HAL Id: inserm-02177273
https://www.hal.inserm.fr/inserm-02177273
Submitted on 8 Jul 2019
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Analysis and expression of a cloned pre-T cell receptor
gene
Claude Saint-Ruf, Katarina Ungewiss, Markus Groettrup, Ludovica Bruno,
Hans Fehling, Harald von Boehmer
To cite this version:
Claude Saint-Ruf, Katarina Ungewiss, Markus Groettrup, Ludovica Bruno, Hans Fehling, et al.. Analysis and expression of a cloned pre-T cell receptor gene. Science, American Association for the Advancement of Science, 1994, 266 (5188), pp.1208-1212. �10.1126/science.7973703�. �inserm-02177273�
18. Russian Federation, statute 2667-1 (4 April 1992). 19. L.Grinberg, personal communication.
20. V. M. Popugaylo, R. P. Sukhanova, M.I. Kukhto, in Current Problems of Anthrax Prophylaxis in the USSR (Moscow, 1974), p. 50.
21. S. R. Hanna, G. A.Briggs, R. P. Hosker, Handbook onAtmospheric Diffusion (U.S. Department of Ener-gy Report No. DOE/TIC-1 1223, Washington, DC, 1982).
22. D.E. Davids and A. R.Lejeune, Secondary Aerosol Hazard in the Field (Defence Research Establish-mentSuffield Report No. 321, Ralston, Alberta, Can-ada, 1981).
23. A.Birenzvige, Inhalation Hazard from Reaerosolized Biological Agents: A Review(U.S. Army Chemical Research,Development and Engineering Center Re-portNo.TR-413, Aberdeen, MD, 1992).
24. P.S. Brachman, S. A. Plotkin, F. H. Bumford, M. M. Atchison, Am. J. Hyg. 72, 6 (1960).
25. D. W.Henderson,S. Peacock, F. C. Belton, J. Hyg. 54, 28(1956); C. A. Gleiser, C. C. Berdjis, H. A. Hartman,W.S. Gochenour, Br. J. Exp. Pathol. 44, 416(1963).
26. H. N.Glassman, Bacteriol. Rev. 30, 657 (1966). 27. A. V.Elkina,Zh. Mikrobiol.Epidemiol.Immunobiol.
1971(no. 9), 112 (1971).
28. J. V.Jemski and G. B.Phillips, in Methods ofAnimal Experimentation, W. 1. Gay, Ed. (Academic Press, NewYork, 1965), pp. 273-341.
29. A.C. Chamberlain, Proc. R. Soc. London A 296, 45 (1967).
30. T.W. Horst, in AtmosphericSulfur Deposition, D. S. Shriner, C. R. Richmond, S. E. Lindberg, Eds. (Ann ArborScience, Ann Arbor,Ml,1980),pp.275-283. 31. A.Bovallius and P.Anas,inProceedings of the 1st International Conference onAerobiology, Interna-tional Association for Aerobiology, Munich, West Germany, 13 to 15 August 1978, A. W. Frankland, E. Stix, H. Ziegler, Eds. (Erich Schmidt, Berlin, 1980), pp. 227-231.
32. G. A. Cristy and C. V. Chester, Emergency Protec-tionAgainst Aerosols (Report No. ORNL-5519, Oak Ridge National Laboratory, Oak Ridge, TN, July 1981).
33. D.S. Ditmer and R. M. Grebe, Eds., Handbook of Respiration(Saunders, Philadelphia, 1958). 34. G.A.Young, M.R.Zelle, R.E. Lincoln, J. Infect. Dis.
79, 233 (1946).
35. J. V.Jemski, personal communication. 36. C.I. Bliss, Ann. Appl. Biol. 22,134 (1935). 37. H. A. Druett, D. W. Henderson, L. Packman, S. J.
Peacock, J. Hyg. 51, 359 (1953).
38. H. A.Druett, Nature 170, 288 (1952).
39. R. Scherrer and V. E. Shull, Can. J. Microbiol. 33, 304 (1987).
40. We thank A. V. Yablokov, Counsellor to the Presi-dentof Russia for Ecology and Health, for letters of introduction; Ural State University and its then rec-tor,P. E. Suetin, for inviting us to Ekaterinburg; S. F.Borisov, V. A. Shchepetkin, and A. P. Tiutiunnik forassistance and advice; members of the Ekater-inburg medicalcommunity and the Sverdlovskaya Oblast SanitaryEpidemiological Service for discus-sions, notes, and documents; interview respon-dents for theircooperation; P. N. Burgasov, USSR Deputy Minister of Health at the time of the out-break, fordocuments regarding livestock deaths; andPeople's Deputy L. P. Mishustina for the ad-ministrative list of those who died. I. V. Belaeva assisted with interviews. We also thank B. Ring, W. H.Bossert, P. J. M. Cannone, M. T. Collins, S. R. Hanna, J. V.Jemski, D. Joseph, H. F. Judson, M. M.Kaplan, J. Medema, C. R. Replogle, R. Stafford, J. H. Steele, and E. D. Sverdlov. Supported by grants to M.M. from the John D. and Catherine T. MacArthurFoundation and the Carnegie Corpora-tion of NewYork.This article is dedicated to Alex-anderLangmuir.
