Zeitschrift fürPhysikalischeChemie, Bd. 199, S. 87-98(1997)
© by Oldenbourg Verlag,München 1997
Formation of
Superclusters
from Metallic Clusters
By
Hans-GerhardFritsche1,
Hans Müller1 andBenjamin
Fehrensen21 Institut für
Physikalische
ChemiederChemisch-Geowissenschaftlichen Fakultät derFriedrich-Schiller-Universität, AmSteiger3,D-07743 Jena,Germany2 Zürich,Institut fürPhysikalische
Chemie, Zentrum, Universitätsstraße22, CH-8092Zürich, Switzerland
(ReceivedAugust 23, 1996)
Mesoscopic
particles
ISupercluster
ISize
effects
ISurface
dependent
properties
I Embeddedatomapproximation
Schmidetal. [3] reported on the
possibility
to build upexperimentally microcrystallinemodificationsofmetals with icosahedral clusters M„ asthebasic units of the structure.
Wetheoretically investigatetwo
possibilities
to constructsuchsuperclusters
: a) a forma-tionof shell-like superclusters, andb)agenerationofsuperclustersfromsuperclusters.Thenumericallyobtained resultsagree verywell with many
experimental
dataofthenewmetallic modification.They explainthegrowthofsuperclusters
by
asimpleanalyti-cal model. Size effects ofsome mechanical propertiesofsuch superclusterscanbe
pre-dictedby
analytical
relations.Schmidetal. [3] berichtetenvonderexperimentellen Erzeugungmikrokristalliner Modi-fikationenvonMetallen mit ikosaedrischen ClusternMaals strukturellenGrundeinheiten.
Wir untersuchten daraufhin mit theoretischen Methoden zwei Wege der Konstruktion solcher Supercluster: a) die Bildung schalenförmiger Supercluster und b) die Bildung
vonSuperclusternausSuperclustern.
Die numerisch gewonnenenErgebnissestimmen rechtgutmitexperimentellenDaten derneuenmetallischenModifikation überein.AufihrerGrundlage ergibtsich ein
analyti-sches Modell des Wachstums von Superclustern, welches die Vorhersage der
Größen-abhängigkeit mechanischerEigenschaftenvonSuperclusternerlaubt.
1. Introduction
The
high
stability
of transition metal clustercompounds
with a core ofgeometrically
closed shells of metal atoms is wellknown[1, 2].
Suchcom-pounds
can be used toproduce ligand-free
ML1
clustersby degradation
of88 Hans-GerhardFritsche, Hans Müller and BenjaminFehrensen
M13
clusters do notdecompose
into smallerparticles
(as
supposed
[4]),
butthey
aggregate
tosuperclusters.
Two lines of the formation ofsuperclusters
have been foundexperimentally
:1)
thegeneration
ofsuperclusters
of one or two full shells of clustersM13
[5, 6]
13
M13
->(M13),3
(a)
55
M13
->(M13)55
(b)
(1)
and
2)
thegeneration
ofsuperclusters
fromclusters/superclusters
[3,
6,
9]
13
M13
->(Mt3)13
(a)
13
(M13)13
->((M13)13),3
(b)
13
((M13)13)13
->((( 13)13) 3) 3
(c)
(2)
Here,
only
the firststeps
of both lines are identical.Some years ago we derived from a
simple
jellium
model within an iterative version thisaufbauprinciple
as adisign principle
for new metals[7, 8],
The formation of
superclusters
fromsuperclusters
can berepeated
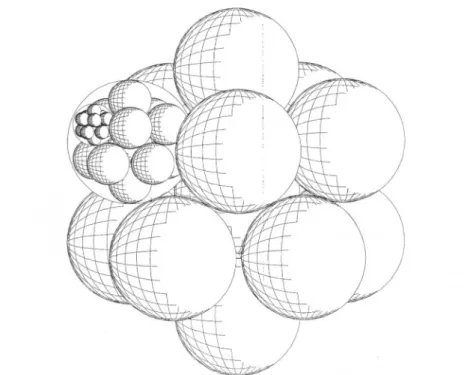
step-wise(Fig.
1).
Theresulting microcrystalline products
of the metalsAu, Co,
Pt,
Rh,
and Ru have beenstructurally
analizedby
variousexperimental
techniques
(time
offlight secondary
ion massspectrometry,
X-ray powder
diffraction,
Mössbauerspectrometry).
