2015 ESC Guidelines for the management
of infective endocarditis
The Task Force for the Management of Infective Endocarditis of the
European Society of Cardiology (ESC)
Endorsed by: European Association for Cardio-Thoracic Surgery
(EACTS), the European Association of Nuclear Medicine (EANM)
Authors/Task Force Members: Gilbert Habib
*
(Chairperson) (France),
Patrizio Lancellotti
*
(co-Chairperson) (Belgium), Manuel J. Antunes (Portugal),
Maria Grazia Bongiorni (Italy), Jean-Paul Casalta (France), Francesco Del Zotti (Italy),
Raluca Dulgheru (Belgium), Gebrine El Khoury (Belgium), Paola Anna Erba
a(Italy),
Bernard Iung (France), Jose M. Miro
b(Spain), Barbara J. Mulder (The Netherlands),
Edyta Plonska-Gosciniak (Poland), Susanna Price (UK), Jolien Roos-Hesselink
(The Netherlands), Ulrika Snygg-Martin (Sweden), Franck Thuny (France),
Pilar Tornos Mas (Spain), Isidre Vilacosta (Spain), and Jose Luis Zamorano (Spain)
Document Reviewers: Çetin Erol (CPG Review Coordinator) (Turkey), Petros Nihoyannopoulos (CPG Review Coordinator) (UK), Victor Aboyans (France), Stefan Agewall (Norway), George Athanassopoulos (Greece), Saide Aytekin (Turkey), Werner Benzer (Austria), He´ctor Bueno (Spain), Lidewij Broekhuizen (The Netherlands), Scipione Carerj (Italy), Bernard Cosyns (Belgium), Julie De Backer (Belgium), Michele De Bonis (Italy),Konstantinos Dimopoulos (UK), Erwan Donal (France), Heinz Drexel (Austria), Frank Arnold Flachskampf (Sweden),
Roger Hall (UK), Sigrun Halvorsen (Norway), Bruno Hoenb(France), Paulus Kirchhof (UK/Germany),
*Corresponding authors: Gilbert Habib, Service de Cardiologie, C.H.U. De La Timone, Bd Jean Moulin, 13005 Marseille, France, Tel:+33 4 91 38 75 88, Fax: +33 4 91 38 47 64,
Email:gilbert.habib2@gmail.com
Patrizio Lancellotti, University of Lie`ge Hospital, GIGA Cardiovascular Sciences, Departments of Cardiology, Heart Valve Clinic, CHU Sart Tilman, Lie`ge, Belgium – GVM Care and
Research, E.S. Health Science Foundation, Lugo (RA), Italy, Tel:+3243667196, Fax: +3243667194, Email:plancellotti@chu.ulg.ac.be
ESC Committee for Practice Guidelines (CPG) and National Cardiac Societies document reviewers: listed in the Appendix ESC entities having participated in the development of this document:
ESC Associations: Acute Cardiovascular Care Association (ACCA), European Association for Cardiovascular Prevention & Rehabilitation (EACPR), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA).
ESC Councils: Council for Cardiology Practice (CCP), Council on Cardiovascular Nursing and Allied Professions (CCNAP), Council on Cardiovascular Primary Care (CCPC). ESC Working Groups: Cardiovascular Pharmacotherapy, Cardiovascular Surgery, Grown-up Congenital Heart Disease, Myocardial and Pericardial Diseases, Pulmonary Circulation and Right Ventricular Function, Thrombosis, Valvular Heart Disease.
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford Uni-versity Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC.
Disclaimer. The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their publication. The ESC is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recom-mendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encour-aged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consultation with that patient and, where appropriate and/or necessary, the patient’s caregiver. Nor do the ESC Guidelines exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional’s responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
&The European Society of Cardiology 2015. All rights reserved. For permissions please email: journals.permissions@oup.com.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
Mitja Lainscak (Slovenia), Adelino F. Leite-Moreira (Portugal), Gregory Y.H. Lip (UK), Carlos A. Mestresc (Spain/United Arab Emirates), Massimo F. Piepoli (Italy), Prakash P. Punjabi (UK), Claudio Rapezzi (Italy), Raphael Rosenhek (Austria), Kaat Siebens (Belgium), Juan Tamargo (Spain), and David M. Walker (UK)
The disclosure forms of all experts involved in the development of these guidelines are available on the ESC website
http://www.escardio.org/guidelines.
a
Representing the European Association of Nuclear Medicine (EANM);bRepresenting the European Society of Clinical Microbiology and Infectious Diseases (ESCMID); and
c
Representing the European Association for Cardio-Thoracic Surgery (EACTS). Online publish-ahead-of-print 29 August 2015
-Keywords Endocarditis † Cardiac imaging † Valve disease † Echocardiography † Prognosis † Guidelines † Infection † Nuclear imaging † Cardiac surgery † Cardiac device † Prosthetic heart valves † Congenital heart disease † Pregnancy † Prophylaxis † Prevention
Table of Contents
Abbreviations and acronyms . . . .3077
1. Preamble . . . .3078
2. Justification/scope of the problem . . . .3079
3. Prevention . . . .3079
3.1 Rationale . . . .3079
3.2 Population at risk . . . .3080
3.3 Situations and procedures at risk . . . .3081
3.3.1 Dental procedures . . . .3081
3.3.2 Other at-risk procedures . . . .3081
3.4 Prophylaxis for dental procedures . . . .3081
3.5 Prophylaxis for non-dental procedures . . . .3082
3.5.1 Respiratory tract procedures . . . .3082
3.5.2 Gastrointestinal or genitourinary procedures . . . . .3082
3.5.3 Dermatological or musculoskeletal procedures . . . .3082
3.5.4 Body piercing and tattooing . . . .3082
3.5.5 Cardiac or vascular interventions . . . .3082
3.5.6 Healthcare-associated infective endocarditis . . . .3082
4. The ‘Endocarditis Team’ . . . .3083
5. Diagnosis . . . .3084
5.1 Clinical features . . . .3084
5.2 Laboratory findings . . . .3084
5.3 Imaging techniques . . . .3084
5.3.1 Echocardiography . . . .3084
5.3.2 Multislice computed tomography . . . .3086
5.3.3 Magnetic resonance imaging . . . .3087
5.3.4 Nuclear imaging . . . .3087
5.4 Microbiological diagnosis . . . .3087
5.4.1 Blood culture– positive infective endocarditis . . . . .3087
5.4.2 Blood culture– negative infective endocarditis . . . . .3088
5.4.3 Histological diagnosis of infective endocarditis . . . .3088
5.4.4 Proposed strategy for a microbiological diagnostic algorithm in suspected IE . . . .3088
5.5 Diagnostic criteria . . . .3089
6. Prognostic assessment at admission . . . .3090
7. Antimicrobial therapy: principles and methods . . . .3091
7.1 General principles . . . .3091
7.2 Penicillin-susceptible oral streptococci and Streptococcus bovis group . . . .3092
7.3 Penicillin-resistant oral streptococci and Streptococcus bovis group . . . .3092
7.4 Streptococcus pneumoniae, beta-haemolytic streptococci (groups A, B, C, and G) . . . .3092
7.5 Granulicatella and Abiotrophia (formerly nutritionally variant streptococci) . . . .3094
7.6 Staphylococcus aureus and coagulase-negative staphylococci . . . .3094
7.7 Methicillin-resistant and vancomycin-resistant staphylococci . . . .3094
7.8 Enterococcus spp. . . .3094
7.9 Gram-negative bacteria . . . .3096
7.9.1 HACEK-related species . . . .3096
7.9.2 Non-HACEK species . . . .3097
7.10 Blood culture– negative infective endocarditis . . . .3097
7.11 Fungi . . . .3097
7.12 Empirical therapy . . . .3097
7.13 Outpatient parenteral antibiotic therapy for infective endocarditis . . . .3098
