Carbon Fluxes from Tropical Peatlands:
Methane, Carbon Dioxide, and Peatland Subsidence
by
Alison May Hoyt
B.S., Yale University (2009)
M.Phil., University of Cambridge (2011)
Submitted to the Department of Civil and Environmental Engineering
in partial fulfillment of the requirements for the degree of
Doctor of Philosophy
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
September 2017
Massachusetts Institute of Technology 2017. All rights reserved.
Author...Signature
redacted
Department of Civil an
4Environmental Engineering
Signature redacted
August 18, 2017
Certified by ...
...
Charles F. Harvey
Professor of Civil and Environmental Engineering
Thesis Supervisor
Signature redacted
A ccepted by...
...
/
Jesse Kroll
Professr of Civil and Environmental Engineering
ARCHNVES
Chair, Graduate Program Committee
MASSACHUTT INSITUTE OF TECHNOLOGY
Carbon Fluxes from Tropical Peatlands:
Methane, Carbon Dioxide and Peatland Subsidence
by
Alison May Hoyt
Submitted to the Department of Civil and Environmental Engineering on August 18, 2017 in Partial Fulfillment of the
Requirements for the Degree of Doctor of Philosophy in Civil and Environmental Engineering
ABSTRACT
Tropical peatlands in Southeast Asia have sequestered carbon over thousands of years and are an important global carbon stock. In natural peat swamp forests, high water levels inhibit decomposition due to anoxic conditions. However, they are being rapidly deforested and drained, releasing stored carbon to the atmosphere. In this thesis, we investigate the carbon dioxide and methane fluxes from both pristine and degraded peat swamp forests in Borneo using field measurements, modeling and remote sensing.
We first study methane fluxes from natural peatlands. We use an isotope-based mass transport model to evaluate the extent of methane production, transport and oxidation. We find an order of magnitude more methane is produced than surface fluxes suggest. This dissolved methane is transported belowground to the rivers and streams draining peatlands. However, much of this methane is oxidized before reaching the atmosphere.
We then study CO2 emissions from peatlands. At the local scale, we use automated soil
respiration chambers to assess how CO2 emissions depend on temperature and water table. At a regional scale, we use remote sensing to investigate carbon losses due to peatland degradation. Drainage of peatlands enables peat decomposition and results in subsidence of the land surface. We track this subsidence using InSAR satellite data and use it to quantify regional CO2
emissions. The spatial resolution of our technique allows us to uncover correlations with past and present land uses and peatland hydrology.
Thesis Supervisor: Charles F. Harvey
Acknowledgements
First and foremost, I would like to thank my advisor Charlie Harvey for his enthusiasm and guidance throughout my time at MIT. I truly appreciate the countless hours of creative research discussions and fieldwork planning. I am also grateful for the freedom he gave me to pursue many exciting collaborations beyond MIT. I would also like to thank the members of my
committee -Shuhei Ono, Laure Gandois, Ben Kocar, and Harry Hemond - for their input over
the years.
This work would not have been possible without the help of many collaborators from around the world. Alex Cobb, Laure Gandois, Rene Dommain and Charlie Harvey have been excellent sources of wisdom on all topics peat-related, and have shared hours of conversation, planning, fieldwork and analysis across many different projects, both in this thesis and beyond.
I would also like to thank the collaborators involved in the individual projects in this
thesis. Estelle Chaussard and Sandra Seppalainen were important collaborators on our shared work on peatland subsidence. Thank you also to Anne Graham and Daniel Sheehan at the MIT
GIS Lab for their many hours of assistance with this project.
The work on methane benefitted greatly from collaboration with Sunitha Pangala, Laure Gandois, Alex Cobb, Charlie Harvey, Vince Gauci, Liz Corbett, Jeff Chanton, Ed Hornibrook, Xiaomei Xu, and Kai Fuu Ming. Additionally, the following collaborators were instrumental in our investigations of the anaerobic oxidation of methane in tropical peat: Xiaolei Liu, Roger Summons, Shane O'Reilly, Andrey Vieira, Guangchao Zhuang, Shuhei Ono, David Wang, Dave VanInsberghe, and Martin Polz. I would particularly like to thank Alex Cobb for managing our field site in Brunei, Xiaolei Liu for his patience and encouragement in the lab, and Sunitha Pangala and Laure Gandois for their friendship during long camping trips in the peat forest.
This work would not have been possible without the dedicated work of the field team
-Jang, Boy, Pudek and Haji Bohari - who worked alongside me in the Mendaram and Damit peat
swamp forests. I would also like to thank the many people who have helped with field trips in 2012, 2013, 2014, 2015, and 2016 as well as other aspects of the research: Alex Cobb, Laure Gandois, Amy Chua, Idaly Ali, Sunitha Pangala, Kai Fuu Ming, Khalish Hafizhah Ideris, Gusti Anshari, Muhammad Nuriman, Lahiru Wijedasa, Maria Lee Ai Lan, Judy Pu, Aloysius Teo, Siew Chin Chua, Lucy Hutyra and Ha Nguyen.
I would also like to thank my friends and family for all their support over the years. In
particular, my husband Adam Bouland has been a great partner throughout our time at MIT. My experience would not have been complete without my cohort, fondly known as "Oobergroup". Finally, thank you to the wonderful Parsons community for many years full of friendship and support.
Table of Contents
Page 3 Abstract 5 Acknowledgments 7 Table of Contents 9 Introduction17 Chapter 1: Development of a Model of CH4 Production and Transport
for Peatlands
39 Chapter 2: Methane in Tropical Peatlands
61 Chapter 3: Trends in CH4 Production and Transport across Latitudes
85 Chapter 4: Hydrologic Control of Soil CO2 Emissions from Tropical Peat
99 Chapter 5: Subsidence in Tropical Peatlands: New Methods for Estimating CO2 Fluxes from Peatlands in Southeast Asia
Introduction
Peat Accumulation
Southeast Asia is home to the world's largest share of tropical peatlands (28.4 Mha).
These peatlands, which can be over 10m deep, have accumulated carbon over thousands of
years. The total tropical peat carbon pool is estimated at 90GtC, which is larger than the carbon
stored worldwide in temperate forest vegetation and is even significant in the context of total
worldwide tropical forest vegetation (Page et al., 2011 a). Across Southeast Asia, this large store
of carbon is being released to the atmosphere as peat swamps are deforested and drained.
