HAL Id: tel-03229991
https://tel.archives-ouvertes.fr/tel-03229991
Submitted on 19 May 2021HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
High rate anaerobic treatment of LCFA-containing
wastewater at low temperature
Suniti Singh
To cite this version:
Suniti Singh. High rate anaerobic treatment of LCFA-containing wastewater at low temperature. Environmental Engineering. Université Paris-Est; Tampereen yliopisto, 2019. English. �NNT : 2019PESC2042�. �tel-03229991�
Joint PhD degree in Environmental Technology
Docteur de l’Université Paris-Est
Spécialité : Science et Technique de l’Environnement
Dottore di Ricerca in Tecnologie Ambientali
Degree of Doctor in Environmental Technology
Thesis for the degree of Doctor of Philosophy in Environmental Technology
PhD thesis –Väitöskirja – Proefschrift – Tesi di Dottorato – Thèse
Suniti Singh
High rate anaerobic treatment of LCFA-containing wastewater at low temperature
11
thDec 2019, Paris
Defended in front of the PhD evaluation committee
Prof. Charles Banks
Chairperson, Reviewer
Prof. Alla Nozhevnikova
Reviewer
Assoc. Prof. Marta Carballa
Reviewer
Prof. Jukka Rintala
Promotor
Asst. Prof. Marika Kokko
Co-Promotor
Prof. Piet N.L. Lens
Co-Promotor
Prof. Giovanni Esposito
Co-Promotor
Prof. Eric D. van Hullebusch
Co-Promotor
Marie Skłodowska-Curie European Joint Doctorate Advanced Biological
Waste-to-Energy Technologies (ABWET)
Evaluation Committee
Chairperson
Prof. Charles Banks
Engineering
University of Southampton United Kingdom
Reviewers/Examiners
Prof. Alla Nozhevnikova
Federal Research Center of Biotechnology Russian Academy of Sciences
Russia
Prof. Charles Banks
Engineering
University of Southampton United Kingdom
Assoc. Prof. Marta Carballa
Department of Chemical Engineering Universidade de Santiago de Compostela Spain
Thesis Promotor
Prof. Jukka Rintala
Faculty of Engineering and Natural Sciences Tampere University
Finland
Thesis Co-Promotors
Asst. Prof. Marika Kokko
Faculty of Engineering and Natural Sciences Tampere University
Finland
Prof. Piet N.L. Lens
Department of Environmental Engineering and Water Technology IHE Delft Institute for Water Education
Delft, The Netherlands
Prof. Giovanni Esposito
Department of Civil and Mechanical Engineering University of Cassino and Southern Lazio
Cassino, Italy
Prof. Eric D. van Hullebusch
Laboratoire Géomatériaux et Environnement Université Paris-Est
Supervisory Team
Thesis Supervisor
Prof. Jukka Rintala
Faculty of Engineering and Natural Sciences Tampere University
Finland
Thesis Co-Supervisors
Asst. Prof. Marika Kokko
Faculty of Engineering and Natural Sciences Tampere University
Finland
Prof. Piet N.L. Lens
Department of Environmental Engineering and Water Technology IHE Delft Institute for Water Education
Delft, The Netherlands
Dr. Gavin Collins
School of Natural Sciences National University of Ireland Ireland
Prof. Vincent O’Flaherty
School of Natural Sciences National University of Ireland Ireland
Dr. Johanna M. Rinta-Kanto
Faculty of Engineering and Natural Sciences Tampere University
Finland
Dr. Riitta Kettunen
Faculty of Engineering and Natural Sciences Tampere University
Finland
This research was conducted in the framework of the Marie Skłodowska-Curie European Joint Doctorate (EJD) in Advanced Biological Waste-To-Energy Technologies (ABWET) and supported by Horizon 2020 under grant agreement no. 643071.
Abstract
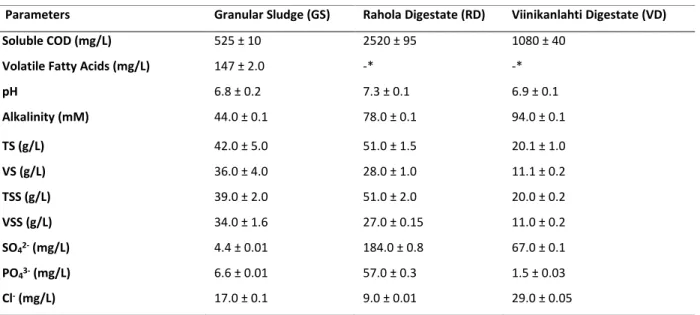
Fats, oil and grease (FOG) are a significant constituent in numerous wastewaters such as those in dairy industry. The hydrolysis of FOG result in the production of long chain fatty acids (LCFA) which destabilize the anaerobic treatment process due to their physico-chemical and microbial toxicity effects. Harnessing the high methanogenic potential of FOG necessitates effective treatment of high LCFA loads, wherein the feasibility of LCFA treatment at low temperatures has been not investigated up to now. The aim of this thesis was to study the feasibility of high-rate anaerobic treatment of LCFA-rich wastewaters at low ambient temperatures using dairy wastewater.
The screening of mesophilic inocula for treatment of mixed LCFA containing synthetic dairy wastewater (SDW) in batch studies showed that granular sludge inoculum achieved faster and higher methane yields (76-82% of theoretical yield) than the two municipal digestates (1-72%) at both 20 and 10°C. The LCFA degradation capacity in the granular sludge inoculum was attributed to the presence of β-oxidizing bacteria from the family Syntrophaceae (Syntrophus and uncultured taxa), the acetotrophic activity of
Methanosaeta and the putative syntrophic acetate oxidizing bacteria (SAOB).
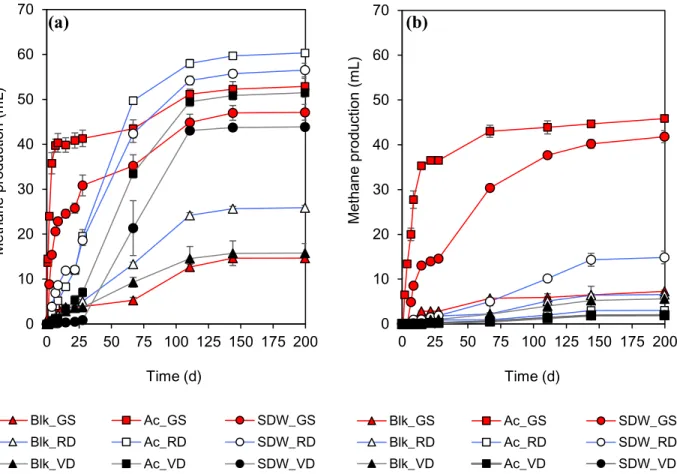
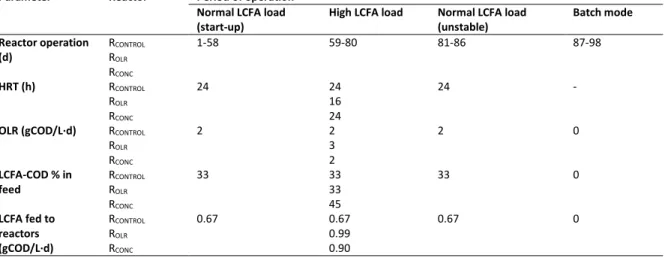
Continuous high-rate treatment of SDW was found to be feasible in expanded granular sludge bed (EGSB) reactors at 20°C (hydraulic retention time (HRT) 24 h, LCFA loading rate (OLR) 670 mgCOD-LCFA/L·d) with a soluble COD (sCOD) removal of 84–91% and methane yield of 44–51%. SDW feeding for longer than two months resulted in LCFA accumulation, which led to granular sludge flotation (36-57%) and disintegration (reduction in d50 of 24–33% and 75–84% in settled and washed-out granules, respectively). To counter the
LCFA induced granular sludge disintegration and flotation, a novel reactor type, dynamic sludge chamber-fixed film (DSC-FF), was designed and achieved sCOD removal of 87-98% at HRTs from 12-72 h (LCFA loading rate 220-1333 mgCOD-LCFA/L·d) at 20°C. Moreover, even at the 12 h HRT, the unsaturated LCFAs (linoleate and oleate) were treated and only part of saturated LCFAs (stearate, palmitate) remained after treatment in the DSC-FF reactors. An increased methanogenic activity was established in the reactor sludges during reactor runs, which was evidenced by a higher acetotrophic activity in the granular sludge (from DSC), and a higher hydrogenotrophic activity in the biofilm (from FF) indicating development of distinct metabolic capabilities in the different reactor compartments.
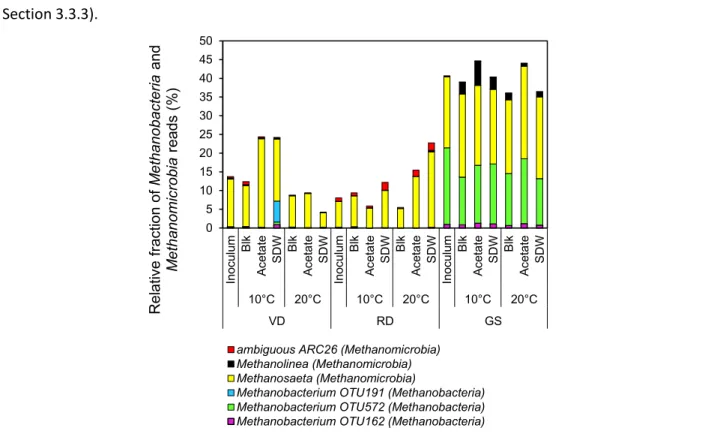
High throughput 16S rRNA sequencing showed that the relative abundance of the acetoclastic methanogen, Methanosaeta, increased in EGSB reactors and in the active microbiomes of granules (from
DSC) and biofilm (from FF) when fed with increasing LCFA concentrations. This suggested acetoclastic methanogenesis as the predominant methanogenesis pathway for SDW and presumably, LCFA degradation at 20°C. Relative abundances of the taxa known to have β-oxidizing and methanogenic activity were high in the active microbiomes during SDW treatment in DSC-FF reactors at 20°C. The biofilm microbiome (from FF) had a prominent presence of the β-oxidizing bacteria Syntrophus and of the hydrogenotrophic methanogen Methanospirillum in comparison to the presence of the acetogenic bacteria, Syntrophobacter, Desulfobulbus, and Geobacter, and of the acetoclastic methanogen in the granular sludge microbiome, suggesting a role of these different taxa during LCFA degradation.