8. Main complications of left-sided valve infective endocarditis and their management . . . .3099
8.1 Heart failure . . . .3099
8.1.1 Heart failure in infective endocarditis . . . .3099
8.1.2 Indications and timing of surgery in the presence of heart failure in infective endocarditis . . . .3100
8.2 Uncontrolled infection . . . .3100
8.2.1 Persisting infection . . . .3100
8.2.2 Perivalvular extension in infective endocarditis . . . .3100
8.2.3 Indications and timing of surgery in the presence of uncontrolled infection in infective endocarditis . . . .3101
8.2.3.1 Persistent infection . . . .3101
8.2.3.2 Signs of locally uncontrolled infection . . . .3101
8.2.3.3 Infection by microorganisms at low likelihood of being controlled by antimicrobial therapy . . . .3101
8.3 Prevention of systemic embolism . . . .3101
8.3.1 Embolic events in infective endocarditis . . . .3101
8.3.2 Predicting the risk of embolism . . . .3101
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
8.3.3 Indications and timing of surgery to prevent embolism
in infective endocarditis . . . .3101
9. Other complications of infective endocarditis . . . .3102
9.1 Neurological complications . . . .3102
9.2 Infectious aneurysms . . . .3103
9.3 Splenic complications . . . .3103
9.4 Myocarditis and pericarditis . . . .3104
9.5 Heart rhythm and conduction disturbances . . . .3104
9.6 Musculoskeletal manifestations . . . .3104
9.7 Acute renal failure . . . .3104
10. Surgical therapy: principles and methods . . . .3105
10.1 Operative risk assessment . . . .3105
10.2 Preoperative and perioperative management . . . .3105
10.2.1 Coronary angiography . . . .3105
10.2.2 Extracardiac infection . . . .3105
10.2.3 Intraoperative echocardiography . . . .3105
10.3 Surgical approach and techniques . . . .3105
10.4 Postoperative complications . . . .3106
11. Outcome after discharge: follow-up and long-term prognosis3106 11.1 Recurrences: relapses and reinfections . . . .3106
11.2 Short-term follow-up . . . .3107
11.3 Long-term prognosis . . . .3107
12. Management of specific situations . . . .3107
12.1 Prosthetic valve endocarditis . . . .3107
12.1.1 Definition and pathophysiology . . . .3107
12.1.2 Diagnosis . . . .3107
12.1.3 Prognosis and treatment . . . .3108
12.2 Infective endocarditis affecting cardiac implantable electronic devices . . . .3108
12.2.1 Introduction . . . .3108
12.2.2 Definitions of cardiac device infections . . . .3108
12.2.3 Pathophysiology . . . .3108 12.2.4 Risk factors . . . .3109 12.2.5 Microbiology . . . .3109 12.2.6 Diagnosis . . . .3109 12.2.7 Treatment . . . .3109 12.2.8 Antimicrobial therapy . . . .3109
12.2.9 Complete hardware removal (device and lead extraction) . . . .3109
12.2.10 Reimplantation . . . .3110
12.2.11 Prophylaxis . . . .3110
12.3 Infective endocarditis in the intensive care unit . . . .3111
12.3.1 Organisms . . . .3111
12.3.2 Diagnosis . . . .3111
12.3.3 Management . . . .3111
12.4 Right-sided infective endocarditis . . . .3111
12.4.1 Diagnosis and complications . . . .3112
12.4.2 Prognosis and treatment . . . .3112
12.4.2.1 Antimicrobial therapy . . . .3112
12.4.2.2 Surgery . . . .3112
12.5 Infective endocarditis in congenital heart disease . . . . .3113
12.6 Infective endocarditis during pregnancy . . . .3113
12.7 Antithrombotic therapy in infective endocarditis . . . . .3114
12.8 Non-bacterial thrombotic endocarditis and endocarditis associated with cancers . . . .3114
12.8.1 Non-bacterial thrombotic endocarditis . . . .3114
12.8.2 Infective endocarditis associated with cancer . . . .3115
13. To do and not to do messages from the guidelines . . . .3115
14. Appendix . . . .3116
15. References . . . .3117
Abbreviations and acronyms
3D three-dimensional
AIDS acquired immune deficiency syndrome b.i.d. bis in die (twice daily)
BCNIE blood culture-negative infective endocarditis CDRIE cardiac device-related infective endocarditis CHD congenital heart disease
CIED cardiac implantable electronic device CoNS coagulase-negative staphylococci CPG Committee for Practice Guidelines CRP C-reactive protein
CT computed tomography E. Enterococcus
ESC European Society of Cardiology ESR erythrocyte sedimentation rate EuroSCORE European System for Cardiac Operative
Risk Evaluation FDG fluorodeoxyglucose HF heart failure
HIV human immunodeficiency virus HLAR high-level aminoglycoside resistance i.m. intramuscular
i.v. intravenous
ICE International Collaboration on Endocarditis ICU intensive care unit
ID infectious disease IE infective endocarditis Ig immunoglobulin IVDA intravenous drug abuser
MIC minimum inhibitory concentration MR magnetic resonance
MRI magnetic resonance imaging
MRSA methicillin-resistant Staphylococcus aureus MSCT multislice computed tomography
MSSA methicillin-susceptible Staphylococcus aureus NBTE non-bacterial thrombotic endocarditis
NICE National Institute for Health and Care Excellence NVE native valve endocarditis
OPAT outpatient parenteral antibiotic therapy PBP penicillin binding protein
PCR polymerase chain reaction PET positron emission tomography PVE prosthetic valve endocarditis SOFA Sequential Organ Failure Assessment
SPECT single-photon emission computed tomography TOE transoesophageal echocardiography
TTE transthoracic echocardiography WBC white blood cell
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
1. Preamble
Guidelines summarize and evaluate all available evidence on a par-ticular issue at the time of the writing process, with the aim of assist-ing health professionals in selectassist-ing the best management strategies for an individual patient with a given condition, taking into account the impact on outcome, as well as the risk – benefit ratio of particu-lar diagnostic or therapeutic means. Guidelines and recommenda-tions should help health professionals to make decisions in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of Guidelines have been issued in recent years by the European Society of Cardiology (ESC) as well as by other soci-eties and organisations. Because of the impact on clinical practice, quality criteria for the development of guidelines have been estab-lished in order to make all decisions transparent to the user. The re-commendations for formulating and issuing ESC Guidelines can be found on the ESC website ( http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines/Guidelines-development/ Writing-ESC-Guidelines). ESC Guidelines represent the official pos-ition of the ESC on a given topic and are regularly updated.
Members of this Task Force were selected by the ESC to re-present professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management (including diagnosis, treatment, prevention and rehabilitation) of a given condition according to ESC Committee for Practice Guidelines (CPG) policy. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk – benefit ratio. Estimates of expected health outcomes for larger populations were included, where data exist. The level of evidence and the strength of the recommendation of particular
management options were weighed and graded according to prede-fined scales, as outlined in Tables1and2.
