HAL Id: hal-01823492
https://hal.archives-ouvertes.fr/hal-01823492
Submitted on 26 Jun 2018
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Geochemistry of Aquifer in Contact with Alum Shale:
Evidence of Limited Contaminant Transfers
Jérôme Sterpenich, Eric. C Gaucher, Jacques Pironon, Jeremy Lerat, Régine
Mosser-Ruck, Niels. H Schovsbo
To cite this version:
Jérôme Sterpenich, Eric. C Gaucher, Jacques Pironon, Jeremy Lerat, Régine Mosser-Ruck, et al..
Geo-chemistry of Aquifer in Contact with Alum Shale: Evidence of Limited Contaminant Transfers.
Proce-dia Earth and Planetary Science, Elsevier, 2017, 17 (5), pp.786 - 789. �10.1016/j.proeps.2017.01.029�.
�hal-01823492�
Procedia Earth and Planetary Science 17 ( 2017 ) 786 – 789
1878-5220 © 2017 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Peer-review under responsibility of the organizing committee of WRI-15 doi: 10.1016/j.proeps.2017.01.029
Available online at www.sciencedirect.com
ScienceDirect
15th Water-Rock Interaction International Symposium, WRI-15
Geochemistry of Aquifer in Contact with Alum Shale: Evidence of
Limited Contaminant Transfers
J. Sterpenich
a,1, E.C. Gaucher
b, N.H. Schovsbo
c, J.G. Lerat
a, R. Mosser-Ruck
a, J.
Pironon
aa
Université de Lorraine, CNRS, CREGU, GeoRessources laboratory, BP 70239, 54506 Vandoeuvre-lès-Nancy, France
b
Total CSTJF, Avenue Larribau, Pau, F-64000, France
c
, Geological Survey of Denmark and Greenland, Øster Voldgade 10, DK-1350 Copenhagen K, Denmark
Abstract
An aquifer and an aquitard in contact with the Alum Shale Formation (Bornholm Island, Denmark) have been sampled and analyzed for their contents in metals and radionuclides. High concentrations of some metals have been found in the aquitard within the formation. However, the overlying aquifer shows low concentration of metals and this provides evidence that the transfer of contaminants into the aquifer is naturally limited.
© 2017 The Authors. Published by Elsevier B.V.
Peer-review under responsibility of the organizing committee of WRI-15.
Keywords: water, toxic elements, Alum Shale, geochemistry, PHREEQC
1.Introduction
The Scandinavian Alum Shale Formation (Middle Cambrian to Lower Ordovician) contains high levels of organic carbon (up to 25 wt.%) and syngenetic enriched trace elements. This formation is also known for its high uranium content1. At the present time, Alum Shale is studied for its shale gas potential in Denmark2,3. Potential release of metals and radionuclides during hydraulic fracking is mentioned by4 and has been specifically studied for the Alum Shale5. However, the natural release of toxic elements (transition elements, heavy metals, radionuclides) from low permeability shales into surrounding aquifers has been only poorly documented. This paper documents the complete chemical analysis of waters sampled in a borehole (Sommerode-1: S-1 well) of the Bornholm Island (Baltic Sea, Denmark) and discusses the composition in terms of possible transfer of elements from the Alum Shale.
