Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Internal Report (National Research Council of Canada. Division of Building
Research), 1965-06-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=1ebe920f-ec9e-4f1a-a2d8-ef3c3baba815 https://publications-cnrc.canada.ca/fra/voir/objet/?id=1ebe920f-ec9e-4f1a-a2d8-ef3c3baba815
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20337960
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Study of efflorescence in clay bricks
NATIONAL RESEARCH COUNCIL CANADA
DIVISION O F BUILDING RESEARCH
A STUDY O F E F F L O R E S C E N C E IN CLAY BRICKS by J. I. Davison I n t e r n a l Report. No. 3 1 3 of t h e Division of Building R e s e a r c h OTTAWA J u n e 1965
PREFACE
The appearance of efflorescence on a b r i c k m a s o n r y building i s usually d i s t r e s s i n g t o t h e designer, t h e owner and the manufacturer of t h e bricks. At a minimum it constitutes an a e s t h e t i c f a i l u r e for a s long a s i t p e r s i s t s , but i s a l s o c a u s e for s o m e concern that i t m a y r e f l e c t a situation in which deterioration of t h e m a s o n r y may occur. F o r t h e s e and other r e a s o n s t h e Division h a s had a long-time i n t e r e s t in efflorescence problems. The p r e s e n t study c a r r i e d out at Halifax h a s been b a s e d on l o c a l m a t e r i a l s and h a s provided t h e opportunity t o c o m p a r e l a b o r a t o r y and field experience and t o extend knowledge of t h e efflorescence phenomenon.
The author, a c h e m i s t and a r e s e a r c h officer of t h e Division a t t h e Atlantic Regional Station in Halifax, i s engaged in r e s e a r c h on m a s o n r y m a t e r i a l s with e m p h a s i s on regional problems.
Ottawa June 1965
N. B. Hutcheon A s s i s t a n t D i r e c t o r
A STUDY O F E F F L O F E S C E N C E IN CLAY BRICKS
J. I. Davison
During t h e winter of 1962-63, field o b s e r v a t i o n s i n s e v e r a l locations i n t h e Atlantic a r e a r e v e a l e d a h i g h e r t h a n a v e r a g e o c c u r r e n c e of e f f l o r e s c e n c e on w a l l s of c l a y b r i c k m a s o n r y . T h e s e v e r i t y of t h e e f f l o r e s c e n c e s e e m e d m o r e c l o s e l y r e l a t e d t o s p e c i f i c b r i c k s t h a n t o m o r t a r composition, t y p e of c o n s t r u c t i o n , etc. It w a s s u g g e s t e d that b r i c k s w e r e contributing t o t h e p r o b l e m e i t h e r by providing (a) a s o u r c e of soluble s a l t s , o r (b) s a t i s f a c t o r y m e d i a f o r
e a s y p a s s a g e of s a l t s i n solution f r o m m o r t a r and/or back-up m a t e r i a l t h r o u g h t h e walls. A study w a s , t h e r e f o r e , u n d e r t a k e n t o i n v e s t i g a t e t h e e f f l o r e s c i n g t e n d e n c i e s of c l a y b r i c k s commonly u s e d i n m a s o n r y c o n s t r u c t i o n i n t h e a r e a .
GENERAL
E f f l o r e s c e n c e , defined a s a deposit of w a t e r -soluble s a l t s e i t h e r (a) on t h e s u r f a c e of a m a s o n r y wall, o r (b) behind t h e s u r f a c e , within t h e p o r e s of t h e u n i t s of t h e s t r u c t u r e , h a s been s t u d i e d extensively i n r e c e n t y e a r s (1, 2,
3,
4). E f f l o r e s c i n g s a l t s , unsightly on m a s o n r y w a l l s , f r e q u e n t l y a p p e a r on new buildings and a r e a s o u r c e of c h a g r i n and e m b a r r a s s m e n t t o t h e owner, t h e a r c h i t e c t , and t h e builder. P a r a d o x i c a l l y , although t h e s a l t d e p o s i t s a r e a e s t h e t i c a l l y objectionable, t h e y a r e h a r m l e s s and u s u a l l y d i s a p p e a r during w a r m , d r y w e a t h e r , while s a l t s deposited behind t h e f a c e and within t h e p o r e s of t h e u n i t s of t h e walls, f r e q u e n t l y i g n o r e d b e c a u s e t h e y cannot be s e e n , h a v e b e c o m e r e c o g n i z e d a s a p o t e n t i a l c o n t r i b u t o r t o m a s o n r y d e t e r i o r-
ation ( 1 ) . The build-up of s a l t s within t h e wall i s accompanied by i n c r e a s i n g p r e s s u r e which c a n eventually r e s u l t i n a s p a l l i n g a w a y of t h e f a c e of ( a ) t h e unit, o r (b) t h e m o r t a r joint. Among t h e f a c t o r s t h a t a r e e s s e n t i a l i n c a u s i n g e f f l o r e s c e n c e a r e : 1, w a t e r
,
2. a s o u r c e of soluble s a l t s , 3, a w a l l s t r u c t u r e conducive t o t h e r e a d y p a s s a g e of w a t e r , and 4, s u i t a b l e w e a t h e r conditions.No e f f l o r e s c e n c e c a n o c c u r in t h e a b s e n c e of w a t e r . It i s t h e w a t e r that c a r r i e s t h e s a l t s in solution t h r o u g h t h e m a s o n r y , and when i t e v a p o r a t e s , t h e s a l t s r e m a i n e i t h e r on t h e s u r f a c e o r within t h e m a s onr y.