The experts of the writing and reviewing panels provided declara-tions of interest forms for all reladeclara-tionships that might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC website (http:// www.escardio.org/guidelines). Any changes in declarations of inter-est that arise during the writing period must be notified to the ESC and updated. The Task Force received its entire financial support from the ESC without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new Guidelines produced by task forces, expert groups or consensus pa-nels. The Committee is also responsible for the endorsement pro-cess of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revi-sions the Guidelines are approved by all the experts involved in the Task Force. The finalized document is approved by the CPG for publication in the European Heart Journal. The Guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC Guidelines covers not only integra-tion of the most recent research, but also the creaintegra-tion of educaintegra-tion- education-al tools and implementation programmes for the recommendations. To implement the guidelines, condensed pocket guidelines versions, summary slides, booklets with essential messages, summary cards for non-specialists, and an electronic version for digital applications (smartphones, etc.) are produced. These versions are abridged and thus, if needed, one should always refer to the full text version, which is freely available on the ESC website. The National Societies of the ESC are encouraged to endorse, translate and implement all ESC Guidelines. Implementation programmes are needed because it
Table 1 Classes of recommendations Classes of recommendations
Suggested wording to use
Class I Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, effective.
Is recommended/is indicated
Class II
divergence of opinion about the Conflicting evidence and/or a usefulness/efficacy of the given favour of usefulness/efficacy. Usefulness/efficacy is less well treatment or procedure.
Class IIa Weight of evidence/opinion is in Should be considered
Class IIb
established by evidence/opinion.
May be considered Class III Evidence or general agreement
that the given treatment or procedure is not useful/effective, and in some cases may be harmful.
Is not recommended
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
has been shown that the outcome of disease may be favourably in-fluenced by the thorough application of clinical recommendations. Surveys and registries are needed to verify that real-life daily prac-tice is in keeping with what is recommended in the guidelines, thus completing the loop between clinical research, writing of guidelines, disseminating them and implementing them into clinical practice.
Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnos-tic or therapeudiagnos-tic medical strategies. However, the ESC Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consult-ation with that patient and the patient’s caregiver where appropriate and/or necessary. It is also the health professional’s responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.
2. Justification/scope of the
problem
Infective endocarditis (IE) is a deadly disease.1,2Despite improve-ments in its management, IE remains associated with high mortality and severe complications. Until recently, guidelines on IE were mostly based on expert opinion because of the low incidence of the disease, the absence of randomized trials and the limited num-ber of meta-analyses.3–7
The 2009 ESC Guidelines on the prevention, diagnosis and treat-ment of IE8introduced several innovative concepts, including limita-tion of antibiotic prophylaxis to the highest-risk patients, a focus on healthcare-associated IE and identification of the optimal timing for surgery. However, several reasons justify the decision of the ESC to update the previous guidelines: the publication of new large series of IE, including the first randomized study regarding surgical therapy;9 important improvements in imaging procedures,10particularly in the field of nuclear imaging; and discrepancies between previous guide-lines.5–8In addition, the need for a collaborative approach involving primary care physicians, cardiologists, surgeons, microbiologists, infectious disease (ID) specialists and frequently other specialists— namely the ‘Endocarditis Team’—has been underlined recently11,12 and will be developed in these new guidelines.
The main objective of the current Task Force was to provide clear and simple recommendations, assisting healthcare providers in their clinical decision making. These recommendations were obtained by expert consensus after thorough review of the available literature. An evidence-based scoring system was used, based on a classifica-tion of the strength of recommendaclassifica-tions and the levels of evidence.
3. Prevention
3.1 Rationale
The principle of antibiotic prophylaxis for IE was developed on the basis of observational studies and animal models and aimed at pre-venting the attachment of bacteria onto the endocardium after tran-sient bacteraemia following invasive procedures. This concept led to the recommendation for antibiotic prophylaxis in a large number of patients with predisposing cardiac conditions undergoing a wide range of procedures.13
The restriction of indications for antibiotic prophylaxis was in-itiated in 2002 because of changes in pathophysiological concep-tions and risk – benefit analyses as follows:14
† Low-grade but repeated bacteraemia occurs more frequently dur-ing daily routine activities such as toothbrushdur-ing, flossdur-ing or chew-ing, and even more frequently in patients with poor dental health.15 The accountability of low-grade bacteraemia was demonstrated in an animal model.16The risk of IE may therefore be related more to cumulative low-grade bacteraemia during daily life rather than spor-adic high-grade bacteraemia after dental procedures.
† Most case –control studies did not report an association between invasive dental procedures and the occurrence of IE.17–19 † The estimated risk of IE following dental procedures is very low.
Antibiotic prophylaxis may therefore avoid only a small number of IE cases, as shown by estimations of 1 case of IE per 150 000 dental procedures with antibiotics and 1 per 46 000 for proce-dures unprotected by antibiotics.20
† Antibiotic administration carries a small risk of anaphylaxis, which may become significant in the event of widespread use. However, the lethal risk of anaphylaxis seems very low when using oral amoxicillin.21
† Widespread use of antibiotics may result in the emergence of resistant microorganisms.13
† The efficacy of antibiotic prophylaxis on bacteraemia and the oc-currence of IE has only been proven in animal models. The effect on bacteraemia in humans is controversial.15
† No prospective randomized controlled trial has investigated the efficacy of antibiotic prophylaxis on the occurrence of IE and it is unlikely that such a trial will be conducted given the number of subjects needed.22
These points have been progressively taken into account in most guidelines, including the 2009 ESC guidelines,5,8,23–26and led to the restriction of antibiotic prophylaxis to the highest-risk patients (patients with the highest incidence of IE and/or highest risk of adverse outcome from IE).
In 2008 the National Institute for Health and Care Excellence (NICE) guidelines went a step further and advised against any anti-biotic prophylaxis for dental and non-dental procedures whatever Table 2 Levels of evidence
Level of evidence A
Data derived from multiple randomized clinical trials or meta-analyses. Level of
evidence B
Data derived from a single randomized clinical trial or large non-randomized studies.
Level of evidence C
Consensus of opinion of the experts and/ or small studies, retrospective studies, registries.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
the patient’s risk.27The authors concluded there was an absence of benefit of antibiotic prophylaxis, which was also highly cost-ineffective. These conclusions have been challenged since estima-tions of the risks of IE are based on low levels of evidence due to multiple extrapolations.28,29
Four epidemiological studies have analysed the incidence of IE fol-lowing restricted indications for antibiotic prophylaxis. The analysis of 2000 – 2010 national hospital discharge codes in the UK did not show an increase in the incidence of streptococcal IE after the re-lease of NICE guidelines in 2008.30The restriction of antibiotic prophylaxis was seen in a 78% decrease in antibiotic prescriptions before dental care. However, residual prescriptions raised concerns regarding a persisting use of antibiotic prophylaxis. A survey performed in 2012 in the UK showed that the majority of cardiolo-gists and cardiac surgeons felt that antibiotic prophylaxis was necessary in patients with valve prosthesis or prior IE.31Recently an analysis of UK data collected from 2000 to 2013 showed a signifi-cant increase in the incidence of IE in both high-risk and lower-risk patients in the UK starting in 2008.32However, this temporal relation-ship should not be interpreted as a direct consequence of the NICE guidelines. These findings may be influenced by confounding factors, in particular changes in the number of patients at risk of hospitaliza-tions and healthcare-associated IE. Moreover, microbiological data were not available. Thus we cannot know whether that increase is due to the microbiological species covered by antibiotic prophylaxis. A repeated prospective 1-year population-based French survey did not show an increase in the incidence of IE, in particular strepto-coccal IE, between 1999 and 2008, whereas antibiotic prophylaxis had been restricted for native valve disease since 2002.33
Two studies from the USA did not find a negative impact of the abandonment of antibiotic prophylaxis in native valve disease in the 2007 American Heart Association guidelines.34,35A more recent analysis on an administrative database found an increase in the inci-dence of IE hospitalizations between 2000 and 2011, with no signifi-cant change after the change of American guidelines in 2007.36The increase in IE incidence was observed for all types of microorgan-isms, but was significant for streptococci after 2007.36It was not sta-ted whether this was due to oral streptococci and if intermediate-or high-risk patients were involved.