The accumulation and loss of carbon in peatlands is tightly coupled to peatland
hydrology. Tropical peat accumulates under waterlogged conditions as the rate of plant
productivity exceeds the rate of decomposition due to a lack of oxygen available for
decomposition. When the water table is lowered, either seasonally or through drainage projects,
oxygen becomes available for aerobic decomposition of organic matter, and CO
2is released to
the atmosphere. In natural peatlands, carbon also leaves the peat as methane (CH
4), dissolved
organic carbon (DOC), particulate organic carbon (POC) and dissolved inorganic carbon (DIC)
(Figure 1). In drained peatlands, CO
2emissions from peat decomposition and fires are the largest
fluxes of carbon to the atmosphere.
Mneral subsoil
5" N
0*
-Peatland western Indonesia I N D 0 N E S I A
I Peatland outside Indonesia
Basal peat radiocarbon ages
a Carbon accumulation sutes
* Eddy flux tower site
+ Paleoclimate proxy sitea
0 250 500 k[
-95* E 100* E 105* E 110* E 115' E 120' E
Figure 2: Distribution of tropical peatlands in Sumatra, Borneo and Peninsular Malaysia (Dommain et al., 2014) Drainage, Deforestation, Subsidence and Fire
In the 1950s Borneo was covered in forests, but today huge areas have been deforested, particularly in lowland areas along the coasts where the majority of peatlands are located. As of 2007, only 10% of peat swamps in Peninsular Malaysia, Sumatra and Borneo remained in pristine condition, and by 2015 this had been reduced to 6% (Miettinen & Liew, 2010; Miettinen
et al., 2016). This has been driven by a demand for timber and the expansion of agriculture,
including acacia and oil palm plantations. Oil palm is extremely productive and accounts for 45% of edible oil worldwide (Singh et al., 2013). As the cheapest food oil, it is mainly consumed in developing countries. A huge increase in demand in recent years has been met largely by Indonesia, which doubled its production from 2000-2009 (USDA, 2009). The resulting deforestation and drainage of peat swamp forests are major contributors to Indonesia's
subsidence on a variety of land uses on peat. Total annual emissions due to peat decomposition are estimated to be between 355-855 MtCO2/yr, equivalent to 1-3% of global emissions from
fossil fuel (Hooijer et al., 2010a). Loss of sequestered peat carbon also results in subsidence of the land surface in vulnerable coastal areas. Subsidence will eventually result in the loss of productive land, with serious economic consequences. Additionally, many poor coastal areas in Southeast Asia lack the resources to cope with the expected increases in flooding that will accompany widespread subsidence.
In addition to continuous fluxes due to peat decomposition in drained peatlands, sporadic emissions from peatland fires can contribute CO2 emissions of a similar magnitude. These fires
are seasonal and have large interannual variability. Average annual emissions from fire are estimated at 469 187 MtCO2/yr (van der Werf et al., 2008). Fires in El Nifio years contribute
much larger emissions. The 1997-1998 El Nifio fire was estimated to release between 3000-9400 MtCO2 in Indonesia (Page et al., 2002). Such fire events also have widespread health and
economic impacts due to the persistent haze that spreads through the region as a result of smoldering peat fires.
Summary of Research and Suggestions for Future Work
In this thesis we use a combination of field measurements, modeling, and remote sensing
to improve our understanding of the processes driving CO2 and CH4 fluxes from tropical
peatlands, as well as to quantify these emissions. We find CO2 emissions dominate carbon losses
from drained tropical peatlands, and play an important role in natural peat swamp forests under dry conditions. We examine the relationship between the water table and CO2 fluxes at short
timescales before quantifying the total carbon loss due to peatland drainage at a regional scale.
We also examine CH4 fluxes from natural peatlands. Although these are much smaller than CO
2
emissions, the processes which drive them are more poorly understood. We present a complete CH4 budget for a tropical peatland for the first time. We find the dominant transport pathways
are very different than those in northern peatlands. Our work focuses on understanding the processes which drive CH4 and CO2 fluxes from tropical peatlands. A summary of the work and
directions for future research to build upon these findings follows.
(I) Methane Fluxes
Wet, stagnant, anoxic conditions in tropical peatlands facilitate CH4 production.
However, the processes which govern methane emissions from tropical peatlands are poorly
understood. Although there have been many measurements of CH4 emissions from the peat
surface, research on the processes driving CH4 production and oxidation is much more limited.
Here we develop a comprehensive process-based understanding of CH4 production, transport,
quantify CH4 production, and to understand the fate of this CH4. We seek to answer the
following questions:
(a) Do tropical peatlands produce more CH4 than we realize?
(b) If so, how is it transported? Where is it oxidized? Does it reach the atmosphere?
We address these questions by developing an isotope-based mass transport model to quantify CH4 production (Chapter 1). We use this model, along with extensive field
measurements to develop a complete CH4 budget for a tropical peatland (Chapter 2). Finally, we
identify fundamental differences in CH4 transport processes between northern and tropical
peatlands (Chapter 3).
In Chapter 1, we present the development of an isotope-based mass transport model to
quantify peatland CH4 production. The model captures the unique hydrology of the peat dome,
the advection and diffusion of dissolved CH4 and DIC, and the fractionation that occurs during
CH4 production. It individually models the transport of each carbon isotope of CH4 and DIC,
allowing it to successfully reproduce patterns in the isotope and concentration profiles in the peat. This model is applied to quantify CH4 production in tropical peatlands (Chapter 2) and to
compare that with rates of methanogenesis in northern peatlands (Chapter 3).
In Chapter 2, we provide a complete characterization of the CH4 budget in a tropical
peatland for the first time. We use the model presented in Chapter 1, in combination with
detailed porewater measurements to quantify CH4 production from a pristine peatland in Brunei
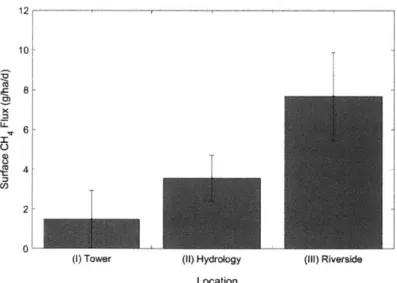
Darussalam. We find an order of magnitude more methane is produced in the permanently saturated peat than surface fluxes suggest. We then use further isotope measurements, in combination with flux chamber data, to study the transport and oxidation of this CH4. We find
the majority of this CH4 is transported as dissolved CH4 toward the rivers draining the peatland.