S i m i l a r l y , soluble s a l t s m u s t be p r e s e n t . A v a r i e t y h a v e b e e n identified, including a l k a l i (Na and K) s u l p h a t e s and c a r b o n a t e s ,
a l k a l i n e e a r t h (Ca, Mg, Al) s u l p h a t e s and, i n s m a l l e r a m o u n t s , c a r b o n a t e s . NaCl h a s b e e n identified in c o a s t a l a r e a s w h e r e unwashed b e a c h s a n d s a r e f r e q u e n t l y used, and C a C l m a y o c c u r 2 if t h i s m a t e r i a l is u s e d e x c e s s i v e l y a s an additive in t h e m o r t a r . T h e s e s a l t s a r e a l l white i n colour. Vanadium and molybdenum compounds, which a r e o c c a s i o n a l l y found i n e f f l o r e s c e n c e , p r o d u c e a g r e e n colour. T h e s o u r c e s of t h e s e soluble s a l t s in m a s o n r y m a y be: 1. back-up m a t e r i a l s , 2. m o r t a r , 3. b r i c k s , and 4. w a t e r . T h e r e l a t i v e c o n t r i b u t i o n s of back-up m a t e r i a l s and m o r t a r s h a v e been e x t e n s i v e l y studied (5, 6, 7 ) , and it is g e n e r a l l y a g r e e d t h a t
e f f l o r e s c e n c e f r o m t h e s e s o u r c e s i n c r e a s e s with i n c r e a s i n g a l k a l i n e content in t h e m a t e r i a l s . T h e p r e s e n c e of a c i d s and soluble s a l t s i n r a i n w a t e r e n t e r i n g m a s o n r y w a l l s h a s a l s o b e e n e s t a b l i s h e d (1) and should not be discounted.
T h e e x i s t e n c e of s o u r c e m a t e r i a l i n b r i c k s h a s b e e n s i m i l a r l y d e m o n s t r a t e d . A "wick" t e s t h a s b e e n developed t o a s s e s s t h e tendency of b r i c k s t o w a r d e f f l o r e s c e n c e . T h i s i s included in t h e ASTM S t a n d a r d Method of Sampling and T e s t i n g B r i c k s , C-67. In a n e x t e n s i v e s t u d y t h a t included 684 b r i c k s m a n u f a c t u r e d i n t h e United S t a t e s , M c B u r n e y and P a r s o n s (8) found t h a t 8 3 p e r . c e n t did not c o n s t i t u t e a s o u r c e of e f f l o r e s c e n c e . C o r r e s p o n d i n g l y , Bonnell and B u t t e r w o r t h (9) found t h a t l e s s t h a n 1 1 of 175 b r i c k s f r o m p l a n t s in G r e a t B r i t a i n and N o r t h e r n I r e l a n d gave h e a v y or s e r i o u s e f f l o r e s c e n c e . B r o w n e l l ( 3 ) s t a t e s t h a t a c e r a m i c p r o d u c t can contain about 0 . 2% of e x t r e m e l y soluble s a l t b e f o r e e f f l o r e s c e n c e b e c o m e s a p p a r e n t .
It is difficult for w a t e r t o m i g r a t e through a w a l l i f t h e w a l l contains d e n s e i m p e r m e a b l e u n i t s and h a s been c o n s t r u c t e d with s u p e r i o r workmanship. I n f e r i o r w o r k m a n s h i p , however, m a y r e s u l t i n r e a d y p a t h s f o r w a t e r t o t r a v e l along, and even with good w o r k m a n s h i p , i t i s p o s s i b l e t h a t t h e p o r o s i t y of t h e u n i t s m a y be in a r a n g e t h a t w i l l f a c i l i t a t e m o i s t u r e movement. B r o w n e l l ( 3 ) f u r t h e r s t a t e s t h a t a c e r a m i c body with z e r o p e r cent a b s o r p t i o n cannot p r o d u c e e f f l o r e s c e n c e , and t h e r e i s p r o b a b l y s o m e p r a c t i c a l l i m i t between z e r o and s i x p e r c e n t a t which e f f l o r e s c e n c e will not o c c u r .
Weather conditions a1s.o p l a y a n i m p o r t a n t p a r t i n t h e e f f l o r e s c e n c e phenomenon. T h e y d e t e r m i n e , i n f a c t , whether
e f f l o r e s c e n c e will occur on the s u r f a c e or within t h e unit. During t h e l a t e winter months, t h e combination of r e l a t i v e l y low t e m p e r a t u r e s and high h u m i d i t i e s p r o d u c e s a slow r a t e of. evaporation. Throughout
t h i s period, any w a t e r in a s t r u c t u r e r e a c h e s the s u r f a c e b e f o r e evaporation, leaving the u n a t t r a c t i v e s a l t s on t h e wall, T h i s is a s e a s o n of poor drying weather when t h e incidence of unsightly e f f l o r e s c e n c e is a t i t s peak. Upon t h e advent of w a r m , d r y s u m m e r w e a t h e r , however, t h e w a t e r m a y change t o vapour b e f o r e r e a c h i n g t h e s u r f a c e of t h e m a s o n r y , and t h e s a l t s a r e
deposited within t h e wall, hidden, and g e n e r a l l y unsuspected. A s p r e v i o u s l y mentioned, t h e danger in t h i s c a s e i s t h a t s a l t s will a c c u m u l a t e in l a r g e enough amounts t o c r e a t e p r e s s u r e s sufficient t o spa11 t h e s u r f a c e f r o m m o r t a r or m a s o n r y uniis.
EXPERIMENTAL
The method u s e d in t h i s investigation w a s e s s e n t i a l l y t h a t followed by R i t c h i e i n which h e studied e f f l o r e s c e n c e on c e r a m i c wicks embedded in c y l i n d e r s of m a s o n r y m o r t a r s of different composition,
which w e r e a l t e r n a t e l y wetted and d r i e d a f t e r t h e m o r t a r had h a r d e n e d . (5). As t h e p u r p o s e of our study w a s t o a s c e r t a i n t h e influence of t h e b r i c k s in t h e e f f l o r e s c e n c e p r o c e s s , t h e wick w a s t h e v a r i a b l e i n s t e a d of t h e m o r t a r . Wicks w e r e cut f r o m t h e v a r i o u s u n i t s and embedded i n .cylinders of a 1:3 p o r t l a n d cement:sand m o r t a r .
T h e wicks w e r e a p p r o x i m a t e l y 3 in. long by 12 in. wide and 3/16 in. thick. T h e r e w e r e 8 different wicks cut f r o m 7 b r i c k s and a sandstone. Throughout t h i s p a p e r , t h e u s e of t h e g e n e r a l t e r m "bricks" will e n c o m p a s s a l l s a m p l e s , including t h e sandstone. T h e 7 b r i c k s r e p r e s e n t e d 6 v a r i e t i e s . Wicks w e r e cut f r o m one b r i c k a t two distinct l e v e l s of i n i t i a l r a t e of absorption.