The present guidelines maintain the principle of antibiotic prophylaxis in high-risk patients for the following reasons: † The remaining uncertainties regarding estimations of the risk of
IE, which play an important role in the rationale of NICE guidelines.
† The worse prognosis of IE in high-risk patients, in particular those with prosthetic IE.
† The fact that high-risk patients account for a much smaller num-ber than patients at intermediate risk, thereby reducing potential harm due to adverse events of antibiotic prophylaxis.
3.2 Population at risk
Patients with the highest risk of IE can be placed in three categories (Table3):
(1) Patients with a prosthetic valve or with prosthetic material used for cardiac valve repair: these patients have a higher risk of IE, a
higher mortality from IE and more often develop complications of the disease than patients with native valves and an identical pathogen.37This also applies to transcatheter-implanted pros-theses and homografts.
(2) Patients with previous IE: they also have a greater risk of new IE, higher mortality and higher incidence of complications than pa-tients with a first episode of IE.38
(3) Patients with untreated cyanotic congenital heart disease (CHD) and those with CHD who have postoperative palliative shunts, conduits or other prostheses.39,40After surgical repair with no residual defects, the Task Force recommends prophy-laxis for the first 6 months after the procedure until endothelia-lisation of the prosthetic material has occurred.
Although American Heart Association/American College of Cardiology guidelines recommend prophylaxis in cardiac transplant recipients who develop cardiac valvulopathy, this is not supported by strong evidence5,25,41 and is not recommended by the ESC Task Force.
Antibiotic prophylaxis is not recommended for patients at intermediate risk of IE, i.e. any other form of native valve disease (including the most commonly identified conditions: bicuspid aortic valve, mitral valve prolapse and calcific aortic stenosis). Nevertheless, both intermediate- and high-risk patients should be advised of the importance of dental and cutaneous hygiene13 (Table4). These measures of general hygiene apply to patients and healthcare workers and should ideally be applied to the general population, as IE frequently occurs without known cardiac disease.
Table 3 Cardiac conditions at highest risk of infective endocarditis for which prophylaxis should be
considered when a high-risk procedure is performed
Recommendations Classa Levelb
Antibiotic prophylaxis should be considered for patients at highest risk for IE:
(1) Patients with any prosthetic valve, including a transcatheter valve, or those in whom any prosthetic material was used for cardiac valve repair.
(2) Patients with a previous episode of IE. (3) Patients with CHD:
(a) Any type of cyanotic CHD.
(b) Any type of CHD repaired with a prosthetic material, whether placed surgically or by percutaneous techniques, up to 6 months after the procedure or lifelong if residual shunt or valvular regurgitation remains.
IIa C
Antibiotic prophylaxis is not recommended in
other forms of valvular or CHD. III C
CHD ¼ congenital heart disease; IE ¼ infective endocarditis. a
Class of recommendation. b
Level of evidence. c
Reference(s) supporting recommendations.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
3.3 Situations and procedures at risk
3.3.1 Dental procedures
At-risk procedures involve manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa (including scal-ing and root canal procedures) (Table5).15,20The use of dental im-plants raises concerns with regard to potential risk due to foreign material at the interface between the buccal cavity and blood. Very few data are available.42The opinion of the Task Force is that there is no evidence to contraindicate implants in all patients at risk. The indication should be discussed on a case-by-case basis. The patient should be informed of the uncertainties and the need for close follow-up.
3.3.2 Other at-risk procedures
There is no compelling evidence that bacteraemia resulting from re-spiratory tract procedures, gastrointestinal or genitourinary proce-dures, including vaginal and caesarean delivery, or dermatological or musculoskeletal procedures causes IE (Table5).
3.4 Prophylaxis for dental procedures
Antibiotic prophylaxis should only be considered for patients at highest risk for endocarditis, as described in Table3, undergoing at-risk dental procedures listed in Table5, and is not recommended in other situations. The main targets for antibiotic prophylaxis in these patients are oral streptococci. Table6summarizes the main regimens of antibiotic prophylaxis recommended before dental procedures. Fluoroquinolones and glycopeptides are not recommended due to their unclear efficacy and the potential induction of resistance. Table 5 Recommendations for prophylaxis of
infective endocarditis in the highest-risk patients according to the type of at-risk procedure
Recommendations Classa Levelb
A. Dental procedures
† Antibiotic prophylaxis should only be considered for dental procedures requiring manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa
IIa C
† Antibiotic prophylaxis is not recommended for local anaesthetic injections in non-infected tissues, treatment of superficial caries, removal of sutures, dental X-rays, placement or adjustment of removable prosthodontic or orthodontic appliances or braces or following the shedding of deciduous teeth or trauma to the lips and oral mucosa
III C
Continued Table 4 Non-specific prevention measures to be followed in high-risk and intermediate-risk patients
These measures should ideally be applied to the general population and particularly reinforced in high-risk patients:
• Strict dental and cutaneous hygiene. Dental follow-up should be performed twice a year in high-risk patients and yearly in the others. • Disinfection of wounds.
• Eradication or decrease of chronic bacterial carriage: skin, urine. • Curative antibiotics for any focus of bacterial infection. • No self-medication with antibiotics.
• Strict infection control measures for any at-risk procedure. • Discourage piercing and tattooing.
• Limit the use of infusion catheters and invasive procedure when possible. Favour peripheral over central catheters, and systematic replacement of the peripheral catheter every 3–4 days. Strict adherence to care bundles for central and peripheral cannulae should be performed.
Table 5 Continued
Recommendations Classa Levelb
B. Respiratory tract proceduresc † Antibiotic prophylaxis is not recommended
for respiratory tract procedures, including bronchoscopy or laryngoscopy, or transnasal or endotracheal intubation
III C
C. Gastrointestinal or urogenital procedures or TOEc † Antibiotic prophylaxis is not recommended
for gastroscopy, colonoscopy, cystoscopy, vaginal or caesarean delivery or TOE
III C
D. Skin and soft tissue proceduresc † Antibiotic prophylaxis is not recommended
for any procedure III C
TOE ¼ transoesophageal echocardiography. a
Class of recommendation. b
Level of evidence. c
For management when infections are present, please refer to Section 3.5.3.
Table 6 Recommended prophylaxis for high-risk dental procedures in high-risk patients
Situation Antibiotic Single-dose 30–60 minutes before procedure Adults Children No allergy to penicillin or ampicillin Amoxicillin or ampicillina
2 g orally or i.v. 50 mg/kg orally or i.v. Allergy to penicillin or ampicillin Clindamycin 600 mg orally or i.v. 20 mg/kg orally or i.v. a
Alternatively, cephalexin 2 g i.v. for adults or 50 mg/kg i.v. for children, cefazolin or ceftriaxone 1 g i.v. for adults or 50 mg/kg i.v. for children.
Cephalosporins should not be used in patients with anaphylaxis, angio-oedema, or urticaria after intake of penicillin or ampicillin due to cross-sensitivity.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
Cephalosporins should not be used in patients with anaphylaxis, angio-oedema or urticaria after intake of penicillin or ampicillin due to cross-sensitivity.