* Corresponding author. Tel.: +33 3 83 68 49 40; fax: +33 3 83 68 47 01. E-mail address: Jerome.sterpenich@univ-lorraine.fr
© 2017 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
787
J. Sterpenich et al. / Procedia Earth and Planetary Science 17 ( 2017 ) 786 – 789
2.Geological setting, mineralogy and water chemistry
The S-1 well was drilled by GEUS in November 20126 until 250.3 m. The top of the Alum Shale Formation is at 218 m depth, for a total thickness of 28 m. The formation consists of dark organic rich shale with fractures cemented by calcite. The well was terminated at 250.3m in the Læså Formation (4.3m thick). This lower formation consists of tight sandstone. In May 2013 water samples in the S-1 well were collected for analyses. Several authors6 have measured the water flow in the well (1.4 m3/h). Most of the inflow took place within the uppermost 75m of the well. The inflow rates decrease rapidly with depth and the section below 80m contributes with less than 20% of the total inflow. In order to characterize the water in contact with the Alum shale, a packer was placed at a depth between 207m and 210m. This packer separates the main aquifer above the Alum Shale (S1 sample) and the aquitard under 210m where productive series are limestone banks and the terminal sandstone (Alum Shale + top of Laeså Formation) (S3 Sample). Baltic Sea has also been sampled on the beach near the well (distance 160m). Direct measurements of non-conservative parameters were made on water after the sampling (redox, pH, alkalinity by Gran titration, conductivity and temperature, Fe(II)). Filtration (0.1 µm) of water was done to separate solid particles. Water was conditioned in plastic containers, split in two sub-samples: the first one was acidified in order to stabilize cations and metals; the second one was only filtered for anions analysis. The mineralogy of the Alum Shale is extensively described in7. A mean mineralogical composition is used in this paper to perform geochemical simulations. The shale is manly composed of clay minerals (illite, chlorite, micas and mixed-layer clays), pyrite, quartz and feldspars, carbonates, rutile and accessory minerals such as phosphate minerals (not considered here). The study of Lerat et al. has shown that mainly clay minerals and sulfides are metal- and radionuclide-bearing phases. The elements of interest in sulfides minerals such as pyrite are Hg (up to 2700 ppm), As (up to 2600 ppm), Co (up to 1000 ppm), Cu (up to 5500 ppm), Mo (up to 1300 ppm), Ni (up to 4200 ppm), Pb (up to 1100 ppm) and Zn (up to 4100 ppm). Clay minerals can contain up to 3500 ppm of V. The chemical composition of S1 and S3 waters and seawater are given in Table 1. Major cations are analyzed by ICP-OES and trace elements by ICP-MS. Anions are measured by colorimetry or chromatography.
Table 1: Chemical composition and physic-chemical parameters of water of formation (S1 and S3) and seawater sampled in Sommerodde-1 well. L.D.: limit of determination. N.D.: not determined.
Major elements (mg/L) S1 S3 Seawater Trace elements (µg/L) S1 S3 Seawater Si 5.89 3.87 0.17 Ba 52.78 811.27 15.12 Al 0.0033 0.0010 0.0011 Be 0.048 0.040 < L.D. Fe tot 1.17 < L.D. < L.D. Bi < L.D. 0.009 0.022 Mn 0.54 0.044 0.0016 Cd < L.D. 0.011 0.020 Mg 4.23 12.03 278.3 Co 0.0919 0.018 0.032 Ca 78.91 35.09 110.5 Cr 1.20 1.96 0.48 Na 37.05 1194 2387 Cs 0.17 1.11 0.04 K 2.13 15.19 85.49 Cu 0.08 0.42 1.67 Ti < L.D. < L.D. < L.D. Ga 0.01 0.006 < L.D. P < L.D. < L.D. < L.D. In < L.D. < L.D. < L.D. SO4 2-50 3.7 582 Ni 2.30 1.07 1.57 HCO3 -205 417 99 Pb 0.06 0.069 0.10 Cl -44 1610 3945 Rb 3.11 35.22 21.01 Fe2+ 3 0.4 < L.D. Sr 188.8 900.9 1628.4 (ng/L) S1 S3 Seawater Th 0.018 < L.D. < L.D. Ce 27.78 < L.D. < L.D. U 0.060 1.17 0.39 Dy 19.32 < L.D. < L.D. V 0.69 0.57 0.16 Er 16.04 < L.D. < L.D. Y 0.14 0.014 0.014 Eu 5.15 19.65 < L.D. Zn 3.37 48.85 4.02 Gd 20.82 5.99 < L.D. Ho 5.01 < L.D. < L.D. (ng/L) S1 S3 Seawater La 8.53 3.88 < L.D. Lu 2.94 < L.D. < L.D. pH (25°C) 7.6 7.9 7.9 Nd 32.42 < L.D. < L.D. pH (reservoir T) 8.04 8.42 7.84 Pr 5.23 < L.D. < L.D. T°C 10.8 11 11.3 Sm 12.81 < L.D. < L.D. Conductivity (µs/cm) 566 5290 11850 Tb 3.01 < L.D. < L.D. Eh (mV) -115.5 N.D. N.D. Tm 2.34 < L.D. < L.D. Total alkalinity (meq/L) 3.36 6.83 1.62 Yb 13.90 < L.D. < L.D.