A s noted previously, t h e m o r t a r contained one p a r t portland cement t o t h r e e p a r t s sand by volume. A pit sand was used along with distilled w a t e r . T h e m o r t a r w a s mixed i n a H o b a r t N-50 m i x e r for 5 min, final flow being a d j u s t e d t o f a l l between 110 and 1 2 5 p e r cent.
Two- by four-in. cylinder m o l d s w e r e used, with a wooden plug i n s e r t e d i n t h e lower p a r t of t h e mold s o that t h e r e s u l t i n g m o r t a r cylinder was 2$ in. high.
T h e m o r t a r w a s placed i n t h e mold i m m e d i a t e l y a f t e r mixing w a s comp1e:t:d and a wick, p r e v i o u s l y wetted i n d i s t i l l e d w a t e r t o i m p r o v e i t s contact, w a s i n s e r t e d t o a depth of 1 in. A deep s c r a t c h had been m a d e on t h e wicks 1 in. f r o m t h e end, and s e r v e d a s a guide in i n s e r t i n g t h e m t o t h e p r o p e r depth i n t h e m o r t a r . L a t e r , t h e s c r a t c h w a s u s e f u l i n facilitating s e p a r a t i o n of wick and m o r t a r by breaking. T h e wick- m o r t a r a s s e m b l a g e w a s r e m o v e d f r o m t h e mold a f t e r 24 h r and s t o r e d i n controlled l a b o r a t o r y a i r (70" F and 50 p e r cent
R.
H. ) for 7 days b e f o r e s t a r t i n g t h e wetting-drying cycles.F o r wetting and drying, s a m p l e s w e r e p l a c e d i n s m a l l j a r s ( c a p a c i t y about 7 oz. ) and distilled w a t e r w a s added t o b r i n g t h e l e v e l just below t h e top s u r f a c e of t h e m o r t a r . M e t a l c o v e r s , i n which s l o t s w e r e cut t o p e r m i t t h e wicks t o p r o t r u d e , w e r e p l a c e d on t h e j a r s . E v a p o r a t i o n of w a t e r through t h e gap between t h e wick and edges of t h e slot w a s r e d u c e d by placing thin g l a s s p l a t e s on t h e c o v e r a g a i n s t t h e wick. T o s t a r t t h e cycling, s a m p l e s w e r e i m m e r s e d i n w a t e r , a s
d e s c r i b e d , for a 24-hr period. T h e n t h e y w e r e r e m o v e d f r o m t h e w a t e r and allowed t o d r y i n l a b o r a t o r y a i r f o r 7 h r . T h e y w e r e then r e p l a c e d in t h e j a r s , using t h e o r i g i n a l w a t e r , which w a s r e p l e n i s h e d i n o r d e r t o b r i n g t h e l e v e l t o its o r i g i n a l height. After 17 h r i n t h e w a t e r , t h e drying p r o c e d u r e w a s repeated. Thus, in 24 h r , s a m p l e s w e r e wetted for 17 h r and d r i e d f o r 7.
During t h e wetting p e r i o d , w a t e r m i g r a t e d through t h e m o r t a r and into t h e wick, u l t i m a t e l y evaporating f r o m t h e wick s u r f a c e s . During i t s m i g r a t i o n , t h e w a t e r picked up soluble s a l t s f r o m t h e m o r t a r and/or t h e wick, and t h e s e s a l t s w e r e left behind when t h e w a t e r evaporated, e i t h e r on t h e s u r f a c e o r within t h e wick.
After completing 10 wetting-drying c y c l e s , t h e s a m p l e s w e r e s t o r e d f o r 7 days i n c o n t r o l l e d l a b o r a t o r y a i r . A s s e s s m e n t s by v i s u a l
o b s e r v a t i o n w e r e made, and t h e s a m p l e s photographed. Wicks w e r e then c a r e f u l l y b r o k e n away f r o m t h e m o r t a r cylinder (along t h e s c r a t c h line), d r i e d i n an oven f o r 48 h r a t 100" C, cooled and weighed. T h e n t h e y w e r e e x t r a c t e d with distilled w a t e r . E a c h wick w a s p l a c e d i n 50 m l of distilled w a t e r and allowed t o s o a k f o r 24 h r . T h e w a t e r w a s t h e n d r a i n e d f r o m t h e wick and r e p l a c e d with another 50 ml. An hour l a t e r t h e p r o c e d u r e w a s r e p e a t e d , and t h i s continued until t h e wick h a d been washed i n 250 m l of water. T h e w a s h w a t e r w a s l a t e r evaporated, and t h e s a l t s r e c o v e r e d w e r e weighed and r e t a i n e d f o r a n a l y s i s .
After t h e washing t r e a t m e n t , wicks w e r e d r i e d for 48 h r at 100" C, cooled and weighed. T h e d i f f e r e n c e in weight, b e f o r e and a f t e r washing, r e p r e s e n t e d t h e weight of soluble s a l t deposited in t h e wick. Blank r u n s had p r e v i o u s l y been conducted on wicks f r o m a l l of t h e b r i c k s , and soluble s a l t t o t a l s , r e c o v e r e d f r o m wicks i n s e r t e d i n m o r t a r , w e r e c o r r e c t e d accordingly. As the wicks v a r i e d slightly in s i z e , f i n a l r e s u l t s w e r e e x p r e s s e d a s a p e r c e n t a g e of t h e i r d r y weight. All s a m p l e p r e p a r a t i o n , wetting-drying cycling, etc.