In summary, this work demonstrated successful inoculum selection at low temperatures (10 and 20°C), and high-rate anaerobic LCFA degradation at 20°C using novel reactor design (here, DSC-FF). The key bacterial and archaeal taxa involved in the anaerobic conversion of LCFA to methane at 20°C were also deduced.
Yhteenveto
Rasvat, öljyt ja rasvat (FOG) ovat merkittävä aineosa monissa jätevesissä, kuten elintarviketeollisuuden jätevesissä. Näiden hydrolyysi tuottaa pitkäketjuisia rasvahappoja (LCFA), jotka vaikuttavat anaerobisen jätevedenkäsittelyprosessin stabiilisuuteen fysikaalis-kemiallisten ja mikrobiologisten toksisuusvaikutustensa vuoksi. FOG:n korkean metaanintuottopotentiaalin hyödyntäminen edellyttää biokaasuprosessilta kykyä käsitellä suuria LCFA-kuormituksia, jota ei ole vielä tutkittu matalissa lämpötiloissa. Tämän opinnäytetyön tavoitteena oli tutkia suuria LCFA-pitoisuuksia omaavien elintarvikejätevesien anaerobista käsittelyä korkeakuormitteisissa reaktoreissa matalissa lämpötiloissa. Synteettisen elintarviketeollisuuden jätevesien, jotka sisälsivät neljää eri LCFA:ta, anaerobista käsittelyä eri mesofiilisilla mikrobiyhteisöillä tutkittiin panoskokeissa. Granulalietteellä tuotettiin metaania nopeammin sekä saatiin suurempi metaanisaanto (76-82% teoreettisesta saannosta) kuin kahdella yhdyskuntamädätteellä (1-72%) 10°C:ssa ja 20°C:ssa. Granulalietteessä LCFA:iden anaerobista hajoamista edistivät β-hapettavat Syntrophaceae-heimon bakteerit (Syntrophus ja tuntemattomat lajit),
Metanosaeta-suvun asetotrofiset metanogeenit, aktiivisuus ja oletetut syntrofiset asetaattia hapettavat
bakteerit (SAOB).
Synteettistä elintarviketeollisuuden jätevettä käsiteltiin anaerobisesti jatkuvatoimisessa korkeakuormitteisessa EGSB-reaktorissa 20°C:ssa (viipymällä 24 h, LCFA-kuormituksella 670 mgCOD-LCFA/L·d). Kemiallisen hapenkulutuksen (COD) poisto oli 84-91% ja metaanisaanto 44-51%. Kahden kuukauden koeajojen aikana LCFA:ita kertyi reaktoriin, mikä johti granulalietteen nousemiseen reaktorin yläosaan (36-57%) ja hajoamiseen. LCFA:n aiheuttamaa granulalietteen hajoamista ja kellumista pyrittiin estämään suunnittelemalla uudenlainen reaktori, jossa oli dynaaminen lietereaktori yhdistettynä biofilmireaktoriin (DSC-FF). Tällä reaktorilla saavutettiin 20°C:ssa 87-98% liukoisen COD:n poisto viipymän ollessa välillä 12-72 h (LCFA-kuormituksella 220-1333 mgCOD-LCFA/L·d). Myös lyhimmällä 12 tunnin viipymällä tyydyttymättömät LCFA:aat (linoleaatti ja oleaatti) poistettiin syötetystä jätevedestä, kun taas osa tyydyttyneistä LCFA:ista (stearaatti ja palmitaatti) poistui reaktorista.
Asetotrofisen metanogeenin, Methanosaeta:n, suhteellinen runsaus (perustuen suurikapasiteettiseen 16S rRNA sekvensointiin) lisääntyi EGSB-reaktorissa sekä granulalietteessä (DSC) että biofilmissä (FF) LCFA-kuormituksen noustessa. Tämä osoittaa, että LCFA:n hajoamisen seurauksena metaania tuotettiin pääasiassa asetaatin kautta 20°C:ssa. Biofilmiin (FF) rikastui β-hapettavia bakteereja, Syntrophus-suvusta, sekä hydrogenotrofisia metanogeeneja, Methanospirillum-suvusta, kun taas granulalietteeseen rikastui
asetaattia hapettavia bakteereita suvuista Syntrophobacter, Desulfobulbus ja Geobacter, sekä asetotrofisia metanogeeneja. Tämä osoittaa, että reaktorin eri osiin rikastuvilla mikrobiyhteisöillä oli erilaiset roolit LCFA:n anaerobisessa käsittelyssä.
Yhteenvetona voidaan todeta, että tämä väitöstyö osoitti mikrobiyhteisön valinnan tärkeyden matalissa lämpötiloissa (10 ja 20°C:ssa). Lisäksi LCFA:ta pystyttiin onnistuneesti käsittelemään anaerobisissa korkeakuormitteisissa reaktoreissa 20°C:ssa käyttämällä uudenlaista reaktorityyppiä (DSC-FF). Väitöstyössä selvitettiin myös pääasialliset bakteerit ja arkit, jotka ottavat aktiivisesti osaa LCFA:iden anaerobiseen käsittelyyn.
Sommario
Grassi, olio e unto (fats, oil and grease, FOG) sono componenti significativi in numerose tipologie di acque reflue come quelle del settore lattiero-caseario. L'idrolisi dei FOG provoca la produzione di acidi grassi a catena lunga (long-chain fatty acids, LCFA) che destabilizzano il processo di trattamento anaerobico a causa della loro tossicità fisico-chimica e microbiologica. Lo sfruttamento dell'alto potenziale metanogeno dei FOG richiede un trattamento efficace di elevati carichi di LCFA, di cui finora non è stata studiata la fattibilità a basse temperature. Lo scopo di questa tesi è stato quello di studiare la fattibilità del trattamento anaerobico di acque reflue ricche di LCFA a basse temperature utilizzando effluenti caseari. Lo screening dell'inoculo mesofilo per il trattamento di LCFA misti in effluenti caseari sintetici (synthetic dairy wastewater, SDW) effettuato in condizioni batch ha mostrato come l'inoculo di fanghi granulari abbia prodotto una maggiore quantità di metano (76-82% della produzione teorica) rispetto ai due digestati municipali (1-72% ) a 20 e 10 °C. La capacità degradativa degli LCFA da parte dell'inoculo di fanghi granulari è stata attribuita alla presenza di batteri β-ossidanti della famiglia Syntrophaceae (Syntrophus e taxa non coltivati), all'attività acetotrofica dei Methanosaeta e ai batteri putativi sintropici che ossidano l'acetato (SAOB).
Il trattamento in continuo di SDW è stato effettuato con elevate rese in reattori a letto granulare espanso (EGSB) a 20°C (tempo di ritenzione idraulica (HRT) 24 h, carico organico (OLR) 670 mgCOD-LCFA / L · d, 33% COD-LCFA) con una rimozione del COD dell'84–91% e una percentuale di metano nel gas prodotto del 44–51%. L'alimentazione di SDW per più di due mesi ha provocato un accumulo di LCFA, che ha portato alla flottazione (36-57%) ed alla disintegrazione dei fanghi granulari. Per contrastare ciò, è stato progettato un nuovo tipo di reattore, denominato dynamic sludge chamber-fixed film (DSC-FF), che ha ottenuto una rimozione di sCOD dell'87-98% con HRT da 12 a 72 h (carico organico 220-1333 mg COD-LCFA / L · d) a 20°C. Inoltre, persino ad un HRT di 12 ore, gli COD-LCFA insaturi (linoleato e oleato) sono stati digeriti e solo una parte degli LCFA saturi (stearato, palmitato) è rimasta dopo il trattamento nei reattori DSC-FF. Una maggiore attività metanogenica nei fanghi è stata ottenuta durante l’esercizio del reattore; ciò è stato evidenziato da una maggiore attività acetotrofica nel fango granulare (DSC) e da una maggiore attività idrogenotrofica nel biofilm (FF), che indica lo sviluppo di capacità metaboliche distinte nei diversi compartimenti del reattore.
Il sequenziamento dell'rRNA 16S ad alto rendimento ha mostrato che l'abbondanza relativa del metanogenico acetoclastico, Methanosaeta, è aumentata nei reattori EGSB e nei microbiomi attivi di
granuli (da DSC) e biofilm (da FF) quando alimentati con concentrazioni di LCFA crescenti. Ciò ha suggerito la metanogenesi acetoclastica come via di metanogenesi predominante per SDW e presumibilmente, degradazione dell'LCFA a 20°C. Le abbondanze relative dei taxa noti per avere attività β-ossidante e metanogenica erano elevate nei microbiomi attivi durante il trattamento SDW nei reattori DSC-FF a 20°C. Il microbioma del biofilm (da FF) ha avuto una presenza preminente dei batteri β-ossidanti Syntrophus e del metanogenico idrogenotrofico, Metanospirillum in confronto alla presenza dei batteri acetogenici,
Syntrophobacter, Desulfobulbus e Geobacter e methanogen del fango acetoclastico, suggerendo un ruolo
di questi diversi taxa durante il degrado della LCFA.