3.5 Prophylaxis for non-dental
procedures
Systematic antibiotic prophylaxis is not recommended for non-dental procedures. Antibiotic therapy is only needed when invasive procedures are performed in the context of infection.
3.5.1 Respiratory tract procedures
Patients listed in Table3who undergo an invasive respiratory tract procedure to treat an established infection (i.e. drainage of an ab-scess) should receive an antibiotic regimen that contains an anti-staphylococcal drug.
3.5.2 Gastrointestinal or genitourinary procedures In the case of an established infection or if antibiotic therapy is in-dicated to prevent wound infection or sepsis associated with a gastrointestinal or genitourinary tract procedure in patients de-scribed in Table3, it is reasonable that the antibiotic regimen in-cludes an agent active against enterococci (i.e. ampicillin, amoxicillin or vancomycin; only in patients unable to tolerate beta-lactams). The use of intrauterine devices was regarded as contra-indicated, but this was based on low levels of evidence. Use of an intrauterine device is now considered acceptable, in particular when other contraceptive methods are not possible and in women at low risk of genital infections.43
3.5.3 Dermatological or musculoskeletal procedures For patients described in Table3undergoing surgical procedures involving infected skin (including oral abscesses), skin structure or musculoskeletal tissue, it is reasonable that the therapeutic regimen contains an agent active against staphylococci and beta-haemolytic streptococci.
3.5.4 Body piercing and tattooing
These growing societal trends are a cause for concern, particularly for individuals with CHD who are at increased susceptibility for the acquisition of IE. Case reports of IE after piercing and tattooing are increasing, particularly when piercing involves the tongue,44 al-though publication bias may over- or underestimate the problem. Currently no data are available on the incidence of IE after such pro-cedures and the efficacy of antibiotics for prevention. Education of patients at risk of IE is paramount. They should be informed about the hazards of piercing and tattooing and these procedures should be discouraged not only in high-risk patients, but also in those with native valve disease. If undertaken, procedures should be performed under strictly sterile conditions, though antibiotic prophylaxis is not recommended.
3.5.5 Cardiac or vascular interventions
In patients undergoing implantation of a prosthetic valve, any type of prosthetic graft or pacemakers, perioperative antibiotic prophylaxis
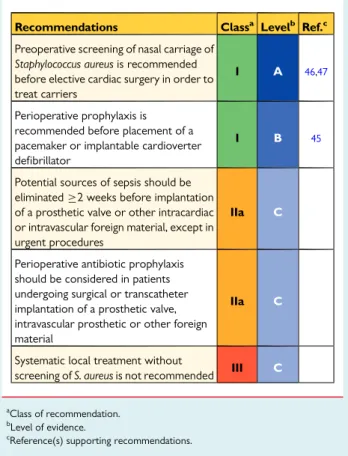
should be considered due to the increased risk and adverse outcome of an infection45–49(Table7). The most frequent microor-ganisms underlying early (1 year after surgery) prosthetic valve infections are coagulase-negative staphylococci (CoNS) and Staphylococcus aureus. Prophylaxis should be started immediately be-fore the procedure, repeated if the procedure is prolonged and ter-minated 48 h afterwards. A randomized trial has shown the efficacy of 1 g intravenous (i.v.) cefazolin on the prevention of local and sys-temic infections before pacemaker implantation.45Preoperative screening of nasal carriage of S. aureus is recommended before elect-ive cardiac surgery in order to treat carriers using local mupirocin and chlorhexidine.46,47Rapid identification techniques using gene amplification are useful to avoid delaying urgent surgery. Systematic local treatment without screening is not recommended. It is strongly recommended that potential sources of dental sepsis should be eliminated at least 2 weeks before implantation of a prosthetic valve or other intracardiac or intravascular foreign material, unless the lat-ter procedure is urgent.48
3.5.6 Healthcare-associated infective endocarditis Healthcare-associated IE represents up to 30% of all cases of IE and is characterized by an increasing incidence and a severe prognosis, thus presenting an important health problem.50,51Although routine antimicrobial prophylaxis administered before most invasive
Table 7 Recommendations for antibiotic
prophylaxis for the prevention of local and systemic infections before cardiac or vascular interventions
Recommendations Classa Levelb Ref.c
Preoperative screening of nasal carriage of Staphylococcus aureus is recommended before elective cardiac surgery in order to treat carriers
I A 46,47
Perioperative prophylaxis is recommended before placement of a pacemaker or implantable cardioverter defibrillator
I B 45
Potential sources of sepsis should be eliminated≥2 weeks before implantation of a prosthetic valve or other intracardiac or intravascular foreign material, except in urgent procedures
IIa C
Perioperative antibiotic prophylaxis should be considered in patients undergoing surgical or transcatheter implantation of a prosthetic valve, intravascular prosthetic or other foreign material
IIa C
Systematic local treatment without
screening of S. aureus is not recommended III C
a
Class of recommendation. b
Level of evidence. c
Reference(s) supporting recommendations.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
procedures is not recommended, aseptic measures during the inser-tion and manipulainser-tion of venous catheters and during any invasive procedures, including in outpatients, are mandatory to reduce the rate of this healthcare-associated IE.52
In summary, these guidelines propose continuing to limit antibiot-ic prophylaxis to patients at high risk of IE undergoing the highest-risk dental procedures. They highlight the importance of hygiene measures, in particular oral and cutaneous hygiene. Epi-demiological changes are marked by an increase in IE due to staphylococcus and of healthcare-associated IE, thereby high-lighting the importance of non-specific infection control mea-sures.51,53This should concern not only high-risk patients, but should also be part of routine care in all patients since IE occur-ring in patients without previously known heart disease now ac-counts for a substantial and increasing incidence. This means that although antibiotic prophylaxis should be restricted to the highest-risk patients, preventive measures should be maintained or extended to all patients with cardiac disease.
Although this section of the guidelines on IE prophylaxis is based on weak evidence, they have been strengthened recently by epidemiological surveys, most of which did not show an in-creased incidence of IE due to oral streptococci.33–35Their ap-plication by patients should follow a shared decision-making process. Future challenges are to gain a better understanding of the mechanisms associated with valve infection, the adaptation of prophylaxis to the ongoing epidemiological changes and the performance of specific prospective surveys on the incidence and characteristics of IE.
4. The ‘Endocarditis Team’
IE is a disease that needs a collaborative approach for the following reasons:
† First, IE is not a single disease, but rather may present with very different aspects depending on the first organ involved, the underlying cardiac disease (if any), the microorganism involved, the presence or absence of complications and the patient’s char-acteristics.8No single practitioner will be able to manage and treat a patient in whom the main clinical symptoms might be car-diac, rheumatological, infectious, neurological or other. † Second, a very high level of expertise is needed from practitioners
from several specialties, including cardiologists, cardiac surgeons, ID specialists, microbiologists, neurologists, neurosurgeons, ex-perts in CHD and others. Echocardiography is known to have a major importance in the diagnosis and management of IE. How-ever, other imaging techniques, including magnetic resonance im-aging (MRI), multislice computed tomography (MSCT), and nuclear imaging, have also been shown to be useful for diagnosis, follow-up and decision making in patients with IE.10Including all of these specialists in the team is becoming increasingly important. † Finally, about half of the patients with IE undergo surgery during
the hospital course.54Early discussion with the surgical team is important and is considered mandatory in all cases of compli-cated IE [i.e. endocarditis with heart failure (HF), abscess or em-bolic or neurological complications].