,
was conducted i n c o n t r o l l e d l a b o r a t o r y a i r (70" F and 50 p e r cent R.H.).BLANK TESTS ON MATERIALS
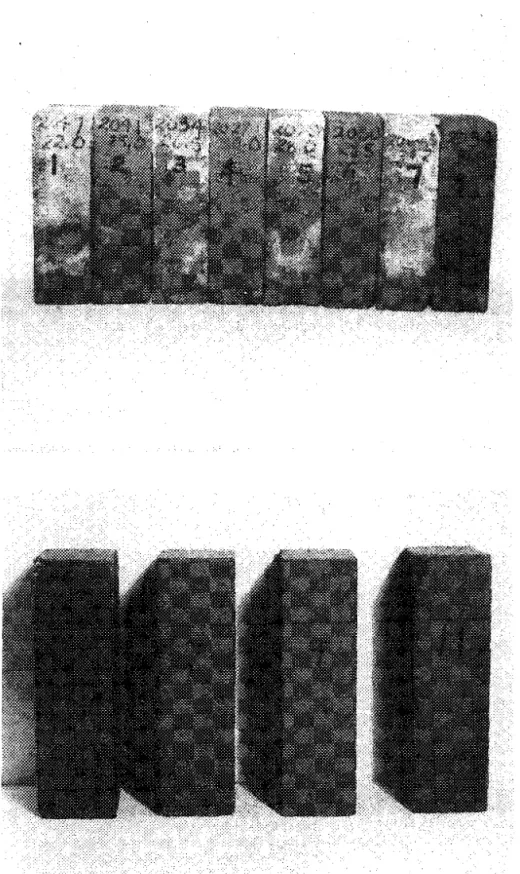
All t h e b r i c k s w e r e subjected t o t h e e f f l o r e s c e n c e t e s t outlined in ASTM C67-60, in which t h e s p e c i m e n s a r e s e t on end i n distilled w a t e r , i m m e r s e d t o a depth of 1 in., and left for 7 days. They w e r e then d r i e d and c o m p a r e d visually with duplicate c o n t r o l s p e c i m e n s . F i v e of the 7 b r i c k s t e s t e d w e r e f r e e of efflorescence. Heavy e f f l o r e s c e n c e o c c u r r e d on one of t h e b r i c k s (No. 5) and t h e r e was a minor o c c u r r e n c e on b r i c k No. 7 , w h e r e a t r a c e of s a l t a p p e a r e d on t h e top f r o n t c o r n e r s of one s a m p l e . R e s u l t s f o r t h e s e two b r i c k s a r e shown i n F i g u r e 1.
Blank r u n s w e r e a l s o conducted t o d e t e r m i n e t h e soluble s a l t content of t h e wicks. The p r o c e d u r e followed i n washing t h e m with distilled w a t e r was outlined previously. The amount of s a l t s r e c o v e r e d is shown in T a b l e I. Seven of t h e eight wicks contained 0. 08 p e r cent
o r l e s s , while wicks f r o m b r i c k No. 5, which exhibited s e v e r e e f f l o r e s c e n c e in t h e t e s t above, contained 0.26 p e r cent.
A c h e m i c a l a n a l y s i s of t h e s a l t r e c o v e r e d f r o m t h i s b r i c k is included in Table IV, and it indicates that the m a j o r constituent i s magnesium sulphate.
C h e m i c a l a n a l y s e s w e r e a l s o c a r r i e d out on t h e m o r t a r constituents, R e s u l t s f o r portland cement and a w a t e r e x t r a c t of t h e sand a r e shown in T a b l e 11. It will be noted that only negligible amounts of s a l t s a r e p r e s e n t i n t h e sand,
SELECTION O F MORTAR MIX
In a p r e l i m i n a r y study, wicks f r o m b r i c k NO, 8 w e r e s e t i n m o r t a r s of v a r i o u s compositions, including a r a n g e of c e m e n t - l i m e combinations and two m a s o n r y c e m e n t s .
R e s u 1 . t ~ w e r e s i m i l a r t b t h o s e of R i t c h i e in establishing g r e a t e s t e f f l o r e s c e n c e with m o r t a r s having highest portland c e m e n t contents (5).
A 1:3 portland cement:sand m o r t a r was, t h e r e f o r e , s e l e c t e d a s t h e m o r t a r in which wicks w e r e t o b e embedded.
RESULTS O F TESTS ON WICKS EMBEDDED IN MORTAR A. Visual
Visual o b s e r v a t i o n s and photographs ( F i g u r e s 2 t o 4)
of wicks f r o m t h e v a r i o u s b r i c k s embedded in m o r t a r c y l i n d e r s indicated f a i r l y heavy deposits on some, while o t h e r s w e r e c o m p a r a t i v e l y f r e e of s a l t s . The m o s t pronounced e f f l o r e s c e n c e was o b s e r v e d on wicks Nos. 3 and
6,
with wick No. 8 c l o s e behind. A c o m p a r i s o n of v i s u a l r e s u l t s proved difficult. Specimens w e r e not a l l s e e n at t h e s a m e t i m e , but w e r eevaluated a t i n t e r v a l s of s e v e r a l days. It should a l s o b e noted t h a t r e d , brown, buff, and g r e y wicks w e r e used. Thus, 4 different colour b a c k - grounds added another complication t o a difficult problem. P h o t o g r a p h s of wicks Nos. 1 and 5, on which v i s i b l e e f f l o r e s c e n c e w a s negligible, a r e not shown.
B. Quantitative
T h e amounts of s a l t s e x t r a c t e d f r o m t h e wicks a r e
r e c o r d e d in T a b l e 111. The f i r s t column i n d i c a t e s t o t a l s a l t s r e c o v e r e d , and t h e second, t h e c o r r e c t e d amounts (value f o r t o t a l s a l t s l e s s t h e amounts originally p r e s e n t in t h e wicks
-
T a b l e I). L a r g e s t amounts of s a l t s (2. 58 p e r cent) w e r e r e c o v e r e d f r o m wicks Nos. 2 and 5, with 2.37 and 2.27 p e r cent being r e c o v e r e d f r o m wicks Nos. 7 and 8. L e s s than 1 p e r cent was r e c o v e r e d f r o m each of t h e r e m a i n i n g 4 wicks.T h e c o r r e c t e d t o t a l s indicate a h i g h e r f i g u r e for wick No. 2 than for wick No. 5. T h e significance of t h e c o r r e c t e d t o t a l s m a y not be great. P o s s i b l y t h e 2. 58 p e r cent m a y r e p r e s e n t t h e capacity of t h e wicks t o hold s a l t s . Any additional s a l t could have been "lost" f r o m t h e e x t e r i o r of t h e wick, e i t h e r by m e c h a n i c a l action in i n s e r t i n g and removing t h e wick f r o m t h e slotted c o v e r on t h e j a r s , o r s i m p l y by a "flaking off" of t h e s a l t f r o m t h e s u r f a c e .