Less than 5% of the produced CH4 escapes by diffusion from the peat surface, and most of the
CH4 is oxidized before reaching the atmosphere. However, tree transport and degassing of CH4
from the river are found to be the most important conduits of CH4 to the atmosphere. These
pathways have received little attention, and should be the focus of future measurements aimed at
identifying CH4 emissions from tropical peatlands.
In Chapter 3, we explore differences in CH4 production and transport between northern
provides ample opportunity for oxidation before the CH4 reaches the atmosphere. As a result, only a small fraction of the total CH4 produced in tropical peatlands is emitted as CH4.
In contrast, northern peatlands are much more stagnant, allowing CH4 concentrations to build up
to the ebullition threshold. CH4 bubbles provide a direct pathway for CH4 to the atmosphere, and
a much larger fraction of the produced CH4 is emitted before being oxidized. Our work illustrates
that relatively small differences in recharge rates and CH4 production rates can dramatically alter CH4 transport and rates of emission to the atmosphere.
Future work aimed at quantifying CH4 emissions from tropical peatlands should focus on
better quantification of understudied processes such as lateral transport, oxidation, degassing from the river, and tree transport. In particular, a better understanding and model of controls on CH4 oxidation could allow better predictions of when and where the produced CH4 will reach the
atmosphere. Our works suggests there may be particular places (e.g. seepage faces close to the river's edge) or times (wet season) when a much larger fraction of CH4 escapes without being
oxidized. A better understanding of the processes which facilitate oxidation may help to explain these spatial and temporal trends. For example, surface oxidation is likely to vary throughout the year based on the level of the water table. Data indicates there is large spatial variability at a
small-scale, which is likely controlled by microtopography, but may also be influenced by the presence of tree roots or preferential flow paths in the peat. Oxidation in the river may also have
strong seasonality. Additionally, anaerobic oxidation of CH4 may play an important role in
tropical peatlands and requires further investigation.
Future work is also required to understand what inhibits methanogenesis in tropical peatlands and accounts for their relatively low rate of CH4 production. Although comparable to
the rates of methanogenesis in some northern bogs, CH4 production rates are on the low end of the range observed in northern peatlands. This is surprising, as warmer temperatures, and a
year-round growing season might be expected to result in much higher annual rates of CH4
production. These differences suggest methanogenesis may be inhibited. However, the
fundamental processes responsible for this difference are poorly understood. Possible limitations due to the substrate and microbial community should be examined, and compared with mineral tropical wetland soils, where higher CH4 fluxes have been observed.
Finally, the strong global trends we uncover in Chapter 3 suggest further work should explore the reasons for, and possible processes underlying these similarities. The CH4 and DIC
isotope and concentration profiles for all northern bogs we analyzed were remarkably similar, despite large differences in CH4 production rates. This hints at a possible fundamental feedback
between the recharge rate and the CH4 production rate, which have inverse effects on these
profiles. For example, concentrations may build up until an energetic threshold is reached.
Alternatively, surface derived DOC may drive methanogenesis. Finally, recharge and CH4
systematically quantify production rates for the first time, a better understanding of the fundamental processes which control them, and their relationship to recharge, is required.
(II) Carbon Dioxide Emissions
Peatland drainage results in large CO2 fluxes to the atmosphere. There has been a marked
increase in such emissions in Southeast Asia as a result of rapid land use change. It is important to understand the processes which control these fluxes at a local scale, and also to develop tools and models which allow more accurate estimation of regional fluxes from peatland degradation. Our work addresses these challenges by answering the following questions:
(a) How do CO2 emissions from the peat surface depend on temperature and water table?
(b) How do these emissions vary in space and time across Southeast Asia?
In Chapter 4, we use automated soil respiration chambers to examine how soil respiration responds to changes in temperature and water table at hourly timescales. We find a strong linear dependence on the water table, with CO2 emissions increasing as the peat dries, consistent with
previous studies. We also find CO2 emissions depend on temperature under dry conditions, and
exhibit a strong diurnal signal. These patterns are particularly pronounced in measurements of
CO2 emissions from a sunny open area. These results suggest that manual chamber
measurements, which are commonly made during the warmest daylight hours, likely
overestimate true CO2 emissions, particularly from degraded peatlands.
In Chapter 5, we measure subsidence rates across much larger spatial scales than ever before. Previous subsidence studies were limited to dozens or occasionally hundreds of subsidence poles. For the first time we make millions of remotely sensed subsidence observations on all peatland land use types using an InSAR time series approach. The large spatial extent of this data, in combination with historical land use maps for the region, allow us to uncover spatial trends, and examine the dependence of subsidence on present and historical land use. Finally, as subsidence results from peat decomposition, we use these results to characterize rates of CO2 emissions from different land uses, and to estimate CO2 emissions across the region.
Future work at the local scale should focus less on quantification of surface fluxes, which have already been extensively measured, and should instead be directed at the processes
autotrophic respiration throughout the diurnal cycle and their relative contributions under changing land uses remain poorly characterized.
At a regional scale, our remote sensing approach provides unprecedented spatial
information on peatland subsidence and CO2 fluxes. Systematic groundtruthing efforts would
complement this work by validating key trends and addressing remaining uncertainties. For example, the strong dependence of subsidence rates on distance from the nearest river has never been documented in ground-based subsidence measurements, because measurements have not been made using a transect design or at the large spatial scales necessary to show such trends. Further study is also needed to investigate the underlying processes responsible for this trend. It may result from differences in peat characteristics, peat depth, water table dynamics resulting from drainage, or a combination of these factors. Additionally, ground-based measurements should shift focus from plantations and forests, which have been well characterized, to other understudied land uses, such as smallholder land, which is dominated by mixed agriculture and accounts for a quarter of total peatland area in Southeast Asia.
Finally, our remote sensing approach should be applied across Southeast Asia to better quantify regional CO2 emissions, and identify subsidence hotspots. Future work in this area will be increasingly powerful, with the possibility of tracking subsidence rates in pseudo real time using current ALOS-2 and Sentinel-I satellites. Their improved revisit times over ALOS-I will reduce uncertainties in subsidence estimates and may even make it possible to track seasonal changes in subsidence rates. In the future, large remote peatland expanses in South America and Africa are also likely to face degradation. Tracking of these locations using a similar approach could provide early warning signs and contribute to regional inventories of CO2 fluxes. Finally, it
is important to note that these inventories will be incomplete without accounting for the role of peatland fires, which are also responsible for large subsidence and CO2 emissions in drained
peatlands.
