HAL Id: hal-02273791
https://hal.archives-ouvertes.fr/hal-02273791
Submitted on 29 Aug 2019HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Novel Homoleptic Bis(dipyrrinato) Zinc(II) Complexes
with Long Lifetimes as Potential Photosensitisers for
Photodynamic Therapy
Johannes Karges, Uttara Basu, Olivier Blacque, Hui Chao, Gilles Gasser
To cite this version:
Johannes Karges, Uttara Basu, Olivier Blacque, Hui Chao, Gilles Gasser. Novel Homoleptic Bis(dipyrrinato) Zinc(II) Complexes with Long Lifetimes as Potential Photosensitisers for Photo-dynamic Therapy. Angewandte Chemie, Wiley-VCH Verlag, In press. �hal-02273791�
Novel Homoleptic Bis(dipyrrinato) Zinc(II) Complexes with Long Lifetimes as Potential Photosensitisers for Photodynamic Therapy
Johannes Karges,a Uttara Basu,a Olivier Blacque,b Hui Chao,c,* and Gilles Gassera,*
a Chimie ParisTech, PSL University, CNRS, Institute of Chemistry for Life and Health
Sciences, Laboratory for Inorganic Chemical Biology, 75005 Paris, France.
b Department of Chemistry, University of Zurich, Winterthurerstrasse 190, CH-8057, Zurich,
Switzerland.
c MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun
Yat-sen University, 510275 Guangzhou, People’s Republic of China.
*Email: ceschh@mail.sysu.edu.cn, Tel. +86 20 84110613, Email:
gilles.gasser@chimieparistech.psl.eu; Tel. +33 1 44 27 56 02; WWW: www.gassergroup.com.
Abstract:
The use of photodynamic therapy (PDT) to treat various types of cancer has received increasing attention over the last years. However, the clinically used photosensitisers (PSs) have some limitations that include poor aqueous solubility, hepatotoxicity, photobleaching, aggregation and slow clearance from the body, leading to photosensitivity. To circumvent these drawbacks, the design of new classes of PSs is of high interest. Herein, we present the first example of the use of bis(dipyrrinato)zinc(II) complexes with exceptionally long lifetimes as efficient PDT PSs. Using the heavy atom effect, we could promote the intersystem crossing (ISC) of these complexes to change the character of the excited state from singlet to a triplet state, enabling singlet oxygen (1O2) generation. To overcome the limitation of quenching effects in water as
well as to improve water solubility, the lead compound of this study 3 was encapsulated in a polymer matrix. While having no observed dark toxicity, it showed an impressive phototoxicity upon irradiation at 500 nm in various monolayer cancer cells as well as 3D multicellular tumour spheroids (MCTS).
Keywords:
Bioinorganic Chemistry, Medicinal Inorganic Chemistry, Metals in Medicine, Photodynamic Therapy, Photosensitizers.
Graphical Abstract:
Synopsis:
To circumvent limitations of current photodynamic therary photosensitizers, we propose the use of bis(dipyrrinato)zinc(II) complexes. These compounds were found to have no dark toxicity, while having an impressive phototoxicity upon irradiation in various monolayer cancer cells as well as 3D multicellular tumour spheroids (MCTS).
Photodynamic Therapy (PDT) is a minimally-invasive medical technique, which has received increasing attention in the recent years with applications in oncology, dermatology and ophthalmology. Photofrin® was the first clinically approved photosensitiser (PS). It is used to
treat various types of cancer (i.e., non-small lung, bladder, oesophageal, or brain cancer). The second generation of PSs including, for example, Temoporfin (Foscan®), Motexafin lutetium,
Palladium bacteriopheophorbide (Tookad® soluble), Purlytin®, Verteporfin (Visudyne®),
Talaporfin (Laserphyrin®) are either clinically approved or were/are currently undertaking
clinical trials. Despite several advanced research and preclinical studies using the first and second generation of PSs, the translational status of PDT still remains unsatisfactory. Ideal photosensitizers must be chemically pure, stable in biological fluids while being efficient reactive oxygen species (ROS) generators.[1]
PDT relies on the photoactivation of a preferably non-toxic PS in the presence of oxygen to create ROS. During this process, the PS is excited to a singlet state (1PS) from which it
undergoes intersystem crossing (ISC) to a longer-lived triplet state (3PS). In a type I mechanism,
an electron or proton is transferred from or to the excited 3PS to or from the biological
environment involving commonly •O
2-, •OOH or •OH radicals. In contrast, in a type II
mechanism, the energy of the excited 3PS is transferred to molecular oxygen (3O
2) to generate
singlet oxygen (1O
2). These species are highly reactive and can directly react with its biological
surroundings to trigger cell death. Porphyrins, chlorins and pthalocyanines form the basic structure of many PSs, which are not only difficult to synthesise and to purify but also suffer from other drawbacks, including poor aqueous solubility, aggregation, slow clearance from the body and hepatotoxicity.[2] It would be therefore of high interest to develop new PSs based on
a half-porphyrin unit. This design would retain the excited state properties of porphyrins while being synthetically less challenging. Moreover, to overcome the limitations of the current PSs, the use of metal complexes has emerged as an interesting alternative due to their attractive photo-photophysical properties (e.g., photostability, high 1O2 production), high water solubility
and chemical stability. In this field, most well-studied transition metal complexes are those of Ru(II)[3], Os(II)[4], Rh(III)[4b, 5] and Ir(III)[3l, 4c, 6]. Such complexes have shown some extremely
promising results with one of such having completed phase I clinical trial.[3c] However, the
metal ions employed in these complexes are not abundant and are often seen unfavourably by industrial partners due to potential toxicity. It is therefore essential to develop complexes based on cheaper, abundant, biologically-relevant metals that have similar or superior photophysical and pharmacological properties.