Quantitative r e s u l t s thus definitely e s t a b l i s h t h e higher e f f l o r e s c e n c e potential of wicks Nos. 2, 5, 7, and 8.
ANALYSIS O F SALTS
S a l t s r e c o v e r e d f r o m wicks Nos. 2, 5, and 8, f r o m which t h e l a r g e s t amounts w e r e obtained, and f r o m wick No. 6, which r a t e d " s e v e r e 1 ' i n t h e v i s u a l a s s e s s m e n t , w e r e analyzed t o d e t e r m i n e t h e m a j o r constituents, and r e s u l t s a r e shown i n T a b l e IV. A l s o included i n t h i s t a b l e i s an a n a l y s i s of s a l t obtained f r o m b r i c k No. 5 during the blank t e s t on it.
R e s u l t s i n d i c a t e that s a l t s obtained f r o m wicks Nos. 2, 6, and 8, contain p r i m a r i l y p o t a s s i u m carbonate, with a s m a l l amount of sulphate. The m a i n constituents of No. 5 a r e p o t a s s i u m and sulphate, with s m a l l e r amounts of c a l c i u m and carbonate. It is a l s o i n t e r e s t i n g t o note that m a g n e s i u m sulphate, t h e m a j o r constituent of s a l t s o r i g i n a l l y i n b r i c k No. 5, becomes potassium sulphate i n t h e wick, a f t e r it h a s been i n contact with t h e m o r t a r .
DISCUSSION
R e s u l t s of v i s u a l a s s e s s m e n t of e f f l o r e s c e n c e on wicks w e r e inconclusive and did not a g r e e with quantitative r e s u l t s obtained when soluble s a l t s w e r e e x t r a c t e d f r o m t h e wicks. T h e l a t t e r r e s u l t s w e r e in good a g r e e m e n t with field o b s e r v a t i o n s w h e r e t h e 3 b r i c k s , f r o m which t h e wicks containing g r e a t e s t a m o u n t s of soluble s a l t s w e r e obtained, a r e identified with t h e m o s t s e v e r e e f f l o r e s c e n c e p r o b l e m s . E x a m p l e s f r o m t h e field involving 2 of t h e 3 b r i c k s a r e shown in F i g u r e 5.
C h e m i c a l a n a l y s e s of s a l t s f r o m wicks and b r i c k , coupled with t h e s e v e r e e f f l o r e s c e n c e o c c u r r i n g during t h e blank t e s t on t h e b r i c k , e s t a b l i s h t h e s o u r c e m a t e r i a l for wick No. 5 as magnesium sulphate
located in t h e brick. It is i n t e r e s t i n g t o note that s a l t s r e c o v e r e d i n t h e blank t e s t w e r e 0, 26 p e r cent of t h e weight of t h e wick. T h i s s u b s t a n t i a t e s Brownell's a s s e r t i o n that e f f l o r e s c e n c e i s likely t o occur when t h e c e r a m i c product contains in e x c e s s of 0. 2 p e r cent of e x t r e m e l y soluble s a l t s ( 3 ) .
T h e s o u r c e m a t e r i a l f o r t h e soluble s a l t s r e c o v e r e d f r o m t h e r e m a i n i n g wicks is a s s u m e d t o h a v e o r i g i n a t e d in t h e m o r t a r . No a p p r e c i a b l e amounts of s a l t s w e r e r e c o v e r e d f r o m blank t e s t s on t h e wicks, while t h e p r e s e n c e of a l k a l i n e s a l t s w a s indicated by a c h e m i c a l a n a l y s i s on t h e portland c e m e n t u s e d i n t h e m o r t a r ( T a b l e 11). The s a m e m o r t a r combination w a s u s e d for a l l s p e c i m e n s , however, and d i f f e r e n c e s i n t h e r e s u l t s , both visual and quantitative, obtained with t h e v a r i o u s wicks, m u s t r e f l e c t d i f f e r e n c e s i n t h e i r p r o p e r t i e s .
Initial r a t e s of absorpt"ion for the b r i c k s , and a b s o r p t i o n p r o p e r t i e s of wicks cut f r o m t h e b r i c k s and u s e d i n t h e study, having p r e v i o u s l y been d e t e r m i n e d , a r e l i s t e d in T a b l e V. A c o m p a r i s o n w a s m a d e between t h e quantitative r e s u l t s and t h e s e p r o p e r t i e s , and is shown g r a p h i c a l l y in F i g u r e
6.
T h e r e i s no c o n c r e t e evidence of a d i r e c t r e l a t i o n s h i p between IRA of t h e b r i c k s and t h e a m o u n t s of s a l t s r e c o v e r e d f r o m t h e wicks, although t h e r e i s a t r e n d indicating l a r g e r amounts of s a l t s a s IRA v a l u e s i n c r e a s e . T h i s t r e n d is c o n t r a d i c t e d by r e s u l t s f o r b r i c k No. 2, with a n R A of 13 gm/30 sq in. /min f r o m which 2.58 p e r cent soluble s a l t s was r e c o v e r e d , while b r i c k s Nos. 7 and 8, with much higher and quite different IRA values, had lower s a l t contents. Again, b r i c k No. 3, with a n LRA value c l o s e t o t h e value for b r i c k No. 2, h a d a much lower s a l t content.
A c o m p a r i s o n of 24-hr i m m e r s i o n and 5-hr boiling a b s o r p t i o n values with s a l t s r e c o v e r e d , s u g g e s t s a m o r e definite relationship. T h e r e s u l t s in F i g u r e
6
indicate a distinct difference between t h e high s a l t content wicks and t h e low ones. T h e s e l i m i t e d r e s u l t s a r e in a c c o r d with t h e second of B r o w n e l l s s conclusions mentioned p r e v i o u s l y-
t h a t a c e r a m i c body with z e r o p e r centa b s o r p t i o n cannot produce e f f l o r e s c e n c e , and t h e r e i s p r o b a b l y s o m e p r a c t i c a l l i m i t between z e r o and s i x p e r cent at which e f f l o r e s c e n c e will not o c c u r ( 3 ) . .