In sintesi, questo lavoro ha dimostrato la riuscita selezione dell'inoculo a basse temperature (10 e 20°C) e una degradazione anaerobica LCFA ad alta velocità a 20°C utilizzando un nuovo design del reattore (qui, DSC-FF). Sono stati dedotti anche i principali taxa batterici e arcaici coinvolti nella conversione anaerobica di LCFA in metano a 20°C.
Résumé
Les gras, huiles et graisses (fats, oil and grease, FOG) constituent une fraction importante de nombreuses eaux usées, telles que celles de l'industrie laitière. L'hydrolyse des FOG entraîne la production d'acides gras à longues chaînes (long chain fatty acid, LCFA) qui déstabilisent le processus de traitement anaérobie en raison de leurs effets toxicologiques physico-chimiques et microbiens. L’exploitation du potentiel méthanogène élevé des FOG nécessite un traitement efficace des charges élevées en LCFA et la faisabilité d’un tel traitement à basse température n’a à ce jour pas été étudiée. L’objectif de cette thèse était ainsi d'étudier la faisabilité d'un traitement anaérobie à haute vitesse à basse température d'eaux usées riches en LCFA à l'aide d'une matrice d'eaux usées laitières.
Le criblage d’inoculas mésophiles dans le traitement d’eaux usées synthétiques reproduisants les caractéristiques d’eaux usées de l’industrie laitière (synthetic dairy wastewater, SDW) et chargées d’une variété d’LCFA au cours d’études par lots a montré que l’inoculum de boues granulaires obtenait des rendements en méthane plus rapides et supérieurs (76 à 82% du rendement théorique) que les deux digestâts municipaux (1 à 72%) à 20 et 10°C. La capacité de dégradation d’LCFA par l'inoculum provenant de boues granulaires a été attribuée aux présences de bactéries β-oxydantes de la famille Syntrophaceae (Syntrophus et taxons non cultivés), de l'activité acétotrophe de Methanosaeta et de bactéries oxydantes putatives d'acétate syntrophique (SAOB).
Un traitement continu à haute vitesse des SDW s'est avéré réalisable dans des réacteurs à lit de boue granulaire expansé (EGSB) à 20°C (temps de rétention hydraulique (HRT) 24 h, taux de charge en LCFA (OLR) 670 mgCOD-LCFA/L·d, 33% COD-LCFA) avec une élimination de la DCO soluble (soluble chemical oxygen demand, sCOD) de 84–91% et un rendement en méthane de 44–51%. Cependant, l'alimentation en SDW pendant plus de deux mois a entraîné une accumulation d’LCFA, ce qui a entraîné la flottaison des boues granulaires (36-57%) et leur désintégration. Pour contrer la flottaison et la désintégration des boues granulaires induites par les LCFA, un nouveau type de réacteur, constitué d’une chambre à
rétention dynamique des boues et d’un film fixe (dynamic sludge chamber – fixed film, DSC-FF),a été
conçu et a permis d’éliminer la sCOD de 87 à 98% avec HRT de 12 à 72 h et un taux de chargement de LCFA de 220-1333 mgCOD-LCFA/L·d à 20°C. De plus, même à 12 heures de HRT, les LCFA insaturés (linoléate et oléate) ont été traités et il ne subsistait après traitement qu'une partie des LCFA saturés (stéarate, palmitate) dans les réacteurs DSC-FF.
Une activité méthanogène accrue a été établie dans les boues du réacteur pendant les essais en réacteur; mise en évidence par une activité acétotrophe plus élevée dans les boues granulaires du DSC et par une activité plus élevée d'hydrogénotrophe dans le biofilm (FF), indiquant le développement de capacités métaboliques distinctes dans les différents compartiments du réacteur.
Le séquençage à haut débit du 16S rRNA a montré que l'abondance relative du méthanogène
acétoclastique, Methanosaeta, augmentait dans les réacteurs EGSBet dans les microbiomes actifs des
granules (de DSC) et du biofilm (de FF) lorsqu'ils sont nourris avec des concentrations croissantes d’LCFA. Ceci suggère que la méthanogenèse acétoclastique est la voie de méthanogenèse prédominante dans la dégradation des SDW et, vraisemblablement, des LCFA à 20°C. L’abondance relative des taxa réputés pour leur activités β-oxydante et méthanogène était élevée dans les microbiomes actifs au cours du traitement des SDW dans les réacteurs DSC-FF à 20°C. Ainsi, le microbiome du biofilm (de FF) présentait d’importantes concentrations de la bactérie β-oxydante Syntrophus et du méthanogène hydrogénotrophe
Methanospirillum, par rapport à la présence des bactéries acétogènes, Syntrophobacter, Desulfobulbus et Geobacter et du méthanogène acétoclastique dans le microbiome des boues granulaires, suggérant que
ces différents taxa ont un rôle spécifique lors de la dégradation des LCFA.
En résumé, ce travail a permis de démontrer la sélection réussie d’'inocula à basses températures (10 et 20°C) et la dégradation anaérobie à haute vitesse des LCFA à 20°C en utilisant une conception novatrice de réacteur (ici, le DSC-FF). Les principaux taxa bactériens et archéens impliqués dans la conversion anaérobie des LCFA en méthane à 20°C en ont également été déduits.
Samenvatting
Vetten, oliën en vetachtige stoffen (fats, oil and grease, FOG) zijn een belangrijk bestanddeel van tal van afvalwaters, zoals van de zuivelindustrie. De hydrolyse van FOG resulteert in de productie van vetzuren met lange ketens (long chain fatty acids, LCFA) die het anaërobe behandelingsproces destabiliseren vanwege hun fysisch-chemische eigenschappen en microbiële toxiciteit. Het benutten van het hoge methanogene potentieel van FOG vereist een effectieve behandeling van hoge LCFA-belastingen, waarbij de haalbaarheid van de LCFA behandeling bij lage temperaturen tot nu toe niet is onderzocht. Het doel van dit proefschrift was het onderzoeken van de haalbaarheid van hoogwaardige anaërobe behandeling van LCFA rijk afvalwater bij lage omgevingstemperaturen met behulp van zuivelafvalwater.
Het screenen van mesofiele inocula voor de behandeling van gemengd LCFA met synthetisch zuivelafvalwater (synthetic dairy wastewater, SDW) in batch studies toonde aan dat korrelig slibinoculum snellere en hogere methaanopbrengsten (76-82% van de theoretische opbrengst) behaalde dan de twee digestaten van huishoudelijk afval (1-72%) bij zowel 20 als 10°C. De afbraakcapaciteit van LCFA in het korrelige slibinoculum werd toegeschreven aan de aanwezigheid van β-oxiderende bacteriën uit de familie Syntrophaceae (Syntrophus en niet-gekweekte taxa), de acetotrofe activiteit van Methanosaeta en vermeende syntrofische acetaat oxiderende bacteriën (syntrophic acetate oxidizing bacteria, SAOB).
Een behandeling van SDW met continue hoge snelheid bleek haalbaar te zijn in geëxpandeerde granulair slibbed (expanded granular sludge bed, EGSB) reactoren bij 20°C, hydraulische retentietijd (HRT) 24 uur, LCFA belasting 670 mgCZV-LCFA/L·d met een oplosbare CZV-verwijdering van 84-91% en methaanopbrengst van 44-51%. SDW-voeding langer dan twee maanden resulteerde in accumulatie, wat leidde tot flotatie (36-57%) en desintegratie van het korrelslib. Om de LCFA-geïnduceerde korrelslib-desintegratie en flotatie tegen te gaan, werd een nieuw reactortype - het dynamische slibkamer-gefixeerde film (dynamic sludge chamber-fixed film, DSC-FF) – ontworpen, welke een oplosbare CZV-verwijdering van 87-98% behaalden bij HRT's van 12-72 uur (LCFA laadsnelheid 220-1333 mg CZV-LCFA/L·d) bij 20°C. Bovendien werden zelfs na 12 uur HRT de onverzadigde LCFA's (linoleaat en oleaat) behandeld en bleef slechts een deel van de verzadigde LCFA's (stearaat, palmitaat) achter na behandeling in de DSC-FF-reactoren. Een verhoogde methanogene activiteit van het reactorslib werd vastgesteld tijdens de reactor runs; bewezen door een hogere acetotrofe activiteit van het korrelige slib (van DSC), en een hogere hydrogenotrofe activiteit in de biofilm (van FF) die de ontwikkeling van verschillende metabole capaciteiten in de verschillende reactorcompartimenten aangeeft.
16S rRNA-sequentiebepaling met hoge doorvoer toonde aan dat de relatieve abundantie van het acetoclastische methanogeen, Methanosaeta, toenam in EGSB-reactoren en in de actieve microbiomen van korrels (van DSC) en biofilm (van FF) wanneer gevoed met toenemende LCFA-concentraties. Dit suggereerde acetoclastische methanogenese als de overheersende methanogenese-route voor SDW en vermoedelijk LCFA-afbraak bij 20°C. Relatieve hoeveelheden van de taxa waarvan bekend is dat ze β-oxiderende en methanogene activiteit hebben, waren hoog in de actieve microbiomen tijdens SDW-behandeling in DSC-FF-reactoren bij 20°C. Het biofilm microbioom (van FF) had een prominente aanwezigheid van de β-oxiderende bacteriën Syntrophus en de hydrogenotrofe methanogeen
Methanospirillum in vergelijking met de aanwezigheid van de acetogene bacteriën, Syntrophobacter, Desulfobulbus en Geobacter, en acetoclastisch methanogen in het korrelslibmicrobioom, hetgeen een rol
van deze verschillende taxa tijdens de LCFA-degradatie suggereert.