During the last decade, the use of compounds with a 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene core commonly called boron-dipyrromethene (BODIPY) have emerged as promising PDT agents. BODIPY complexes are well known to be excited to a singlet state (1PS) and to
relax from there in very high quantum yields causing a strong fluorescence. This allows them to be majorly known as fluorescence imaging probes.[7] However, this feature is undesirable for
PDT as the majority of the absorbed energy does not cross to the required triplet state (3PS). A
comprehensive review on the development of these dyes as potential PDT agents discusses in detail the strategies that have been adopted to attenuate their fluorescence and enhance their triplet excited state lifetime.[8] These compounds were found to be amenable to extensive
modification around the central core to feature many attractive properties including high extinction coefficients, resistance to photobleaching, high cellular uptake as well as low toxicity for PDT applications.[8-9] For use in PDT, the dipyrrin core structure can be modified by
utilising the spin-orbit coupling of heavy atoms, such as iodine, to promote the ISC.[8-9, 10]
Importantly, a recent study has shown that the position of substitution on the BODIPY core has a drastic effect on the ISC. For example, iodination at positions 3 and 5 increases the fluorescence process.[10c] To promote the ISC, the heavy atom is typically placed without the
disruption of the planarity and conjugation of the system.[8] Very importantly, the introduction
of heavy atoms also shifts the absorption of the PS to longer wavelengths, which can be used to achieve deeper tissue penetration making them potentially be applicable for treating deep-seated tumours or large tumours.[11] Worthy of note, the majority of investigated metal
complexes for PDT applications use blue or UV-A light, which limits the penetration inside the tissue.[12]
Apart from boron, the dipyrrin scaffold can coordinate to other metals including Ni(II), Zn(II), Cu(II), Fe(III), Rh(III) and Co(III). These compounds have been studied since nearly a century in view of material science for generation of suitable nanoarchitectures including coordination polymers, one-dimensional nanowires, two-dimensional nanosheets and metal – organic frameworks (MOFs).[13] As an unrepresented class of compounds, in the recent years, the study
of bis(dipyrrinato)zinc(II) complexes have gained interest in view of their luminescence properties.[13a, 14]
With this knowledge in mind, in this work, we have designed homoleptic bis(dipyrrinato)zinc(II) complexes as potential PDT agents. As the atom with the strongest heavy atom effect of the halogens, we attached iodine in position 2 and 6 to avoid interfering with the planarity of the conjugated system. To improve the photophysical properties, peripheral bulky aryl groups were introduced in position 8 of the dipyrrin core. As shown for BODIPY complexes as well as
bis(dipyrrinato)zinc(II) complexes, these groups are able to inhibit the internal rotation and highly increase their photophysical properties.[7a, 13a, 14a] Herein, we present the synthesis as well
as in-depth photophysical and biological evaluation of a series of homoleptic bis(dipyrrinato)zinc(II) complexes 1-4 (Figure 1), which were found to accumulate in the cytoplasm. As the excited state of the bis(dipyrrinato)zinc(II) complexes is able to be significantly quenched in a polar environment, the lead compound 3 was encapsulated in a polymer matrix to overcome this drawback. The complexes as well as the encapsulated version
3-NP were found to efficiently generate singlet oxygen upon light exposure causing cell death
in various monolayer cancer cell lines as well as 3D multicellular tumour spheroids (MCTS), while not showing any dark toxicity. To the best of our knowledge, these are the first examples of bis(dipyrrinato)zinc(II) complexes for potential applications in PDT.
Results and Discussion
The dipyrrin ligands were synthesised starting from 2, 4-dimethylmethylpyrrole with the corresponding aldehydes in the presence of catalytic amounts of trifluoracetic acid and then oxidised with tetrachloro-p-benzoquinone. Subsequently, the positions 2 and 6 were iodinated using a mixture of iodine and iodic acid. Finally, the complexes 1-4 were synthesised by complexation of the dipyrrin ligands with Zn(OAc)2.2H2O using Et3N as a base. All complexes
were analysed using 1H, 13C-NMR, HRMS and the purity of the compounds were verified by
elemental analysis. Additionally, the complexes 1-3 have been characterised using single crystal X-ray crystallography. Details on the synthesis and characterisation of the complexes can be found in the SI (Scheme S1, Table S1, Figure S1-S15).
Figure 1. Chemical structures of the Zn(II) complexes 1-4 investigated in this work.
The photophysical properties of the investigated compounds are presented in Table S2. As expected from previous literature, the complexes have a strong absorbance peak at 516 nm (Figure S16), which is sligthly red shifted in comparison to other reported homoleptic bis(dipyrrinato)zinc(II) complexes due to the heavy atom effect.[13a, 14a, 14b, 14d] The maximum
of the luminescence signal was measured between 535-550 nm (Figure S17) resulting in a Stokes shift of 18-34 nm, demonstrating an overlap between absorption and emission spectra. Compound 4 (1.1%) and especially compounds 2 and 3 (4.2-4.5%) were found to have high luminescence quantum yields in comparison to the model compound 1 (0.3%) due to the hindrance of the internal rotation of the aryl group in position 8. This effect has been documented for various BODIPY complexes and other bis(dipyrrinato)metal complexes.[7a, 13a, 14a] All complexes were found to have exceptionally long lifetimes (Figure S18-S21, Table S2)
in comparison to other bis(dipyrrinato)metal complexes in the range of 207-559 ns.[13a, 14] These
long lifetimes indicate that the complexes are majorly luminescent due to their longer lived triplet state, which is being formed due to the heavy atom effect. Importantly, the presence of air drastically decreased the lifetime, illustrating that the excited state can interact with a component of the air. The type of ROS generated was confirmed using electron spin resonance spectroscopy (ESR) with 2,2,6,6–tetramethylpiperidine (TEMP) as a singlet oxygen 1O
2
scavenger and 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a •OOH or •OH radical scavenger.
