Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018), w w w . e l s e v i e r . e s / b s e c v
Mullite
fabrication
from
natural
kaolin
and
aluminium
slag
Fouzia
Chargui
a,b,
Mohamed
Hamidouche
a,b,∗,
Hocine
Belhouchet
c,d,
Yves
Jorand
e,
Rachida
Doufnoune
a,f,
Gilbert
Fantozzi
eaEmergentMaterialsResearchUnit,Setif1University,19000Setif,Algeria
bOpticalandPrecisionMechanicalInstitute,Setif1University,19000Setif,Algeria
cNon-metallicMaterialsLaboratory,OpticalandPrecisionMechanicalInstitute,Setif1University,19000Setif,Algeria
dPhysicalDepartment,FacultyofScience,MohamedBoudiafM’silaUniversity,28000M’Sila,Algeria
eLyonUniversity,INSA-Lyon,MATEISLaboratoryCNRS-UMR5510,69621Villeurbanne,France
fProcessEngineeringDepartment,FacultyofTechnology,Setif1University,19000Setif,Algeria
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received4August2017 Accepted10January2018 Availableonlinexxx Keywords: Mullite Kaolin Aluminumslag Phasetransformationa
b
s
t
r
a
c
t
The structuraltransformationsofkaolin–aluminiumslagmixturesduring heatingwere studied using differential thermal analysis (DTA), thermal gravimetric analysis (TGA), Fouriertransforminfrared(FTIR)spectroscopy,X-raydiffractionanalysis(XRD)andscanning electronmicroscopy(SEM).Theamountofformedmulliteincreaseswiththefiring temper-ature.At1500◦C,themullitizationofthemixtureisalmostcomplete.Themorphologyof theformedmulliteisbimodal(primaryandsecondaryphases).Theprimarymullite,formed fromprocessingofkaolinbythegradualcollapseofmetakaolinfrom990◦C,hasashapeof elongatedcrystals.Theotherhand,thesecondarymulliteformedbysolution-precipitation fromtheglassphaseinthepresenceofaluminaparticleshasashapeofaciculargrains.
©2018SECV.PublishedbyElsevierEspa ˜na,S.L.U.Thisisanopenaccessarticleunderthe CCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Producción
de
mullita
a
partir
de
caolín
natural
y
escoria
de
aluminio
Palabrasclave: Mullita Caolín Escoriadealuminio Transformacióndefase
r
e
s
u
m
e
n
Seestudiaron lastransformacionesestructuralesdelasmezclasdecaolín yescoria de aluminioduranteelcalentamientomedianteanálisistérmicodiferencial(ATD),análisis ter-mogravimétrico(ATG),espectroscopíainfrarrojaportransformadadeFourier(EITF),análisis por difracciónde rayos X(XRD, por sus siglas eninglés) y microscopiaelectrónica de barrido (MEB). Lacantidadde mullita creadaaumenta conla temperaturade cocción. A 1.500◦C,lamullitizaciónde lamezcla escasi completa. Lamorfologíade lamullita creadaesbimodal(fasesprimariaysecundaria).Lamullitaprimaria,creadaapartirdel
∗ Correspondingauthor.
E-mailaddresses:mhamidouche@univ-setif.dz,mhamidouche@yahoo.fr(M.Hamidouche).
https://doi.org/10.1016/j.bsecv.2018.01.001
0366-3175/©2018SECV.PublishedbyElsevier Espa ˜na,S.L.U.Thisisanopen accessarticleundertheCCBY-NC-NDlicense (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018),
procesamientodelcaolínporlapaulatinadesintegracióndelmetacaolínapartirde990◦C, tieneunaformadecristalesalargados.Además,lamullitasecundariacreadapor disolución-precipitaciónapartirdelafasedevidrioenpresenciadepartículasdealúminatieneuna formadegranosaciculares.