Samenvattend toonde dit werk succesvolle inoculum selectie aan bij lage temperaturen (10 en 20°C), en hoge anaërobe LCFA-afbraak bij 20°C met behulp van een nieuw reactorontwerp (hier DSC-FF). De belangrijkste bacteriële en archaeale taxa die betrokken zijn bij de anaërobe omzetting van LCFA in methaan bij 20°C werden ook vastgesteld.
Acknowledgements
About 3.5 years ago, I flew across to carry out research in the area of low temperature anaerobic digestion of industrial wastewaters during which, me and my experimental anaerobes, both optimally mesophiles were trying to adapt under the various selective pressures in temperate conditions. It has been an interesting journey unravelling a minuscule of the black box during my affiliations with Tampere University (TU), Finland, NUI Galway, Ireland and UNESCO-IHE Delft, Netherlands. I would like to acknowledge the financial support provided by Horizon 2020 (grant agreement no. 643071) to Advanced Biological
Waste-to-Energy Technologies (ABWET) program under the Marie Skłodowska-Curie European Joint Doctorate
(EJD) program for making this research work possible.
As anticipated the process was time-taking, and I gratefully acknowledge the support received during these taxing years. I would like to thank my PhD supervisor Prof. Jukka Rintala for his guidance through these years and for his valuable comments on my work. I am very grateful to my instructor Asst Prof. Marika Kokko for the support through the plentiful revisions. I am thankful to my co-supervisors Prof Piet Lens, Dr. Johannna Rinta-Kanto and Dr. Riitta Kettunen for their insights within the never-ending tight deadlines. Thank you, Dr. Gavin Collins and Prof. Vincent O’Flaherty for the stimulating discussions and your guidance during my research exchange in Ireland. I acknowledge my colleagues at Tampere University, NUI Galway, UNESCO-IHE Delft and from the ABWET cohort for their invaluable help. It was a great learning experience on- and off-work exploring science with these diverse research groups. I am very grateful to my friends for being available in time and space with their kind words, encouragement, and positivity, and for always making me believe more in myself.
The final thanks goes to my parents and brother for their constant support and tolerance to my continual absence in the past years. A very special thanks to Kabir, I am eternally grateful for your support, this would not have been possible or worth it without you. It is the journey (with its highs and lows) that makes you - the destination is incidental, and an outcome of the choices made.
Contents
Abstract ... i
Acknowledgements ... xi
List of publications ... xvi
Author’s contribution... xvii
List of Symbols and Abbreviations ... xviii
1 GENERAL INTRODUCTION AND THESIS OUTLINE ... 1
1.1 Introduction ... 1
1.2 Objectives and scope of the study ... 3
1.3 Thesis Outline ... 4
References ... 6
2 ANAEROBIC LOW TEMPERATURE TREATMENT OF LCFA-RICH WASTEWATER... 10
2.1 LCFA-rich wastewaters and anaerobic degradation ... 10
2.1.1 LCFA-rich wastewaters ... 10
2.1.2 Principles of anaerobic degradation ... 11
2.1.3 Anaerobic degradation of LCFAs ... 12
2.2 Anaerobic wastewater treatment at low temperatures ... 14
2.2.1 Anaerobic high rate reactors and low temperatures ... 14
2.2.2 Anaerobic microbiology at low temperature ... 16
2.3 Anaerobic treatment of LCFA rich wastewaters at low temperature ... 20
References ... 25
3 ACETOTROPHIC ACTIVITY FACILITATES METHANOGENESIS FROM LCFA AT LOW TEMPERATURES: SCREENING FROM MESOPHILIC INOCULA ... 31
Abstract ... 31
3.1 Introduction ... 32
3.2 Materials and Methods ... 34
3.2.1 Inoculum and substrate ... 34
3.2.2 Methane production in batch assays ... 36
3.2.3 Analytical methods and calculations ... 36
3.2.4 DNA extraction, quantification and 16S rRNA sequencing ... 38
3.2.5 Bioinformatics and Statistical tools ... 38
3.3 Results and Discussion ... 39
3.3.1 Inoculum characteristics and microbial community composition ... 39
3.3.2 Methane production at low temperature from SDW and Acetate ... 41
3.3.3 Effect of Low Temperature and SDW on Microbial Community Composition ... 43
3.4 Conclusions ... 51
References ... 52
4 ANAEROBIC TREATMENT OF LCFA-CONTAINING SYNTHETIC DAIRY WASTEWATER AT 20°C: PROCESS PERFORMANCE AND MICROBIAL COMMUNITY DYNAMICS ... 59
4.1 Introduction ... 60
4.2 Materials and Methods ... 62
4.2.1 Inoculum and synthetic wastewater ... 62
4.2.2 EGSB reactor experiments ... 62
4.2.3 Methane production from granular sludge from the EGSB reactors ... 63
4.2.4 Analytical methods and calculations ... 63
4.2.5 16S rRNA amplicon sequencing and bioinformatics ... 64
4.3 Results and Discussion ... 66
4.3.1 Anaerobic treatment of LCFA-containing SDW at 20°C ... 66
4.3.2 Effect of LCFA loading rate on anaerobic treatment of SDW ... 67
4.3.3 Effect of LCFA loading rate on EGSB granule characteristics ... 68
4.3.4 Microbial community evolution during SDW treatment ... 69
4.4 Conclusions ... 74
References ... 75
5 RAPID GRANULATION AND ENHANCED METHANISATION AT 20˚C IN A NOVEL, DYNAMIC-SLUDGE-CHAMBER - FIXED-FILM (DSC-FF) BIOREACTOR TREATING LCFA WASTEWATER ... 80
Abstract ... 80
5.1 Introduction ... 81
5.2 Material and Methods ... 84
5.2.1 Inoculum and synthetic wastewater ... 84
5.2.2 Reactor design and experimental set-up ... 84
5.2.3 Analytical methods and calculations ... 85
5.3 Results ... 86
5.3.1 Reactor performance: COD removal and methane production ... 86
5.3.2 Profiles of metabolic intermediates from LCFA degradation ... 88
5.3.3 Comparative role of DSC and FF compartments in organics (COD) fractionation ... 88
5.3.4 Sludge washout and flotation ... 90
5.3.5 De novo granulation in DSC ... 91
5.4 Discussion ... 91
5.4.1 Advancement from the state-of-the art ... 91
5.4.2 Novel reactor configuration: proof-of-concept ... 92
5.4.3 Methane yields and dissolution ... 94
5.4.4 Degradation of LCFAs at 20°C ... 95
5.4.5 Granulation and development of distinct metabolic activity ... 95
5.5 Conclusions ... 96
References ... 97
6 DYNAMICS AND ASSEMBLY IN ACTIVE MICROBIOME OF GRANULES AND BIOFILMS TREATING LCFA-RICH WASTEWATER IN HIGH-RATE REACTORS ... 100
Abstract ... 100
6.1 Introduction ... 101
6.2 Materials and Methods ... 102
6.2.1 DSC-FF reactor operation and analytical methods ... 102
6.2.2 Sampling, nucleic acid extraction and 16S rRNA gene amplicon sequencing... 103
6.3 Results ... 104
6.3.1 Microbial community diversity in reactor sludges ... 107
6.3.2 Microbial community composition in reactor microbiome ... 107
6.4 Discussion ... 111
6.4.1 Synchronized microbial community dynamics in high-rate reactors ... 111
6.4.2 Microbial community assembly in granular sludge and biofilm microbiome ... 114
6.5 Conclusions ... 114
References ... 116
7 GENERAL DISCUSSION AND CONCLUSIONS ... 119
7.1 General discussion ... 119
7.2 Recommendations for future research ... 124
7.3 Conclusions ... 125
References ... 127
APPENDIXES: SUPPORTING INFORMATION ... 129
Supplementary Methods ... 129
Supplementary Figures ... 130
List of publications
I. Singh, S., Rinta-Kanto, J.M., Kettunen, R., Lens, P., Collins, G., Kokko, M., Rintala, J., 2019. Acetotrophic activity facilitates methanogenesis from LCFA at low temperatures: screening from mesophilic inocula. Archaea, 2019, 1–16.
II. Singh, S., Rinta-Kanto, J.M., Kettunen, R., Tolvanen, H., Lens, P., Collins, G., Kokko, M., Rintala, J., 2019. Anaerobic treatment of LCFA-containing synthetic dairy wastewater at 20°C: Process performance and microbial community dynamics. Science of the Total Environment, 2019, 960-968.
III. Singh, S., Holohan, C., Mills, S., Castilla-Archilla, J., Kokko, M., Rintala, J., Lens, P., Collins, G., O’Flaherty,V., 2020. Rapid granulation and enhanced methanisation at 20˚C in a novel, dynamic-sludge-chamber - fixed-film (DSC-FF) bioreactor treating LCFA wastewater. Submitted for publication.
IV. Singh, S., Rinta-Kanto, J.M., Lens, P., Kokko, M., Rintala, J., O’Flaherty,V, Collins, G., 2020. Dynamics and assembly in active microbiome of granules and biofilms treating LCFA-rich wastewater in high-rate reactors. Submitted for publication.
Author’s contribution
Paper I, Chapter 3:
Suniti Singh planned and performed the batch experiments, analyzed the parameters, and results, and wrote the manuscript. Jukka Rintala and Riitta Kettunen were involved in planning the experiment. Marika Kokko, Gavin Collins, Johanna Rinta-Kanto, and Piet Lens participated in the preparation and correction of the manuscript.
Paper II, Chapter 4:
Suniti Singh planned and performed the experiments, physical-chemical and bioinformatics analyses, and wrote the manuscript. Jukka Rintala and Riitta Kettunen were involved in planning the experiment. Henrik Tolvanen was involved in data analysis for particle size measurement. Marika Kokko, Gavin Collins, Johanna Rinta-Kanto, Piet Lens and Jukka Rintala participated in the preparation and correction of the manuscript.