While no signal for the formation of a •OOH or •OH radical was detected, the formation of 1O 2
in MeOH and PBS was confirmed by the characteristic 1O
2,2,6,6-tetramethylpiperidinyloxyl (TEMPO) in the ESR spectrum (Figure S22-S25). Worthy of note, the majority of clinically used PSs act by the generation of 1O2.[15] The generation of 1O2 of the
investigated compounds has been quantified by two methods; i) directly by measuring the phosphorescence signal of 1O2 around 1270 nm upon irradiation at 505 nm and ii) indirectly by
capturing 1O2 with a reporter molecule and following its production by absorbance spectroscopy
upon irradiation at 510 and 540 nm.[16] Compounds 2-4 were found to have 1O2 quantum yields
(Table S3) between 43-57% in MeOH and between 2-5% in aqueous solution. Worthy of note, the generation of 1O2 in an aqueous environment is much lower as the excited state of the
bis(dipyrrinato) metal complexes is quenched due to the formation of a symmetry-breaking charge transfer state in a polar environment.[14c] In comparison, the 1O2 quantum yield of 1 was
drastically lower, which was expected due to its poorer luminescence properties.
The stability of the complexes in different solvents for up to 48 h in the dark and upon photo-exposure was studied as it is an important pharmacological parameter for biological applications. [17] The complexes were incubated for different times (0, 1, 2, 4, 8, 12, 24, 48 h)
in toluene, water and cell culture media (DMEM) and the corresponding absorbance spectra were recorded. No significant difference (Figure S26-S37) was observed, indicating the stability of the complexes under the experimental conditions. The stability upon irradiation was investigated by constant LED irradiation at 510 nm and monitoring their absorbance spectra. All complexes were found with minimal changes in their absorbance spectra (Figure S38-S41), demonstrating their photostability.
The lipophilicity/hydrophilicity was investigated by determination of their distribution coefficients between phosphate buffer saline (PBS) and octanol phases. All complexes 1-4 were found majorly in the organic phase with logP (Table S4) values between +1.3 - +2.0. This is expected as the complexes are neutral and bear large organic lipophilic aromatic systems. This study was followed by the assessment of the cellular uptake of complexes 1-4 by determining the amount of Zn after incubating them in human cervical carcinoma (HeLa) cells for different times (0.5, 1, 2, 4, 8 h) using inductively coupled plasma mass spectrometry (ICP-MS). Within 4 h, (Figure S42-S45) the maximum metal concentration was reached and from there on it increased asymptotically. Worthy of note, structurally related Zn(II) porphyrin derivatives have been shown to take a much longer incubation time to reach their uptake maximum.[18] As
expected, the comparison of the uptake between 1-4 shows a slightly better uptake from compound 4 (4 > 3 ~ 2 > 1, Figure S46) in agreement with the lipophilicity values. The uptake mechanism was then investigated by blocking different pathways by pre-incubation with various uptake inhibitors. Blocking the transport mechanisms with metabolic
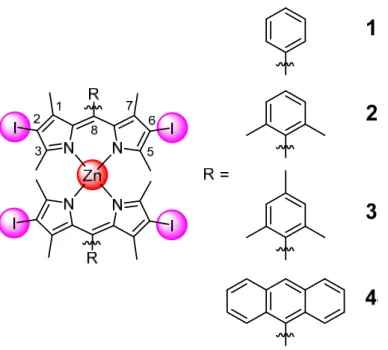
(2-deoxy-D-glucose and oligomycin), cationic transporter (tetraethylammonium chloride) and endocytotic (ammonium chloride or chloroquine) inhibitors had only a negligible effect (Figure S47-S50) on the internalisation of the complexes and therefore the involvement of these pathways was ruled out. The incubation at lower temperature (4 °C, Figure S47-S50) showed only a slight decrease in uptake caused by the slower diffusion processes at lower temperature. Overall, these results indicate an energy independent cellular uptake through passive diffusion for all the complexes. The cellular localisation was then examined using confocal laser scanning microscopy (Figure 2a) in HeLa cells. The compounds were incubated with the commercial dyes MitoTracker® Deep Red, LysoTracker Deep Red and Hoechst 33342 and their distribution
pattern compared. No significant congruency was detected, indicating that the compounds 1-4 do not majorly localise in these organelles. The cellular localisation in Hela cells was then quantified in the major cellular organelles (i.e., nucleus, mitochondria, lysosome, cytoplasm) using ICP-MS (Figure 2b) and subtraction of the natural occurring Zn. All complexes 1-4 were found to be present mostly in the cytoplasm with a small amount of unselective accumulation in the mitochondria, whereas no signal was detected inside the nucleus and lysosomes. Interestingly in previous studies, structurally related Zn(II) porphyrin complexes were also found to be localising unselectively within the cell with some interactions with membrane proteins.[18b, 19]
Figure 2. a) Confocal luminescence image of HeLa cells incubated with compounds 1-4 (25
μM, 2% DMSO, v%) for 6 h at 37°C in the dark. The investigated Zn complexes were detected using their luminescence properties (Zn, λex = 514 nm, λem = 530 - 610 nm). b) Sub-cellular
Nuc. = Nucleus) of 1-4 (25 μM, 2% DMSO, v%) in HeLa cells after 6 h incubation in the dark determined after organelle extraction and determination of the amount of Zn inside each organelle by ICP-MS.