T h e r e i s a l s o a g e n e r a l r e l a t i o n s h i p between s o l u b l e s a l t contents and a p p a r e n t p o r o s i t y with l a r g e r s a l t c o n c e n t r a t i o n s o c c u r r i n g in wicks with g r e a t e s t p o r o s i t i e s . A s in t h e c a s e of t h e LRA c o m p a r i s o n , t h e r e a r e s o m e c o n f l i c ~ i n g r e s u l t s t o t h i s t r e n d . Wicks with 17. 2 p e r cent p o r o s i t y had soluble s a l t contents m o r e i n l i n e with
9.
4 p e r cent p o r o s i t y wicks than with t h o s e at t h e 2 2 . 0 p e r cent level. T h u s , t h e r e is a g e n e r a l indication of i n c r e a s i n g s a l t content with i n c r e a s i n g a b s o r p t i o n values f o r t h e wicks u s e d in t h e study, but l i m i t e d , and s o m e conflicting r e s u l t s , m a k e definite conclusions difficult,C o n s i d e r a t i o n w a s a l s o given t o t h e effect of t h e n a t u r e of t h e s t r u c t u r e on t h e e f f l o r e s c e n c e p r o c e s s . C l e a r l y , a b r i c k
containing a l a r g e p e r c e n t a g e of p o r e s running through t h e unit would be m o r e s u s c e p t i b l e t o e f f l o r e s c e n c e than a unit containing a l a r g e p e r c e n t a g e of closed o r "dead-end" p o r e s . S i m i l a r l y , it i s r e a s o n a b l e t o a s s u m e t h a t t h e r e m u s t b e a c e r t a i n r a n g e of p o r e s i z e s t h a t w i l l f a c i l i t a t e m a x i m u m m o i s t u r e m i g r a t i o n and t h u s p r o m o t e e f f l o r e s c e n c e .
R e c o r d s of p o r e - s i z e d i s t r i b u t i o n s f o r 6 of t h e 8 b r i c k s included in t h e study, obtained with a m e r c u r y p o r o s i m e t e r , a r e r e c o r d e d in T a b l e VI and shown g r a p h i c a l l y in F i g u r e 7. T h e s e r e s u l t s a r e f o r
s a m p l e s r e p r e s e n t a t i v e of the t y p e s of b r i c k s u s e d in t h e study, but a r e not a c t u a l l y r e s u l t s f o r t h e p a r t i c u l a r s p e c i m e n s used. In s o m e i n s t a n c e s t h e r e i s a c o n s i d e r a b l e d i f f e r e n c e between t h e p o r o s i t y v a l u e s in T a b l e VI
and t h e v a l u e s l i s t e d f o r t h e c o r r e s p o n d i n g wicks in T a b l e V, F o r t h e p u r p o s e of t h i s d i s c u s s i o n , however, t h e v a l u e s in T a b l e VI w i l l b e
c o n s i d e r e d r e p r e s e n t a t i v e f o r t h e different wicks u s e d in t h e study. P o r e s i z e s in b r i c k s Nos. 1 and 4 a r e s m a l l ; o v e r 80 p e r cent of t h o s e in b r i c k No. 1 w e r e u n d e r 1 p a n d 75 p e r c e n t of t h o s e in b r i c k No, 4 w e r e under 2. 5 p . Twenty-five p e r cent of t h e p o r e s i n b r i c k No. 3 w e r e between 1 znd 10 p , with t h e r e m a i n d e r being f a i r l y evenly divided between u n d e r 1 p and over 10 p
.
T h e r e m a i n i n g 3 b r i c k s had a l a r g e p e r c e n t a g e of p o r e s in t h e 1 t o 10 p s i z e range. A l m o s t 70 p e r cent of t h e p o r e s i n b r i c k No. 2 w e r e between 1 and 10 p, Most of t h e r e m a i n d e r w e r e under 1 p . Relatively, t h e s a m e d i s t r i b u t i o n o c c u r r e d i n b r i c k No. 6. B r i c k No. 8 had o v e r 60 p e r cent of i t s p o r e s in t h e 1 t o 10 p r a n g e , with t h e r e m a i n d e r
divided between under 1 p and over 10 LL
.
It differed f r o m b r i c k s Nos. 2 and6
in having a g r e a t e r p e r c e n t a g e of p o r e s over 10 p i n s i z e . No d a t a a r e a v a i l a b l e f o r b r i c k No. 5, nor for b r i c k No. 7, although t h e l a t t e r i s c o n s i d e r e d e s s e n t i a l l y t h e s a m e f o r t h i s p u r p o s e a s b r i c k No. 8.Thus, two of t h e b r i c k s with h i s t o r i e s of r a t h e r s e v e r e e f f l o r e s c e n c e in t h e field, and which yielded l a r g e soluble s a l t contents during wick t e s t s d e s p i t e low s a l t c o n c e n t r a t i o n s e s t a b l i s h e d during blank t e s t s , h a v e p o r e s i z e s p r e d o m i n a n t l y i n t h e 1 t o 10 p r a n g e . T h r e e of t h e four r e m a i n i n g b r i c k s f o r which p o r e - s i z e data a r e available, had
d i s t r i b u t i o n s p r e d o m i n a n t l y below 1 p and o v e r 10 p. T h e f o u r t h b r i c k , No. 6, had a p o r e - s i z e distribution s i m i l a r t o t h a t i n t h e two u n i t s with an e f f l o r e s c e n c e h i s t o r y , but i t i s not a s s o c i a t e d with s e r i o u s e f f l o r e s c e n c e i n t h e field, nor w e r e l a r g e a m o u n t s of soluble s a l t s r e c o v e r e d f r o m i t s wicks. B r i c k No. 6 was t h e s a n d s t o n e ; t h e o t h e r s w e r e f i r e d c l a y b r i c k s . P e r h a p s i t s different behaviour m a y r e s u l t f r o m d i f f e r e n c e s i n p o r e s h a p e s and s t r u c t u r e , r a t h e r than s i z e . T h i s b r i e f study u s i n g p o r e - s i z e d i s t r i b u t i o n s of r e p r e s e n t a t i v e s a m p l e s of u n i t s u s e d i n t h e e x p e r i m e n t a l w o r k s u g g e s t s a n i n t e r e s t i n g a r e a f o r f u t u r e i n v e s t i g a t i o n s of t h e e f f l o r e s c e n c e phenomenon.
