dependence may affect TL parameters, as genetics, ecology and other spatio-temporal factors do [10,17,20,39–41]. Kilpatrick and Ives [42] predicted that the strength of competition between species would affect the slope in tem-poral versions of TL. A previous study tested a modification of this prediction (for spatial TL instead of temporal TL) with free-living bacteria that were grown alone or in competition in artificial environments of variable nutrient richness [9]. Though competition did occur between the tested bacteria, it did not change the slopes of the spatial TL from 2, with or with-out competition. Here, we pursued this investigation further with symbiotic microorganisms in ecologically realistic conditions that facilitated each other rather than competed. Our results showed the variance of bacterial population density in different replicates related to the mean of bacterial population density by a power law consistent with TL. As in the case of competi-tion [9], we found no significant effect of facilitation on the form or parameters of a spatial TL
(Fig 3). The discrepancy between our results for a spatial TL and Kilpatrick and Ives’s
predic-tions [42] about the effect of competition on a temporal TL highlights the importance of details in experimental tests of theoretical predictions and leaves open the challenge of finding experi-mental conditions suitable to test Kilpatrick and Ives’s predictions.
Another theoretical study showed that biological replicate numbers affect the parameters of TL [43]. TL’s slopes different from 2 may be undetectable if a stochastic multiplicative growth process in a Markovian environment (e.g. bacterial multiplication) is observed for a duration that exceeds the natural logarithm of the number of biological replicates. In our experiment, bacterial numbers were on average 1000 times that of the inoculum, implying that bacteria replicated at least ten times, since 210� 1000. Cell divisions likely exceeded 10 cycles as most bacteria in a closed system quickly move from an exponential growth phase to a plateau phase, where bacterial density is constant because appearing cells and dying cells are in equal num-bers. We had seven replicates, and a minimum of 10 replication cycles is larger than ln7 � 1.95. The slopes indistinguishable from 2 that were estimated in our study, and those of Ram-sayer et al. [9], may thus be statistical artifacts due to the long duration of the experiments rela-tive to the number of replicates. Computing a power analysis with TL parameters from our study revealed the minimum number of replicates needed to observe a significant change in the intercept and the slope would have been approximately 100 and 20 000 respectively. Such replication is very challenging to reach in most experimental systems. It is possible that inter-specific interactions such as competition [9] and facilitation (the present study) affect TL’s slope during relatively short time frames (e.g. a few hours for rapidly multiplying organisms
such as bacteria). Unveiling the ecological mechanisms that alter TL’s parameters using micro-bial organisms remains an exciting area of research that will necessitate experimental designs adapted to the specific demographic features of microorganisms.
Our study explored interactions between microbial symbionts associated withD. melanoga-ster larvae in ecologically realistic conditions. Screening twenty natural isolates of bacteria in the presence and absence of a freshly collected strain of the yeastH. uvarum, we found yeast had a small facilitative effect (Fig 1A) that did not differ statistically among bacteria (Fig 1B). Bacterial identity had a large influence on yeast multiplication (Fig 2). These interactions between symbionts demonstrate this phenomenon in natural conditions inD. melanogaster, a key model system of symbiosis studies. Effects of yeast on bacteria did not significantly affect the parameters of the spatial version of TL. TL is a powerful means of investigating the forces that affect the demography of many species, however challenging it may be to use TL in micro-bial systems.
Acknowledgments
We thank Guido Favia and two anonymous reviewers for comments on an earlier version of the manuscript. We thank Edouard Jurkevitch and Elodie Vercken for insightful comments. We thank Anne Xue´reb for her help at the beginning of the experiment and Laure Benoit for her help with the molecular identification of the bacterial strains.
Author Contributions
Conceptualization: Robin Guilhot, Simon Fellous. Data curation: Robin Guilhot, Joel E. Cohen.
Formal analysis: Robin Guilhot, Simon Fellous, Joel E. Cohen. Funding acquisition: Simon Fellous.
Investigation: Robin Guilhot, Simon Fellous, Joel E. Cohen. Methodology: Robin Guilhot, Simon Fellous, Joel E. Cohen. Project administration: Robin Guilhot, Simon Fellous. Supervision: Simon Fellous, Joel E. Cohen.
Validation: Simon Fellous, Joel E. Cohen.
Writing – original draft: Robin Guilhot, Simon Fellous, Joel E. Cohen. Writing – review & editing: Robin Guilhot, Simon Fellous, Joel E. Cohen.
References
1. Ferrari J, Vavre F. Bacterial symbionts in insects or the story of communities affecting communities. Phil Trans R Soc B Biol Sci. 2011; 366:1389–1400.