©2018SECV.PublicadoporElsevierEspa ˜na,S.L.U.Esteesunart´ıculoOpenAccessbajo lalicenciaCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Mullite, whose chemical composition is found between 3Al2O3-2SiO2and2Al2O3-SiO2,isoneofthemoststudiedand
usedceramics.Itsapplicationsareverydiverseandcoverfrom refractoryfieldtotechnicalapplications[1–3].Inairandunder atmosphericpressure,it isthermally andchemicallystable fromroomtemperaturetomeltingone[4].Ithasverygood thermo-mechanicalproperties,whichallowsustouseit as structuralelementssuchasthermalengines.Indeed, sensitiv-itytocreepisverylimited[5].Itsthermalexpansioncoefficient isrelativelylowleadingtoagoodthermalshockresistance[6]. Weuse different precursors and methodsto synthesize mullite.Wemanufactureitthroughseveralreactiveprocesses atdifferent temperatures [7]. Eachfabrication method has specificfeaturesdepending on thewanted applicationand productperformance.Solidphasereaction oxides alumina-silicamixturesathightemperature,generallyabove1400◦C
[8],canleadtomulliteformation.Bysol–gelmethods[9],or co-precipitationofmixturesofsaltsinsolutionbasedonsilicon andaluminium,wesynthesizelow-temperaturemullite[11]. Onthecontrary,whenthe precursorsare natural alumino-silicateclays,weformthemullitebythermalprocesseslower than1300◦C[12].
Kaolin, whose main constituent is kaolinite (Al2Si2O5
(OH)4), undergoessuccessivestructuraland microstructural
transformationsduringits firing[13].Thedecompositionof kaolinite in metakaolinite takes place between 400◦C and 630◦C. The gradual collapse of metakaolin promotes the formationofnanoscalecrystalsofmullite,inadditionto for-mationoffreeamorphoussilica.At1200◦C,thecrystallization ofcristobaliteistriggeredfromamorphoussilica,anda con-siderableamountofmullitecrystalsisdeveloped[14].Thelast transformationstepistheverificationofcristobalite,which occursatatemperaturegenerallyabove1400◦C.Thepresence ofsomeimpurities,suchasCaO,Na2OandK2O,intheinitial
kaolinfavoursvitrificationofcristobaliteatlower tempera-tures.Toobtainasecondarymullite,thissilicaglasscanbe ‘mullitized’byaddingalumina,aluminiumhydroxidesor alu-miniumslag[15].Secondarymulliteisdifferenttotheprimary onebythemorphology andgrainsize[16].Primarymullite formsneedles,duetothepresenceofthevitreousphase[17]. Whilesecondarymulliteisintheformofaggregatesof needle-likesmallgrains[18],weformitbyasolution-precipitation oftheglassphaseincontactwiththeparticlesofalumina. According toPascual et al., addition of 1–3wt% ofMgO to thekaolin-aluminamixturepromotesgraingrowthofprimary andsecondarymullite,andtheadditionof1–5%Y2O3inhibits
graingrowthofthesecondarymullite[19].Wedetectno dif-ferenceintheX-raydiffractionspectraofthetwomullites.
However,weobservedifferencesbetweeninfraredabsorption spectra.
The aim of the present work is the mullitization of two types of Algerian kaolin by adding aluminium slag. We have sintered stoichiometric mixturesat temperatures between1000◦Cand1500◦Cfor2h.Inordertounderstand microstructuraltransformationsandchemicalreactions,we performeddifferentialthermalanalysiscoupledwiththermal gravimetricanalysis(DTA/TGA).Wehaveanalysed,byX-ray diffraction(XRD),thephasetransformationsduringthe var-iousthermaltreatments.Wedeterminedthemorphologyof mullite byscanning electronmicroscopy(SEM).Westudied thechemicalbondsofthephasesformedduringthefiringof aluminiumkaolin-slagmixturesatdifferenttemperaturesby Fouriertransforminfrared(FTIR)spectroscopy.
Experimental
procedure
Wehaveusedtwonaturalkaolinextractedfromtwovarious sitesofDjebelDebbaghnearGuelma(North-EastofAlgeria). Theamountofaluminaintheircompositionandcolour dif-ferentiate the twokaolin.Thefirst compositionnotedDD1 iswhitecolouredwhereasthesecondone(DD3)ingreyish coloured.Thedifferenceintheircoloursisduetothekindof theircontainingimpurities.
Their absolute densities, measured with a helium pyc-nometer apparatusare respectively2.63g/cm3 forDD1and
2.61g/cm3forDD3.Theusedaluminiumslag,waste of
alu-miniumindustry,isprovidedbyALGALcompany(Algeria).It iswhitecolouredandcomposedby87%(inmass)ofalumina, itsabsolutedensityisabout4.03g/cm3.
