© byAkademischeVerlagsgesellschaft,
The
Formation
of ThRh
Hydride
and the
Synthesis
of Methane
by
its Reaction with CO
By
H. Berner, H.
Oesterreicher1,
K. EnsslenFakultat fiirPhysik,UniversitatKonstanz, D-7750Konstanz,WestGermany
and L.
Schlapbach
Laboratorium fiirFestkorperphysik,ETH, CH-8093Zurich,Switzerland
(ReceivedJuly26, 1982)
Metalhydride / Surfacesegregation/ Catalysis /Methanation
Thecompound ThRh forms ThRhH3A under 1bar H2 pressure at room temperature. Thermodynamic analysis yieldsAH°=
—
58.7kJ/mole H2and AS"=
—
128J/K moleH2.A methanation reaction is observed whenThRhH3[isexposedtoCO. Apreferentialreaction of CO with thehydrogenstored in the metalhydriderather than with thehydrogenin the ambient
gas phase is shown by use ofhydrogen-isotopes. XPS experiments ofThRh and ThRhHz
demonstrate surfacesegregationintoTh02 and Rhclusters,whenexposedto02 orCO.
Die intermetallischeVerbindungThRhbildetbeieinemH2-Gleichgewichtsdruckvon1 bar undZimmertemperaturdasHydrid ThRhH3dessen BildungsenthalpieAH"= —58.7kJ/mol
H2 und Bildungsentropie AS°=-128J/K mol H2 betragt. Wird ThRhH3
, einer
CO-Atmosphareausgesetzt,ist dieEntstehungvonMethan nachzuweisen. Mit Hilfevon
Wasser-stoffisotopen wird gezeigt, daB bei dieser Reaktion der Wasserstoff, der im Metallhydrid gespeichertist,gegeniiberdem WasserstoffausderGasphase bevorzugt eingebautwird.
XPS-UntersuchungenvonThRh undThRhHz zeigenbeiZugabevon02bzw. CO eine
Oberflachen-zersetzung,bei derTh02- und Rh-Teilchenentstehen.
Introduction
Metals andintermetallic
compounds
consisting
ofrareearthsoractinidesincombinationwith transition metalscanabsorb
large
amountsofhydrogen.
1
GuestProfessor, permanentaddressatUC-SanDiego,La Jolla where work issupported by
76 H. Berner,H.Oesterreicher, K. Ensslen and L.Schlapbach
Onestep of thisprocessis the dissociation of the
H2
moleculeatthe surface. As shownby
theexample
ofLaNi5 [1]
asegregation
of therareearth elementatthe surface takes
place, binding
theoxidizing impurities
andkeeping
the transition metal in the metallicstate. Inthiswaysitesarecreated,
whichareabletodissociate molecules. On the other hand these
compounds
candeliverlarge quantities
ofatomichydrogen
from the bulk.They
therefore should begood
candidates ascatalysts
forhydrogenation
reactions.Wallaceetal.
[2
—
4] investigated
various
compounds
ofthistypeintheirunhydrided
statewithregard
totheircatalytic activity
in theNH3
andCH4
synthesis. They
found,
that under thesynthesis
gas CO andH2
thecom-pounds decompose
intocrystallites consisting
of the transition metal and ofanoxide of therareearth/actinide
which showedatleastinone casehigher
activity
than aconventional oxidesupported catalyst
of thesame elements.Soga
etal.[5]
studied thehydrogenation
ofethylene
overLaNi5
andfound,
that the rate of
hydrogenation
over thehydride
waslarger
than over theunhydrided
material. Oesterreicheretal.[6]
showed thecatalytic
formation ofwater, whenhydrides
ofintermetalliccompounds
wereexposed
toair.Inthis workwestudied the
CH4 synthesis
from carbon monoxideoverThRh
hydride.
We choose ThRhas amodelcompound
type
"rareearth oractinide/transition
metal" for several reasons:a)
the material isexpected
to form ahydride,
which is even stable attemperatures
of 200-300°
C.
b)
Rhodium could becomecatalytically
active in clustersize on thesurface,
if theexpected segregation
ofThorium occurs.c)
Thorium isreported [4]
toforma moreactivesupportthanLa,UorZr.In addition to a
study
ofthehydriding
behaviour of ThRh and thereactionof ThRh
hydride
withCO,
XPSwasused tohelp understanding
ofthe surface processes.
Experimental
details and results1. Material
ThRhwasprepared by meltingthecomponentstogetheron awatercooledcopperhearth of
anarcfurnace under argonatmosphere.The buttonwasflippedand remelted several timesto
improve homogeneity.