To study the efficacy of the complexes 1-4 as PDT PSs, their cytotoxicity in the dark and upon irradiation at 510 nm (20 min, 5.0 J/cm2) and 540 nm (40 min, 9.5 J/cm2) towards non-cancerous
retinal pigment epithelium (RPE-1), human cervical carcinoma (HeLa), mouse colon carcinoma (CT-26) and human glioblastoma astrocytoma (U373) cells was investigated (Table S5-S6). All complexes were found to be non-toxic in the dark, which is an important requirement for a PDT agent (IC50 > 100 μM). Upon exposure to light at both 510 nm or 540 nm, all complexes were
able to generate 1O2 and trigger cell death in all investigated cell lines with IC50 values in the
low micromolar range. Complexes 2 and 3 showed a greater cytotoxicity without any significant selectivity between cancerous and non-cancerous cells with IC50 values in the low micromolar
range (4.3 – 13.4 μM). Complexes 2 and 3 were found to be superior in comparison to 1 and 4 due to their better photophysical properties including 1O
2 production (Table S3). Under the
same conditions, the effect of the complexes was compared with the anticancer drug cisplatin and the PS protoporphyrin IX (PpIX). The comparison shows that the toxicity of the complexes
2 and 3 is in a similar range as cisplatin whereas PpIX is slightly more toxic upon light exposure.
The phototoxic index (PI) is defined as the ratio between the IC50 value in the dark and the IC50
value upon light exposure. Complexes 2 and 3, being the most phototoxic complexes, were found to have PI values between 7.5 - 23.3 in various cell lines.
After evaluating the photocytotoxicity on cell monolayers, the cytotoxic effect of the complexes was investigated in 3D multicellular tumor spheroids (MCTS). MCTS are a widely used tissue model for the assessment of the delivery of drugs as it is a closer model to clinically treated tumours. It is important to mention that many investigated anticancer agents have failed during translation from cancer monolayer cells to in vivo models. This has been partially attributed to the compromised drug delivery through the penetration of extracellular barriers. It has been shown that small MCTS with diameters of 200 μm are able to mimic intercellular interactions. They can therefore be used to investigate drug delivery. Recent studies have shown that larger MCTS can also mimic pathological conditions found in solid tumours such as hypoxia at the tumour centre and its proliferation gradients.[20] Consequently, we have chosen to investigate
MCTS with diameters of 800 μm as tumor model. For this purpose, HeLa MCTS were incubated with complexes 1-4 and tetraphenylporphyrin (H2TPP) was used as a representative
for the clinically used porphyrin-based compound. The penetration of the compounds was analysed via Z-stack imaging microscopy. After 12 h of incubation (Figure S51-S54, Figure 3), all complexes showed a luminescence signal at every section depth corresponding with a complete penetration of the compounds in the MCTS whereas H2TPP only showed a weak
signal.
Figure 3. a) Images of 3D HeLa MCTS after incubation with 3 for 12 h (50 μM, 2% DMSO,
v%), from left to right: Brightfield, Zn complex luminescence, Overlay. b) Excited Z-axis
images scanning from the top to the bottom of an intact spheroid. c) 3D z-stack of an intact spheroid. The investigated Zn complexes were detected using their luminescence properties (λex
For further investigation of the PDT ability in MCTS, the photocytotoxicity was determined by measurement of the ATP concentration in the tumor spheroids. All complexes were found to be non-toxic in dark in the MCTS. (Table S7). On the contrary, the MCTS treated with compounds 2, 3 or 4 and exposed to light at 500 nm (16.7 min, 10.0 J/cm2) showed a significant
effect on the cell viability with IC50 values in the micromolar range (IC50 for 2: 26.5 ± 2.3 μM,
3: 23.1 ± 3.9 μM, 4: 89.6 ± 5.4 μM) while complex 1 and H2TPP did not show any significant toxicity (IC50 >100 μM) under identical experimental conditions. As expected from experiments
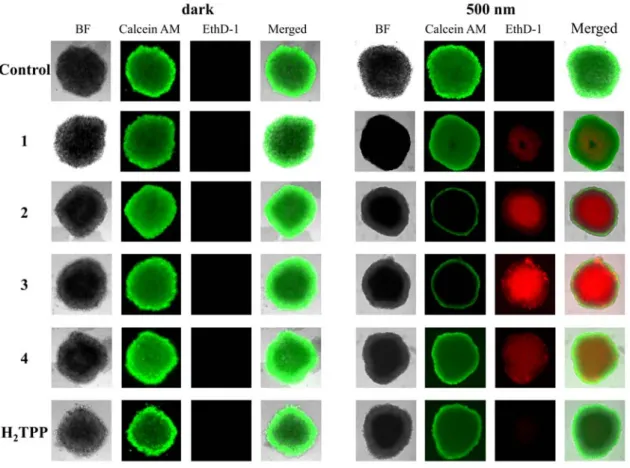
in cell monolayers, complexes 2 and 3 showed a higher phototoxicity than the other investigated compounds with PI values between 3.8 – 4.3. It was observed that a higher concentration of the compounds is needed to have the desired phototoxic effect in MCTS. This was previously observed for various other drug candidates.[21] We assume that this effect is due to the hypoxic
and reducing microenvironment of MCTS, which can strongly influence the efficiency of a PDT treatment as well as the limitation of the light to reach inside the MCTS and generate the required ROS for the treatment. These factors are ignored in a 2D monolayer cancer cell model. Therefore, experiments in the MCTS show a more realistic effect of our complexes in the 3D MCTS model. To further establish the effect of the complexes on cell viability, HeLa MCTS were treated with complexes 1- 4 (50 μM) and stained with calcein AM and EthD-1, 48 h after the light treatment. (Figure 4) This stain can distinguish between the living (green fluorescence signal) and the dead (red fluorescence signal) cells. All MCTS treated with the complexes 1-4 in the dark, complex 1 and H2TPP exposed to the light source showed a strong green
fluorescence signal while having no or a very weak red fluorescence signal indicating that the MCTS were still intact. The MCTS treated with 4 showed both red and green signals suggesting that the MCTS is partly intact. Contrary to this, the light treatment had a drastic effect on MCTS, which were treated with 2 and 3 where a strong red fluorescence signal was measured indicating that the MCTS were mostly eradicated.