It has beenfound,
that the newme-tallic modification is stableat low temperatures.At
higher
temperatures(for
((AU|3),3)13
at700—
800
K),
it is transferredinto the structure of normal bulkmetal
[9].
It can be
expected,
that this newmicrocrystalline
modification hasproperties
different from that ones of thecorresponding
bulk metal. Some ofthemwill beinvestigated
inthis papertheoretically by
useof the Embed-ded Atom Method(EAM).
2.
The
embedded
atommethod
(EAM)
The embedded atom method has been described in detail in many papers
[10—13].
The method wassuccessfully
applied
to manyproblems
of metals(clusters
andbulk).
For areview,
see[13].
Here,
weonly
report
on some fewaspects
of the method.We start with an
approximation
of the totalpotential
energy ofinterac-tion
by
additive attractiveembedding energies,
Fh
andpairwise
additiveenergies
ofrepulsion,
of the atoms of the cluster
MN\
=
F,
( ,,,)
+„
( )
·
(3)
Formation ofSuperclustersfrom Metallic Clusters 89
Fig.1.
Supercluster
((M,,),,),,:generation line2, productofEq.(2b).In this
expression,
,,, is the host electrondensity
at atom i due to theremaining
atoms,/\( )
is the energy to embed atom i into thebackground
electron
density
, and(7?,,)
is the core-corepair repulsion
betweenatoms i and
j
separated by
the distance/?„.
The host electrondensity
isapproximated by
thesuperposition
of densities of valenceelectronsQ„,=
Q'iRg),
(4)
where
'
(Ru)
is the electrondensity
at atom i contributedby
atomj.
The atomic densities will be taken from the tables of Clementi etal.[14],
and McLean et al.[15].
Values of the functions F and can be calculated from the formal definitions of thesequantities
within thedensity-functional
framework[16],
but it is usualtoestablishF andempirically
fromphysi-cal
properties
of solids(lattice
constant, elastic constants, cohesive energy, andvacancy-formation
energy)
[10, 12].
Numerical calculations have been carried out here for M =
Li, Ni,
andAu. We used the
EAM-parametrization
ofZhang
etal.(lithium, [17]),
and of Voteretal.(nickel,
[18]).
Forgold,
wedeterminednewEAM-parameters
90 Hans-GerhardFritsche, Hans Müller and BenjaminFehrensen Table 1. Equilibrium dataof icosahedral clusters M,, (M = Li, Ni, Au) optimized by
EAM calculations: r, = shortest interatomic
distance, E/13 = binding
energy peratom.
rm = cut-off radius of EAM-interactions.
M,3 rc„,
[Á]
r,[Á]
-E/13 [eV] rcJr,Li„ 4.9629 2.823 1.279 1.758
Ni„ 4.7895 2.382 3.034 2.011
Au,, 3.4739 2.630 3.101 1.321
The contributions to the energy of interaction of atoms
separated
fromeach other morethan adistancercut arevery small
(contributions
tothe host electrondensity
,,., as well as contributions to the core-corerepulsion
).
Usually, they
areneglected.
(Their
neglection
isincorporated
into the deter-mination of theEAM-parameters
by
theadaption
ofexperimental
data ofsolids.)
3.
Results
3.1 Icosahedral basic clusters
M13
First,
weoptimized
the basic icosahedral clustersMI3.
The resultsaregiven
in Table 1. The shortest interatomic
distances,
ru and thebinding energies
per atom,
£713,
arequalitatively
correct(between
theexperimental
valuesof bulk and thatones of the diatomic
molecule).
For detaileddiscussion, see[19].
The last column of Table 1 shows the maximum range ofinteratomic interactions in units of the shortest interatomicdistance,
r,, of theicosa-hedral.
3.2 The dimeric
supercluster
(M13)2
It has been
verified,
that two icosahedral clustersM,3
at theirequilibrium
distance,
r2, have arigid
structure to ahigh degree
of accuracy[20].
As anexample, Fig.