Therefore the presence of an Endocarditis Team is crucial. This multidisciplinary approach has already been shown to be useful
in the management of valve disease11(the ‘Heart Valve Clinic’), particularly in the selection of patients for transcatheter aortic valve implantation procedures (‘Heart Team’ approach).55In the field of IE, the team approach adopted in France, including standardized medical therapy, surgical indications following guideline recommen-dations and 1 year of close follow-up, has been shown to significant-ly reduce the 1-year mortality, from 18.5% to 8.2%.12Other authors have recently reported similar results.56Taking these reports to-gether, such a team approach has been recommended recently as class IB in the 2014 American Heart Association/American College of Cardiology guideline for the management of patients with valvular heart disease.25
The present Task Force on the management of IE of the ESC strongly supports the management of patients with IE in refer-ence centres by a specialized team (the ‘Endocarditis Team’). The main characteristics of the Endocarditis Team and the referring indications are summarized in Tables8and9.
Table 8 Characteristics of the ‘Endocarditis Team’
When to refer a patient with IE to an ‘Endocarditis Team’ in a reference centre
1. Patients with complicated IE (i.e. endocarditis with HF, abscess, or embolic or neurological complication or CHD), should be referred early and managed in a reference centre with immediate surgical facilities.
2. Patients with complicated IE can be initially managed in a non-reference centre, but with regular communication with the non-reference centre, consultations with the multidisciplinary ‘Endocarditis Team’, and, when needed, with external visit to the reference centre.
Characteristics of the reference centre
1. Immediate access to diagnostic procedures should be possible, including TTE, TOE, multislice CT, MRI, and nuclear imaging.
2. Immediate access to cardiac surgery should be possible during the early stage of the disease, particularly in case of complicated IE (HF, abscess, large vegetation, neurological, and embolic complications). 3. Several specialists should be present on site (the ‘Endocarditis Team’),
including at least cardiac surgeons, cardiologists, anaesthesiologists, ID specialists, microbiologists and, when available, specialists in valve diseases, CHD, pacemaker extraction, echocardiography and other cardiac imaging techniques, neurologists, and facilities for neurosurgery and interventional neuroradiology . Role of the ‘Endocarditis Team’
1. The ‘Endocarditis Team’ should have meetings on a regular basis in order to discuss cases, take surgical decisions, and define the type of follow-up.
2. The ‘Endocarditis Team’ chooses the type, duration, and mode of follow up of antibiotic therapy, according to a standardized protocol, following the current guidelines.
3. The ‘Endocarditis Team’ should participate in national or international registries, publicly report the mortality and morbidity of their centre, and be involved in a quality improvement programme, as well as in a patient education programme.
4. The follow-up should be organized on an outpatient visit basis at a frequency depending on the patient’s clinical status (ideally at 1, 3, 6, and 12 months after hospital discharge, since the majority of events occur during this period57).
CHD ¼ Congenital heart disease; CT ¼ computed tomography; HF ¼ heart failure; ID ¼ Infectious disease; IE ¼ infective endocarditis; MRI ¼ magnetic resonance imaging; TOE ¼ transoesophageal echocardiography; TTE ¼ transthoracic echocardiography.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
5. Diagnosis
5.1 Clinical features
The diverse nature and evolving epidemiological profile of IE ensure that it remains a diagnostic challenge. The clinical history of IE is highly variable according to the causative microorganism, the pres-ence or abspres-ence of pre-existing cardiac disease, the prespres-ence or ab-sence of prosthetic valves or cardiac devices and the mode of presentation. Thus IE should be suspected in a variety of very differ-ent clinical situations. It may presdiffer-ent as an acute, rapidly progressive infection, but also as a subacute or chronic disease with low-grade fever and non-specific symptoms that may mislead or confuse initial assessment. Patients may therefore present to a variety of specialists who may consider a range of alternative diagnoses, including chronic infection; rheumatological, neurological and autoimmune diseases; or malignancy. The early involvement of a cardiologist and an ID specialist to guide management is highly recommended.
Up to 90% of patients present with fever, often associated with sys-temic symptoms of chills, poor appetite and weight loss. Heart mur-murs are found in up to 85% of patients. Up to 25% of patients have embolic complications at the time of diagnosis. Therefore IE has to be suspected in any patient presenting with fever and embolic phenom-ena. Classic signs may still be seen in the developing world in subacute forms of IE, although peripheral stigmata of IE are increasingly uncom-mon elsewhere, as patients generally present at an early stage of the disease. However, vascular and immunological phenomena such as splinter haemorrhages, Roth spots and glomerulonephritis remain common. Emboli to the brain, lung or spleen occur in 30% of patients and are often the presenting feature.58In a febrile patient, diagnostic suspicion may be strengthened by laboratory signs of infection, such as elevated C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR), leucocytosis, anaemia and microscopic haematuria.
However, these signs lack specificity and have not been integrated into current diagnostic criteria. Atypical presentation is common in elderly or immunocompromised patients,59in whom fever is less common than in younger individuals. A high index of suspicion and low threshold for investigation are therefore essential in these and other high-risk groups, such as those with CHD or prosthetic valves, to exclude IE or avoid delays in diagnosis.
5.2 Laboratory findings
In addition to specialized microbiological and imaging investigations, a number of laboratory investigations and biomarkers have been evaluated in sepsis/sepsis syndromes and endocarditis. The large number of proposed potential biomarkers reflects the complex pathophysiology of the disease process, involving pro- and anti-inflammatory processes, humoral and cellular reactions and both circulatory and end-organ abnormalities.60However, owing to their poor positive predictive value for the diagnosis of sepsis and lack of specificity for endocarditis, these biomarkers have been excluded from being major diagnostic criteria and are only used to facilitate risk stratification.
Sepsis severity may be indicated by the demonstration of a number of laboratory investigations, including the degree of leucocytosis/leu-copoenia, the number of immature white cell forms, concentrations of CRP and procalcitonin, ESR and markers of end-organ dysfunction (lactataemia, elevated bilirubin, thrombocytopaenia and changes in serum creatinine concentration); however, none are diagnostic for IE.61Further, certain laboratory investigations are used in surgical scoring systems relevant to risk stratification in patients with IE, in-cluding bilirubin, creatinine and platelet count [Sequential Organ Fail-ure Assessment (SOFA) score] and creatinine clearance [European System for Cardiac Operative Risk Evaluation (EuroSCORE) II]. Final-ly, the pattern of increase in inflammatory mediators or immune complexes may support, but not prove, the diagnosis of IE, including the finding of hypocomplementaemia in the presence of elevated antineutrophil cytoplasmic antibody in endocarditis-associated vas-culitis or, where lead infection is suspected clinically, the laboratory finding of a normal procalcitonin and white cell count in the presence of significantly elevated CRP and/or ESR.62
5.3 Imaging techniques
Imaging, particularly echocardiography, plays a key role in both the diagnosis and management of IE. Echocardiography is also useful for the prognostic assessment of patients with IE, for its follow-up under therapy and during and after surgery.63Echocardiography is particularly useful for initial assessment of the embolic risk and in decision making in IE. Transoesophageal echocardiography (TOE) plays a major role both before and during surgery (intraoperative echocardiography). However, the evaluation of patients with IE is no longer limited to conventional echocardiography, but should include several other imaging techniques such as MSCT, MRI,18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) or other functional imaging modalities.10
5.3.1 Echocardiography
Echocardiography, either transthoracic echocardiography (TTE) or TOE, is the technique of choice for the diagnosis of IE, and plays a Table 9 Recommendations for referring patients to
the reference centre
Recommendations Classa Levelb Ref.c
Patients with complicated IE should be evaluated and managed at an early stage in a reference centre, with immediate surgical facilities and the presence of a multidisciplinary ‘Endocarditis Team’, including an ID specialist, a microbiologist, a cardiologist, imaging specialists, a cardiac surgeon and, if needed, a specialist in CHD
IIa B 12,56
For patients with uncomplicated IE managed in a non-reference centre, early and regular communication with the reference centre and, when needed, visits to the reference centre should be made
IIa B 12,56
CHD ¼ congenital heart disease; ID ¼ infectious disease; IE ¼ infective endocarditis. a Class of recommendation. b Level of evidence. c
Reference(s) supporting recommendations.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
key role in the management and monitoring of these patients.64,65 Echocardiography must be performed as soon as IE is suspected. TOE must be performed in case of negative TTE when there is a high index of suspicion for IE, particularly when TTE is of suboptimal quality. TOE should also be performed in patients with positive TTE to rule out local complications. The indications of echocardiograph-ic examination for diagnosis and follow-up of patients with sus-pected IE are summarized in Table10 and Figure1. In patients with S. aureus bacteraemia, echocardiography is justified in view of the frequency of IE in this setting, the virulence of this organism and its devastating effects once intracardiac infection is estab-lished.66,67In these patients, TTE or TOE should be considered ac-cording to individual patient risk factors and the mode of acquisition of S. aureus bacteraemia.66,67
Table 10 Role of echocardiography in infective endocarditis
Recommendations Classa Levelb Ref.c
A. Diagnosis
† TTE is recommended as the first-line imaging modality in suspected IE.