*
RESEARCH ARTICLE
Analysis and Expression of
a
Cloned Pre-T Cell
Receptor Gene
Claude
Saint-Ruf,*
Katharina
Ungewiss,*
Marcus
Groettrup,
Ludovica Bruno, Hans Joerg
Fehling,
Harald von Boehmer
The T cell antigen receptor (TCR)P3
chain regulates early Tcell development in the absence of the TCRoL chain. The developmentally controlled gene described here encodesthe pre-TCRa (pTcx)chain, which covalently associates with TCRf andwith the CD3 proteins forms a pre-TCR complex that transduces signals in immature thymocytes. Unlike the X5 pre-B cell receptor protein, the pTcx chain is a type I transmembraneproteinwhosecytoplasmictailcontainstwopotentialphosphorylation sites and aSrc homology3 (SH3)-domain bindingsequence. Pre-TCRa transfection experiments indicated that surfaceexpression of the pre-TCR is controlled by addi-tionaldevelopmentallyregulatedproteins. Identification ofthepTagenerepresentsanessential step in the structure-function analysisof the pre-TCR complex.
T
celldevelopmenttakes place indiscrete stepsduring which theTCRgenesare rear-ranged and expressed in temporal order. During development ofTCRax-expressing
cells the
TCRP
gene isrearrangedandex-pressedbefore the TCRao gene(1,2). With-outTCRrearrangement thedevelopmentof Tcells isarrestedatanearly stage(3-5).By introducing
TCRP
transgenes into mice thatare defectivefor rearrangement of an-tigen receptor genes, it was shown thatTCRP
proteins, in the absence of TCRaC. Saint-Ruf is at the Unite INSERM 373, Institut Necker, 75730Paris, Cedex15,France.K.Ungewiss,
M.Groettrup,L.Bruno,H.J.Fehling,andH.von Boe-hmer are at the Basel Institute forImmunology, CH-4005Basel, Switzerland.
'The first two authorscontributedequallytothis work.
chains,aresufficienttopromoteearlyTcell development (6-8). Although such mice
are still rearrangement-defective, their
im-mature thymocytes (which express neither
theCD4norCD8proteins) beginto express
CD4andCD8coreceptors,transcripts of the TCRcxlocus become detectable (7),and the number ofthymocytes increases (6-8). In-troduction of
TCRP
transgenesintonormalmice suppresses rearrangement of
endoge-nous
TCRP
genes(9, 10).The TCRI trans-gene is expressed on the cell surface intheabsence of TCRaLproteins inboth normal (11) as well as in rearrangement-defective
mice (7, 8, 12) inan80-kD disulfied-linked
complexand as a
glycosyl-phosphatidylino-sitol(GPI)-linked 40-kDmonomer.
The presenceof theTCRBchaininthe
80-kD complex suggested that either the
complex was a homodimer or that an un-known TCR chain was involved that may affect T cell maturation. A glycosylated chainof33kD(gp33) is paired with
TCRP
proteinsinaTCR,-transfected immatureT cell line (SCB.29) from severe combined immunodeficient (SCID) mice (12), but could not be identified in normal thymo-cytes(12, 13). Thegp33-TCR3
complex of SCB.29 cells is associated with CD3 pro-teins (8, 12) and cross-linking of TCR chains initiatesCa2+
mobilization. Thissuggestedthat this TCR, complex could be responsible for the developmental progres-sion observed in
TCRP
transgenic, rear-rangement-deficient mice, whereas theTCRP
GPI-linked monomer could repre-sentatransgenicartifact(14, 15).Wehave now cloned the gene encoding gp33 and examined its structure and expression. Be-causeofitsproperties, the gp33 proteinwas named the pre-TCRa (pTax) chain.Pre-Tcell receptora
(pTa)
expression inimmature Tcells. The pTa chaincanbe identified by two-dimensional (diagonal) gel electrophoresis, in whichthe disulfide-linkedpTao
protein under reducing condi-tionsmigrates away from the diagonaljust underneath the TCR, protein(12) (Figs. 1 and 2). The analytical method was scaled up to obtain sufficient amounts ofpTa
protein for microsequencing. In a first at-tempt a 20-amino-acid-long NH2-terminal sequence wasobtained; apeptide of the 18
NH2-terminalresidues wassynthesized and
injected into rabbits to obtain a
pTa-spe-cific antiserum. The antiserum was tested forbindingtothepTao protein.Tothis endlysates
from theTCRax-negative
SCB.29 cell line as well as theTCRcx3-expressing
B6.2.16BW hybridoma (12) were precipi-tated with themonoclonalantibody(mAb)F23.1 to
V38
proteins (16). Precipitates.M ... ..l ...