2 shows the cluster-cluster interaction energy,AE(L
=2)
=E(2
·13)
-2
£(13),
ofthe dimericsupercluster
(Au,3)2
independence
onthe distance between the bothclusters,
r2. The values at theequilibrium
are: = -0.45eV,
r2 = 7.89 A. Inharmonic
approximation,
the
frequency
of the relative oscillation of the both clusters is =3.8 · 1011
Hz,
thecorresponding
wavelength
is = 0.08 cm.3.3 Formation of
bigger superclusters
Now,
westudy
the formation ofsuperclusters
(M13)L
from Lrigid
Formation ofSuperclustersfrom Metallic Clusters 91
In
fact,
the energy of the cluster-cluster interaction= E
(L
13)
-L
£(13)
(5)
will
depend
on the relative orientation of theclusters,
but it will be shown in section3.3.1,
thatchanges
of this orientation will influence thequantity
only by
a constant factor.Interesting qualitative
results canbe obtainedby
theonly optimization
of the distance r2 between twoneighbouring
clus-ters of the
supercluster,
too. In this sense, we define here the structure of thesupercluster
tobe fixed. All clustersM,3
inside of thesupercluster
have the same orientation oftheir local z-axis.3.3.1 Generation line 1
As an
example
of thegeneration
line1,
wereport
on the formation of icosahedralsuperclusters QJ.i3)L consisting
of closed icosahedral shells of icosahedral cluster unitsLi13.
Investigations
of other structures ofsuper-clusters
(linear,
squared, simple
cubic,
body-centered
cubic,
face-centeredcubic)
gave similar results. In thisnotation,
the centres of the basic clustersLi,,
define thepolyhedron
of thesupercluster.
We studiedsuperclusters
of =1,2,...,8
shells with L =13,55,...,2057
cluster unitsor =
169,715,...,26741
atoms and determined theoptimum
shortest distance r2oftwo
neighbouring
clustersLi,
,for eachsupercluster.
The resultsaregiven
92 Hans-GerhardFritsche.Hans Müller and BenjaminFehrensen Table2. Equilibrium data of shell-like icosahedral superclusters (Li13)¿ (generation
line1):r2 = shortest interclusterpairdistance, /L =
cluster-cluster interaction energy per cluster unit, AE/(L CN,) = interaction energy ofone
pair
of clusters of the super-cluster.L r2
[k]
-AE/L [eV] -AEI(L CN,) [eV]1 13 8.3199 0.3906 0.1209 2 55 8.3814 0.4969 0.1168 3 147 8.3969 0.5458 0.1153 4 309 8.4057 0.5737 0.1145 5 561 8.4113 0.5916 0.1140 6 923 8.4152 0.6040 0.1137 7 1415 8.4181 0.6132 0.1135 8 2057 8.4203 0.6203 0.1134
Line 1 :
Supercluster
(Li,3)L
1-0\.
^ 0.8
on\ I.I.I
0.00 0.10 0.20 0.30 0.40 0.50
L-"
Fig.3. Sizeeffect ofthecohesiveenergy,zl£/L, of icosahedral
superclusters
(Li,,),,with= 1,2,...,8closed shells(generation line 1).
We see:
1)
The shortest interclusterdistance, r2, isnearly
constant.2)
The cluster-cluster interaction energy percluster, /L,
increases with the size of thesupercluster. Fig.
3shows,
that the size-effect of /L can be describedby
theequation
/L«a +
b
·Lrm.
(6)
This relation is a first indication ofthe
fact,
that there is no onprinciple
a difference between size effects ofsuperclusters
and size effectsof clusters.However,
animportant quantitative
difference exists: The distances betweenFormation ofSuperclustersfrom Metallic Clusters 93
thecentresoftwo
neighbouring
clusters in thesupercluster
aremuchgreaterthan the distances between two
neighbouring
atoms in thecorresponding
clusters. Due to
it,
insuperclusters
notonly
thecluster-pair approximation
of the cluster-cluster interactionenergy
»
,
(7)
/< j
is
justified,
but also the restriction to theonly
contributions of the nearestneighbouring
pairs
of clusters:r>
¡EÁRÚ
forRu=r2
EU{RU)
=\
(7 a)
L
0 otherwise(R,,
= distance between the centres of the cluster / and7,
r2 = nearest
neighbouring
distance oftwoclusters).