In summary, this thesis provides an improved understanding of the processes responsible
for CO2 and CH4 emissions from tropical peatlands. We also provide new tools for improved
quantification of these greenhouse gas budgets, both through the development of a model to quantify CH4 production, and by the application of a novel remote sensing approach. This work
makes a significant contribution to reducing uncertainties in our understanding of the processes underlying CO2 and CH4 emissions from tropical peatlands, and highlights areas where further
Chapter 1:
Development of a Model of CH
4Production and Transport for Peatlands
Introduction
Wetlands are an important source of CH4 globally (Saunois et al., 2016). However, there
is large variability in peatland CH4 fluxes across latitudes (Couwenberg et al., 2010), and
between mineral and organic wetland soils in the tropics (Sjigersten et al., 2014). Although CH4
surface fluxes from tropical peatlands are 1-2 orders of magnitude lower than those from
northern peatlands (Couwenberg et al., 2010), in situ dissolved CH4 concentrations can be
similar (e.g. tropical: Holmes et al., 2015 and Chapter 3 of this thesis; northern: Corbett et al.,
2015). Thus, it remains unclear how CH4 production rates vary across these ecosystems.
In this chapter (Chapter 1) we develop a model which allows us to quantify CH4
production throughout the peat profile, and to better understand its transport out of the peatland. We then use this approach to quantify CH4 production rates in tropical peatlands (Chapter 2) and
compare these rates with CH4 production in northern peatlands (Chapter 3).
The modeling approach presented here relies on stable isotope (613C) and DIC
measurements from the peat porewater. CH4 has a low solubility in water, and is likely to degas or bubble in peatland ecosystems with high rates of methanogenesis. This makes direct
measurements of CH4 concentrations an unreliable indicator of methanogenesis. In contrast,
isotopic measurements are not affected by ebullition, and retain the signatures of CH4 production
and oxidation. Unlike flux measurements, which are highly variable in space and time, and can give insights into seasonal trends, isotopic signatures provide an integrated signal of CH4
production, appropriate for use with a steady-state model.
Models of CH4 Production, Isotope Fractionation and Transport in Peatlands
To interpret isotope values, fractionation during methanogenesis and transport must be considered. Existing models of CH4 production and transport within the catotelm (the
permanently saturated peat) represent various subsets of the following processes:
methanogenesis, methane oxidation, diffusive transport, ebullition and vertical advection (Beer
& Blodau, 2007 based on Berg et al., 1998; Steinmann et al., 2008; Shoemaker & Schrag, 2010;
Corbett et al., 2013a)
Each of these models applies the principles of fractionation during methanogenesis to calculate total CH4 production from the 613C of DIC, in addition to concentration profiles. Our
model implementation of fractionation during methanogenesis follows the conceptual framework for CH4 production presented by Steinmann et al., (2008). Shoemaker & Schrag (2010) also
include fractionation in a transient model of CH4 production, in which CH4 production is
(2008) have a more tightly constrained CH4 production rate, as CH4 production is assumed to
decrease exponentially with depth. Finally, Corbett et al. (2013a) calculate CH4 production
directly from DIC concentrations and 613C values at each depth throughout the peat profile. Although each of the models includes some transport processes, none of the models includes lateral advection of dissolved CH4. Beer & Blodau (2007) and Shoemaker & Schrag
(2010) both include diffusive but not advective transport. Corbett et al. (2013a) implicitly include vertical transport, but not lateral flow. Finally, Steinmann et al. (2008) explicitly model diffusion and vertical advection, presenting the framework most similar to our own.
Here we present an isotope-based model of CH4 production which accounts for both
vertical and lateral transport for the first time. This is particularly important to accurately
represent coastal ombrotrophic peat domes in Southeast Asia which are underlain by marine clay (Dommain et al., 2015; Baird et al., 2017). In these peatlands, most water runs off the peat surface, or through the shallow near-surface zone (Cobb et al., 2017). However, some of the flow recharges into the deeper peat, where CH4 is produced, even during dry conditions when the
water table is low. This flow is evident because groundwater discharge from the peat continues to feed rivers and streams that are sourced only by local peatlands, when conditions are relatively dry and the water table is low. Furthermore, the presence of modern dissolved organic carbon in the porewater to depths of 4.5m (Corbett et al., 2013b; Gandois et al., 2014) confirms that recharge penetrates into the deep peat. Downward flow is eventually obstructed by the
underlying marine clay. To maintain downward flow in the presence of this barrier, lateral flow must accelerate towards the peat boundaries (Figure 1) and this lateral flow carries dissolved methane produced within the peat to be released to adjacent streams (Hope et al., 2001; Billett et
al., 2004; Billett & Moore, 2008).
An accurate model of lateral transport is also relevant to northern raised bogs, where peat deposits often overlay mineral soil or marine clay which is relatively impermeable (Fraser et al., 2001). In some cases, porous sand deposits are beneath the peat, in which case a model of strictly downward flow, without lateral flow, may be more appropriate (Steinmann et al., 2008).
Although we developed our model to include lateral transport, our implementation is highly flexible, and can easily accommodate systems where vertical transport completely dominates lateral transport.
signatures integrate information over months to decades, in contrast to CH4 concentrations,
which may be affected by short-term fluctuations in production or degassing. Depending on the rate of water flowing into the deep peat, the residence time of DIC in the peat porewater can be tens of years to over 100years. Since the isotope signature is not affected by degassing, the 613C
of DIC or CH4 at depth reflects years to decades of CH4 production, and can be a reasonable
approximation of the steady-state of the system.
In contrast, transient models of CH4 production are more common (Beer & Blodau, 2007;
Steinmann et al., 2008; Shoemaker & Schrag, 2010). These models have the advantage of being able to interpret seasonal changes in production rates. They may be able to capture changes in the CH4 production and oxidation rates near the peat surface, where the largest changes are
expected, and which are not included in our model. However, this approach requires a much larger temporal dataset, limiting the number of sites where this approach can be applied. It also makes the model results much more susceptible to noise in the data. Natural heterogeneity or measurement noise may be over-interpreted by a transient model as short-term or local changes in the CH4 production rate.