Figure 4. Representative image of viability assay with HeLa MCTS kept in the dark and
exposed to light. MCTS were treated with compounds 1-4 (50 μM, 2% DMSO, v%) in the dark for 12 h. After this time, MCTS were exposed to a 500 nm (16.7 min, 10 J/cm2) irradiation.
After 2 days, the cell survival was assessed by measurement of the fluorescence of calcein (λex
= 495 nm, λem = 515 nm) or cell death by measurement of the fluorescence of EthD-1 (λex =
495 nm, λem = 635 nm).
As the excited state of the investigated bis(dipyrrinato)zinc(II) complexes is significantly quenched in a polar environment due to the formation of a symmetry-breaking charge transfer state in a polar environment[14c], the lead compound 3 with the best photophysical properties as
well as phototoxic effect was encapsulated with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] ammonium salt (DSPE-PEG2000-OCH3). Worthy of note, PEGylated phospholipids are approved by the US Food and
Drug administration (FDA). The generated particles 3-NP were prepared with a well-defined and average size of 119 nm (Figure S55). Importantly, the particles were found to be highly water soluble. Details on the preparation of these particles can be found in the SI. The drug release kinetics of the generated particles were studied upon dialysis against a PBS solution at physiological and low pH. As expected, the particles remained intact at pH 7.4 whereas the
majority was released within 4 h at pH 5.0 (Figure S56). As the absorption and emission spectra of 3-NP and 3 are identical, it indicates that the photophysical properties of 3 is not changed after encapsulation. As expected, the luminescence quantum yield of the particles 3-NP (3.9%) in water were found in the same range than those of the complex 3 in toluene (4.5%), overcoming the quenching effect of the excited state in a polar environment. The cellular localisation was then examined using confocal laser scanning microscopy (Figure 5a) as well as ICP-MS after extraction of the corresponding cell organelle (Figure 5b) in HeLa cells. Interestingly, whereas the pure complex (3) was mainly localising in the cytoplasm, the generated particles 3-NP selectively accumulated in the lysosomes. Worthy of note, a small amount of the particles were found in in the surrounding cytoplasm. Recent investigations on the encapsulation of large aromatic systems in the same polymer matrix have also reported that the generated particles were majorly localising in the lysosomes.[22] The uptake mechanism was
then examined by preincubation with various inhibitors (Figure S57). As the incubation at lower temperature (4°C) as well as with metabolic inhibitors significantly reduced the amount of the complex inside the cell, an energy depended mechanism is suggested. Additionally, the incubation with endocytotic inhibitors had a drastic effect. Overall, an energy depended endocytosis pathway is suggested for 3-NP.
Figure 5. a) Confocal luminescence image of HeLa cells incubated for 4 h with 3-NP (Zn, 10
μM, λex = 514 nm, λem = 530 - 610 nm) and LysoTracker Deep Red (LTR, 500 nM, λex = 633
nm, λem = 650 - 720 nm) at 37°C in the dark. b) Sub-cellular distribution (Cell = Whole cell,
Cyto. = Cytoplasm, Mito. = Mitochondria, Lys. = Lysosome, Nuc. = Nucleus) of 3-NP in HeLa cells after 4 h incubation in the dark determined after organelle extraction and determination of the amount of Zn inside each organelle by ICP-MS.
To study the PDT efficacy of 3-NP, its cytotoxicity in the dark and upon irradiation at 500 nm (16.7 min, 10.0 J/cm2) on HeLa cells was investigated. While showing no dark cytotoxicity
(IC50 > 100 μM), the particles were found to be highly toxic upon light exposure with an IC50
value of 1.2 ± 0.3 μM and therefore a PI of 83.3, which is much higher than for 3 itself. This clearly highligths the success of this encapsulation. In addition, the generated particles were tested on HeLa MCTS as a 3D model. The luminescence signal of 3-NP was measurable at every section depth corresponding with a complete penetration of the compounds in the MCTS (Figure S58). Cytotoxicity studies of 3-NP indicate no observed dark toxicity (IC50 > 100 μM)
while being highly phototoxic with an IC50 value of 5.3 ± 1.2 μM and having therefore a PI of
18.9. This once again demonstrate the interest of the encapsulation since the complex alone 3 had an IC50 value of 23.1 ± 3.9 μM while H2TPP as a representative of the porphyrin-based
compounds sowed no observed effect (IC50 > 100 μM) under the same conditions.