Paper III, Chapter 5:
Suniti Singh and Vincent O’Flaherty were involved in the planning of experiments and design of reactors. Suniti Singh performed the experiments, and the related physico-chemical and data analysis, and wrote the manuscript. Conall Holohan performed the SMA batch experiments. Juan Castilla-Archilla helped in the set-up of reactors. Simon Mills helped in reactor operation. Vincent O’Flaherty, Jukka Rintala, Marika Kokko, Piet Lens, Conall Holohan and Gavin Collins participated in the preparation and correction of the manuscript. Paper IV,
Chapter 6:
Suniti Singh and Gavin Collins planned the experiments and analysis. Suniti Singh performed the experiments and the bioinformatics analysis, and, wrote the manuscript. Vincent O’Flaherty, Piet Lens, Johanna Rinta-Kanto, Marika Kokko and Jukka Rintala participated in the preparation and correction of the manuscript.
List of Symbols and Abbreviations
AD Anaerobic digestion AF Anaerobic filter
COD Chemical oxygen demand CSTR Completely stirred tank reactor
DGGE Denaturing gradient gel electrophoresis DSC Dynamic sludge chamber
EGSB Expanded granular sludge reactor FOG Fat, oil, and grease
FF Fixed film
HRT Hydraulic retention time LCFA Long chain fatty acids MBR Membrane bioreactor mRNA Messenger RNA
OTU Operational taxonomic unit OLR Organic loading rate
PERMANOVA Permutational multivariate analysis of variance PCoA Principal coordinate analysis
PCR Polymerase chain reaction
QIIME Quantitative insights into microbial ecology rRNA Ribosomal RNA
sCOD Soluble chemical oxygen demand SRT Sludge retention time
SAOB Syntrophic acetate oxidising bacteria SDW Synthetic dairy wastewater
STP Standard temperature and pressure tCOD Total chemical oxygen demand
TS Total solids
UASB Upflow anaerobic sludge blanket VFA Volatile fatty acids
1
GENERAL INTRODUCTION AND THESIS OUTLINE
1.1
Introduction
The United Nations 2030 Agenda for Sustainable Development mandated seventeen Sustainable Development Goals (SDGs), of which, four that belong to the focus areas of clean water, clean energy, sustainable communities, and climate action are inherently linked to the development of sustainable wastewater treatment and resource recovery systems. Utilization of water in domestic, agricultural or industrial sectors results in polluted wastewaters due to the introduction of biodegradable organics, nutrients, and inert; that would adversely impact the environment and human health if discharged as such (Crini and Lichtfouse, 2018). Wastewater treatment generally involves a combination of physical, chemical and biological processes. Several factors, for example, the wastewater and process characteristics, determine the selection of the effective treatment method. The physicochemical methods are typically used for pre- and primary treatment steps, whereas biological treatment is used typically as the secondary treatment step for the removal of organic matter. Within the biological treatment, the activated sludge processes implementing treatment at aerobic conditions are most widely used (Salsabil et al., 2010), but result in the generation of a considerable amount of excess sludge and high energy requirement for aeration. Anaerobic treatment is another treatment option, which converts organic compounds into biogas (methane and carbon dioxide) with the generation of a low amount of excess sludge; and eliminates the energy requirements expended on aeration. Currently, anaerobic treatment using high rate
reactors are widely used for warm industrial wastewaters and sewage treatment in moderate climates (Batstone and Jensen, 2011).
Several industrial wastewaters are emitted at low temperatures (≤20°C), including wastewaters from the bottling, malting, and brewing industries, soft drinks processes, as well as from the food, poultry, and dairy processing. Anaerobic high rate treatment of such cold wastewaters at the discharge temperatures would steer the treatment processes towards achievement of energy neutrality (Martin et al., 2011; Petropoulos et al., 2019).
The wastewaters produced from the food and dairy industries have a significant fat, oil, and grease (FOG) fraction, which should be harnessed for biogas production owing to the high methane production potential of lipids compared to that of carbohydrates and proteins (Alves et al., 2009). FOG hydrolysis results in the production of a mixture of long-chain fatty acids (LCFAs) (carbon length C12 - C18). Single LCFAs have frequently been used for investigating the anaerobic treatment of LCFA-rich wastewaters, despite the synergistic toxic effect exerted by LCFAs. LCFA accumulation may destabilize the anaerobic treatment process due to the physicochemical and microbial toxicity effects of LCFA and instigate operational limitations during the long-term anaerobic treatment of FOG or LCFA-rich wastewaters. Furthermore, LCFAs may inhibit the activity of different microbial groups in anaerobic consortia – hydrolytic bacteria, syntrophic bacteria, and methanogenic archaea (Davidsson et al., 2008; Hwu and Lettinga, 1997; Lalman and Bagley, 2001, 2000; Sun et al., 2013).
The physicochemical factors affecting the performance involve : (i) Mass-transfer limitations due to the formation of hydrophobic LCFA layer around the sludge aggregates due to LCFA sorption and entrapment, (ii) Sludge flotation due to the decrease in sludge density arising from LCFA accumulation on sludge aggregates, (iii) Sludge wash-out due to the disintegration of the granular sludge structure, (iv) Increased solubilization of the lipid bilayer (Desbois and Smith, 2010; Koster and Cramer, 1987) and the membrane proteins leading to direct cell toxicity and lysis (Hanaki et al., 1981; Rinzema et al., 1994), (v) Decreased cell permeability (Zhou et al., 2013), (vi) Disruption of cellular energy functions by disrupting the electron transport chain and uncoupling oxidative phosphorylation (Desbois and Smith, 2010), and, (vii) Enzyme activity inhibition (Zheng et al., 2005). These challenges associated with LCFA degradation are aggravated at low temperatures due to the alterations in the physicochemical characteristics, kinetics, thermodynamics, and hydrodynamics associated with reduced temperature, and the physiological adaptations needed by microbes at low temperatures.
High rate anaerobic FOG treatment has been investigated at mesophilic conditions (Cavaleiro et al., 2016; Dereli et al., 2015; Duarte et al., 2018; Jeganathan et al., 2006; Jensen et al., 2015; Kundu et al., 2013; Leal
et al., 2006; Passeggi et al., 2009; Ramos et al., 2014; Saatci et al., 2003; Silva et al., 2014) and thermophilic conditions (Hwu et al., 1997a, 1997b; Poh and Chong, 2014), while low-temperature anaerobic treatment has been studied using substrates with low FOG content (Bialek et al., 2012; Connaughton et al., 2006a; Esparza-Soto et al., 2013; McHugh et al., 2006; Sheldon and Erdogan, 2016). Thus, the possibility of high rate anaerobic treatment of LCFA-rich industrial wastewaters at low temperatures remains unknown. In anaerobic high rate reactors, microbes are enriched from the inoculum during the operation wherein numerous factors, as operational temperature, substrate characteristics and loading among others may affect the active population and microbial interactions in the reactor (Braz et al., 2019; Lin et al., 2017). As the growth of anaerobic consortia is derived from the energy obtained from organics degradation, the populations of the different microbial groups may be used as an indicator of bioreactor performance (Carballa et al., 2015; Gonzalez-Fernandez et al., 2015). The active microbial populations in anaerobic reactors have been monitored by using both culture-dependent methods and culture-independent methods for linking the microbial community to interactions and functions (Vanwonterghem et al., 2014). The culture-dependent methods have been used for the enrichment and identification of FOG-and LCFA-degraders from anaerobic consortia (Grabowski et al., 2005b, 2005a; Hatamoto et al., 2007; Sousa et al., 2007). Multiple culture-independent methods such as denaturing gradient gel electrophoresis (DGGE), polymerase chain reaction (PCR), fluorescence in situ hybridization (FISH), restriction fragment length polymorphism (RFLP) and 16S RNA sequencing have been used for the profiling and identification of FOG- and LCFA-degraders (De Francisci et al., 2015; Grabowski et al., 2005b; Petropoulos et al., 2018, 2019; Treu et al., 2016; Ziels et al., 2017a), with a more recent transition towards the use of stable isotope probing (SIP) and advanced -omics platforms as metagenomics and transcriptomics to decipher the microbial interactions and functions (Hatamoto et al., 2007; Kougias et al., 2016; Treu et al., 2016; Ziels et al., 2017b). As FOG and LCFA degradation can be maximized by enriching and maintaining optimal concentrations of hydrolytic bacteria, synergistic (acidogenic and acetogenic) bacteria, and hydrogenotrophic and acetoclastic archaea; the identification, and enumeration of these FOG- and LCFA-degrading microbes would suggest new microbial functions, interactions and correlations to environmental parameters during low temperature LCFA degradation.
1.2
Objectives and scope of the study
The main objective of this thesis was to evaluate the feasibility of high rate anaerobic treatment of LCFA-rich feed at low ambient temperatures. The feasibility was evaluated in batch assays and continuous
anaerobic reactors using synthetic dairy wastewater (SDW) as a substrate. The specific objectives, which were studied using various experimental designs, were to evaluate:
1. The effects of inoculum source on methane production from LCFA-rich SDW in batch assays at low temperatures (Chapter 3),
2. The feasibility of using high-rate anaerobic reactors for treating LCFA-rich wastewater at low temperatures, and evaluating the effects of LCFA loading on sludge retention, granular sludge characteristics and reactor performance
a) expanded granular sludge bed (EGSB) reactors (Chapter 4) b) dynamic sludge bed-fixed film (DSC-FF) reactors (Chapter 5)
3. The temporal microbial community dynamics and assembly in the sludge microbiomes in batch (Chapter 3) and continuous reactors treating LCFA-rich wastewater (Chapters 4 and 6).