3)
Toverify
the relations(7)
and(7 a),
we now define a mean first coordinationnumber,
C/V,(L),
of a clusterLi,,
inside of thesupercluster
(Li13)¿
as the total number of all nearestneighbouring
clusters devidedby
number L.Here,
for the sake ofsimplicity,
we suppose the nearestperiph-eral and the nearestradial distances between two clusters
Li13
of theicosa-hedral
supercluster
to be the same.The last column of Table 2
shows,
that the cluster-cluster interaction energy of onepair
ofinteracting
clusters, E,
=AE/(L
C7V,(L)),
isnearly
constant for all icosahedral
superclusters.
That means: the formation ofsuperclusters
issimply
controlledby
anoptimization
of the first coordi-nation of the cluster units inside of thesupercluster:
AE/L « 0.5
E,
CNAL)
.
(8)
Itis
obvious,
thatequation
(8)
isequivalent
with theequations
(7)
and(7 a).
The
validity
of the relations(6)
and(8)
has beenproved
for variousstructures of
superclusters
with different relative orientations of theicosa-hedral units
Li13:
linearchains,
squared
structures,simple
cubes,
body-cen-tered cubes, and face-centered cubes. In every case, the
description by
thesimple analytical
relations(6)
and(8)
was excellent.Therefore,
we con-clude:The size effect of the cluster-cluster interaction energy in
superclusters
ismainly
a size effect of theaveraged
first coordination number of the cluster units. The chemical nature of thespecific
metal,
the internal struc-ture of the cluster units aswell as the relative distances and orientations ofthe clusters inside of the
supercluster only
determine the values of thecon-stants a, b, and
£,
in theEqs.
(6)
and(8).
This result
explains, why
massspectra
ofsuperclusters
(Au,3)L
just
showthe
preferred
occurrence ofsuperclusters
with L = 13 and L = 55 clusterunits
[5,
6]:
Thecorresponding
structure of a full shell icosahedron94 Hans-GerhardFritsche,HansMüller andBenjaminFehrensen
Ni,3
_(Ni„)„
r,
[Â]
r4[Á]
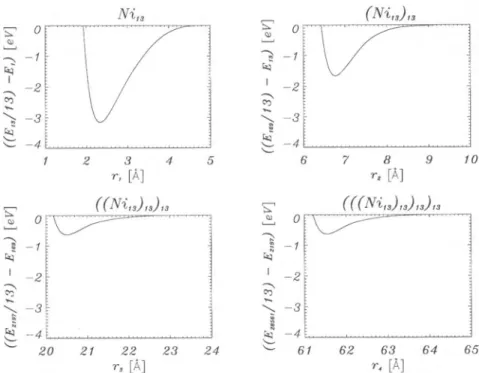
Fig.4. Formation energies of icosahedral superclusters ofnickel, AEk, for 4 steps of
generation
line 2.3.3.2 Generation line 2
Asa second
possibility,
weinvestigate
now astepwise generation
of icosa-hedral structures ofclusters/superclusters
of the 3 elementsLi, Ni,
and Au:step 1: 13M—»
M13
(see
section3.1.)
r, =
optimum
distance ofM—Mstep
2: 13M13
—»(M13),3
(see
section 3.3.1 for =1)
r2 =
optimum
distance ofM,3—M,3
step
3: 13(M13),3
->((M,3)13)13
r3 =
optimum
distance of(M13)13—(A/13),3
step
4: 13((M13)13)13
->(((M13)13)13)13
r4 =
optimum
distance of((M,3),3)13-((M13),3)13.
Each
step,
k,
uses theoptimized product
of thepreceding
step,
k—
1,
as a
rigid
unit to build up the next icosahedral structure with a new op-timized cluster-cluster distance rk.Formation ofSuperclustersfrom Metallic Clusters 95
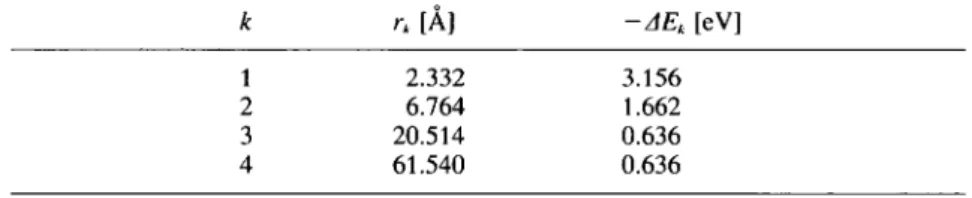
Table 3. Equilibrium data of k = 1, 2, 3, 4 steps ofgeneration ofsuperclusters from
superclusters ofNil3 (line 2): rk = shortest supercluster-supercluster
distance, AEk =
binding
energyofsuperclusterspersupercluster-unit.
k rk
[À]
-AEk [eV]1 2.332 3.156
2 6.764 1.662
3 20.514 0.636
4 61.540 0.636
Fig.