on July 9, 2019
http://science.sciencemag.org/
S ~
lail~
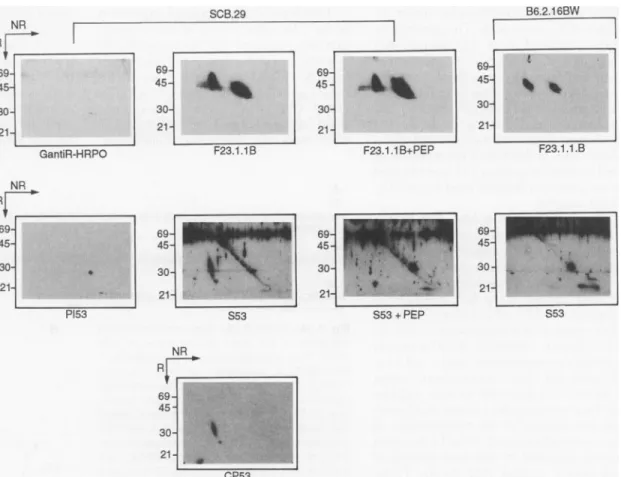
Fig. 1. Identification of the pTa chain by antibodies. Im-munoprecipitations ofTCR3
from either SCB.29 or B6.2.16BW cells were im-munoblotted with control re-agents or antibodies to
TCRI orpTot, with or with-out peptide (PEP) from the NH2-terminus of pTa (38). SCB.29, cell line from SCID mouse transfected with
TCRj3;B6.2.1 6BW,
TCRao3-expressing T cell hybridoma; GantiR-HRPO, goat anti-bodies to rabbit immuno-globulin conjugated to horseradish peroxidase; F23.1, mAb toTCRB
(V.8);
P153, pre-immune serum from rabbit 53; S53, im-muneserum from rabbit 53 injected with NH2-terminal peptide ofpTax;CP53, affin-ity column-purified S53. Molecular sizes are given in kilodaltons. SCB.29 B6.2.16BW RR ~~~~~~~~~~~~~~~~~~~~~~~969
: 'r' 69- :~~~~~4545j : 69t16= ~~~30-21 212121
GantiR-HRPO F23.1.1B F23.1.1 B+PEPF23.1.1 .B NR RV 69 4- t 45- 21-P153 69 69 -69-45 45 45 30 30 30-21- 21 - 21 S53 S53+PEP S53 NRr
69-45 -30fl21f-were separated on diagonal gels and the proteinsanalyzed in Western immunoblots by staining with either mAb F23.1 or serum from immunized rabbit 53 (Fig. 1). The
TCRP
mAbstained proteins off and on thediagonal, corresponding to disulfide-linked and-unlinked
TCRP
proteins,respectively, in the lysates of both SCB.29 and B6.2.16.BW cells (Fig. 1, top) whereas the control GantiR-HRPO [goat anti-rabbit immunoglobulin (Ig) conjugated to horse-radish peroxidase]produced no signal. The pre-immune serum from rabbit 53 (PI53) likewise produced no signal (except for someweak reactivity with the 30-kD mark-er on the diagonal) whereas the immune serum (S53) stained in SCB.29 lysates a proteinoffthediagonal beneath theTCR,3protein thatwasnot stainedin B6.2.16BW lysates (Fig. 1, middle). The staining of the
pTac
proteincould be completely inhibitedby thespecific peptide, whereas the exten-sive background stainingcould not. Affin-itypurificationon apeptide column finally yielded areagent ofexquisitespecificity for the pTax protein that became visible as a streak ofdifferentiallyglycosylatedproteins as well as a nonglycosylated dot (Fig. 1, bottom). These results established that the protein sequence was obtained from the
pTa protein and that the antiserum could be used to analyze expression ofthe
pTa-TCRP
complex invarious cells.BecausethepTot-TCRBcomplexin
thy-mocytes was notpreviously identified, we used the above approach to examine this
complex in thymocytes from TCRa,-defi-cient
(TCRct-/')
mice (Fig. 2).Wecould detectthepTaL-TCRP
complex by precip-itation with the mAb H57 to allTCRP
proteins (17) from lysates of TCRC-'-thymocytes. Thus thepTa-TCRP
com-plex does not represent a peculiarity of a single cell line, but is also formed and glycosylated in immature thymocytes.Cloning of the pTa chain complemen-taryDNA(cDNA).ToobtainapTachain cDNA sequence we prepared a cDNA li-brary from the SCB.29 cell line in lambda ZAP II (Stratagene). Alysateof the library was prepared and amplified by PCR (poly-merase chain reaction) with degenerate or nondegenerateoligonucleotides,
correspond-ing to the most 3' part of the pTcx
NH2-Fig. 2. Identification of the pTa
chain inTCR-'-thymocytes(4 x SC 109 cells) and SCB.29 (2 x 107 C cells). Two-dimensional
SDS-PAGE of immunoprecipitates with H57(17). The pTa chainisrevealed by CP53 serum. The spot onthe diagonal may represent
nondisul-fide-linked pTa in the precipitate.
Stainingwith mAb F23.1 wassimi- '4 lar to that inFig.1.Methodswereas
in Fig. 1. Molecular sizesare indi-catedinkilodaltons.
terminal protein sequence and an oligonu-cleotide complementary to the lambda ZAP vector-sequence atthe 5' end of the cDNA inserts. Specific PCR products were identi-fiedby Southern (DNA) hybridization with another set of degenerate oligonucleotides
correspondingtothe pTcx sequence between the PCR primers. Hybridizing PCR frag-mentsweresubcloned and sequenced. In this way afragment ofapproximately 180 bp was obtained that encoded part of the 5' un-translated region, the leader sequence, and the sequencecorresponding tothe first five amino acids of thepTao protein. This frag-ment was thenused as a probe for conven-tional screening of the SCB.29 cDNA
li-brary. Several independent lambda clones were isolated that contained thefull-length
codingsequence ofpTcx. The sequence re-vealedan openreading frame of618
nucle-'B.29 -200 -97 -69 -45 -30 -21 200- 45- 30- 21-TCRa# thymocytes
SCIENCE VOL.266 * 18NOVEMBER 1994 1209
on July 9, 2019
http://science.sciencemag.org/
otides encoding a hydrophobic leader se-quence of 23 amino acids. The extracellular region comprised about 130 residues with similarity to the constant (C)domain ofthe immunoglobulin supergene family with two cysteines in position 31 and 91 correspond-ingto the invariant cysteines thatform the intrachaindisulfide bond in the Ig domain. Inaddition there were two potential glyco-sylation sites in this part of the molecule.A third cysteine inposition 119 could be used for aninterchain disulfide bond in the
pTa-TCRP
complex.The extracellular Ig-like domain has onlysomeweak (20to25 percent) homol-ogy with the Ig-like domain of TCRox, Ig lightand Ig heavy chain constant domains, aswellasx5,being mosthomologous to the constant domain of the IgA molecule. In contrastto X5(20),pTa doesnotcontain a J-like sequence but strictly ends at the Ig-like C domain. UnIg-like X5, thepTa protein containsatransmembrane region and a cy-toplasmic tail. The transmembrane region of about 20 hydrophobic residues includes twobasicaminoacids(arginine andlysine)
that are identical to the polar residues in the transmembrane part of the TCRoQ chain and are separated by the same number of hydrophobic residues (18). In the TCRa chain, these polarresidues are essential for the assembly and transport of the
TCR43-CD3 complex(18). The cytoplasmic region ofabout 31 residues isrich in proline resi-dues and could constitute a SH3-domain binding region. In addition, there are PPSRK and PPTHR sequences similar to the PPGHRmotif present in the
cytoplas-mictail of CD2, known to be involved in
CD2-dependentTcellactivation (19) and containing two potential phosphorylation
sitesfor protein kinase C(Fig. 3).