The remaining point f o r discussion concerns t h e conflicting evidence of visual hnd quantitative r e s u l t s f o r wicks Nos. 3 and
6,
f r o m which comparatively s m a l l amounts of s a l t s w e r e r e c o v e r e d despite pronounced deposits observed visually. It i s suggested that t h i s was t h e r e s u l t of different s u r f a c e t e x t u r e s for t h e s e wicks; that s a l t deposits w e r e left when water moved up the s u r f a c e of the wick by c a p i l l a r y attraction, and then evaporated. Smoother t e x t u r e of the other wicks did notfacilitate s u r f a c e capillarity, and s a l t s recovered f r o m them w e r e
introduced a s water p a s s e d through the p o r e s of the wicks and evaporated. COMPARISON OF SULPHATE AND CARBONATE EFFLORESCENCE
Field observations have indicated a m o r e s e v e r e and somewhat different mappearing efflorescence on m a s o n r y containing b r i c k No. 5, than on m a s o n r y containing b r i c k s Nos. 2 or 8. The f o r m e r h a s the appearance of a loose, fluffy powder and o c c u r s in l a r g e r amounts than the l a t t e r , which a p p e a r s a s a fine, clinging powder. Although
chemical analysis was not done on s a l t s observed in the field, a visual comparison was made with l a b o r a t o r y e f f l o r e s c e n c e c r e a t e d by i m m e r s i n g b r i c k s in solutions of equal s t r e n g t h of potassium sulphate and potassium
carbonate. T h r e e s a m p l e s of b r i c k No, 7 w e r e i m m e r s e d end-wise t o a depth of 1 in. in a 5 p e r cent solution of potassium sulphate. An equal number of s a m p l e s w e r e i m m e r s e d in a s i m i l a r manner in a 5 p e r cent
solution of potassium carbonate, and l e f t f o r 7 days. A heavy efflorescence appeared on the b r i c k s i m m e r s e d in the potassium sulphate solution s h o r t l y after the s t a r t of t h e t e s t , but only a minor showing o c c u r r e d on t h e b r i c k s in the potassium carbonate solution. At the end of the 7 days, the bricks w e r e removed f r o m the solutions and allowed t o dry. Drying resulted in a much m o r e pronounced showing on both bricks. T h i s i s c l e a r l y shown in F i g u r e 8, where a heavier deposit with a different appearance o c c u r s on the b r i c k s that w e r e i m m e r s e d in the sulphate.
Significantly, the efflorescence noted in the top photograph of F i g u r e 8 i s quite s i m i l a r t o that seen in the top photograph of Figure 5, while t h e r e i s a corresponding s i m i l a r i t y between t h e bottom p i c t u r e s in F i g u r e s 5 and 8.
C ONC LUSIONS
1. Blank efflorescence t e s t s on b r i c k s f r o m which wicks w e r e cut w e r e negative in a l l but one instance. Heavy efflorescence o c c u r r e d on b r i c k No. 5. Chemical analysis revealed the major constituent of t h e efflorescent s a l t to be magnesium sulphate.
2. Blank t e s t s on wicks iridicated l e s s than 0 . 1 p e r cent soluble s a l t contents f o r a l l but No. 5, which contained 0. 26 p e r cent. T h e l a t t e r wicks w e r e cut f r o m t h e b r i c k r e f e r r e d t o i n t h e p r e v i o u s p a r a g r a p h .
3. T e s t s on wicks cut f r o m different b r i c k s and embedded i n 1:3 p o r t l a n d cement:sand m o r t a r w e r e u n s u c c e s s f u l i n r e p r o d u c i n g
e f f l o r e s c e n c e s i m i l a r t o t h a t o b s e r v e d i n t h e field.
4. Soluble s a l t t o t a l s , obtained by washing wicks a t t h e conclusion of t h e e f f l o r e s c e n c e t e s t s , r e v e a l e d highest c o n c e n t r a t i o n s i n 4 wicks cut f r o m b r i c k s found t o b e p a r t i c u l a r l y s u s c e p t i b l e t o
e f f l o r e s c e n c e in t h e field.
5. C h e m i c a l a n a l y s e s of s a l t s r e c o v e r e d f r o m wicks embedded i n m o r t a r indicated p o t a s s i u m c a r b o n a t e t o b e t h e m a i n
constituent in all but wick No. 5, w h e r e p o t a s s i u m s u l p h a t e predominated.
6.
Soluble s a l t s o c c u r r i n g in wick No. 5 originated with s a l t s o r i g i n a l l y found i n t h e b r i c k itself, while f o r a l l o t h e r s p e c i m e n s , t h e s o u r c e m a t e r i a l a p p e a r s t o have been a l k a l i s a l t s in the p o r t l a n d c e m e n t u s e d in t h e m o r t a r .7. A c o m p a r i s o n of t h e a b s o r p t i o n p r o p e r t i e s of t h e wicks and t h e i r soluble s a l t contents i n d i c a t e s a gener a1 r e l a t i o n s h i p with i n c r e a s i n g s a l t contents a s a b s o r p t i o n values r o s e .
8. Examination of p o r e - s i z e d i s t r i b u t i o n s f o r t y p i c a l s p e c i m e n s of
6
of t h e 8 b r i c k s included in t h e study r e v e a l e d p o r es i z e s in t h e 1 t o 10 p r a n g e f o r wicks having l a r g e s t soluble s a l t contents, while wicks with lower s a l t contents had p o r e s in t h e r a n g e of under 1 p and over 10 p. An exception was t h e sandstone, which had low s a l t concentration and a p o r e - s i z e distribution in t h e 1 t o 10 p range. An i n t e r e s t i n g a r e a f o r f u t u r e e f f l o r e s c e n c e s t u d i e s i s indicated.
ACKNOWLEDGEMENTS
T h e author w i s h e s t o thank h i s colleague, T , Ritchie, of t h e Inorganic M a t e r i a l s Section, who c a r r i e d out t h e d e t e r m i n a t i o n s of p o r e - s i z e distribution. He i s a l s o indebted t o t h e Division of
Applied C h e m i s t r y and e s p e c i a l l y t o G. R. Duval, A. Mykytiuk, and E. C. Goodhue, who conducted t h e c h e m i c a l a n a l y s e s .