The sample of the Baltic Sea shows the known dilution with fresh waters coming from rivers by comparison with the standard seawater8. S1 (aquifer) is slightly salty (Na: 37 mg/L) as attested by the low electrical conductivity. It is rich in Ca (79 mg/L) and Fe (1 to 3 mg/L). A notable discrepancy is observed between total iron and reduced iron in the analyses: this is likely due to iron oxide precipitation between sampling and ICP analyses. Concerning the trace elements, Sr is present, as well as Mn (0.54 mg/L) which is just above the potability limit (0.4 mg/L). Rare Earth Elements can be quantified in S1 when they are mainly below the detection limit in the other samples. For S3 (formation water), the salinity is about half that of the Baltic Sea as shown by the electrical conductivity and the NaCl content. The composition in trace elements is complex and enriched for Ba, Cr, Cs, Rb, U, and Zn.
All the waters are slightly basic as confirmed by the pH between 7.5 and 8.0 at 25°C. The redox state, when it was possible to measure, is rather reduced for S1 and S3 and considered as oxidizing for the seawater in contact with the atmosphere.
3. Discussion: Comparison of aquifer and formation waters with seawater (Baltic Sea)
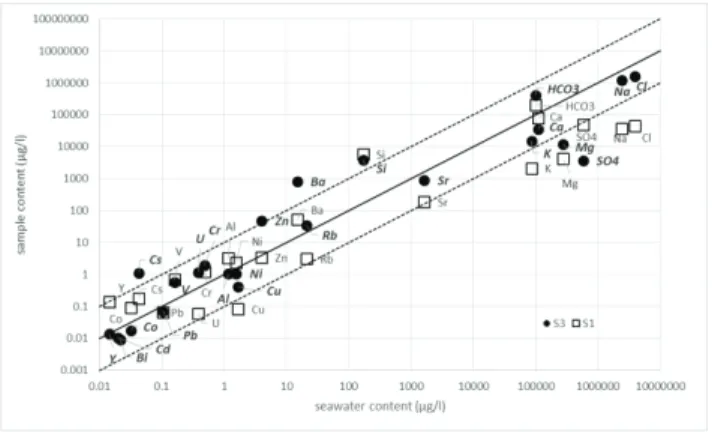
The compositions of S1 (aquifer) and S3 (formation water) samples are plotted as a function of seawater in Figure 1. The plain and dashed lines correspond to 1:1 and 1:10 concentration ratios respectively. Note that elements below the detection limit in seawater (all the trace elements measured in ng/L) are not included in this graph. In a general manner, seawater is enriched in alkali and alkaline-earth elements (except Cs and Rb for S3), Cl- and SO4
2-anions, Pb and Cu by comparison with both S1 and S3 samples. S1 and S3 are rather enriched in HCO3
-, Si-, Ba-, Cs-, V and Cr. The comparison between S1 and S3 shows that the upper aquifer (S1) is strongly enriched in Fe, Mn, SO4
2-, and Y. S3 is strongly enriched in Ba2-, Sr2-, Rb2-, Cs2-, Zn2-, U2-, and in a lower extent in Cu and Eu. A contribution of V, Cs, and Cr from clay minerals is proposed.
The composition of waters was studied in terms of saturation index with respect to different minerals (Phreeqc Thermoddem database), either constituting the Alum Shale, or susceptible to precipitate. S1 and S3 oversaturated with respect to clays, iron oxides, feldspars, quartz, calcite and aragonite, magnesite. S1 and S3 are under-saturated with respect to sulphates, amorphous silica, brucite. Gibbsite is oversaturated in S1 and close to equilibrium in S3. Dolomite is close to equilibrium in S1, oversaturated in S3. A strong undersaturation of sulphates is shown for S3. Uraninite is oversaturated in S3 (log (Saturation Index)=1.02) but not in S1 log (Saturation Index)=-0.23.