The sequenceanalysis indicates that the
pTaprotein is well suited for pairing with theTCR, chain. Eventhoughthereisonly
moderateidentitytoanyparticularmember of theIgsupergenefamily, this protein bears thehallmarks of thisfamily.The cysteinein position 119 as well as the transmembrane partseemdesignedtoform acomplexwith
TCRI and CD3 proteins. ThepTa-TCRB
heterodimer hasanasymmetrical shape be-causethe
TCRP
has twoand thepTcxonly
oneextracellularIg-likedomain.Onemight
expect therefore that another short protein maybenoncovalentlyattached tothe
mol-ecule, perhaps like the VpreB domain (20)
that is supposed to bind to the pre-B cell receptor. The relatively long cytoplasmic
tail ofpTa isdifferent from the short cyto-plasmic tail ofthe TCRa and
P
chains and theIg heavychains.Becauseofthepotential
SH3-domainbindingsequence,
phosphoryl-ationsites, andthe CD2 likesequences,the
pTa chainmay bedirectly involvedin sig-nal transductionbythepre-TCR.
1210
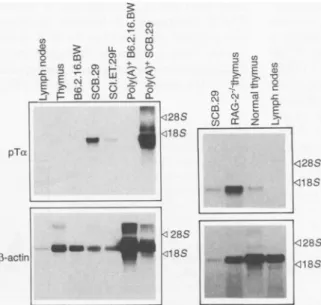
Developmentally regulated expression of the pTot gene. The pTax RNA expres-sion was analyzed by Northern (RNA) blotting: Total and polyadenylated [poly(A)
'I
RNA was isolated from various tissues or cell lines and probed with pTa cDNA (Fig. 4, left). The pTa message could be detected in total and poly(A)+ RNA from the immature,TCRI-trans-fected cell line SCB.29 as well as in the nontransfected parental line SCI.ET.27F
(12), but not at all in the
TCRao3-express-inghybridomaB6.2.16.BW (12). In mice, there was some expression in the thymus, whereas lymph node (Fig. 4) and spleen cells werenegative. Expression of thepTa
gene did not require rearrangement, as full-sizeRNAwasabundant in the thymus
A
-23 1 V
MLLHEWAMARTWLLLLLGVRCQALPSGIAGTPFPSLAPPITLLVDGRQHMLVVCLVLDAAPPGLDNPVWFSAGNGSALDA
-SIGNALPtElF= 0-CO
58 V V
FTYGPSLAPDGTWTSLAQLSLPSEELEAWEPLVCHTRPGAGGQNRBTHPLQLSGESSTARSCFPEPLGGTQRQVLWLSLL
138
RLLLFKLLLLDVLLTCSHLRLHVLAGQHLQPPPSRKSLPPTERIWTZ
+ I IM- -PKC-
-PKC-Fig. 3.(A) Predicted pTat sequence. Amino acid B
NH2
sequence of the open reading frame of pTa2cDNA.The nucleotide sequence is available from
the GenBank database, accession number ; U16958. Aminoacid numberingisgivenatthe left.
Locations of the potential N-linked glycosylation
sites(CHO), thepredicted leader (peptide signal)
NH2I-and transmembrane (TM) sequences, and the
potential protein kinase Cphosphorylation sites S S
(PKC) are shown. Thesequences PPSRK(169) pIa
and PPTHR(176)aresimilartothePPGHR motif pTa s S foundinCD2. The threecysteinesinpositions 31,
91, and119 are marked(V). DNA sequence anal-
7-S-S
ysiswasdonewith theGCG program (Geneticscomputer group, program manual for the GCG
package, version 7, April 1991). Homology
3
SU
R+searches in the EMBL(release 33.0, March 1994), ( /(1(1(
GenBank (release 83.0, February 1994) and UU+U SWISSPROT(release 28.0, February 1994) data 0000
bankswere done with theFASTA(39) program. COOH Sequence comparisonsandalignmentswith
in-munoglobulin superfamily domains were done COOH
withthe PILEUP(40) program.Single-letter abbreviations for the amino acid residuesare:A, Ala;C, Cys;
D,Asp; E,Glu; F, Phe; G, Gly; H, His;I,lie;K, Lys; L, Leu; M, Met; N, Asn; P,Pro;Q,Gin;R,Arg;S,Ser;
T, Thr; V, Val; W, Trp; and Y,Tyr. (B) The pTa-TCR,3 complex. _, Corresponds tothe N-linked glycosylation sites.P,representsproteinkinaseCphosphorylation sitesinthepTacytoplasmic tail. Two basicaminoacids,arginine(R') andlysine (K'),arelocatedin thepTatransmembraneregion.