The orthorhombiccrystalstructure[7]wasverifiedby analysisofX-ray powder diagrams
(Guiniercamera,Cu-A'aradiation).Therewasalso evidence ofTh02presence, butnotof Thor
Rh.
2. Hydriding
ThRhwashydridedin thereactorofahigh-pressurestainless steelapparatuswith various reservoirs of known volume and facilitiestoworkatdifferentpressure ranges.Thetemperature
was monitored by a NiCr —Ni thermocouple in the reactor bed. For the variation of the temperature,anexternal heaterwasused.
ThesamplewasexposedtoH2 gas(99.999%), additionally purifiedfrom02and H20 by
chemisorption and molecular sieve filtration, respectively, accomplished by passing the gas
through a commercially availablecartridge (Oxisorb, Messer Griessheim). The amount of
absorbedhydrogenwascalculated from thepressuredrop.The first reaction withH2(28 bar) occurredspontaneouslyatroomtemperaturewithoutpreviousactivation.Atthistemperature
and1barequilibrium H2pressurethehydride ThRhH3,is formed. Thepressure-composition
isotherms,shown inFig.1weremeasured for fourtemperaturesby monitoringtheequilibrium
H2pressurestartingfromapoint,whereThRh isinthehydride phaseandpumpingoutknown
quantitiesofhydrogengas. Threeof thesedesorptionisotherms showplateaus,associatedwith the transformation of thehydride (/?) phaseinto the solid solution(a)phase. Thermodynamic
parametersderived fromalogarithmic plotofplateaupressurevs. reciprocaltemperatureare
AH"= -58.7kJ/mole H2 and AS"= -128J/K molr H2.The X-ray pattern of the ThRh
hydridecouldnotbe indexedby enlargingthe orthorhombic cell ofThRh,norbythepresenceof
ThH2orTh4H15.
3.
Catalysis
3.1.Apparatus
The
catalysis
experiments
wereperformed
inthehydriding
apparatus
by
useofcorresponding
parts
of itas asingle
passreactor(volume
5cm3)
or as a78 H. Berner, H.Oesterreicher, K. Ensslen and L.Schlapbach
closed
system.
This contained several chambers forfilling
in knownamountsof different gases. In addition to CO
(99.97%), H2 (99.999%)
andD2
(99.4 %)
required
for theCH4 synthesis,
Ar(99.997
%)
wasusedas areferencegastocheck
changes
inthehomogeneity
ofthegasmixture and in thepartial
pressures ofthecomponents.
A
quadrupole
massspectrometer
(Balzers,
QMS)
builttogether
with aseparate
high
vacuum station was used. Thecomponents
of the gas werequantitatively
analysed by considering
the known ratios ofsingly
anddoubly
ionized andfragmentary
molecules andby
alsoincluding
a factor for theprobability
ofionization,
which isdifferentfordifferentgases[8,9].
Since thepeak
atthe atomicmassunity
(amu)
16iscomposed
notonly
ofCH4
but alsoof CO and
H20,
thepeak
atamu15(CH3-fragment)
wasusedtodetect the presence ofCH4.
3.2. Procedure
AThRh
piece
of about 0.75gwashydrided
atroomtemperature,
cooledto
—
50 °C and after
removing
thegaseoushydrogen, exposed
toCO and Ar. Then thereactorwasheatedto100°Cand
part
ofthehydrogen
inthehydride
allowedtodesorb. Thistemperature
washeld for about 12h whilethegaseswere circulated in the closed
system,
drivenby
convection,
to reachequilibrium
andhomogeneity.
This was confirmedby observing
constanttotalpressureand
obtaining
thesameratios of thepartial
pressuresasthoseratios determined from the filled in
quantities.
Either the closedsystemwasused
during
thewholeexperiment
orthereactorwasswitchedovertoasingle
passreactorand thegases
passed
overthe ThRhhydride
ata constantfeedrate of1 ml
gas/min (standard pressure).
This ratewas limitedby
the massspectrometer,
which was run at 1 •10~4mbar.
At the end of eachsingle
experiment
the reactor wasevacuated,
the gas furtheranalysed by
massspectrometry
and thehydride
wasoutgassed
at200 °C for about 1h. Thisprocedure
wasrepeated
several times with the same ThRhsample.
3.3. Results
In all
experiments
withexposing
ThRhhydride
toCO,
CH4
wastheonly
detectable
product.
The presence ofH20
wasnot detected eitherduring
theexperiment
orduring
evacuation at the end of theexperiment.