Fig. 1. Comparison of major and trace elements content of waters from S1 (upper aquifer) and S3 (Alum Shale water) with seawater from Baltic Sea. The solid line corresponds to equality of concentrations and the dashed lines correspond to a ten times enrichment.
789
J. Sterpenich et al. / Procedia Earth and Planetary Science 17 ( 2017 ) 786 – 789
Fig. 2. PHREEQC geochemical modeling of the influence of the W/R ratio on the behavior of the Alum Shale in contact with seawater (Baltic Sea).
We have modeled leaching of the Alum Shale with increasing quantities of seawater (without oxygen). First Water/Rock ratio (W/R) corresponds to the porosity water. Slight dissolution of pyrite is observed whatever W/R. When W/R increases, clay minerals tend to precipitate. Carbonates dissolved only for W/R greater than 10.
The metal bearing minerals, pyrites and clays, do not dissolve if considering an intrusion of seawater, whatever the W/R ratio. There is no possibility of a massive release of potentially toxic elements from the Alum Shale in the Baltic Sea as long as the system remains reduced.
4.Conclusion
The study of waters in contact with the Alum Shale shows that the transfer of elements, and especially transition elements, heavy metals and radionuclides, between the rock and the overlying aquifers is limited. The S1 water is not in equilibrium with the shale because it exhibits oversaturation with respect to clay minerals which are some of the metal bearing phases. Given this context, equilibration between water and clays can affect ionic exchange but will not lead to dissolution nor large releases of elements. Pyrite, which bears some transition elements and heavy metals, is susceptible to dissolve but the geochemical simulations show that this dissolution remains very limited as long as the system remains under reduced conditions.
References
1. Schovsbo, N.H., Uranium enrichment shorewards in black shales: A case study from the Scandinavian Alum Shale. GFF, 2002. 124(2): p. 107-115.
2. Gautier, D.L., N.H. Schovsbo, and A.T. Nielsen. Resource potential of the alum shale in Denmark. in Society of Petroleum Engineers - SPE/AAPG/SEG Unconventional Resources Technology Conference. 2016.
3. Schovsbo, N.H., A.T. Nielsen, and D.L. Gautier, The Lower Palaeozoic shale gas play in Denmark, in Geological Survey of Denmark and Greenland Bulletin. 2014. p. 19-22.
4. Gregory, K.B., R.D. Vidic, and D.A. Dzombak, Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements, 2011. 7(3): p. 181-186.
5. Gaucher, E.C., et al., Toxic Metals in Shales: Questions and Methods for a Better Management of Flow-Back Waters, in Unconventional Resources Technology Conference (URTEC) 2014: Denver (Co).
6. Schovsbo, N.H., A.T. Nielsen, and K. Klitten, The lower palaeozoic now fully cored and logged on Bornholm, Denmark, in Geological Survey of Denmark and Greenland Bulletin. 2015. p. 9-12.
7. Lerat, J., et al., Metals and radionuclides in the Alum shale of Denmark: identification of the bearing phases for a better management of the hydraulic fracturing waters. Journal of Unconventional Oil and Gas Resources, submitted.
8. Feistel, R., et al., Density and absolute salinity of the baltic sea 2006-2009. Ocean Science, 2010. 6(1): p. 3-24. -2.00E-02 -1.50E-02 -1.00E-02 -5.00E-03 0.00E+00 5.00E-03 1.00E-02 1.50E-02
1.00E-02 1.00E-01 1.00E+00 1.00E+01 1.00E+02 1.00E+03
'
mo
l
W/R Dolomite Calcite Siderite
-2.00E-03 0.00E+00 2.00E-03 4.00E-03 6.00E-03 8.00E-03 1.00E-02 1.20E-02 1.40E-02
1.00E-02 1.00E-01 1.00E+00 1.00E+01 1.00E+02 1.00E+03
'
mo
l
W/R Pyrite Clinochlore Illite(Al)