Modeling 14C: Insights into the C source for methanogenesis
Although only stable isotope measurements are needed to fit CH4 production rates, 14C
can be added to the model following a similar framework, and allows the model to track the C source for methanogenesis. Modeling the impact of fractionation during methanogenesis and transport on 14C throughout the peat profile allows us to disentangle the relative contributions of peat and DOC to CH4 production. The 14C content of respiration products has been measured in a
number of studies and shows a consistent trend across bogs (Aravena et al., 1993; Charman et al., 1999; Chasar et al., 2000a; Chanton et al., 2005, 2008a; Corbett et al., 2013b). These profiles have traditionally been interpreted using mixing models, which neglect the influence of transport on isotope signatures. By including 14C in our modeling framework, we can better understand these profiles in the context of flow through the peat (Hoyt et al., 2015). This is the first time a reactive-transport model has been applied to understand 14C profiles in peatlands.
Model Development
The model presented here captures the fractionation during CH4 production and transport,
and uses isotope and concentration profiles to quantify the rate of methanogenesis throughout the peat profile. It uses a steady-state ID reactive transport model with a non-uniform vertical
velocity to capture the both vertical and lateral advection as well as diffusive transport. It generates not only concentration profiles, but also models the profiles of 613
C and A14C of both
CO2 and CH4. This is possible with the individual modelling of different isotopes. We
individually track six carbon pools: 1CO2, 13 CO
2, 14CO2, 12CH4, 'CH 4, and 14CH4 and solve for
their concentrations according to the general transport equation, which captures advective transport, diffusive transport and production:
dc d2c
it- -D =P
where the source term (P), is modified to reflect fractionation during production for each isotope. The coupled equations for each of the six carbon pools are solved iteratively using a finite
difference approach.
In the following sections, we cover the hydrologic framework for the model, CH4
transport, and the formulation of fractionation during methanogenesis in more detail.
Hydrologic Framework
Our goal was to build the simplest mathematical model of groundwater flow and solute
transport through peat that provides reasonable quantitative estimates of both lateral and vertical CH4 fluxes from the peat when combined with field data. Vogel (1968) provided a model of
lateral and vertical groundwater flow that has been widely applied to interpret isotopic tracers in aquifers. His model represents flow through a homogeneous domain of constant thickness subject to uniform recharge along the surface and discharge at the lateral boundaries. Under these assumptions, the vertical and horizontal components of groundwater velocity along a stream tube are:
r r
VZ = -- z; vX = -x (1)
Ob Ob
where z is the elevation above the base of peat with a thickness b; x is the distance from a groundwater divide; r is the rate of recharge, and; 0 is the effective porosity.
Vogel's model implies that groundwater age depends only on depth, not on lateral
position. This implication is extremely useful, but perhaps counterintuitive. For example, a solute molecule that infiltrates in the central area of the domain, near the groundwater divide, will reach a give depth at the same time as a solute molecule that enters the domain closer to the edge. The molecule in the center will move slowly along a nearly vertical path until it nears the bottom of the peat, whereas the molecule entering towards the edge will move much faster horizontally, covering a much larger horizontal distance over the same interval of time. Both molecules, however, will move at the same vertical velocity (eq. 1), obtaining the same depths at the same times. This characteristic of the flow system enables us to construct a relatively simple model for methane transport that we use as a tool for interpreting field data.
I
bi
Figure 1. Schematic of vertical and lateral transport in a peat dome. Top panel is vertically exaggerated to show
curvature of a peat dome, which can be kilometers wide, and a few meters high. However, at any point along the peat dome, the peat surface is nearly flat, and the peat can be approximated as a domain of constant thickness (bottom panel).
CH
4Transport
Because groundwater age is laterally uniform, CH4 concentrations will also be laterally
uniform where the rate of CH4 production is uniform. Consequentially, the advective-diffusive
equation reduces to:
r _b ac(z) a2C(Z)
+ Da = P (2)
where D is the apparent diffusion coefficient and P is the source or sink rate. The key difference between the form of this equation, and that of differential equations used in a previous study of CH4 fluxes from peat which included advection (Steinmann et al., 2008), is that this equation
enforces a no-flow condition at the bottom boundary of the peat (z=0) where the peat is underlain
by a thick layer of clay.
Over the long-term, fluxes of CH4 leaving the peat must balance methane production
within the peat. The upward diffusive flux from the stream tube and the lateral advective flux at the discharge boundary must sum to the rate that CH4 is produced along the entire stream tube:
-LD Cb + b fb C(z)dz = bLP (3)
where L is the length of the stream tube from the divide. We employ both analytic and numerical
approaches to solve equations 2 and 3. We provide analytic solutions for equations 2, and each
term in 3, that apply when P is uniform with depth, such as the case of uniform CH
4production
(see SI, Chapter 1). We employ a numerical solution to equation 2 when modeling the isotopic
signature of methane because P, the rate of production, varies with depth for different isotopes
even when the overall rate of methane production is uniform. The isotopic signature of DIC
varies with depth and hence the rate of production of
12CH4 and
13CH
4
also varies with depth
correspondingly. The transport terms (left hand side of equation 1), however, remain the same.
Model of Methanogenesis 2cH2O+2H1202CC2+4H
CQ2 + 4H2 - CH4 + 2HO by CO2 Reduction Pathway: Net pathway: 2CH20 -- CO2 + CH4 CO2 Sources: Organic C 2n COn PeatDOC C2CH
2CH20 +2HO -2CO2 + 4H, gg O+H >~ 2 4
(-30%9) (fractionawingpathway
eC
enied DIC Processes:
diffulsingy fromi clavK'
diffsiiu
trlfllaxFractionation
during methanogenesis.(0"00) 12
CH4 is preferentially produced, resulting in light CH4.
Heavy CO2 is left behind, enriching the bulk CO2 Pool
C(-)2 advected ... .
from surface (-30000) Mixing and Fractionation during transport:
(product of aerobic respiration) CH4 and CO2 are diffused and advected through the peat column
Heavier 13C02 and 13
CH4 diffuse more slowly than 12CO2 and 12
CH4,
but are advected at the same rate.
Figure 2. Conceptual model of methanogenesis by the CO2 reduction pathway. CO2 is first produced from the organic source material (peat or DOC). Then, CH4 is produced from this bulk CO2 pool. As a result, the isotopic signature of the CH4 will reflect the bulk CO2 pool.