1.3
Thesis Outline
This Ph.D. dissertation comprises of seven chapters, the main topics of which are shown in Fig. 1.1. The first chapter presents a general introduction regarding the background, problem statement, and research objectives. Chapter 2 provides the state of the art on the anaerobic treatment of LCFA at low temperatures by unifying the theoretical backgrounds from the low-temperature anaerobic treatment of industrial wastewaters, and, the anaerobic conversion of FOG and LCFA-rich wastewaters. In Chapter 3, the biochemical and microbiological effects of inoculum sources on the methane production from LCFA-rich SDW are evaluated in batch assays at low temperatures (10 and 20°C). Chapter 4 assesses the feasibility of EGSB reactors and the effects of OLR and LCFA concentration during continuous high-rate treatment of LCFA-rich wastewater at 20°C using biochemical, morphological, and temporal microbial community analysis. In Chapter 5, the feasibility of novel reactor design (DSC-FF) for continuous high-rate treatment of LCFA-rich wastewater is evaluated at 20°C by a gradual increase in OLR and LCFA loading loads with a decrease in HRT (72 to 12 h). Simultaneously, the use of a sludge mixture is evaluated to engineer microbial consortium suitable for SDW treatment. Chapter 6 evaluates the temporal microbial community dynamics and assembly in the active microbiomes of granular sludge, biofilm, and effluent; and the use of sludge mixture (as inoculum) on the microbial diversity during the high-rate treatment of LCFA-rich SDW in DSC-FF reactors at HRTs ranging from 72-12 h. Chapter 7 presents a comprehensive discussion of the results based on the experimental work in this thesis. This chapter presents an overview of the
practical applications of this research and provides recommendations and perspectives for future research.
References
Alves, M.M., Pereira, M.A., Sousa, D.Z., Cavaleiro, A.J., Picavet, M., Smidt, H., Stams, A.J.M., 2009. Waste lipids to energy : how to optimize methane production from long-chain fatty acids ( LCFA ). Microb. Biotechnol. 2, 538–550. doi:10.1111/j.1751-7915.2009.00100.x
Batstone, D.J., Jensen, P.D., 2011. Anaerobic Processes, in: Treatise on Water Science, Vol 4. pp. 615– 639.
Bialek, K., Kumar, A., Mahony, T., Lens, P.N.L., O’Flaherty, V., 2012. Microbial community structure and dynamics in anaerobic fluidized-bed and granular sludge-bed reactors: influence of operational temperature and reactor configuration. Microb. Biotechnol. 5, 738–752. doi:10.1111/j.1751-7915.2012.00364.x
Braz, G.H.R., Fernandez-Gonzalez, N., Lema, J.M., Carballa, M., 2019. Organic overloading affects the microbial interactions during anaerobic digestion in sewage sludge reactors. Chemosphere 222, 323–332. doi:10.1016/j.chemosphere.2019.01.124
Carballa, M., Regueiro, L., Lema, J.M., 2015. Microbial management of anaerobic digestion: Exploiting the microbiome-functionality nexus. Curr. Opin. Biotechnol. 198, 896–906.
doi:10.1016/j.copbio.2015.01.008
Cavaleiro, A.J., Pereira, M.A., Guedes, A.P., Stams, A.J.M., Alves, M.M., Sousa, D.Z., 2016. Conversion of Cn-Unsaturated into Cn-2-Saturated LCFA Can Occur Uncoupled from Methanogenesis in
Anaerobic Bioreactors. Environ. Sci. Technol. 50, 3082–3090. doi:10.1021/acs.est.5b03204 Connaughton, S., Collins, G., O’Flaherty, V., 2006. Psychrophilic and mesophilic anaerobic digestion of
brewery effluent: a comparative study. Water Res. 40, 2503–2510. doi:10.1016/j.watres.2006.04.044
Crini G., Lichtfouse E., 2018. Wastewater Treatment: An Overview. In: Green Adsorbents for Pollutant Removal. Environmental Chemistry for a Sustainable World. Springer, Cham. 1-21. doi:
https://doi.org/10.1007/978-3-319-92111-2_1
Davidsson, Å., Lövstedt, C., la Cour Jansen, J., Gruvberger, C., Aspegren, H., 2008. Co-digestion of grease trap sludge and sewage sludge. Waste Manag. 28, 986–992. doi:10.1016/j.wasman.2007.03.024 De Francisci, D., Kougias, P.G., Treu, L., Campanaro, S., Angelidaki, I., 2015. Microbial diversity and
dynamicity of biogas reactors due to radical changes of feedstock composition. Bioresour. Technol. 176, 56–64. doi:10.1016/j.biortech.2014.10.126
Dereli, R.K., Heffernan, B., Grelot, A., van der Zee, F.P., van Lier, J.B., 2015. Influence of high lipid containing wastewater on filtration performance and fouling in AnMBRs operated at different solids retention times. Sep. Purif. Technol. 139, 43–52. doi:10.1016/j.seppur.2014.10.029 Desbois, A.P., Smith, V.J., 2010. Antibacterial free fatty acids: Activities, mechanisms of action and
biotechnological potential. Appl. Microbiol. Biotechnol. 85, 1629–1642. doi:10.1007/s00253-009-2355-3
Duarte, M.S., Silva, S.A., Salvador, A.F., Cavaleiro, A.J., Stams, A.J.M., Alves, M.M., Pereira, M.A., 2018. Insight into the role of facultative bacteria stimulated by micro-aeration in continuous bioreactors converting LCFA to methane. Environ. Sci. Technol. 52, 6947–6507. doi:10.1021/acs.est.8b00894
Esparza-Soto, M., Arzate-Archundia, O., Solis-Morelos, C., Fall, C., 2013. Treatment of a chocolate industry wastewater in a pilot-scale low-temperature UASB reactor operated at short hydraulic and sludge retention time. Water Sci. Technolgy 67, 1353–1361. doi:10.2166/wst.2013.010
Gonzalez-Fernandez, C., Sialve, B., Molinuevo-Salces, B., 2015. Anaerobic digestion of microalgal biomass: Challenges, opportunities and research needs. Bioresour. Technol. 198, 896–906. doi:10.1016/j.biortech.2015.09.095
Grabowski, A., Blanchet, D., Jeanthon, C., 2005a. Characterization of long-chain fatty-acid-degrading syntrophic associations from a biodegraded oil reservoir. Res. Microbiol. 156, 814–821.
doi:10.1016/j.resmic.2005.03.009
Grabowski, A., Nercessian, O., Fayolle, F., Blanchet, D., Jeanthon, C., 2005b. Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol. Ecol. 54, 427– 443. doi:10.1016/j.femsec.2005.05.007
Hanaki, K., Matsuo, T., Nagase, M., 1981. Mechanism of inhibition caused by long-chain fatty acids in anaerobic digestion process. Biotechnol. Bioeng. 23, 1591–1610. doi:10.1002/bit.260230717 Hatamoto, M., Imachi, H., Harada, H., 2007. Identification and Cultivation of Anaerobic, Syntrophic
Long-Chain Fatty Acid-Degrading Microbes from Mesophilic and Thermophilic Methanogenic Sludges. Appl. Environ. Microbiol. 73, 1332–1340. doi:10.1128/AEM.02053-06
Hwu, C.S., Lettinga, G., 1997. Acute toxicity of oleate to acetate-utilizing methanogens in mesophilic and thermophilic anaerobic sludges. Enzyme Microb. Technol. 21, 297–301.
doi:10.1177/0047117805050061
Hwu, C.S., Molenaar, G., Garthoff, J., Van Lier, J.B., Lettinga, G., 1997a. Thermophilic high-rate anaerobic treatment of wastewater containing long-chain fatty acids: Impact of reactor hydrodynamics. Biotechnol. Lett. 19, 447–451. doi:10.1023/A:1018344127057
Hwu, C.S., Van Beek, B., Van Lier, J.B., Lettinga, G., 1997b. Thermophilic high-rate anaerobic treatment of wastewater containing long-chain fatty acids: Effect of washed out biomass recirculation. Biotechnol. Lett. 19, 453–456. doi:10.1023/A:1018396111127
Jeganathan, J., Nakhla, G., Bassi, A., 2006. Long-term performance of high-rate anaerobic reactors for the treatment of oily wastewater. Environ. Sci. Technol. 40, 6466–6472. doi:10.1021/es061071m Jensen, P.D., Yap, S.D., Boyle-Gotla, A., Janoschka, J., Carney, C., Pidou, M., Batstone, D.J., 2015.