Further aprogressive
decrease ofthehydrogen
storagecapacity
was observed. Thisfact,
together
with the absence of oxygencontaining
gaseousproducts
and therecordedpressure decreaseduring CH4
synthesis
suggest,
that the ThRhsample
became more and more oxidized.Ineach successive
experiment
the time and thetemperature,
atwhich thebeginning
of theCH4
synthesis
could bedetected,
was lower than in the
300-L,
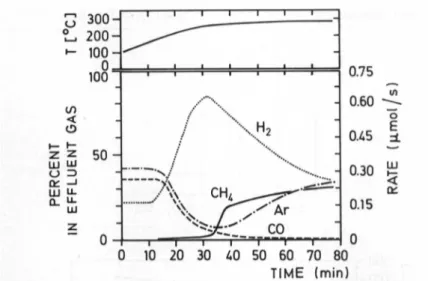
200-•- 100-0, 100 t/1 < UJ LU o => en -J LU D_ Ui 50H
vH2
0.75 0.60 QMS-I
0.15 0 o £-I
0.30ffi
< i i i—i T—i-—r 0 10 20 30 40 50 60 70 80 TIME (min)Fig.2. Rate ofCH4formationfrom COoverThRhHzduringincreaseof thetemperature.H2
evolves from thehydride.Ar isusedas a"feed rate" reference
amount of the
analysed
gas as a measure for the onset. In thevirgin
hydrogenation cycle
of ThRh theonsetis found after12hat100"Cplus
3.5 h at200°C. Inthe8th
catalysis
cycle
itcouldbealready
seenafter12 hat95°C. The total pressure was 1.1 and 1.2
bar,
respectively.
Westudiedmoredetailed the influence of the
desorption
of thehydrogen
from the metal
hydride
ontheCH4
formation.InFig.
2thegasmixture andtemperature
course in asingle
passexperiment
are shown. Atemperature
increase leadstoincreased
hydrogen desorption,
initiating instantaneously
ahigher
rateofCH4
formationcompared
with the fraction ofargon,which isareference of thefeedrate
during
desorption.
Toshow thepreference
in themethane
synthesis
between thehydrogen
evolved from thehydride
and the ambientH2
gas,anisotope experiment
wascarriedout. ThRhwashydrided
and after
removing
most of the gaseoushydrogen,
exposed
to carbon monoxide and deuterium in the closedsystem.
During
evacuation of thereactorthemass
spectrum
showedpeaks
atamu2,
3and4in the ratio 7:6:8arising
fromH2,
HD andD2
andpeaks
atamu12to20,
which refertoseveralC
—
H
—
D
compounds
listedinTable1.Although
the presence ofmoreD2
gas than
H2
gasisevident,
CH4
andCH3D
are dominant overCD4.
Fromthese results we
conclude,
that informing
methane,
hydrogen
from thehydride
ispreferred
overhydrogen
from thegasphase.
After7
experiments
with CO and 10hydriding cycles,
the ThRhpiece
haddisintegrated
topowder
and had increased itsweight by 7%. X-ray
diffraction
analysis
showedTh02, ThRh3
and Rh. Thissample
was thenexposed
tooxygenat200°80 H. Berner,H.Oesterreicher, K.Ensslen and L. Schlapbach Table1. Distributionofreactionproducts ofCO andD2 overThRhHzat171°C
_J_I_I_I_
CH*
CH3D CH2D2 CHD3 CD<0 50 100 150 200 250 300
TIME (min)
Fig.3. Formation ofCH4andC02from CO andH2 overthe oxidized ThRhsample during
increaseof thetemperature.H20is alsoformed(seetext).Aris usedas a"feed rate" reference
experiment
is shown inFig.
3.Hydrogen
was admitted inthe gaseousstate, because thematerialdidnottakeupanymorehydrogen.
Thedecreaseof COcombinedwiththe increase in
CH4
implies,
thatCH4
is formed from CO andnot from
C02,
which was alsoproduced.
Water was detected inlarger
amounts
during
evacuation and therefore not marked in thefigure.
TheX-ray
diffractionanalysis
showed that the material consists ofTh02
and Rh. The lastexperiment
carried out without the ThRhsample
or itsdecomposed products
confirmed,
that noproducts
at reaction conditions were formedby experimental
artefacts.4. Surface
analysis
The ThRh surfacewasstudied
by
X-ray
photoemission
spectroscopywith aVG-Escalab-Spectrometer using
Mg-Ka
radiation(hv
=1253.6eV;
Au4fV2
at83.9eV,
FWHM 1.2eV).