Conclusion
In summary, we have designed and synthesised homoleptic iodinated bis(dipyrrinato)zinc(II) complexes with remarkably long excited state lifetimes as potential PDT PSs. The iodine atoms can promote spin-orbit coupling thus enabling an ISC process. ESR spectroscopy confirmed that the excited state of the complexes 3PS is able to interact with molecular oxygen to generate
singlet oxygen upon light exposure at clinically relevant longer wavelengths. The complexes were found to efficiently enter cancer cells within 4 h through passive diffusion where they accumulate in the cytoplasm. While showing no dark toxicity, they were able to trigger cell death upon irradiation in several cancer cell lines at a low micromolar concentration. Additionally, they were also found to be active in 3D tumour model HeLa MCTS. As the excited state of the complexes is quenched in an aqueous environment, the lead compound 3 was encapsulated in a polymer matrix. The generated nanoparticles was found to not only have an improved water solubility and photophysical properties but also to accumulate selectively in lysosomes, contrary to the complex itself which accumulated in the cytoplasm. Upon light exposure, the particles caused cell death at very low micromolar concentrations in monolayer cancer cells as well as 3D tumour model HeLa MCTS. We strongly believe that these compounds as well as their corresponding particles have a great potential for the development of a new class of PDT PSs.
Acknowledgements
We thank Dr. Philippe Goldner for access to state-of-the-art laser apparatus. This work was financially supported by an ERC Consolidator Grant PhotoMedMet to G.G. (GA 681679), has received support under the program “Investissements d’ Avenir” launched by the French Government and implemented by the ANR with the reference ANR-10-IDEX-0001-02 PSL
(G.G.), the National Science Foundation of China (Nos. 21525105 and 21778079 for H.C.) and the 973 Program (No. 2015CB856301 for H.C.).
References
[1] a) D. E. Dolmans, D. Fukumura, R. K. Jain, Nat. Rev. Cancer 2003, 3, 380-387; b) A.
E. O’Connor, W. M. Gallagher, A. T. Byrne, Photochem. Photobiol. 2009, 85, 1053-1074; c) R. Bonnett, Chem. Soc. Rev. 1995, 24, 19-33; d) S. Bonnet, Dalton Trans. 2018,
47, 10330-10343.
[2] a) K. Plaetzer, B. Krammer, J. Berlanda, F. Berr, T. Kiesslich, Lasers Med. Sci. 2009,
24, 259-268; b) T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M.
Korbelik, J. Moan, Q. Peng, JNCI: Journal of the National Cancer Institute 1998, 90, 889-905; c) B. W. Henderson, T. J. Dougherty, Photochem. Photobiol. 1992, 55, 145-157; d) P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, CA Cancer J. Clin. 2011, 61, 250-281; e) S. Callaghan, M. O. Senge, Photochemical & Photobiological Sciences 2018,
17, 1490-1514.
[3] a) F. Heinemann, J. Karges, G. Gasser, Acc. Chem. Res. 2017, 50, 2727-2736; b) M.
Jakubaszek, B. Goud, S. Ferrari, G. Gasser, Chem. Commun. 2018, 54, 13040-13059; c) S. Monro, K. L. Colón, H. Yin, J. Roque III, P. Konda, S. Gujar, R. P. Thummel, L. Lilge, C. G. Cameron, S. A. McFarland, Chem. Rev. 2019, 119, 797-828; d) F. E. Poynton, S. A. Bright, S. Blasco, D. C. Williams, J. M. Kelly, T. Gunnlaugsson, Chem.
Soc. Rev. 2017, 46, 7706-7756; e) J. Shum, P. K.-K. Leung, K. K.-W. Lo, Inorg. Chem.
2019, 58, 2231-2247; f) H. Huang, B. Yu, P. Zhang, J. Huang, Y. Chen, G. Gasser, L.
Ji, H. Chao, Angew. Chem. Int. Ed. 2015, 54, 14049-14052; g) C. Mari, V. Pierroz, R. Rubbiani, M. Patra, J. Hess, B. Spingler, L. Oehninger, J. Schur, I. Ott, L. Salassa,
Chem. Eur. J. 2014, 20, 14421-14436; h) C. Mari, V. Pierroz, A. Leonidova, S. Ferrari,
G. Gasser, Eur. J. Inorg. Chem. 2015, 2015, 3879-3891; i) M. Jakubaszek, J. Rossier, J. Karges, J. Delasoie, B. Goud, G. Gasser, F. Zobi, Helv. Chim. Acta 2019, 0; j) J. Karges, F. Heinemann, F. Maschietto, M. Patra, O. Blacque, I. Ciofini, B. Spingler, G. Gasser, Bioorg. Med. Chem. 2019, 27, 2666-2675; k) R. Lincoln, L. Kohler, S. Monro, H. Yin, M. Stephenson, R. Zong, A. Chouai, C. Dorsey, R. Hennigar, R. P. Thummel, S. A. McFarland, J. Am. Chem. Soc. 2013, 135, 17161-17175; l) K. Qiu, Y. Chen, T. W.
Rees, L. Ji, H. Chao, Coord. Chem. Rev. 2019, 378, 66-86; m) E. Wachter, D. K. Heidary, B. S. Howerton, S. Parkin, E. C. Glazer, Chem. Commun. 2012, 48, 9649-9651; n) B. S. Howerton, D. K. Heidary, E. C. Glazer, J. Am. Chem. Soc. 2012, 134, 8324-8327.
[4] a) Y. Sun, L. E. Joyce, N. M. Dickson, C. Turro, Chem. Commun. 2010, 46, 6759-6761;
b) A. A. Holder, D. F. Zigler, M. T. Tarrago-Trani, B. Storrie, K. J. Brewer, Inorg.
Chem. 2007, 46, 4760-4762; c) L. K. McKenzie, H. E. Bryant, J. A. Weinstein, Coord. Chem. Rev. 2019, 379, 2-29.