Fig. 4. pTot mRNA expression in various cells and tissues. Total or
poly(A)+RNAwasisolated from cell linesandtissuesandthe RNA was separated on agarose gels. After transfer,the RNA wasprobed with alabeled cDNAspecificforpTcx; af-tertheoriginalprobewasstripped
fromthefilter, it wasagain
hybrid-ized with a labeled ,-actin probe
(41).
SCIENCE VOL.266 18NOVEMBER 1994
on July 9, 2019
http://science.sciencemag.org/
BI1mW1BX WAV
of RAG-2-'- (recombination activating gene 2) rearrangement-defective mice (21) and it was expressed higher in
RAG-2-/- thymocytes than in thymocytes from normal mice (Fig. 4). The latter result suggested that the pTco gene was predom-inantly expressed in the more immature thymocytes.
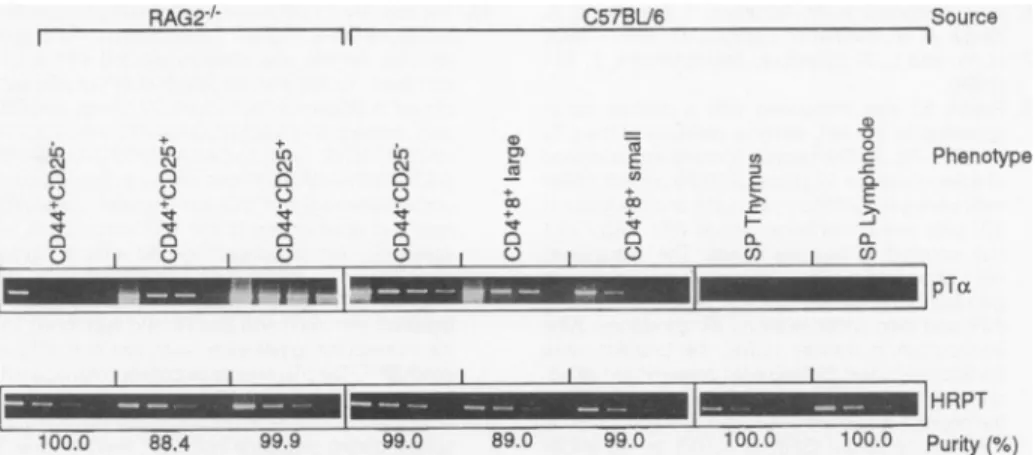
Expression in early thymocytes was confirmed by PCR analysis with
oligonu-cleotides specific for the pTcx gene (Fig. 5). The developmentally regulated pTot message was expressed in different amounts at successive stages of thymic development. There was high expression in CD44-CD25+ and CD44-CD25-double negative (CD4-CD8-) cells but therewasweaker expression inearlier and later stages of development. Small single positive (expressing only CD4 or CD8) thymocytes hadnodetectable pTat RNA. The difference in the intensity of the pTcx RNAbands betweenRAG-2-/- and nor-mal thymocytes in Fig. 4 is explained by the fact that most cells in the normal
thymus are small CD4+CD8+ cells, whereas in the RAG-2-'- thymus most cells are CD44-CD25+ and because CD44- CD25 + cells express more pTao RNAthansmall CD4+CD8+thymocytes.
RAG2-1-U) C14 0 C-) uO ,c
8
c) 1 11~~~~~~~~~~~~I v) C14 0 00*
C4 C) C) I* N 0jThus pTa isexpressed in the tl fore TCRI and TCRoL genes, may be some overlap in the exi pTot and TCRa genes. Coexp pTcx-TCRB and TCRot-TCR[3 he on the cell surface of TCRm-i SCB.29cells has been observed (2 fore this coexpression is theoretic ble inimmature thymocytes.
The transfection of pTa in deficient, mature T cell line. 1 results show that aglycosylated p complexistransported to the cell immature T cells. Because
TCRI
and pTcx only one extracellular ] main, one would expect thatTCRP
complex has an addition; valently associated Ig-like domc like the VpreB domain in the 1receptor (23). One might furth that without suchanadditionalp pTa-TCR3 complex maynotbe edto the cell surface.
To testwhetheror notthe p' complexwas sufficient for cells pression, we transfected a pTot into the
58ot-py.1
mature Tcel which expresses aTCRP
gene proteins that are essential for si pression of the o43 TCR, but lacC57BU6 (D 0) CD
0D
I0
E 0 to 00 0 E n-u,) D 0 c 0. C,) I-El~
il
l 100.0 88.4 99.9 99.0 89.0 99.0 100.0 100.0Fig.5.Semiquantitative analysisofpTa expressiononlymphocytesubsets.Thymocytes frorn
and normalC57BU6micewereseparated byfluorescence-activated cellsorting(FACS)orcc
dependent killing.Subsets of T cellsweresubjectedtopolymerchain reaction(PCR)asdescr
Sampleswerestained with ethidium bromide.SP, single positive(CD4orCD8)Tcells; HPR-thine-guanine phosphoribosyl transferase.