The ThRhwascleft underUHVconditions(2
—
4-
10~10mbar)
and afteranalysing
the clean surface thesamples
wereexposed
in situ toincreasing
doses of02
andCO,
respectively.
Thephotoelectron
energy distribution curves of the core levels Th4/7/2i5/2,
Rh
3J5/2
3/2, O 1s1/2 and C 1s1/2
were measuredto evaluate the chemicalstate and the concentration ratios within the escape
depth
of thephoto-electrons
(~20A).
The chemical state was determinedby comparison
toknown
spectra.
Theconcentration ratio wascalculated from theintegrated
peak height
dividedby
theoretical cross sections[10].
In
Fig.
4thespectra
ofcleft ThRh before and afteroxygentreatmentareshown. The clean
sample
contains metallic Th[11,12]
and metallic Rh[13,14].
Withincreasing
amounts of oxygen,increasing intensity
of thechemically
shifted Th4/peaks
is observed. This isexplained by
formation ofTh02 [12]
inagreement
with the increase andposition
ofthe oxygenpeak.
TheRhpeak
shifts 0.2 eVtolowerbinding energies,
which could beexplained
by
the formation of small Rh clusters with ahigh
amount oftop
surfaceatoms. The C
spectra
do notchange during
exposure to02.
Thecon-centration Th:Rh
changes
from 1.6:1 of the cleftsample
to2.7:1 after 100L02 (1
L= 1Langmuir
= 1•10~6Torr
s).
Therefore02
exposure leads toenrichment of Th at the surface in the form of
Th02.
The Rh remains metallic.330 335 340 345 350 305 310 280 285 290 530 535
BINDING ENERGY (eV| [Ep=0]
-Fig.4.XPSspectra of cleft ThRhexposedto02 at20 C:(a)cleftsample,Th:Rh=1.6:1;
(b)exposedtotL02,Th:Rh=2.1:1;(c)exposedto10LO2,Th:Rh=2.5:1;(d) exposedto
82 H. Berner,H.Oesterreicher, K. Ensslen and L.Schlapbach
Fig.5. XPSspectraof cleft ThRhexposedtoCOat200 C:(a)cleftsample,Th:Rh=1.5:1;
(b) exposed to10LCO,Th:Rh= 1.6:1;
(c)
exposed to1 000 L CO,Th:Rh=1.8:1Further the
spectra
of ThRhwererecorded before and after COexposureatroom
temperature
andat200° C. Some of themareshown inFig.
5. Thesame effect
—
enrichment of Th as
ThOz
and metallic Rh—
as after
02
exposure isseen.
However,
ittakes 1000LCOtoproduce
thesameamountofTh02
as withonly
1L02.
The Cspectra
show new small contributions atenergies
between 282 and 285eV,
especially
at200°C,
which couldoriginate
fromdissociatedCO[15].
The ratio C: O howeverchanges
from1 :0.5 for the cleftsample
to 1 :1.2 after 1000 LCO,
sothat notall of the oxygen canbederived from dissociated CO. To
analyse
theamount ofsurfacecoverageby
oxygen,which diffuses from the bulktothesurface,
wefollowed the surfaceconcentration ofO and C ofa
freshly
cleftsample
at200 °C over2h. Thecarbon
peak
didnotincreaseatall.Theoxygenpeak
increased40%
overthatin the clean
surface,
where the(Th
+Rh)
:0 ratio was 3.2:1.The cleftThRhwas
hydrided
inaseparate
chamber oftheUHV-system
at20 bar
starting
atroomtemperature
and cooled downto —100° Cto avoid strongH2 desorption.
Thehydride
wasexposed
to CO and afterwards warmeduptoremoveallhydrogen.
Thepowdered hydride
had the lowest ofallmeasuredcontentsof
impurities
C and Oas seeninFig.