4presents
thecluster/supercluster—formation energies
of line 2AEk
=£*~^£*'
for * =1,
2, 3,4
,
(9)
in
dependence
of the distance rk for the elementnickel,
Table3 thecorre-sponding
values at theequilibrium.
Qualitatively,
the results of lithium andgold
are similar. Ofcourse, themagnitudes
ofAEk
andrk differ from thatones of nickel.Acareful
analysis
of the structures of thesuperclusters
showsashorten-ing
of the distances between the outercontacting
atoms of theinteracting
units of the
superclusters
incomparison
with the constant value of r,:-0.232
Á
instep 2,
-0.010Á
instep 3,
and -0.002Á
instep
4. In the same sequence, the relative values of the cluster-cluster-formation energy,AEkIAEu
decreases: 0.526 instep 2,
0.201 instep 3,
and0.201 instep
4.Obviously,
thegeneration
line 2 does notstop
atstep
4. Thegrowth
ofsuperclusters
can berepeated stepwise
with a constantenergetic
rate ofgrowth.
4.
Properties
of shell-like
superclusters
(Mi3)L
4.1 Cohesiveenergy of
superclusters
Now,
wediscuss thegeneration
line 1 moregenerally
andassume thesuper-cluster
(Afi3)¿
to have a structure ofgeometrically
closed shells of unitsM13. Then,
the number Lof cluster units canbeexpressed by
acubicpoly-nomial of the number of
shells,
n :L{n)
=a3n3
+a2n2
+ a,n + a0.(10)
The coefficients ak of
Eq. (10)
aregiven
in[21]
for 15highly
symmetrical
particles.
The total energy of interaction between all units
M13
of the supereluster isAE = 0.5 ·L
96 Hans-Gerhard Fritsche,Hans Müller and BenjaminFehrensen
For shell-like
superclusters,
also the meanL-dependent
coordinationnum-bers
CW,(L)
areanalytical
expressions
ofthe number ofshells,
n:CN,(L(n))
=2(b3n3
+b2n2
+btn
+b0)IL.
(12)
The values of the coefficients
b¡
aregiven
in[21].
Thus,
from theEqs.
(11)
and(12)
follows apolynomial representation
ofthe total interaction energy of thesupercluster
AE(n)
=Et
3 3
+b2n2
+b,n
+bn).
(13)
From the
Eqs.
(10)
and(13),
the cohesive energy of thesupercluster,
/L,
can be written as
/L =
A0
+A_,/?-' +A_2n-2
+...
(14)
with the bulk-value of the
supercluster
{AE/L\
=A0
= ,(15)
Ű3 and-1
=(b2a3
—)
E,/(aj),...
4.2 Surface
dependent properties
ofsuperclusters
We define the surface energy,
ES(L),
ofasupercluster
ofLrigid interacting
basic unitsM,3
by
the relationES{L)
=AE(œ)
-AE(L)
.
(16)
Putting
theEqs.
(10,
14, and15)
intoEq.
(16),
we obtainEs(n)
=-[B2n2
+,
+B0
+^, '1
+···],
(17)
with
B2
=a3A-u
ß,
=a3A-2
+a2A^„
...
The
question,
what is the surface ofasupercluster,
is difficultquantitatively
to be answered. As a crude
approximation,
we assume the surface of thesupercluster
tobe the surface ofthepolyhedron
withfacessimply
construct-ed from the centres of the cluster units
M13
at the outershell of thesuper-cluster.
(In
reality,
the surface must begreater.)
Then,
the surface area,A,
follows as
A=fr2,
(18)
with r
being
anedge
length
of thepolyhedron.
Thequantity /
is a form factorspecific
for thespecial polyhedron.
At anedge
ofasupercluster
ofshells,
(n
+1)
cluster unitsMn
arepositioned
with the distance r2betweentwo
neighbouring
units.Thus,
theedge length
can beexpressed by
the shell numberFormation ofSuperclustersfrom Metallic Clusters 97
and the surface area is a
squared polynomial
ofn:A
=fr\
(n2
+ In +1).