R E F E R E N C E S
1. A n d e r egg, F. 0. E f f l o r e s c e n c e . ASTM B u l l e t i n No. 195, O c t o b e r 1952.
2. B u t t e r w o r t h , B. E f f l o r e s c e n c e a n d S t a i n i n g of B r i c k w o r k . T h e B r i c k Bulletin, Vol. 3, No. 5, 1961.
3. B r o w n e l l , W. E. F u n d a m e n t a l F a c t o r s Influencing E f f l o r e s c e n c e of C l a y P r o d u c t s . J o u r n a l of t h e A m e r i c a n C e r a m i c Society, Vol. 32, No. 12, 1949. 4. R i t c h i e , T. Study of E f f l o r e s c e n c e on E x p e r i m e n t a l B r i c k w o r k P i e r s . J o u r n a l of t h e A m e r i c a n C e r a m i c Society, Vol. 38, No. 10, 1955. 5. R i t c h i e , T. S t u d y of E f f l o r e s c e n c e P r o d u c e d on C e r a m i c Wicks by M a s o n r y M o r t a r s . J o u r n a l of t h e A m e r i c a n C e r a m i c S o c i e t y , Vol, 38, No. 10, 1955. 6 . R o g e r s , P. L. A Method of T e s t f o r P o t e n t i a l E f f l o r e s c e n c e of M a s o n r y M o r t a r . ASTM B u l l e t i n , J a n u a r y 1959. 7. Young, J a m e s E. Backup M a t e r i a l s a s a S o u r c e of E f f l o r e s c e n c e . J o u r n a l of t h e A m e r i c a n C e r a m i c S o c i e t y , Vol. 40, No. 7, J u l y 1957. 8. M c B u r n e y , J. W. and D. E, P a r s o n s , T h e Wick T e s t f o r E f f l o r e s c e n c e of Building B r i c k . ASTM P r o c e e d i n g s , Vol. 37,
1937, p. 332.
9. Bonnell, D. G. R. a n d B. B u t t e r w o r t h , C l a y Building B r i c k s of t h e United Kingdom. HM S t a t i o n e r y Office, 1950.
T A B L E I
R E S U L T S O F BLANK T E S T S ON WICKS
S a l t s R e c o v e r e d , Wick f r o m B r i c k No.
70
wt. of w i c kT A B L E I1
CHEMICAL ANALYSIS O F MORTAR MATERIALS
P o r t l a n d C e m e n t .
yo
Wash W a t e r of t h e S a n d ( P a r t sp e r
Million) nn. d.=
n o n e d e t e c t e dT A B L E I11
SALTS RECOVERED FROM WICKS AuFTER E F F L O R E S C E N C E T E S T
Wick No. S a l t s R e c o v e r e d , O/o wt. of wick T ot a1 C o r r e c t e d * 1 0, 2 4 0. 22 2 2. 58 2. 52 3 0 . 9 4 0 - 9 3 4 0.90 0.86 5 2. 58 2. 3 1 6 0. 78 0.71 7 2. 27 2. 2 4 8 2. 37 2. 29
'k T o t a l amount l e s s amount o r i g i n a l l y p r e s e n t i n wicks.
T A B L E IV
CHEMICAL ANALYSIS O F SOLUBLE SALTS 0 UTAINED FROM WICKS AND E F F L O R E S C E N T
SALT REMOVED FROM BRICK NO, 5
S a l t f r o m S a l t f r o m
Wick N u m b e r : (2) (5) (6) (8) B r i c k No. 5
40.68 1. 36 35.32 38.96 1.06
T A B L E VI
B r i c k No.
PORE-SIZE DISTRIBUTION O F SIX
BRICKS
( P o r e Size,
70)
T o t a l > l o
CI
P o r o s i t y ,70
Figure i Efflorescence test on bricks N o s . 5 and '7,
T O P
-
Brick No. 5. H e a v y efflorescence on odd- numbered specimens compared with even-numbered controls.BOTTOM
-
B r i c kNo,
7 - N o t e small salt depositsFigure 2 EffEoseacenee on wicks embedded in mortar.
T O P
-
Wick No. 2. There is some deposit near t o p 0% wicks. W i c k s a r e huff csPour makingefflorescence l e s s obvious.
F i g u r e 3 Efflorescence on wicks e m b e d d e d in mortar.
TOP
- W i c k No. 4. BOTTOM-
W i c k N o .6.
F i g u r e 4 E f f l o r e s c e n c e on wicks embedded in mortar. T O P
-
W i c k No. 7 .Figure 5 Field observations of efflorescence of masonry buildings containing:
'TOP
-
B r i c kNo.
5 .-
-
=W i c k
no.
5
- -0
- 0 0-
- 0-
0 --
0 0-
-
-
I
I
I
0
0-
-
-
O
--
-
-
-
-
0 0-
-
0-
01I
I
-
o 1
-
-
-
-
-
-
-
-
0-
-
-
-
0 0-
0
001S A L T S R E C O V E R E D ,
96
O F W E I G H T
O F
W I C K
F I G U R E 6
C O M P A R I
S O N
O F
S A L T S R E C O V E R E D A N D
P R O P E R T I E S O F W I C K S
OR 3 3 7 7 - 1B r i c k n o .
3
P o r .
-
9.
3%
B r i c k n o .
1
P o r .
-
1 6 .
7%
---
B r i c k n o .
4
P o r ,
-
7 . 2 %
--lo
B r i c k n o . 2
--
P o r .
-
2 3 . 9 %
--
8
- -6
-4
B r i c k n o .
8
P o r .
-
1 6 . 2 %
--
2
-
I
P O R E S I Z E ,
U
,
B r i c k n o . 6
- - l o
P o r .
-
1 1 . 1 %
--
-
8
-
-6
-
--
4
F I G U R E 7
-
--
2
-
--
D I S T R I B U T I O N O F P O R E S I Z E S I N B R I C K S
AR 3 3 77-2 - ( ~ o ~ 1 ~ 0 ~ ~ ~ '1
~ ~ (lcon5 1
2 ~0 . 5 - ~1
~1.0- 2.5 51
2.5- ~5.0 ~1
5.0-10.O1
~ ~ >1
1 0Figure 8 C o ~ p a r i s o n of sulphate and carbonate after
Brick
No.
9w a s immersed on end to a depth of 1 in.
TOP
-
5
per cent sokrltion of K 2SO
4'