[5] a) S. Swavey, K. J. Brewer, Inorg. Chem. 2002, 41, 6196-6198; b) W. Su, Z. Luo, S.
Dong, X. Chen, J.-a. Xiao, B. Peng, P. Li, Photodiagnosis Photodyn. Ther. 2019; c) J. D. Knoll, C. Turro, Coord. Chem. Rev. 2015, 282-283, 110-126; d) A. M. Angeles-Boza, P. M. Bradley, P. K. L. Fu, S. E. Wicke, J. Bacsa, K. R. Dunbar, C. Turro, Inorg.
Chem. 2004, 43, 8510-8519.
[6] a) A. Zamora, G. Vigueras, V. Rodríguez, M. D. Santana, J. Ruiz, Coord. Chem. Rev.
2018, 360, 34-76; b) L. K. McKenzie, I. V. Sazanovich, E. Baggaley, M. Bonneau, V.
Guerchais, J. A. Williams, J. A. Weinstein, H. E. Bryant, Chem. Eur. J. 2017, 23, 234-238; c) H. Huang, S. Banerjee, P. J. Sadler, ChemBioChem 2018, 19, 1574-1589; d) V. Novohradsky, A. Rovira, C. Hally, A. Galindo, G. Vigueras, A. Gandioso, M. Svitelova, R. Bresolí-Obach, H. Kostrhunova, L. Markova, J. Kasparkova, S. Nonell, J. Ruiz, V. Brabec, V. Marchán, Angew. Chem. Int. Ed. 2019, 58, 6311-6315.
[7] a) A. Loudet, K. Burgess, Chem. Rev. 2007, 107, 4891-4932; b) G. Ulrich, R. Ziessel, A. Harriman, Angew. Chem. Int. Ed. 2008, 47, 1184-1201; c) N. Boens, V. Leen, W. Dehaen, Chem. Soc. Rev. 2012, 41, 1130-1172; d) J. Karolin, L. B.-A. Johansson, L. Strandberg, T. Ny, J. Am. Chem. Soc. 1994, 116, 7801-7806.
[8] A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung, K. Burgess, Chem. Soc.
Rev. 2013, 42, 77-88.
[9] a) S. O. McDonnell, M. J. Hall, L. T. Allen, A. Byrne, W. M. Gallagher, D. F. O'Shea,
J. Am. Chem. Soc. 2005, 127, 16360-16361; b) T. Yogo, Y. Urano, Y. Ishitsuka, F.
Maniwa, T. Nagano, J. Am. Chem. Soc. 2005, 127, 12162-12163; c) S. H. Lim, C. Thivierge, P. Nowak-Sliwinska, J. Han, H. van den Bergh, G. Wagnières, K. Burgess, H. B. Lee, J. Med. Chem. 2010, 53, 2865-2874; d) S. Atilgan, Z. Ekmekci, A. L. Dogan, D. Guc, E. U. Akkaya, Chem. Commun. 2006, 4398-4400; e) W. M. Gallagher, L. T. Allen, C. O'Shea, T. Kenna, M. Hall, A. Gorman, J. Killoran, D. F. O'Shea, Br. J. Cancer
Kilic, Z. Kostereli, L. T. Yildirim, A. L. Dogan, D. Guc, E. U. Akkaya, Angew. Chem.
Int. Ed. 2011, 50, 11937-11941; g) L. Huang, X. Yu, W. Wu, J. Zhao, Org. Lett. 2012, 14, 2594-2597; h) M. A. Filatov, S. Karuthedath, P. M. Polestshuk, S. Callaghan, K. J.
Flanagan, M. Telitchko, T. Wiesner, F. Laquai, M. O. Senge, PCCP 2018, 20, 8016-8031; i) S. Callaghan, M. A. Filatov, H. Savoie, R. W. Boyle, M. O. Senge, Photochem.
Photobiol. Sci. 2019, 18, 495-504.
[10] a) A. M. Potocny, J. J. Teesdale, A. Marangoz, G. P. A. Yap, J. Rosenthal, Inorg. Chem.
2019, 58, 5042-5050; b) T. N. Singh-Rachford, A. Haefele, R. Ziessel, F. N. Castellano,
J. Am. Chem. Soc. 2008, 130, 16164-16165; c) M. J. Ortiz, A. R. Agarrabeitia, G.
Duran-Sampedro, J. B. Prieto, T. A. Lopez, W. A. Massad, H. A. Montejano, N. A. García, I. L. Arbeloa, Tetrahedron 2012, 68, 1153-1162; d) W. Wu, H. Guo, W. Wu, S. Ji, J. Zhao,
J. Org. Chem. 2011, 76, 7056-7064; e) Y. Chen, J. Zhao, L. Xie, H. Guo, Q. Li, RSC Advances 2012, 2, 3942-3953; f) H. He, P.-C. Lo, S.-L. Yeung, W.-P. Fong, D. K. Ng, Chem. Commun. 2011, 47, 4748-4750; g) A. Gorman, J. Killoran, C. O'Shea, T. Kenna,
W. M. Gallagher, D. F. O'Shea, J. Am. Chem. Soc. 2004, 126, 10619-10631; h) J. Killoran, L. Allen, J. F. Gallagher, W. M. Gallagher, F. Donal, Chem. Commun. 2002, 1862-1863.
[11] a) B. C. Wilson, W. P. Jeeves, D. M. Lowe, Photochem. Photobiol. 1985, 42, 153-162; b) K. Ogawa, Y. Kobuke, Anti-Cancer Agents Med. Chem. 2008, 8, 269-279; c) S. Bonnet, Comment Inorg. Chem. 2015, 35, 179-213.