(20)
Now,
asimple
relation of thespecific
surface energy, , follows:withc0 =
( )
=B2/(frl),
c_, =
(£?,
-2
B2)/(fr22)
Similarly, analytical
formulae for other mechanicalproperties
of super-clusters like theisotropie
surfacestressorthecompressibility
canbe derived[21, 22].
5.
Concluding
remarks
Icosahedral clusters
M13
of alkalinemetals,
transitionmetals,
and noblemet-als have diameters
greater
than orequal
to the range of the direct interac-tions between two metal atoms. Our numerical calculations haveshown,
that such clusters interact in
superclusters
(M13)t
at theequilibrium by
theonly
attraction of the nearestneighbouring
pairs
ofthe clusters. Because of the collective character of the metallic interaction consideredby
the non-lineardependence
of theembedding
functions Fon the host electronden-sity
(Eqs.
3 and4),
this result is not trivial.Fora defined structure of the
supercluster,
which has been determinedexperimentally
[1,
3, 5, 6,
9],
the cohesive energy of thesupercluster
only
depends
on the sizedependent
mean coordination ofa clusterby
the nextclusters inside of the
supercluster.
This resultjustifies
asimple
topological
model of the cluster-cluster interaction in metallicsuperclusters proposed
eight
years ago[23].
Acknowledgement
The authors are
indepted
to the DeutscheForschungsgemeinschaft
and tothe Fonds der Chemischen Industrie forfinancial support. References
1. G. Schmid, Struct.
Bonding
62(1985) 51. 2. . K.Teo,Polyhedron7 (1988)2317.3. G. Schmid and N.Klein, Angew. Chem. 98 (1986)910.
4. M. R. Hoare and P. Pal,J.
Cryst.
Growth 17(1972)77;Nature(London)236(1972)35.
5. H. Feld, . Leute,D.
Rading,
A. Benninghoven and G. Schmid,J. Am. Chem.Soc. 112(1990)8166;Z. Phys. D17(1990)73.6. G.Schmid, in: Clusters andColloids, ed. by G.Schmid,VerlagChemie,Weinheim, New York,Basel,
Cambridge,
Tokyo, 1994 (p. 205).=
A
[c„
+ c_i«1
98 Hans-GerhardFritsche,Hans Müller andBenjaminFehrensen 7. H. Müller and Ch.Opitz. . Phys. Chem. 187(1994) 179.
8. . Müller and Ch.Opitz,Croat. Chem. Acta 68(1995)721. 9. G. Schmid,Polyhedron 7(1988)2321.
10. M. S. Daw and M. I. Baskes,
Phys.
Rev. 29(1984)6443. 11. S. M.Foiles,Phys.
Rev. 32(1985) 3409.12. S.M.Foiles,M. I. BaskesandM.S.Daw,
Phys.
Rev. 33(1986)7983. 13. M.S. Daw,S. M. Foilesand M. I.Baskes, Mater. Sci.Rep.
9(1993)251. 14. E.Clementi and C. Roetti,At. Data Nucl. Data Tables 14(1974) 177. 15. A. D. McLeanand R.S. McLean,At. Data Nucl. Data Tables 26(1981) 197. 16. M.S. Daw,Phys.
Rev. 39(1989) 7441.17. .Zhang and Y. Ouyang, Phys.Rev. 48(1993) 3022.
18. A. F. Voter, in: Interrnelallic Compounds, Vol. 1, ed. byJ. H. Westbrook, and R. L.
Fleischer,John Wiley SonsLtd, 1994,p. 77. 19. H.-G. Fritsche,Z.
Phys.
Chem. 191 (1995)219. 20. B. Fehrensen,ThesisJena, 1995.21. H.-G. Fritsche,Z.
Phys.
Chem. 187(1994) 195.22. H. Müller,H.-G. Fritsche andL.Skala,in ClustersofAtoms andMolecules,Springer
Series in Chemical
Physics,
Vol. 52,p. 114, Berlin, Heidelberg, NewYork, London, Paris,Tokyo,
Hong Kong,Barcelona, Budapest (1994).23. H.-G. Fritsche, D. Bonchev and O. Mekenyan, J. Less-Common Met. 141 (1988)