2. A´ lvarez-Pe´rez S, Lievens B, Fukami T. Yeast-bacterium interactions: the next frontier in nectar research. Trends Plant Sci. 2019; 24:393–401.https://doi.org/10.1016/j.tplants.2019.01.012PMID:
30792076
3. Callens M, Watanabe H, Kato Y, Miura J, Decaestecker E. Microbiota inoculum composition affects holobiont assembly and host growth in Daphnia. Microbiome. 2018; 6:56.https://doi.org/10.1186/ s40168-018-0444-1PMID:29566771
4. Gould A, Zhang V, Lamberti L, Jones E, Obadia B, Korasidis N, et al. Microbiome interactions shape host fitness. Proc Natl Acad Sci U S A. 2018; 115:e11951–e11960.https://doi.org/10.1073/pnas. 1809349115PMID:30510004
5. Sommer AJ, Newell PD. Metabolic basis for mutualism between gut bacteria and its impact on the Dro-sophila melanogaster host. Appl Environ Microbiol. 2019; 85(2):e01882–18.https://doi.org/10.1128/ AEM.01882-18PMID:30389767
6. Brown JJ, Mihaljevic JR, Des Marteaux L, Hrček J. Metacommunity theory for transmission of heritable symbionts within insect communities. Ecol Evol. 2020; 10:1703–1721.https://doi.org/10.1002/ece3. 5754PMID:32076545
7. Taylor L. Aggregation, variance and the mean. Nature. 1961; 189:732–735. 8. Taylor R. Taylor’s power law: order and pattern in nature. Academic Press; 2019.
9. Ramsayer J, Fellous S, Cohen JE, Hochberg ME. Taylor’s Law holds in experimental bacterial popula-tions but competition does not influence the slope. Biol Lett. 2011; 8:316–319.https://doi.org/10.1098/ rsbl.2011.0895PMID:22072282
10. Kaltz O, Escobar-Pa´ramo P, Hochberg ME, Cohen JE. Bacterial microcosms obey Taylor’s law: effects of abiotic and biotic stress and genetics on mean and variance of population density. Ecol Process. 2012; 1:1–6.
11. Beissinger S, McCullough D. Population viability analysis. University of Chicago Press; 2002. 12. Krasnov B, Morand S, Khokhlova I, Shenbrot G, Hawlena H. Abundance and distribution of fleas on
desert rodents: linking Taylor’s power law to ecological specialization and epidemiology. Parasitology. 2005; 131:825–837.https://doi.org/10.1017/S0031182005008590PMID:16336736
13. Cohen JE, Rodrı´guez-Planes LI, Gaspe MS, Cecere MC, Cardinal M V., Gu¨rtler RE. Chagas disease vector control and Taylor’s law. PLoS Negl Trop Dis. 2017; 11:e0006092.https://doi.org/10.1371/ journal.pntd.0006092PMID:29190728
14. Xu M, Brunborg H, Cohen JE. Evaluating multi-regional population projections with Taylor’s law of mean-variance scaling and its generalisation. J Popul Res. 2017; 34:79–99.
15. Cohen JE, Bohk-Ewald C, Rau R. Gompertz, Makeham, and Siler models explain Taylor’s law in human mortality data. Demogr Res. 2018; 38:773–841.
16. Xu M, Kolding J, Cohen JE. Sequential analysis and design of fixed-precision sampling of Lake Kariba fishes using Taylor’s power law. Can J Fish Aquat Sci. 2018; 76:904–917.
17. Cohen JE, Lai J, Coomes DA, Allen RB. Taylor’s law and related allometric power laws in New Zealand mountain beech forests: the roles of space, time and environment. Oikos. 2016; 125:1342–1357. 18. Do¨ring TF, Knapp S, Cohen JE. Taylor’s power law and the stability of crop yields. F Crop Res. 2015;
183:294–302.
19. Cohen JE. Every variance function, including Taylor’s power law of fluctuation scaling, can be produced by any location-scale family of distributions with positive mean and variance. Theor Ecol. 2019; 13:1–5. 20. Johnson PTJ, Wilber MQ. Biological and statistical processes jointly drive population aggregation:
using host–parasite interactions to understand Taylor’s power law. Proc R Soc B Biol Sci. 2017; 284:20171388.https://doi.org/10.1098/rspb.2017.1388PMID:28931738
21. Becher PG, Flick G, Rozpedowska E, Schmidt A, Hagman A, Lebreton S, et al. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct Ecol. 2012; 26:822– 828.