[12] a) H. Huang, S. Banerjee, P. J. Sadler, ChemBioChem 2018, 19, 1574-1589; b) S. Chakrabortty, B. K. Agrawalla, A. Stumper, N. M. Vegi, S. Fischer, C. Reichardt, M. Kögler, B. Dietzek, M. Feuring-Buske, C. Buske, S. Rau, T. Weil, J. Am. Chem. Soc.
2017, 139, 2512-2519; c) R. E. Doherty, I. V. Sazanovich, L. K. McKenzie, A. S.
Stasheuski, R. Coyle, E. Baggaley, S. Bottomley, J. A. Weinstein, H. E. Bryant, Sci.
Rep. 2016, 6, 22668.
[13] a) R. Sakamoto, T. Iwashima, M. Tsuchiya, R. Toyoda, R. Matsuoka, J. F. Kögel, S. Kusaka, K. Hoshiko, T. Yagi, T. Nagayama, J. Mate. Chem. A 2015, 3, 15357-15371; b) S. A. Baudron, Dalton Trans. 2013, 42, 7498-7509; c) Y. Ding, Y. Tang, W. Zhu, Y. Xie, Chem. Soc. Rev. 2015, 44, 1101-1112; d) T. E. Wood, A. Thompson, Chem. Rev.
2007, 107, 1831-1861.
[14] a) I. V. Sazanovich, C. Kirmaier, E. Hindin, L. Yu, D. F. Bocian, J. S. Lindsey, D. Holten, J. Am. Chem. Soc. 2004, 126, 2664-2665; b) S. Lee, C.-H. Seok, Y. Park, A. Lee, D. H. Jung, S.-H. Choi, J. Park, Mol. Cryst. Liq. Cryst. 2010, 531, 365-372; c) C.
Trinh, K. Kirlikovali, S. Das, M. E. Ener, H. B. Gray, P. Djurovich, S. E. Bradforth, M. E. Thompson, J. Phys. Chem. C 2014, 118, 21834-21845; d) S. Kusaka, R. Sakamoto, Y. Kitagawa, M. Okumura, H. Nishihara, Chem. Asian J. 2012, 7, 907-910; e) R. Sakamoto, S. Kusaka, Y. Kitagawa, M.-a. Kishida, M. Hayashi, Y. Takara, M. Tsuchiya, J. Kakinuma, T. Takeda, K. Hirata, Dalton Trans. 2012, 41, 14035-14037. [15] M. C. DeRosa, R. J. Crutchley, Coord. Chem. Rev. 2002, 233-234, 351-371.
[16] a) J. Karges, P. Goldner, G. Gasser, Inorganics 2019, 7, 4; b) Y. Ellahioui, M. Patra, C. Mari, R. Kaabi, J. Karges, G. Gasser, S. Gómez-Ruiz, Dalton Trans. 2019, 48, 5940-5951.
[17] a) M. Patra, T. Joshi, V. Pierroz, K. Ingram, M. Kaiser, S. Ferrari, B. Spingler, J. Keiser, G. Gasser, Chem. Eur. J. 2013, 19, 14768-14772; b) U. Basu, J. Karges, F. Chotard, C. Balan, P. Le Gendre, G. Gasser, E. Bodio, R. Malacea Kabbara, Polyhedron 2019, in press, doi.org/10.1016/j.poly.2019.1002.1041; c) A. K. Renfrew, J. Karges, R. Scopelliti, F. D. Bobbink, P. Nowak-Sliwinska, G. Gasser, P. Dyson, ChemBioChem
2019, accepted, https://doi.org/10.1002/cbic.201900236.
[18] a) H. Kolarova, J. Macecek, P. Nevrelova, M. Huf, M. Tomecka, R. Bajgar, J. Mosinger, M. Strnad, Toxicol. In Vitro 2005, 19, 971-974; b) J. M. Dąbrowski, B. Pucelik, M. M. Pereira, L. G. Arnaut, G. Stochel, J. Coord. Chem. 2015, 68, 3116-3134.
[19] C. Pavani, A. F. Uchoa, C. S. Oliveira, Y. Iamamoto, M. S. Baptista, Photochem.
Photobiol. Sci. 2009, 8, 233-240.
[20] a) A. Pluen, Y. Boucher, S. Ramanujan, T. D. McKee, T. Gohongi, E. di Tomaso, E. B. Brown, Y. Izumi, R. B. Campbell, D. A. Berk, R. K. Jain, Proc. Natl. Acad. Sci. 2001,
98, 4628-4633; b) P. A. Netti, D. A. Berk, M. A. Swartz, A. J. Grodzinsky, R. K. Jain, Cancer Res. 2000, 60, 2497-2503; c) J. Friedrich, C. Seidel, R. Ebner, L. A.
Kunz-Schughart, Nat. Protoc. 2009, 4, 309; d) T. T. Goodman, C. P. Ng, S. H. Pun,
Bioconjugate Chem. 2008, 19, 1951-1959.
[21] a) J. Liu, Y. Chen, G. Li, P. Zhang, C. Jin, L. Zeng, L. Ji, H. Chao, Biomaterials 2015,
56, 140-153; b) R. R. Allison, G. H. Downie, R. Cuenca, X.-H. Hu, C. J. Childs, C. H.
Sibata, Photodiagnosis Photodyn. Ther. 2004, 1, 27-42.
[22] Q. Zang, J. Yu, W. Yu, J. Qian, R. Hu, B. Z. Tang, Chem. sci. 2018, 9, 5165-5171.