Isotope Fractionation
Here we assume all CH
4is produced via the CO
2reduction pathway, consistent with
isotopic observations at our site in Brunei (Chapter 2) and other observations in tropical peat
(Holmes et al., 2015). Although acetoclastic methanogenesis commonly dominates in freshwater
The CO2reduction pathway is summarized by the following reactions:
2CH20 + 2H20 -+ 2CO2 + 4H2 (4)
CO2 + 4H2 -. CH4 + 2H20 (5)
Net pathway: 2CH20 -+ CO2 + CH4
As a result of this two-step process, CH4 is produced from CO2 that originates from a
variety of sources, not only the CO2 derived from the organic carbon driving methanogenesis.
This is reflected in the isotopic composition of the CH4, and is captured by our model
formulation. As shown in Figure 2, organic carbon is first converted to CO2 at a rate of 2n (eqn 4). This CO2 mixes with CO2 from other sources, including CO2 which is advected from the
surface (produced through aerobic respiration), and DIC with a marine signature diffusing up from the clay. Following eqn 5, half of this CO2 is then converted to CH4 at a rate of n, and half
remains as CO2, resulting in a net CO2 production rate of n. Notably, CH4 is produced from the
bulk CO2 pool, and reflects a mix of sources, rather than solely from the CO2 produced in eqn 4.
The CO2 reduction pathway results in differences in the isotopic signature (61 3C) of CO2 and CH4 of 50-80%o due to the lower reaction rate of the heavier 3C. The corresponding
fractionation factor UCO2-CH4 =(613C02 +1000)/(61 3CH4 +1000), ranges from 1.055-1.090
(Whiticar et al., 1986). (In contrast, acetoclastic methanogenesis is a less fractionating pathway). To represent this fractionation factor in the model, the 1 3C0
2 reacts into CH4 at 0.94 times the
rate of 12CO2. This corresponds to 1/a for a = 1.064 from our dataset (Chapter 2).
R, = r
[12 CO2] 2 2 1 1 CjH4CH4
1CH4 R2 = r2 ["C021 R3= r3['4CO2] aCO22n(i-ftn-fl
S)our 2nf Peat CO2a L-30% -) - -- - -2nf4 14CO2 Additional SourcesFigure 3. 2n = total rate of CO2 produced during methanogenesis. n = RI + R2 + R3 = total rate of CH4 production
Model Formulation of Fractionation During Methanogenesis
Figure 3 illustrates the rates of production of each isotope of CO2 and CH4 in the model,
demonstrating the contribution of model sources and sinks of CO2 and CH4 to fractionation.
During the first reaction step (eqn 4) CO2 is produced from organic material (peat or DOC) at a
total rate of 2n. During this step, produced CO2 reflects the isotopic composition of the source
material, peat or DOC, as isotopic fractionation during fermentation is assumed to be negligible. The valuesfi? andfi 4 represent the fractions of total CO2 production composed of 13C02 and
14CO
2 respectively. These values are assigned to represent the age and isotopic composition of
the peat or DOC. Thefb value is the same for peat and DOC, which have similar 6 1C values,
butf]4 can be adjusted to reflect the radiocarbon content of the peat, which ages with depth, or
the DOC, which is modern.
Half of the total CO2 produced (composed of 12C02, 13CO2 and 14CO2 pools) is then
converted to CH4, which is composed of the 12CH4, "CH4, and 14CH4 pools. Fractionation during
methanogenesis occurs due to the different rates of CH4 production from each of these pools.
Following Figure 3, we can express the variable rates of methane production from the 2C, 1C
and 14C pools as follows: R,=ri[ 1 2
C02]
R2 =r2 [3CO2]
R3 =r3 [14CO2]
where R I+R2+R3 = n = total rate of CH4 production
The relative rates of production are given by the fractionation factor in the model (i.e. the
132 reacts into CH4 at 0.94 times the rate of 1CO2). This corresponds to
I/a
fora =
1.064from our dataset (Chapter 2). Due to the difference in mass, the fractionation for 4C is expected to be twice as large, which we represent by doubling the fractionation factor.
r2 =P
ri,
P
=I/a
= 0.94 (CO2 reduction pathway) r3=-y
ri, y=
0.88 (2x fractionation for 14C)Using these constraints we can solve for rl, r2, and r3:
R I+R2+R3 = n
ric12 + r2c13+ r3c14= n
ric12 +
Pric13+
yric14 = nr1 (c 12 + Pc13 + yC14) =
n
ri= n /( c12 + Pc13 + yC14)r2= np /( c12 +
Pc13
+ yc14)r3= ny /( c12 + Pc13 + yc14)
where the following notation shorthand has been used for simplicity:
C12= [ CO2, c13 = [" CO2, c14 = [ 14C02]
M12=
[
CH4], m13 = [ CH4, M14 = [ 14CH4]The reaction rates r], r2 and r3 are then used to create source and sink terms for the transport equation for each of the CO2 and CH4 pools shown below. For C02, CO2 is produced at a rate of 2n from fermentation of the peat or DOC. This CO2 mixes with CO2 from other sources
(such as advection or diffusion from other depths, respiration or DIC from the clay). Then CO2 is reduced to CH4 at a rate of n. This is expressed as a source term with a rate of 2n produced from
the peat, and a sink term with a rate of n from the bulk CO2. At the same time, CH4 is produced
with a single source term. This formulation allows for mixing of CO2 from other sources, and the production of CH4 from the bulk CO2 pool, rather than directly from the fermentation products of peat. It reflects the stoichiometry of the C02 reduction pathway. A similar formulation was used
by Steinmann et al. (2008) in their model of the isotopic composition of CO2 and CH4
The complete production terms for each of the carbon pools are listed below: Units: (ID) [12CO2], ["CC, [ [14CO2, [12CH4], [CH [ 14 CH4] >> mol/m n, R1, R2, R3 >> mol/m/yr rI, r2, r3 >> 1/yr , y >> unitless 12C02]:
n
2 + 2n (1 -f
1 3-f4)
(c1 2 + 1c1 3 + yc1 4) [13C02]: ( +n/k
1 3 + 2nf13 (C12 + Pc1 3 + yc1 4) [14C 02]:-
nyC+ 2nf14 + C14 (c12 + 1c13 + yc14) 5730[12
CH4]: nc12 (c12 + Pc13 + yc14) [13 CH4]: nflC13 (c12 + Pc13 + yc14) __ y_____4 __ In (}) [14CH4]: nyc14+
M14 (c12 + Pc13 + yc14) 5730In the analysis presented here, the rate of methanogenesis is assumed to be uniform with depth. This assumption is supported by measurements from a tropical peatland in Panama (Wright et al., 2011). However, this model formulation and code has the capacity to vary
production of CH4 and CO2 with depth. Other models have allowed rates of methanogenesis to
vary with depth (Beer & Blodau, 2007; Shoemaker & Schrag, 2010) or have assumed it decays exponentially with depth (Steinmann et al., 2008).