I B 64,65
† TOE is recommended in all patients with clinical suspicion of IE and a negative or non-diagnostic TTE.
I B 64,
68–71
† TOE is recommended in patients with clinical suspicion of IE, when a prosthetic heart valve or an intracardiac device is present.
I B 64,71
† Repeat TTE and /or TOE within 5 – 7 days is recommended in case of initially negative examination when clinical suspicion of IE remains high. I C † Echocardiography should be considered in Staphylococcus aureus bacteraemia. IIa B 66,67
† TOE should be considered in patients with suspected IE, even in cases with positive TTE, except in isolated right-sided native valve IE with good quality TTE examination and unequivocal echocardiographic findings.
IIa C
B. Follow-up under medical therapy † Repeat TTE and/or TOE are
recommended as soon as a new complication of IE is suspected (new murmur, embolism, persisting fever, HF, abscess, atrioventricular block).
I B 64,72
Continued
Table 10 Continued
Recommendations Classa Levelb Ref.c
† Repeat TTE and/or TOE should be considered during follow-up of uncomplicated IE, in order to detect new silent complications and monitor vegetation size. The timing and mode (TTE or TOE) of repeat examination depend on the initial findings, type of microorganism, and initial response to therapy.
IIa B 64,72
C. Intraoperative echocardiography † Intraoperative echocardiography is
recommended in all cases of IE requiring surgery.
I B 64,73
D. Following completion of therapy † TTE is recommended at completion
of antibiotic therapy for evaluation of cardiac and valve morphology and function.
I C
HF ¼ heart failure; IE ¼ infective endocarditis; TOE ¼ transoesophageal echocardiography; TTE ¼ transthoracic echocardiography.
a
Class of recommendation. b
Level of evidence. c
Reference(s) supporting recommendations.
Clinical suspicion of IE
TTE
Prosthetic valve Intracardiac device
If initial TOE is negative but high suspicion for IE remains, repeat TTE and/or TOE within 5–7 days
Non-diagnosis TTE TOEa Stop Low High Positive
TTE NegativeTTE
Clinical suspicion of IE
IE = infective endocarditis; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiography.
aTOE is not mandatory in isolated right-sided native valve IE with good quality TTE examination and unequivocal echocardiographic
Figure 1 Indications for echocardiography in suspected infect-ive endocarditis.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
Three echocardiographic findings are major criteria in the diag-nosis of IE: vegetation, abscess or pseudoaneurysm and new dehis-cence of a prosthetic valve8,64,65(see Table11for anatomical and echocardiographic definitions). Nowadays, the sensitivity for the diagnosis of vegetations in native and prosthetic valves is 70% and 50%, respectively, for TTE and 96% and 92%, respectively, for TOE.64,65Specificity has been reported to be around 90% for both TTE and TOE. Identification of vegetations may be diffi-cult in the presence of pre-existing valvular lesions (mitral valve prolapse, degenerative calcified lesions), prosthetic valves, small vegetations (, 2 – 3 mm), recent embolization and in non-vegetant IE. Diagnosis may be particularly challenging in IE affecting intracardiac devices, even with the use of TOE.
False diagnosis of IE may occur, and in some instances it may be difficult to differentiate vegetations from thrombi, Lambl’s excres-cences, cusp prolapse, chordal rupture, valve fibroelastoma, de-generative or myxomatous valve disease, strands, systemic lupus (Libman – Sacks) lesions, primary antiphospholipid syndrome, rheumatoid lesions or marantic vegetations.74Therefore the re-sults of the echocardiographic study must be interpreted with cau-tion, taking into account the patient’s clinical presentation and the likelihood of IE.
The sensitivity of TTE for the diagnosis of abscesses is about 50%, compared with 90% for TOE. Specificity higher than 90% has been reported for both TTE and TOE.64,65Small abscesses may be diffi-cult to identify, particularly in the earliest stage of the disease, in the postoperative period and in the presence of a prosthetic valve. IE must always be suspected in patients with new periprosthetic regur-gitation, even in the absence of other echocardiographic findings of IE.64
In cases with an initially negative examination, repeat TTE/TOE must be performed 5 – 7 days later if the clinical level of suspicion is still high, or even earlier in the case of S. aureus infection.75Other imaging techniques should also be used in this situation (see section 5.5). Finally, follow-up echocardiography to monitor complications and response to treatment is mandatory (Figure1).
Real-time three-dimensional (3D) TOE allows the analysis of 3D volumes of cardiac structures in any possible plane. A recent study has shown that conventional TOE underestimates vegetation size and that 3D TOE is a feasible technique for the analysis of vegetation morphology and size that may overcome the shortcomings of con-ventional TOE, leading to a better prediction of the embolic risk in IE.763D TOE is particularly useful in the assessment of perivalvular extension of the infection, prosthetic valve dehiscence and valve perforation.77Although in clinical practice 3D TOE is increasingly performed along with conventional TOE in many centres, at present 3D TOE should still be regarded as a supplement to standard echo-cardiography in most cases.
5.3.2 Multislice computed tomography
The potential risks of vegetation embolization and/or haemo-dynamic decompensation during coronary angiography (when in-dicated) have led to proposals to consider MSCT coronary angiography as an alternative technique for some patients with endocarditis.78
MSCT can be used to detect abscesses/pseudoaneurysms with a diagnostic accuracy similar to TOE, and is possibly superior in the provision of information regarding the extent and consequences of any perivalvular extension, including the anatomy of pseudoaneur-ysms, abscesses and fistulae.79In aortic IE, CT may additionally be useful to define the size, anatomy and calcification of the aortic valve, root and ascending aorta, which may be used to inform sur-gical planning. In pulmonary/right-sided endocarditis, CT may re-veal concomitant pulmonary disease, including abscesses and infarcts.
In the evaluation of prosthetic valve dysfunction, one recent study has suggested that MSCT may be equivalent or superior to echocardiography for the demonstration of prostheses-related vegetations, abscesses, pseudoaneurysms and dehiscence.80 How-ever, large comparative studies between the two techniques are missing, and echocardiography should always be performed first.