Fig. 6. Transfection ofa pTa construct into a mature, TCRa-deficient T cell line. Immunopre-cipitationsofTCRI fromSCB.29,SCI.ET.27F(the
nontransfectedSCID cell line), thepTa transfec-tantsof 58a- ,y. 1 (a TCRa-matureTcellline) (43)
named 58a-y.1-33andrepresentedbyclones 7,9,and10, and 58a- y.1 cellswereseparated
undernonreducing (NR)andreducing(R) condi-tions and immunoblottedwith theCP33pTa an-tiserum. Cl) CM. r-Fi U)% U.)U) U)0 NR LL 00 U)CU) 200- 97- 69- 45- 30-21-_
hymus be- chains. When these cellswere supertrans-but there fected with the pTot construct,
intracellu-pression
of larpTa-TCRP
dimers wereobserved, but )ression of in contrast to dimers in the immature T terodimers cell line SCB.29, the disulfide-linkedpTa transfected proteins in the mature T cell line were ?2).There- poorly or not at all glycosylated, indicat--allypossi- ing that most ofthe heterodimer did not leave the endoplasmic reticulum (Fig. 6). a TCRa- Also, surface expression of TCRI in the [he above pTa transfectants was not increased,Tcx-TCRP
whereas TCRca4 surface expression was surfacein observed after TCRQ. transfection. This 13 has two result was consistent with thehypothesis
Ig-like do- that another developmentally regulated the pTa- protein(VpreT) may be required for prop-al, nonco- er assembly, glycosylation, and transport ain, much tothe cell surface of thepTao-TCR13 com-pre-B cell plex. The result is of course equally con-ier expect sistent with the hypothesis that mature T )roteinthe cells may contain proteins that prevent transport-pTa-TCRP
complexes from leaving the endoplasmic reticulum and that thepTa-Tra-TCRP
TCRBcomplexcanonly be transported to ;urfaceex- the cell surface in the absence of theseconstruct retentionmolecules.
11 line (8), Regulation of T cell development by and CD3 the pre-TCR complex.Inconjunctionwith urface ex- experiments in TCR13 transgenic, rear-cks TCRcx rangement-defective SCID mice (6-8) and analogous experimentsin
TCRP
transgenicRAG-/-mice (21, 24), our results are con-Source sistent with the hypothesis that the pre-TCR complex is sufficient to promote T cell development in the absence of other TCR chains encodedby rearranging genes. Phenotype Although the experiments in the TCR,3
transgenic, rearrangement-deficient mice have indicated thatTCR13 proteinsare suf-ficient to induce T cellmaturation, studies pTa in
TCR-/-
mice suggest that aTCRP
proteinisnotessential for the expression of CD4 and CD8 molecules in developmentbecause CD4+CD8+ cells are present in
HRPT
TCRP-/-
mice (24). The possibility wasPurity(°) considered that
CD4+CD8+
thymocytes
inPuRity2(%)
TCR,13 mice wereof they8 lineage(be-npRAG-2-'-
cause they were absent inTCRB-/-omplement-
TCRy-/-mice) and that the TCRB protein,
ihypoxan-
wasessential
forexpression
ofCD4orCD8coreceptors in the
ao3
lineage
(24). There are, however, no experiments supporting thisview. Incontrast,reconstitutionofthy-musesfromSCID(25, 26)orRAG-/-mice (22) with either pro-T cells or ^y8T cells have shown thatthymocytesdevoid of TCR j0 V11 proteins can express CD4 and CD8
mole-culesaslongasotherTCR-bearing cellsare
>>0 present. Thus the pre-TCR complex may notdirectly regulateCD4 andCD8 expres-sionthrough intracellularsignaling.
Intracellular signaling by the pre-TCR R complex may, however, be essentialto ob-___ tainlarge numbers ofimmaturethymocytes.
Thiscanbededuced from experimentsthat showed that the proportion of productive
SCIENCE VOL. 266 18NOVEMBER 1994
.l
1211
on July 9, 2019
http://science.sciencemag.org/
rearrangements in CD44-CD25- (28) and total thymocytes (29) fromTCRa-/' mice were much higher than expected if cells of this phenotype could similarly expand, irre-spective of whether or not they carried productive
TCRPi
genes.Thus the pre-TCR complex may regulate survival and expan-sion of immature Tcells rather than regu-lating directly CD4 and CD8 gene expres-sion in the lineage ofa13 T cells.The role of the pre-TCR complex in T cell development is supported by other studies.InSCID(30) as well as in
TCRP-or RAG-deficient (31, 32) mice, the ef-fects of productiveTCRP
transgenes on thymocyte development can be mimicked by treating thymocytes of these mice with antibodies to CD3e. These results support the hypothesis that theTCRP
protein exerts its function through a pre-TCR complex that associates with CD3 pro-teins. It is not clear from these studies whethersignaling through CD3 is all that is required or whether the cytoplasmic part of the pTot chain plays an essential role. A potential role of the cytoplasmic tail of pTa needs to be analyzed under more physiological conditions.Arole of
p56lk
inthesignaling by the pre-TCR is implicated by studies in mice thatareLck-defective (33)orcarrya dom-inant negative Lck mutation (34). The Lck dominant negativemutationresultsin adevelopmentalarrestthat resembles that of rearrangement-deficient mice. On the other hand the introduction ofan activelck gene in rearrangement-deficient mice (35) or in normal mice (36) causes the same effects as a
TCRP
transgene. Thusp561ck
maybe part of thesignalingcascade ofthe pre-TCRcomplex. Because thecy-toplasmic tail ofthepTachaincontains a
proline-rich, potential SH3-binding do-main (37),
p561Ck
mayberecruited by thepTaL protein into the pre-TCR complex.
The cloning and expression analysis of thepTagene has established that the pre-TCR isexpressed in immature thymocytes
and opens the waytodetermine the precise structureand function ofthepre-TCR
com-plexinTcelldevelopment.