Complete Transport Equations
The complete equations used in the model are shown below. The transport terms (advection and diffusion) are listed on the left hand side. The source and sink terms accounting for CH4and CO2 production are listed on the right hand side.
19c1 2 Da2c 1 2 -nC12+ u
Ox
DOx
22 = + -(c 12+
pc
13+ yc
14)+
2n (1- f
13 - f1 4) Uax
D a2C1()X 2 3 (c+ C13 _ + 2nf13 (C12 + PC13 + yc14) dc14 D 2c14 -nyc 14ax
ax2 (c 1 2 + 13c + yc14) in (}) 2nf14 + 3C14 5730 a iM12 D = ax2 02 M1 3D Ox
aX2 a 2M1 4 D X2 nc1 2 (c1 2 + Pc1 3 + yc14) nf3C1 3 (C 1 2 + PC1 3 + YC1 4) nyc 1 4 (c1 2 + Pc1 3 + yc1 4) In(})
+ 57 5730 1CH4 and DIC Fluxes
Total production of CH4 and DIC within the peat profile, as well as fluxes into and out of
the peat were calculated as follows: Ac
Fdiffusive = -D
Az
Flateral = C * Uhorizontal
Az
Fadvection from surface = Usurf ace * Csurface
Fproduction = P
Az
Diffusion Coefficients
The diffusion coefficients were initially adjusted to reflect the molecular weights of the different isotopes, but this was found to have negligible impact on the results. These calculations
[
12C02]: [13C02]: [ 1 2 CH4]: OM1 2 UOx
Oin
13 U -ax aM14 U oxwere done according to the following equation, adjusting for the mass of each isotope (Appelo & Postma, 2005) (where 18.015 is the molecular weight of water, 44 and 45 are the molecular weights of C02 with 1 2C and 13C isotopes respectively).
D13C02 in water 44 * (45 + 18.015)
= 0.9968
D12C02 in water 45 * ( 44 + 18.015)
Further diffusion effects in liquid water are discussed further in Bourg et al. (2010) but are assumed to be small.
Discretization and boundary conditions
The transport equations for each isotope of DIC and CH4 were discretized using a finite
difference approach, and solved iteratively using Matlab. Dirichlet boundary conditions were used at the top of the model domain (30cm below the peat surface at the position of the water table) to impose fixed values of CH4 and DIC concentrations and isotopic composition. A
Neumann no-flux boundary condition was applied at the bottom of the peat profile to represent the impermeable clay beneath the peat. An alternative model formulation representing DIC from the marine clay diffusing up from beneath the peat was also implemented using a fixed DIC concentration and 613C value in the clay beneath the peat. This formulation resulted in a small
flux of DIC from the clay into the peat, but did not qualitatively change the results. Diffusion is relatively slow, and DIC deep in the peat becomes highly enriched as a result of CH4 production,
Fvapotanspiration Seasonally flooded shallow peat Near-surface runotf flows to nver Permanently water-logged peat column A ---- Laterally flowing water carries dissolved CH4 Fl = u~c A -4 --- Lateral velocity u, compensates for decreases in vertical
velocity u with depth
A
Pools of 2CO,, 3CO,
C02, CH4 2CH4, and "CH4 are
CA represented at every depth
A Upward diffusion of CH4 follows concentration
A gradient Ff ,,= Dc
tarine clav underfie' peat
Figure 4. Schematic of discretization. Black boxes and arrows show pools and fluxes which are explicitly modeled.
Grey boxes and arrows show processes which are implicitly included in the model by the hydrologic framework or values of the boundary conditions.
Determination of recharge rate
We fit concentration profiles of conservative natural tracers to estimate the recharge rate
into the catotelm. In Southeast Asia's coastal peatlands, high concentrations of chloride and
bromide occur naturally in the marine clay underlying the peat, and diffuse up through the peat
profile. This upward diffusion is balanced by downward advection of recharging rainwater with
very low chloride and bromide concentrations. At other sites, such as the Glacial Lake Agassiz
Peatlands, peat deposits are underlain by sandy soils with high calcium and magnesium
concentrations, which are also low in the rainwater (Romanowicz et al., 1995; Glaser et al.,
2016). In both cases, the resulting concentration profile is described by:
C(z) = Cciay -
(
Ccay - Crain) * erf(z2bD
Assuming a linearly decreasing vertical velocity, we used this equation to fit the recharge rate
into the catotelm, U, to the concentration profiles.
Im 2m 3m Increasing A ection 4m 5m 0 50 100 150 200 2 C1 (mg/L)
Figure 5. Conceptual fits to chloride profile show the role of increasing advection.
Model Results: Influence of parameters and surface boundary conditions
In the model formulation parameters and surface boundary conditions can take on fixed values based on data values from the site, which may vary between northern and tropical peatlands, or can be fitted parameters. Some parameters, such as the rates of recharge and methanogenesis, influence both the concentration profiles and isotopic composition, while others, such as the fractionation factor, only influence the isotopic composition. Here we discuss the influence of these parameters on the concentration and isotope profiles.
Influences on concentration profiles: CH4 production and recharge rates
Proportional increases in the recharge rate, U, and CH4 production rate, P, have nearly identical influences on the DIC and CH4 concentration and 613C profiles. This can be noted from
the transport equation:
dc a2C
u-- D-= P
For all but the lowest recharge rates in natural peatlands, the advective term dominates the diffusive term throughout the majority of the profile. Only in the deep peat does diffusion begin to play an important role. Where diffusion is negligible, the transport equation simplifies to:
recharge/high production are nearly identical, except for at the base of the peat profile where
diffusion plays a larger role.