The higher sensitivity of MRI compared with CT for the detection of cerebral lesions is well known and has been confirmed in the con-text of endocarditis. However, in the critically ill patient, CT may be more feasible and practical and is an acceptable alternative when MRI is not available. MSCT angiography allows complete Table 11 Anatomical and echocardiographic
definitions
Surgery/necropsy Echocardiography Vegetation Infected mass attached to
an endocardial structure or on implanted intracardiac material.
Oscillating or non-oscillating intracardiac mass on valve or other endocardial structures, or on implanted intracardiac material. Abscess Perivalvular cavity
with necrosis and purulent material not communicating with the cardiovascular lumen. Thickened, non-homogeneous perivalvular area with echodense or echolucent appearance. Pseudoaneurysm Perivalvular cavity
communicating with the cardiovascular lumen.
Pulsatile perivalvular echo-free space, with colour-Doppler detected. Perforation Interruption of endocardial
tissue continuity.
Interruption of endocardial tissue continuity traversed by colour-Doppler Fistula Communication between
two neighbouring cavities through a perforation. Colour-Doppler communication between two neighbouring cavities through a perforation. Valve aneurysm Saccular outpouching of
valvular tissue. Saccular bulging of valvular tissue. Dehiscence of a prosthetic valve Dehiscence of the prosthesis. Paravalvular regurgitation by TTE/TOE, with or without rocking motion of the prosthesis.
TOE ¼ transoesophageal echocardiography; TTE ¼ transthoracic echocardiography.
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/
visualization of the intracranial vascular tree and carries a lower trast burden and risk of permanent neurological damage than con-ventional digital subtraction angiography, with a sensitivity of 90% and specificity of 86%.81Where subarachnoid and/or intraparench-ymal haemorrhage is detected, other vascular imaging (i.e. angiog-raphy) is required to diagnose or exclude a mycotic aneurysm if not detected on CT.
Contrast-enhanced MSCT has a high sensitivity and specificity for the diagnosis of splenic and other abscesses; however, the differ-entiation with infarction can be challenging. MSCT angiography pro-vides a rapid and comprehensive exploration of the systemic arterial bed. Detailed multiplanar and 3D contrast-enhanced angiographic reconstructions allow vascular mapping with identification and char-acterization of peripheral vascular complications of IE and their follow-up.82
5.3.3 Magnetic resonance imaging
Given its higher sensitivity than CT, MRI increases the likelihood of detecting cerebral consequences of IE. Different studies including systematic cerebral MRI during acute IE have consistently reported frequent lesions, in 60 – 80% of patients.83Regardless of neurological symptoms, most abnormalities are ischaemic lesions (in 50 – 80% of patients), with more frequent small ischaemic lesions than larger territorial infarcts.84Other lesions are found in ,10% of patients and are parenchymal or subarachnoidal haemorrhages, abscesses or mycotic aneurysms.83–86
Systematic cerebral MRI has an impact on the diagnosis of IE since it adds one minor Duke criterion87in patients who have cerebral lesions and no neurological symptoms. In one study, find-ings of cerebral MRI upgraded the diagnosis of IE in 25% of patients presenting initially with non-definite IE, thereby leading to earlier diagnosis.85
Cerebral microbleeds are detected only when using gradient echo T2* sequences and are found in 50 – 60% of patients.85 Micro-bleeds represent small areas of haemosiderin deposits and are considered as an indicator of small vessel disease. The lack of concordance between ischaemic lesions and microbleeds and the differences in their predictive factors suggest that microbleeds are not of embolic origin.86,88Therefore, although IE and the presence of microbleeds are strongly linked, microbleeds should not be considered as a minor criterion in the Duke classification.87
Cerebral MRI is, in the majority of cases, abnormal in IE patients with neurological symptoms.89It has a higher sensitivity than CT in the diagnosis of the culprit lesion, in particular with regards to stroke, transient ischaemic attack and encephalopathy. MRI may also detect additional cerebral lesions that are not related to clinical symptoms. Cerebral MRI has no impact on the diagnosis of IE in pa-tients with neurological symptoms, as they already have one minor Duke criterion, but MRI may impact the therapeutic strategy, par-ticularly the timing of surgery.89In patients without neurological symptoms, MRI shows cerebral lesions in at least half of the patients, most often ischaemic lesions.90Systematic abdominal MRI detects lesions in one of three patients evaluated, most often affecting the spleen.91Ischaemic lesions are most common, followed by ab-scesses and haemorrhagic lesions. Abdominal MRI findings have no incremental impact on the diagnosis of IE when taking into ac-count the findings of cerebral MRI.
To summarize, cerebral MRI allows for a better lesion character-ization in patients with IE and neurological symptoms, whereas its impact on IE diagnosis is marked in patients with non-definite IE and without neurological symptoms.
5.3.4 Nuclear imaging
With the introduction of hybrid equipment for both conventional nuclear medicine [e.g. single-photon emission CT (SPECT)/CT] and PET (i.e. PET/CT), nuclear molecular techniques are evolving as an important supplementary method for patients with sus-pected IE and diagnostic difficulties. SPECT/CT imaging relies on the use of autologous radiolabelled leucocytes (111In-oxine or
99m
Tc-hexamethylpropyleneamine oxime) that accumulate in a time-dependent fashion in late images versus earlier images,92 whereas PET/CT is generally performed using a single acquisition time point (generally at 1 h) after administration of18F-FDG, which is actively incorporated in vivo by activated leucocytes, monocyte-macrophages and CD4+T-lymphocytes accumulating at the sites of infection.
Several reports have shown promising results for radiolabelled white blood cell (WBC) SPECT/CT and18F-FDG PET/CT imaging in IE. The main added value of using these techniques is the reduc-tion in the rate of misdiagnosed IE, classified in the ‘Possible IE’ cat-egory using the Duke criteria, and the detection of peripheral embolic and metastatic infectious events.93Limitations to the use of18F-FDG PET/CT are represented by localization of septic emboli in the brain, due to the high physiological uptake of this tracer in the brain cortex, and to the fact that at this site, metastatic infections are generally ,5 mm, the spatial resolution threshold of current PET/ CT scanners.
Caution must be exercised when interpreting18F-FDG PET/CT results in patients who have recently undergone cardiac surgery, as a postoperative inflammatory response may result in non-specific
18
F-FDG uptake in the immediate postoperative period. Further-more, a number of pathological conditions can mimic the pattern of focally increased18F-FDG uptake that is typically observed in IE, such as active thrombi, soft atherosclerotic plaques, vasculitis, primary cardiac tumours, cardiac metastasis from a non-cardiac tu-mour, post-surgical inflammation and foreign body reactions.94
Radiolabelled WBC SPECT/CT is more specific for the detection of IE and infectious foci than18F-FDG PET/CT and should be pre-ferred in all situations that require enhanced specificity.95 Disadvan-tages of scintigraphy with radiolabelled WBC are the requirement of blood handling for radiopharmaceutical preparation, the duration of the procedure, which is more time consuming than PET/CT, and a slightly lower spatial resolution and photon detection efficiency compared with PET/CT.
An additional promising role of18F-FDG PET/CT may be seen in patients with established IE, in whom it could be employed to moni-tor response to antimicrobial treatment. However, sufficient data are not available at this time to make a general recommendation.
5.4 Microbiological diagnosis
5.4.1 Blood culture – positive infective endocarditis Positive blood cultures remain the cornerstone of diagnosis and pro-vide live bacteria for both identification and susceptibility testing. At
by guest on January 14, 2016
http://eurheartj.oxfordjournals.org/