6. CO exposure ofthat
partially
hydrided sample
showsnofurther differenceascompared
totheunhydrided
material.Discussion
The derived
thermodynamic properties
of ThRhhydride,
AH0 =—
58.7
kJ/mole
H2
and AS°= —128J/K
•moleH2,
arecomparable
tothose of similarhydrides
such as ThNi andThCo,
which arereported
in the330 335 340 345 350/305 310 280 265 290 530 535 BINDING ENERGY IeV)-*>
Fig. 6. XPSspectra of cleft ThRh and ofThRhHz exposedtoCO:(a)cleftsampleat20°C, Th:Rh=1.6:1; (b) hydrided, cooled to -100°C, Th:Rh=1.4:1; (c) hydride exposed to
10 LCO,Th:Rh=1.5:1;(d)hydride exposedto100 LCO,Th:Rh=1.5:1;(c)hydride
ex-posed to 1000 L CO, Th:Rh=1.6:1;(/) hydride exposed to10000 L CO, Th: Rh= 1.8:1;
(g) hydride warmed up to 20 C, Th:Rh=2.5:1; (h) completely dehydrided,
Th:Rh=2.4:1
literature
[16],
Thus ThRhhydride
is stableenough
to be handled withoutstrong
desorption
attemperatures
up to 200 °C. Thistemperature
rangeisnecessary to
study CH4 synthesis
from CO over the metalhydride.
Withthe enrichmentof Thas
Th02
onthesurfaceand the formation ofmetallic
Rh,
whenexposed
to02,
ThRh has theexpected
surfaceproperties
anticipated
from similarexperiments
withLaNi5 [1],
where oxidized La and metallic Ni arefound in the ratio 1 :1 on the surface.When ThRh
hydride
isexposed
toCO,
CH4
is formed belowtempera-tures of 200°C. The
hydrogen evolving
from thehydride
ispreferred
informing CH4, compared
tothehydrogen
in the gasphase,
as shown intheisotope
experiment.
This fact makesevident that theorigin
andstate of thehydrogen
isveryimportant.
Thispreference
appearstobe causedby
thelarge
supply
of atomichydrogen
by
thehydride.
In addition newclean,
i.e.catalytically
active surfaces may be createdduring
hydriding
anddehydriding
of the intermetalliccompound by progressive
disintegration
orsegregation.
However the oxygen of the carbon monoxide is used
subsequently
for oxidation ofTh,
although
a"reducing atmosphere"
is formedby
dehydrid-ing.
But if theCH4
synthesis
from CO would workonly
on the basis of84 H.Berner, H.Oesterreicher, K. Ensslen and L.Schlapbach
synthesis.
This isnotthecase. TheX-ray
patternshows,
that Rhcrystallites
have been formed. These cristallites
together
withTh02
canalsoproduce
CH4
andmight
be consideredasthoriasupported
Rhcatalyst.
The formationof
CH4
from CO andH2
overtheseverely by
CO oxidizedsample
startedatthe lowest
temperature
of allexperiments.
Thisextremely
low value was95°C.
Acknowledgements
We would like tothank Professor E. Bucher for
support
and interest in this work and Mr. G. G. Baumann forhelp
with the massspectrometric
analysis.
References
1. H. C.Siegmann,L.Schlapbach, Phys.Rev. Lett. 40(1978)972.
-L.Schlapbach,A.Seiler,
F. Stucki and H. C. Siegmann,J. Less-Common Met. 73(1980) 145. 2. T. Takeshita,W. E. Wallace and R. S. Craig,J. Catal. 44(1976)236.
-V. T.Coon,T.
Takeshita,W. E. Wallace and R. S.Craig,J. Phys.Chem.80(1976)1878. 3. H. Imamura and W. E.Wallace,J. Catal. 65(1980)127.
4. A. Elattar, W. E. Wallace and R. S. Craig,Adv. Chem. 178(1979)7. 5. K. Soga, H. Imamura and S.Ikeda,J.Phys. Chem. 81(1977) 1762. 6. H.Oesterreicher,K. Ensslen and E.Bucher, Appl.Phys.22(1980)303.
-H. Oesterreicher and F.Spada,Mater. Res. Bull. 15(1980)477.
7. Shunk,Constitution ofBinary Alloys,SupplementNr. 2 McGraw-Hill,New York 1969. 8. Index of MassSpectral Data,AMD11,ASTM.
9. Balzers,PhysikalischeGrundlagenzurParlialdruckanalyse (14.9.1973).
10. J. H.Scofield,J. ElectronSpectrosc.8(1976)129.
11. S.B. Nornesand R. G. Meisenheimer,Surf. Sci. 88(1979) 191.
12. J. C.Fuggle,A. F.Burr,L. M.Watson,D. J. Fabian and W.Lang,J.Phys.F:4(1974)335. 13. R.Nyholmand N. Martensson,J. Phys.C: 13(1980) L279.
14. T. L.Barr,J.Phys. Chem. 82(1978) 1801.
15. J. C. Fuggle,in: Handbookof X-rayand ultravioletphotoelectronspectroscopy(D.Briggs
Ed.),Heyden (1977).