Anaerobic membrane bioreactors enable high rate treatment of slaughterhouse wastewater. Biochem. Eng. J. 97, 132–141. doi:10.1016/j.bej.2015.02.009
Koster, I.W., Cramer, A., 1987. Inhibition of Methanogenesis from Acetate in Granular Sludge by Long-Chain Fatty Acids. Appl. Environ. Microbiol. 53, 403–409. doi:10.1016/j.tibtech.2011.11.003 Kougias, P.G., Treu, L., Campanaro, S., Zhu, X., Angelidaki, I., 2016. Dynamic functional characterization
and phylogenetic changes due to Long Chain Fatty Acids pulses in biogas reactors. Sci. Rep. 1–10. doi:10.1038/srep28810
Kundu, K., Bergmann, I., Hahnke, S., Klocke, M., Sharma, S., Sreekrishnan, T.R., 2013. Carbon source - A strong determinant of microbial community structure and performance of an anaerobic reactor. J. Biotechnol. 168, 616–624. doi:10.1016/j.jbiotec.2013.08.023
Lalman, J.A., Bagley, D.M., 2001. Anaerobic degradation and methanogenic inhibitory effects of oleic and stearic acids. Water Res. 35, 2975–2983. doi:10.1016/S0043-1354(00)00593-5
Lalman, J.A., Bagley, D.M., 2000. Anaerobic degradation and inhibitory effects of Linoleic Acid. Water Res. 34, 4220–4228. doi:10.1016/S0043-1354(00)00180-9
Leal, C.M.R.M., Freire, D.M.G., Cammarota, M.C., Sant’Anna Jr, G.L., 2006. Effect of enzymatic hydrolysis on anaerobic treatment of dairy wastewater. Process Biochem. 41, 1173–1178.
doi:10.1016/j.procbio.2005.12.014
Lin, Q., De Vrieze, J., Li, C., Li, Jiaying, Li, Jiabao, Yao, M., Hedenec, P., Li, H., Li, T., Rui, J., Frouz, J., Li, X., 2017. Temperature regulates deterministic processes and the succession of microbial interactions in anaerobic digestion process. Water Res. 123, 134–143. doi:10.1016/j.watres.2017.06.051 Martin, I., Pidou, M., Soares, A., Judd, S., Jefferson, B., 2011. Modelling the energy demands of aerobic
and anaerobic membrane bioreactors for wastewater treatment. Environ. Technol. 32, 921–932. doi:10.1080/09593330.2011.565806
McHugh, S., Collins, G., O’Flaherty, V., 2006. Long-term, high-rate anaerobic biological treatment of whey wastewaters at psychrophilic temperatures. Bioresour. Technol. 97, 1669–1678.
doi:10.1016/j.biortech.2005.07.020
Passeggi, M., López, I., Borzacconi, L., 2009. Integrated anaerobic treatment of dairy industrial wastewater and sludge. Water Sci. Technol. 59, 501–506. doi:10.2166/wst.2009.010
Petropoulos, E., Dolfing, J., Yu, Y., Wade, M.J., Bowen, E.J., Davenport, R.J., Curtis, T.P., 2018. Lipolysis of domestic wastewater in anaerobic reactors operating at low temperatures. Environ. Sci. Water Res. Technol. 4, 1002–1013. doi:10.1039/c8ew00156a
Petropoulos, E., Yu, Y., Tabraiz, S., Yakubu, A., Curtis, T.P., Dolfing, J., 2019. High rate domestic wastewater treatment at 15°C using anaerobic reactors inoculated with cold-adapted
sediments/soils-shaping robust methanogenic communities. Environ. Sci. Water Res. Technol. 5, 70–82. doi:10.1039/c8ew00410b
Poh, P.E., Chong, M.F., 2014. Upflow anaerobic sludge blanket-hollow centered packed bed (UASB-HCPB) reactor for thermophilic palm oil mill effluent (POME) treatment. Biomass and Bioenergy 67, 231–242. doi:10.1016/j.biombioe.2014.05.007
Ramos, C., García, A., Diez, V., 2014. Performance of an AnMBR pilot plant treating high-strength lipid wastewater: Biological and filtration processes. Water Res. 67, 203–215.
doi:10.1016/j.watres.2014.09.021
Rinzema, A., Boone, M., van Knippenberg, K., Lettinga, G., 1994. Bactericidal effect of long chain fatty acids in anaerobic digestion. Water Environ. Res. 66, 40–49. doi:10.2175/WER.66.1.7
Saatci, Y., Arslan, E.I., Konar, V., 2003. Removal of total lipids and fatty acids from sunflower oil factory effluent by UASB reactor. Bioresour. Technol. 87, 269–272. doi:10.1016/S0960-8524(02)00255-9 Salsabil, M.R., Laurent, J., Casellas, M., Dagot, C., 2010. Techno-economic evaluation of thermal
treatment, ozonation and sonication for the reduction of wastewater biomass volume before aerobic or anaerobic digestion. J. Hazard. Mater. 174, 323–333. doi:10.1016/j.jhazmat.2009.09.054 Sheldon, M.S., Erdogan, I.G., 2016. Multi-stage EGSB/MBR treatment of soft drink industry wastewater.
Chem. Eng. J. 285, 368–377. doi:10.1016/j.cej.2015.10.021
Silva, S.A., Cavaleiro, A.J., Pereira, M.A., Stams, A.J.M., Alves, M.M., Sousa, D.Z., 2014. Long-term acclimation of anaerobic sludges for high-rate methanogenesis from LCFA. Biomass and Bioenergy 67, 297–303. doi:10.1016/j.biombioe.2014.05.012
Sousa, D.Z., Alcina Pereira, M., Stams, A.J.M., Alves, M.M., Smidt, H., 2007. Microbial communities involved in anaerobic degradation of unsaturated or saturated long-chain fatty acids. Appl. Environ. Microbiol. 73, 1054–1064. doi:10.1128/AEM.01723-06
Sun, Y., Wang, D., Qiao, W., Wang, W., Zhu, T., 2013. Anaerobic co-digestion of municipal biomass wastes and waste activated sludge : Dynamic model and material balances.
Treu, L., Campanaro, S., Kougias, P.G., Zhu, X., Angelidaki, I., 2016. Untangling the effect of fatty acid addition at species level revealed different transcriptional responses of the biogas microbial community members. Environ. Sci. Technol. 50, 6079–6090. doi:10.1021/acs.est.6b00296 Vanwonterghem, I., Jensen, P.D., Ho, D.P., Batstone, D.J., Tyson, G.W., 2014. Linking microbial
community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr. Opin. Biotechnol. 27, 55–64. doi:10.1016/j.copbio.2013.11.004
Zheng, C.J., Yoo, J.S., Lee, T.G., Cho, H.Y., Kim, Y.H., Kim, W.G., 2005. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 579, 5157–5162.
doi:10.1016/j.febslet.2005.08.028
Zhou, X., Meile, L., Kreuzer, M., Zeitz, J.O., 2013. The Effect of Saturated Fatty Acids on Methanogenesis and Cell Viability of Methanobrevibacter ruminantium. Archaea 1–9. doi:10.1155/2013/106916. Ziels, R.M., Beck, D.A.C., Stensel, H.D., 2017a. Long-chain fatty acid feeding frequency in anaerobic
codigestion impacts syntrophic community structure and biokinetics. Water Res. 117, 218–229. doi:http://dx.doi.org/10.1016/j.watres.2017.03.060
Ziels, R.M., Sousa, D.Z., Stensel, H.D., Beck, D.A.C., 2017b. DNA-SIP based genome-centric metagenomics identifies key long-chain fatty acid-degrading populations in anaerobic digesters with different feeding frequencies. ISME J. 1–12. doi:10.1038/ismej.2017.143
2
ANAEROBIC LOW TEMPERATURE TREATMENT OF LCFA-RICH
WASTEWATER
2.1 LCFA-rich wastewaters and anaerobic degradation
2.1.1 LCFA-rich wastewaters
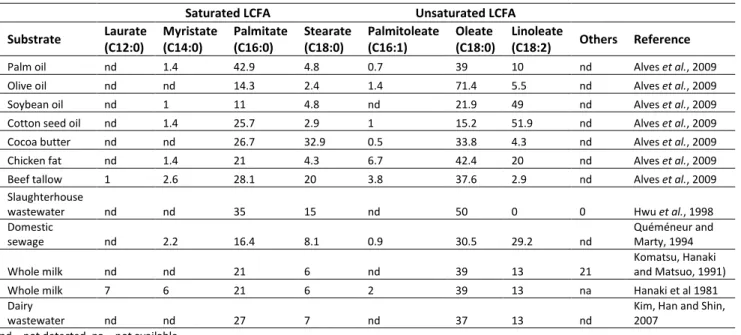
Numerous wastewaters are abundant in FOG content, notably those emitted from the oil mills, slaughterhouses, food processing, and dairy production units (Alves et al., 2009). Lipid catabolism produces LCFAs which have a variable chain length of carbon atoms in their aliphatic tail (C= 12- 18). The composition of various FOG-rich wastewaters is shown in Table 2.1, along with the LCFA concentrations shown in Table 2.2. The most commonly found LCFAs in wastewaters are - palmitate, stearate, oleate, and linoleate; wherein oleate is the most abundant (Alves et al., 2009). LCFAs inherently are carboxylic acids in saturated or unsaturated forms, with their degree of unsaturation dependent on the number of unsaturated (double or triple) bonds. The solubility of LCFAs in water decreases with an increase in the carbon chain length, but, increases with the degree of unsaturation (Bober and Garus, 2006; Yoke, 1958). Almost 90% of the total organic carbon (and thus, the methanogenic potential) of lipids is conserved within the LCFAs (Hanaki et al., 1981).