0 1 2 3 4 CH4 Concentration (mM) 5 6 0 -1 -2 -3 -4 -5 -6 0
0
-1 -2 .S_ 0-4 -5 -6 0 -1 -2 -4Shallow oxidation zone,
CH4
- \
a
\
a
- \
-- -'Frc'O tKontfl Facto~
-100 -80 -60 -40 13C \ 4 % % ricreasin n Recharge Rate P=0.I/U=0.6 - P=0.2/U=1.2 - -P=0.2/U=0.6 - P=0.IIU=1.2 Increasing Production Rate 5 DIC 4)4 Increasing E % Production \ Rate Increasing :2 Recharge Rate 0 -20 0
Figure 6. DIC and CH4 concentration and 813C profiles demonstrate the influence of changing recharge and
production rates. Profiles with both a high rate of recharge and high rate of methanogenesis (blue solid line, P =
0.2mM/yr, U = 1.2m/yr) are nearly indistinguishable from profiles with a lower rate of recharge and a lower rate of methanogenesis (black dotted line, P = 0. 1mM/yr, U = 0.6m/yr). For a given rate of methanogenesis, increasing the recharge rate results in reduced DIC and CH4 concentrations, and more depleted 6'
3
C of DIC and CH4 profiles
(dashed blue, P = 0. 1mM/yr, U = 1.2m/yr). Alternatively, fixing the recharge rate and increasing the rate of methanogenesis results in increased DIC and CH4 concentrations, and more enriched 63C of DIC and CH4 profiles
(dashed black, P = 0.2mM/yr, U = 0.6m/yr)
Influences on isotope profiles:
The isotopic composition of DIC and CH
4are influenced directly by the fractionation
factor, the 613C of the source material, and the surface 613C values of DIC and CH
4. They are
also influenced indirectly by the production and recharge rates, and the surface concentrations of
DIC and CH
4.
'I 1~ rfl 4% I ~ 0 Ia
4% O~ 4% 4% 4%Shaltow oxidation zone'
-6 1 2 3 4 DIC Concentration (mM) 6 20 r
Direct Isotope Influences:
Fractionation factor: Fractionation during methanogenesis is modeled using a
fractionation factor that controls the 613C separation between DIC and CH4. Increased
fractionation leads to a larger separation between the 613C of DIC and CH4, shifting the
profiles apart. Reduced fractionation shifts the profiles together.
513C of the organic source material: Decomposition of carbon in peat and DOC drives
methanogenesis. In Brunei, this organic source material has a signature of -28 to -31 %o
(Chapter 2), while in northern bogs the DOC and peat are often more enriched, from -25 to -27%o (Chapter 3). A shift in the 61 3C of the source material shifts both the 6 3C of DIC
and CH4 in the same direction.
Surface values of(5'3C of DIC and CH
4: DIC and CH4 produced at the peat surface may
have significantly different isotopic signatures than that produced throughout the peat profile. For example, DIC at the surface is produced by aerobic decomposition, a non-fractionating pathway, and reflects the signature of the organic source material. This is much lighter than DIC throughout the remainder of the profile. Correspondingly, CH4 at
the surface may be enriched due to aerobic surface oxidation or acetoclastic
methanogenesis. This would result in CH4 which is significantly more enriched than CH4
produced by CO2 reduction in the peat. These signatures are represented in the model
with a fixed value surface boundary condition, and influence the deeper 613C profiles by
advective and diffusive transport.
Indirect Concentration Influences:
CH4 production and recharge rates: The 613C of DIC becomes more enriched with more CH4 production and/or a reduced recharge rate. This propagates to the CH4, which
becomes correspondingly more enriched.
Surface DIC and CH4 concentrations: The surface 613C signatures of DIC and CH4 are
transported throughout the peat profile by advective and diffusive transport. The degree
to which this signature propagates depends on the surface DIC and CH4 concentrations. If
surface concentrations are high, surface DIC and CH4 will make up a larger fraction of
A similar effect results from increasing CH
4concentrations. The higher the surface CH
4concentration, the deeper the isotopic influence of surface oxidation will propagate into
the peat profile. This results in the "elbow" moving deeper into the peat. In contrast, very
low concentrations of CH
4near the surface can sharpen the "elbow", producing a
strikingly sharp bend as the result of the competing forces of advection and diffusion. In
tropical peatlands, surface concentrations of CH
4are very low (<0.01 to 0. 1mM),
resulting in a dramatic "elbow" of
20-
30%o (Chapter 2). In contrast, in northern bogs,
surface concentrations of CH
4are much higher (-0.5mM), resulting in a smooth bend of
5-10%o (Chapter 3).
1 4 CH13C DIC 2. More dep
5. Bend -surface boundaryconditionreflects oxidation surface DICi
is higher, de
2. Stronger bend if surface CN4conc. is lower, propagation
only shallow propagationof surface values surface valu
E 4. 8IC of organic source material determines position of central axis
4- -0
0 4
3. Shape of 81CH4 closely follows DIC
1. 13C of DIC becomes more enrich 1. Uke DIC, 5"CH4 is more enriched with more production or less recha
with more production or less recharge This propagatesto the 8'CH4
3. Increasedfractionation between DiC and CH4separatesthese curves
-80 -60 -40 813c -20 leted if conc. eper of as ed rge 0 20
Parameters affecting isotopic composition of DIC and CH4
Concentration Influences:
1. Production and recharge rates
2. Surface concentrations of DIC and CH4
Isotope Influences:
3.
Uniform fractionation between DIC and CH4 4. 83C of organic source material5. Surface values of 813C of DIC and CH4
Figure 7. 613C profiles of CH4 and DIC highlighting the influence of model parameters. Parameters are grouped by
those which have a direct isotope influence, and those which more indirectly affect the 6 13C profiles, and
predominantly affect concentrations in the profile.
Alternative flow profiles
Flow through the deep peat is modeled with a linearly decreasing vertical velocity, which
reaches zero at the base of the peat profile. However, neither the flow regime nor the changes in
hydraulic conductivity with depth are well characterized for tropical peatlands. In temperate
bogs, some measurements of hydraulic conductivity have found decreases with depth (Clymo,
2004). To adapt to such a scenario, this model could be modified to include an exponentially
decreasing vertical velocity. Similar profiles would be reproduced if this were paired with an
0 -1 -2 -4 -5 -6 -100