22. Broderick NA, Lemaitre B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes. 2012; 3:307–321.https://doi.org/10.4161/gmic.19896PMID:22572876
23. Fischer CN, Trautman EP, Crawford JM, Stabb E V, Handelsman J, Broderick NA. Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. Elife. 2017; 6: e18855.https://doi.org/10.7554/eLife.18855PMID:28068220
24. Chandler JA, Lang JM, Bhatnagar S, Eisen JA. Bacterial communities of diverse Drosophila species: ecological context of a host–microbe model system. PLoS Genet. 2011; 7:e1002272.https://doi.org/10. 1371/journal.pgen.1002272PMID:21966276
25. Bellutti N, Gallmetzer A, Innerebner G, Schmidt S, Zelger R, Koschier EH. Dietary yeast affects prefer-ence and performance in Drosophila suzukii. J Pest Sci. 2018; 91:651–660.https://doi.org/10.1007/ s10340-017-0932-2PMID:29568250
26. Staubach F, Baines JF, Ku¨nzel S, Bik EM, Petrov DA. Host species and environmental effects on bacte-rial communities associated with Drosophila in the laboratory and in the natural environment. PLoS One. 2013; 8:e70749.https://doi.org/10.1371/journal.pone.0070749PMID:23967097
27. Ryu J-H, Kim S-H, Lee H-Y, Bai JY, Nam Y-D, Bae J-W, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008; 319:777–782.
https://doi.org/10.1126/science.1149357PMID:18218863
28. Behar A, Jurkevitch E, Yuval B. Bringing back the fruit into fruit fly-bacteria interactions. Mol Ecol. 2008; 17:1375–1386.https://doi.org/10.1111/j.1365-294X.2008.03674.xPMID:18302695
29. Compant S, Mitter B, Colli-Mull JG, Gangl H, Sessitsch A. Endophytes of grapevine flowers, berries, and seeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb Ecol. 2011; 62:188–197.https://doi.org/10.1007/s00248-011-9883-y
PMID:21625971
30. Legendre P. 2018. Package ’lmodel2’. Model II Regression. R library. Available from: https://cran.r-project.org/web/packages/lmodel2/index.html.
31. Seth EC, Taga ME. Nutrient cross-feeding in the microbial world. Front Microbiol. 2014; 5:350.https:// doi.org/10.3389/fmicb.2014.00350PMID:25071756
32. Hassani MA, Dura´n P, Hacquard S. Microbial interactions within the plant holobiont. Microbiome. 2018; 6:58.https://doi.org/10.1186/s40168-018-0445-0PMID:29587885
33. Barata A, Santos SC, Malfeito-Ferreira M, Loureiro V. New Insights into the ecological interaction between grape berry microorganisms and Drosophila flies during the development of sour rot. Microb Ecol. 2012; 64:416–430.https://doi.org/10.1007/s00248-012-0041-yPMID:22438040
34. Rombaut A, Guilhot R, Xue´reb A, Benoit L, Chapuis MP, Gibert P, et al. Invasive Drosophila suzukii facilitates Drosophila melanogaster infestation and sour rot outbreaks in the vineyards. R Soc Open Sci. 2017; 4:170117.https://doi.org/10.1098/rsos.170117PMID:28405407
35. Ponomarova O, Gabrielli N, Se´vin D, Mu¨lleder M, Zirngibl K, Bulyha K, et al. Yeast creates a niche for symbiotic lactic acid bacteria through nitrogen overflow. Cell Syst. 2017; 5:345–357.https://doi.org/10. 1016/j.cels.2017.09.002PMID:28964698
36. Ze´le´ F, Magalhães S, Ke´fi S, Duncan A. Ecology and evolution of facilitation among symbionts. Nat Commun. 2018; 9:1–12.https://doi.org/10.1038/s41467-017-02088-wPMID:29317637
37. Gilbert DG. Dispersal of yeasts and bacteria by Drosophila in a temperate forest. Oecologia. 1980; 46:135–137.https://doi.org/10.1007/BF00346979PMID:28310639
38. Buser CC, Newcomb RD, Gaskett AC, Goddard MR. Niche construction initiates the evolution of mutu-alistic interactions. Ecol Lett. 2014; 17:1257–1264.https://doi.org/10.1111/ele.12331PMID:25041133 39. Anderson R, Gordon D, Crawley M, Hassell M. Variability in the abundance of animal and plant-species.
Nature. 1982; 296:245–248.
40. Mellin C, Huchery C, Caley MJ, Meekan MG, Bradshaw CJA. Reef size and isolation determine the tem-poral stability of coral reef fish populations. Ecology. 2010; 91:3138–3145. https://doi.org/10.1890/10-0267.1PMID:21141175
41. Lagrue C, Poulin R, Cohen JE. Parasitism alters three power laws of scaling in a metazoan community: Taylor’s law, density-mass allometry, and variance-mass allometry. Proc Natl Acad Sci U S A. 2015; 112:1791–1796.https://doi.org/10.1073/pnas.1422475112PMID:25550506
42. Kilpatrick A, Ives A. Species interactions can explain Taylor’s power law for ecological time series. Nature. 2003; 422:65–68.https://doi.org/10.1038/nature01471PMID:12621433
43. Giometto A, Formentin M, Rinaldo A, Cohen JE, Maritan A. Sample and population exponents of gener-alized Taylor’s law. Proc Natl Acad Sci U S A. 2015; 112:7755–7760.https://doi.org/10.1073/pnas. 1505882112PMID:25941384