REFERENCESAND NOTES
1. D. H.Raulet,R. D.Garman,H.Saito, S. Tonegawa, Nature314, 101(1985).
2. R. H.Snodgrass,Z.Dembic,M.Steinmetz, H. von Boehmer, ibid.315,232(1985).
3. M.Bosma andA.M.Carrol,Annu.Rev.Immunol.9, 323(1991).
4. Y.Shinkai,Cell68,855(1992). 5. P.Mombaerts etal.,ibid., p.869.
6. H.vonBoehmer,Annu.Rev. Immunol.8,531(1990).
7. H.Kishietal.,EMBO J.10,93(1991).
8. M.Groettrup,A.Baron,G.Griffiths,R. Palacios,H. vonBoehmer, ibid.11, 2735(1992).
9. Y. Uematsu etal.,Cell 52, 831(1988).
10. R.G.Fenton,P.Marrack,J. W.Kappler, 0.
Kana-gawa,J.G. Seidman, Science241, 1089(1988). 11. H.von Boehmer et al., Proc.Natl. Acad. Sci. U.S.A.
85,9729(1988).
12. M. Groettrup etal., Cell 75, 283 (1993).
13. M. Groettrupand H. von Boehmer, Eur. J.Immunol. 23,1393(1993).
14. H.von Boehmer, Cell 76, 219 (1994).
15. M. Groettrupand H. von Boehmer, Immunol. Today 14, 6101 (1993).
16. U. D.Staerz, H.-G. Rammensee,J.D. Benedetto,M. J.Bevan, J. Immunol. 134, 3994 (1985). 17. R.T. Kubo, W. Born, J. Kappler, P. Marrack, M. J.
Pigeon,ibid. 142,2736(1989).
18. P. Cosson, S. P. Lankford, J. S. Bonifacio, R. D. Klausner, Nature 351, 414 (1991).
19. H.C. Chang, J. Exp. Med. 172, 351 (1990). 20. H.Karasuyama,A.Kudo,F.Melchers, ibid.,p. 969. 21. Y.Shinkai et al., Science 259, 822 (1993). 22. M.Groettrup, thesis, UniversityofBasel, Switzerland
(1993).
23. A. KudoandF. Melchers,EMBO J. 6, 2267(1987). 24. P. Mombaerts et al.,Nature 360, 225 (1992). 25. E. W.Shores,S.0. Sharrow, A. Singer, Eur. J.
Im-munol. 21, 973 (1991).
26. F. Lynch and E. M. Shevach,Int. Immunol. 5, 991
(1993).
27. H. R. Rodewald, unpublisheddata.
28. E.C. Dudley,H.T.Petne,L. M.Shah,M. J.Owen,A. C. Hayday, Immunity 1, 83 (1994).
29. C.A.Mallick, E. C. Dudley,J. L.Viney, M.J.Owen, A. C.Hayday,Cell 73,513 (1993).
30. C. N. Levelt, A. Ehrfeld, K. Eichmann, J. Exp. Med. 177, 707(1993).
31. C.N. Levelt,P. Mombaerts,A.Iglesias,S. Tonegawa, K. Eichmann, Proc. Natl. Acad. Sci. U.S.A.90,11401 (1993).
32. H. Jacobs etal.,Eur. J. Immunol.24, 934 (1994). 33. T. J. Molina etal., Nature 357, 161 (1992). 34. S. D. Levin, S. J. Anderson,K. A. Forbush, R. M.
Perlmutter,EMBOJ.12,1671(1993).
35. P. Mombaerts, S.J.Anderson, R.M.Perlmutter,T. W.Mak,S. Tonegawa, Immunity 1, 261 (1994). 36. S.J. Anderson, K. M.Abraham, T. Nakayama, A.
Singer, R.M.Perlmutter,EMBOJ. 11, 4877(1992). 37. H. Yu and L.S. Schreiber, StructuralBiol. 1, 417
(1994).
38. Rabbit 53 was immunized with a peptide corre-spondingtothe NH2-terminal residues of the pTot showninFig. 3. (The peptide containsanAla instead ofaSerinposition12.) Either SCB.29orB6.2.16BW cellswerelysedinTritonlysisbuffer and thelysateof 107cellswasimmunoprecipitated withmAb F23.1 (16) coupled to agarose beads. The precipitates wereseparatedbySDS-1 0percentpolyacrylamide gel electrophoresis(PAGE), first under nonreducing (NR) and then under reducing (R) conditions. After equilibration in transfer buffer, the proteins were transferredbywetblottingontopolyvinyliden difluo-ride membranes. Filterswereincubated witheither the preimmuneserum(P153,at adilutionof1 100), the immune serum (S53, at 1:100) ortheaffinity column-purified (CP53,at1:100)in phosphate-buff-ered saline containing0.4 percentTween and2 per-centfetal calfserum.Forpeptide competition, pep-tide(35pgg/ml)wasfirst incubatedwithS53serum or mAb F23.1for1hour. For detectionofTCRBprotein aF23.1 -biotinconjugatewasused. Afterthe first-stage reagents were removed, GantiR-HRPO at 1:20,000orstreptavidine-HRPO(1:5000)was add-edfor visualization oftherabbit and F23.1 antibod-ies,respectively.