Table 2.1. Composition of FOG-rich wastewaters
Wastewater source pH COD (g/L) Solids (g/L) FOG (g/L) References
Cheese production 5.5-7.7 (6.7#) 0.79-6.55
(2.93#) 1.1-6.4 (2.75#) (TS) 0.1-0.6 (0.29 #)
Gutiérrez, Encina and Fdz-Polanco, 1991 Ice cream production 6.6-7.3 (6.9#) 3.77-6.10
(4.94#) 0.6-.4 (1.1#) (TSS) 0.8#
Hawkes, Donnelly and Anderson, 1995 Cheese production 5.5-9.5 (7.3#) 1.0-7.5 (4.4#) 0.5-2.5 (1.1#) 0.2-1.8 (0.7#) Monroy H. et al., 1995
Whey production 4.3-8.7 5.4-77.3 3.9-58.9 (TS) 0.4-5.7 Kalyuzhnyi, Martinez and Martinez, 1997 Cheese production 5.2# 5.34# 4.21# na Strydom, Britz and
Mostert, 1997 Milk processing 6.9# 4.65# 2.75# na Strydom, Britz and
Mostert, 1997 Butter production 5.8# 1.91# 1.72# na Strydom, Britz and
Mostert, 1997 Milk and cream bottling plant 8-11 2-6 0.4-1.0 (TSS) 0.3-0.5 Ince, 1998 Dairy wastewater na 56.6-140.2(18#) 1.7-12.6 (7.2#) (TSS) 0.1-10.6 (4.8 #) Arbeli et al., 2006
Cheese production 3.7-4.3, (4.0#) 11-29.5 (20.3#) 1.4-9.4 (5#) 0.5-3.3 (1.9#) Vlyssides et al., 2012
# - mean, na – not available, TS - total solids, TSS - total suspended solids
Table 2.2. Composition of LCFAs in FOG-rich raw materials and wastewaters (shown as % of total LCFA)
Saturated LCFA Unsaturated LCFA
Substrate Laurate (C12:0) Myristate (C14:0) Palmitate (C16:0) Stearate (C18:0) Palmitoleate (C16:1) Oleate (C18:0) Linoleate (C18:2) Others Reference
Palm oil nd 1.4 42.9 4.8 0.7 39 10 nd Alves et al., 2009 Olive oil nd nd 14.3 2.4 1.4 71.4 5.5 nd Alves et al., 2009 Soybean oil nd 1 11 4.8 nd 21.9 49 nd Alves et al., 2009 Cotton seed oil nd 1.4 25.7 2.9 1 15.2 51.9 nd Alves et al., 2009 Cocoa butter nd nd 26.7 32.9 0.5 33.8 4.3 nd Alves et al., 2009 Chicken fat nd 1.4 21 4.3 6.7 42.4 20 nd Alves et al., 2009 Beef tallow 1 2.6 28.1 20 3.8 37.6 2.9 nd Alves et al., 2009 Slaughterhouse
wastewater nd nd 35 15 nd 50 0 0 Hwu et al., 1998
Domestic sewage nd 2.2 16.4 8.1 0.9 30.5 29.2 nd Quéméneur and Marty, 1994 Whole milk nd nd 21 6 nd 39 13 21 Komatsu, Hanaki and Matsuo, 1991)
Whole milk 7 6 21 6 2 39 13 na Hanaki et al 1981
Dairy
wastewater nd nd 27 7 nd 37 13 nd
Kim, Han and Shin, 2007
nd – not detected, na – not available
2.1.2 Principles of anaerobic degradation
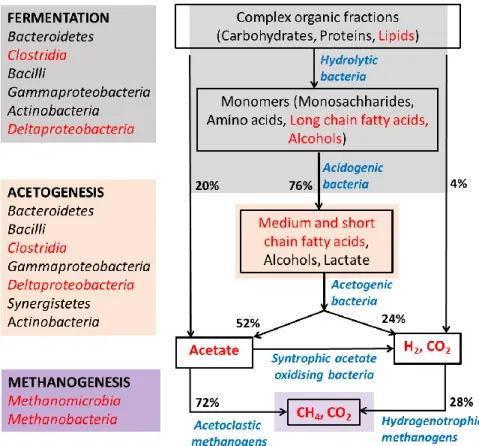
Anaerobic degradation is a microbially-mediated process that involves the degradation of different organic fractions, i.e., carbohydrates, proteins, and lipids, resulting in the production of biogas. In natural and engineered environments, the carbon flow proceeds sequentially through the different groups in an anaerobic consortium, involving the hydrolytic, acidogenic, and acetogenic bacteria, and the methanogenic archaea.
During hydrolysis, complex macromolecules (carbohydrates, proteins, and fats) are hydrolysed to simpler solubilized forms by the action of exocellular enzymes produced by hydrolytic bacteria. This is followed
by acidogenesis, wherein solubilized monomers such as monosaccharides, amino acids, and LCFAs are degraded by acidogenic bacteria into volatile fatty acids (VFAs), alcohols, lactate, carbon dioxide, and hydrogen; mediated at low concentrations of formate and low partial pressures of hydrogen (Fig. 2.1). Subsequently, acetate is produced from the VFAs by acetogenic bacteria during acetogenesis. The terminal step during anaerobic digestion is methanogenesis, wherein, methanogenic archaea convert acetate or hydrogen and carbon dioxide to methane (Fig. 2.1). Major pathways for methanogenesis are:
i. Acetoclastic methanogenesis: The methyl end of acetate gains an electron from the carboxyl end, resulting in one molecule each of methane and carbon dioxide. It is the prevailing pathway and contributes to about 72% of the methane production, mediated by the action of
acetoclastic archaea.
ii. Reductive methanogenesis: Hydrogen molecule donates an electron to carbon dioxide, resulting in the production of one methane and two water molecules. It contributes to about 28% of the methane production, mediated by the action of hydrogenotrophic archaea.
iii. Reductive methanogenesis coupled to syntrophic acetate oxidation: The methyl and the carboxyl ends of the acetate are oxidized to carbon dioxide along with the production of
hydrogen during syntrophic acetate oxidation. The hydrogen is further converted to methane by hydrogenotrophic methanogenesis.
2.1.3 Anaerobic degradation of LCFAs
Hydrolysis of lipids is carried out by exocellular lipases resulting in the production of glycerol and LCFA (Fig. 2.1). Lipid hydrolysis is slower and more challenging than the hydrolysis of carbohydrates, such as lactose (Pavlostathis and Giraldo-Gomez, 1991; Perle et al., 1995; Vidal et al., 2000); due to the low bioavailability of lipids (Petruy and Lettinga, 1997) and the need for a sludge retention time (SRT) long enough for the growth of fatty-acid oxidizing bacteria (Miron et al., 2000). However, during lipid catabolism, LCFA degradation often is the rate-limiting step (Hanaki et al., 1981; Novak and Carlson, 1970; Pavlostathis and Giraldo-Gomez, 1991). LCFA inhibition on acetoclastic methanogens was modelled based on the inhibitory concentration, and the LCFA adsorbed onto sludge with a non-competitive inhibition model and a cell-functionality (inhibition) model (Zonta et al., 2013). These two proposed models suggested a high sensitivity of acetoclastic methanogens to LCFA than the acidogenic bacteria (Zonta et al., 2013). Ma et al. (2015) also found methanogens as the most inhibited by LCFAs based on the kinetics of inhibition factors of the individual AD microbial groups. Under the LCFA inhibition conditions, methanogenesis was the rate-limiting step, whereas lipid hydrolysis was the fastest AD step which was
strongly affected by the inoculum to substrate ratio. In contrast, under the conditions without LCFA inhibition, the lipid hydrolysis was the rate-limiting step (Ma et al., 2015).
Fig. 2.1. Interactions between microbial groups and carbon flow during anaerobic degradation of lipids (Amani et al., 2010; Cai et al., 2016). The microbial classes involved in substrate degradation in fermentation, acetogenesis, and methanogenesis are presented on the left. The microbial classes and metabolic intermediates known to be involved in the degradation of FOG-rich substrates are highlighted in red.
The LCFA degradation proceeds sequentially, with an initial LCFA adsorption to the cell surface, followed by the activation of saturated and unsaturated LCFAs for their transport into the cytosol in prokaryotes. Within the cytosol, the degree of unsaturation affects LCFA degradation. In the case of mono-unsaturated LCFAs (e.g., oleate C18:1), isomerase converts the configuration of unsaturated LCFA (cis) to mono-unsaturated LCFA (trans). In the case of poly-mono-unsaturated LCFAs (e.g., linoleate C18:2), isomerase converts the configuration of di-unsaturated LCFA (cis) to di-unsaturated LCFA (trans) which is further converted to a saturated trans-configuration by the action of reductase (Fig. 2.2) (Sousa et al., 2009). The mono-unsaturated LCFAs in trans configurations are converted to their saturated forms and transported intracellularly for further degradation through β-oxidation (Fig. 2.2). Recently, another pathway has been suggested for the saturation of unsaturated LCFAs (Cavaleiro et al., 2016), that is independent of hydrogen partial pressure and suggested the involvement of non-syntrophic microbes. During each cycle of β-oxidation, the LCFAs are shortened by 2 carbons in chain length, producing one fatty acid molecule with
smaller chain length and one acetate molecule (Fig. 2.2). The conversion of LCFA to lower molecular weight Cn-2 fatty acid proceeds through cyclic β-oxidation up until the production of an equivalent number
of acetate or propionate molecules from the LCFA is achieved (Fig. 2.2).
Fig. 2.2. LCFA catabolism via cyclic β-oxidation in Escherichia coli (even-numbered LCFA) (Sousa et al., 2009).
The hydrolytic bacteria usually belong to the classes Bacteroidetes, Clostridia, Bacilli,
Gammaproteobacteria, and, Actinobacteria (Amani et al., 2010; Cai et al., 2016). To date, only seven
species are known to degrade LCFA (carbon atoms > 12) from the classes Clostridia (family
Syntrophomonadaceae) (Hatamoto et al., 2007; Sousa et al., 2007; Wu et al., 2007) or Deltaproteobacteria
(family Syntrophaceae) (Jackson et al., 1999); and only four species (Syntrophomonas sapovorans,
Syntrophomonas curvata, Syntrophomonas zehnderi, and Thermosyntropha lipolytica) from the class Clostridia are currently known to degrade the unsaturated LCFAs, e.g., oleate (C18:1) and linoleate (C18:2)
(Sousa et al., 2009).
2.2 Anaerobic wastewater treatment at low temperatures
2.2.1 Anaerobic high rate reactors and low temperatures
High treatment capacity of wastewaters is needed in anaerobic reactors to accomplish treatment in smaller reactor volumes and treatment space and improve cost benefits. This can be achieved by the use of high-rate reactors, referring to systems wherein the SRT is uncoupled from the HRT (van Lier et al., 2015); consisting of anaerobic consortia in suspension, biofilm or self-aggregated granular form. A higher