39. W. M. R. Pearson and D. J. Lipman, Proc. Natl. Acad.Sci. U.S.A. 85,2444(1988).
40. D. F.Fengand R.S.Doolittle,J.Mol.Evol.35,359 (1987).
41. TotalRNA waspreparedfromcellsuspensions (thy-mocytesfromC57BU6mice andRAG-2-'- mice, andlymphnodecells fromC57BU6mice)andfrom celllines(SCB.29,SCI.ET.27FandB6.21 6.BWcells)
with RNAzol B(TM Cinna Scientific, Friedswood, TX). ThemRNA waspreparedfromtotalRNA with theuseoftheOligotexTMmRNA Kit(QIAGEN).Both totalRNA and mRNA wereseparatedon1.2percent agarose-formaldehyde gels and transferred to Gene-Screenmembranesaccordingtothemanufacturer's
recommendations (Du Pont). Filters were first hybrid-ized at650Cfor 8 hours andhybridized with the same solution [1 mMEDTA, 0.5 M NaH2PO4, 7 percent SDS, and salmon sperm DNA (100pg/mI)]containing thespecific probe at650Covernight. To remove any unspecifically bound probe, the membranes were washed first with 1 mMEDTA, 40 mMNaH2PO4, 1 percent SDS at650C. Filters were hybridized first with a cDNA probecorresponding to the entire coding sequence ofpTcxandsubsequently, after dishybrid-ization, with aprobe specific for]3-actin message. Washed membranes were exposed to Kodak X-OMAT AR film at-700Cfor 1 to 5 days. 42. CD44+CD25-,CD44+CD25+, and CD44-CD25+
double-negative(CD4-CD8-) thymocytes were ob-tained fromRAG-2-'-mice.CD44-CD25- thymo-cytes wereobtained fromC57BL/6mice by comple-ment-dependent killing of thymocytes with antibod-ies toCD4 and CD8. The thymocytes were then stained with antibodies specific for CD4, CD8, CD44, CD25, andCD3 surface markers to obtain the desiredpopulations by cell sorting. The purity of the subsets wasdeduced from reanalysis of sorted cells. In order toquantitatepTaRNA, cells (5 x 104) were directly sorted in 500 ,u of RNAzol (TM Cinna Scien-tific),and total RNA was extracted according to the manufacturer's protocol. The cDNA was prepared with random hexamer primers and reverse-tran-scribed withSuperscript kit (Gibco BRL). Dilutions (1:3) of cDNA in water were then used in PCR amplification reactions. Primers used were oligonu-cleotides recognizing sequences in the 5' and 3' regions of the pTa
(5'-CTGCAACTGGGTCAT-G0TTC-3' and 5'
TCAGA0GGGTGGGTAAGATC-3')and HRPT(5'-CACAGGACTAGAACACCTGC-3' and5'-GCTGGTGAAAAGGACCTCT-3') genes. Am-plification was done for 35 cycles at an annealing temperature of550Cwith athermal cycling machine (Perkin-Elmer Cetus). A 1 5-,ul portion of each ampli-fiedproduct was separated through a 1.2% agarose gel by electrophoresis and stained with ethidium bromide.
43. The58a- ,y. 1 T cell line was obtained by transfection of the C58TCRat-TCR,-Tcell linewithaTCRPgene (8). This cell line was supertransfected with a pTa construct. To thisend,full-length cDNA ofpTa sub-clonedinBluescriptSK+ (clone D2.1) was amplified with primers 5'-TAGCCTCGAGCTGCMCTGGGT-CATGCTTC-3' and 5'-CAGAGAATTCTCAGACG-GGTGGGTAAGATC-3' that annealinthe 5'and3' untranslatedregion of pTa just upstream oftheATG startand downstream oftheTGA stopcodon, re-spectively, and introduced specific artificial cloning sites: Xho intheformer case, EcoRIinthe latter. The resultingPCR fragment of about 660 bp was purified, digested with Xho andEcoRI,andsubcloned into thecorrespondingpolylinker restriction sitesof Blue-script SK+. ThepTa sequence between the Nco site atthe secondin-frameATG and theoneBglII site downstreamof the stop codonswasreplaced by the corresponding sequence from D2.1inorderto elimi-natepotential mutations introduced duringthePCR amplification. The resultingplasmidwasdigestedwith Xho and Not Itorelease thecDNAfragment encod-ingpTa, which was then insertedinthe correspond-ing sitesof expression vectorBCMGSHyg(20). The construct was transfected into 58a-3y.1 cells by electroporation.
44. Wethank D. Avila,for microsequencing of thepTa protein; J. Kaufman, M. Reth, and J.Cebrianfor help with the analysis of the pTa sequences; C.Benoist, D.Mathis, and M. Wiles for adviceon
pTacloning;J.Garcia-Sanz for adviceontheRNA analysis;S.Goude,V. Stauffer,andC.Geigerfor secretarial help; andK.Hafen and C.Laplacefor expert technical assistance.TheBaselInstitute for Immunology issupported by Hoffmann-LaRoche, Basel.Supportedinpart bytheInstitutNational de laSante and Recherche Medicale, Paris,and by the Faculte Necker Enfants Malades, Descartes Universite,Paris.H.v.B. isaffiliatedwiththeFaculte Necker, Paris, andalsosupported bytheHuman Frontier ScienceProjectOrganization(HFSPO).
9September1994;accepted18October1994
SCIENCE * VOL. 266 * 18 NOVEMBER 1994
m
on July 9, 2019
http://science.sciencemag.org/
Analysis and expression of a cloned pre-T cell receptor gene
C Saint-Ruf, K Ungewiss, M Groettrup, L Bruno, HJ Fehling and H von Boehmer
DOI: 10.1126/science.7973703 (5188), 1208-1212. 266
Science
ARTICLE TOOLS http://science.sciencemag.org/content/266/5188/1208 REFERENCES
http://science.sciencemag.org/content/266/5188/1208#BIBL
This article cites 36 articles, 9 of which you can access for free
PERMISSIONS http://www.sciencemag.org/help/reprints-and-permissions
Terms of Service
Use of this article is subject to the
registered trademark of AAAS.
is a
Science
American Association for the Advancement of Science. No claim to original U.S. Government Works. The title
Science, 1200 New York Avenue NW, Washington, DC 20005. 2017 © The Authors, some rights reserved; exclusive licensee (print ISSN 0036-8075; online ISSN 1095-9203) is published by the American Association for the Advancement of
Science
on July 9, 2019
http://science.sciencemag.org/