Tabla 1representsthechemical compositionsof respec-tivelybothkaolinandaluminiumslag.
Toobtainmulliteweprepared,withastoichiometric com-position,twomixturesofDD1andDD3withaluminiumslag, theyarerespectivelynotedMDD1andMDD3.
Wemilledthemixtureswithaplanetaryballmilling appa-ratusduring5h;withareportpowder/ballsequalto(50/200) gin80mlwater.
We driedthe mixturesat 110◦C and then crushed and sievedto45mmeshes.Thesampleswereuniaxialpressedat 100MPaforobtainingbarsshaped(40mm×10mm×10mm), afterthattheywereheatedupto600◦Catarateof1◦C/min for 1h to avoid cracking. Then we sintered the samples between 1000◦C and 1500◦C for2h, withaheatingrateof 5◦C/min.WesubjectedpowdersmixturesMDD1andMDD3 todifferentialthermalanalysis(DTA)andthermogravimetric analysis (TGA) using Setaram Setsys 16/18 simultaneous TG/DTAanalyzerwith␣-aluminaasthereferencematerial. Weconductedthetest betweentheroomtemperature and
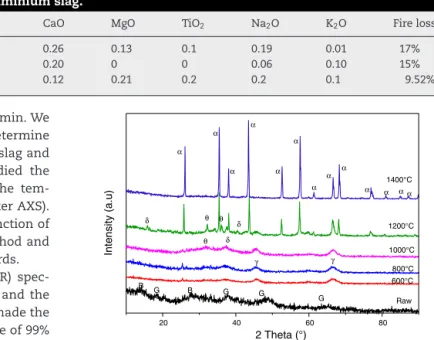
Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018), Tabla1–Chemicalcompositionsofkaolinandaluminiumslag.
Oxides SiO2 Al2O3 Fe2O3 CaO MgO TiO2 Na2O K2O Fireloss(%)
KaolinDD1 44.9 37.49 0.12 0.26 0.13 0.1 0.19 0.01 17%
KaolinDD3 43 39.9 1.9 0.20 0 0 0.06 0.10 15%
Aluminiumslag 2.5 87 0.15 0.12 0.21 0.2 0.2 0.1 9.52%
1400◦Cinairatmospherewithaheatingrateof5◦C/min.We haveusedaheliumpycnometer(AccuPycII1340)todetermine theabsolutedensityofcalcinedkaolin,aluminiumslagand there mixtures at different temperatures. We studied the evolution of the crystalline phases, according to the tem-peraturestreatment,bytheX-raysdiffraction(BrückerAXS). Wecalculatedtherateofmulliteformation,asafunctionof thermaltreatment,usingtheinternalstandardmethodand theI/IcorrelationpresentedintheJCPDSstandardcards.
Weanalysed,by Fouriertransform infrared (FTIR) spec-troscopy,thepresenceofspecificatomicgroupingsandthe characteristicvibrationsofthechemicalbands.Wemadethe testsontranslucentpastillesobtainedbythemixtureof99% mgfrom thematerialtobeanalysedcrushed wellwith1% mgofKbrbymeansofadeviceoftypePerkin-Elmes700.The sampleswerepolishedandthermallyetchedtoobservethe microstructurebyusingascanningelectronmicroscope(SEM) (ZeissSUPRA55VPtype).
Results
and
discussion
TheX-raydiffractionanalysis spectra(Fig.1)revealed that both the used kaolins contain essentially kaolinite (Index JCPDS80-0886)andhalloysite(JCPDS29-1489).
Thealuminiumslagistotallytransformedinto␣-alumina afterathermaltreatmentat1400◦Cwhereitsabsolutedensity isequalto3.41g/cm3(Fig.2).
TheobservationofkaolinpowdersbySEM(Fig.3)revealeda structurecomposedofagglomeratesofleaveslengthenedand orientedinalldirections.Thesecases,randomlyentangled, havefavouredanimportantporosity.
TheobservationofaluminiumslagbySEMshoweda struc-turecomposedofagglomeratesofsphericalgrains.
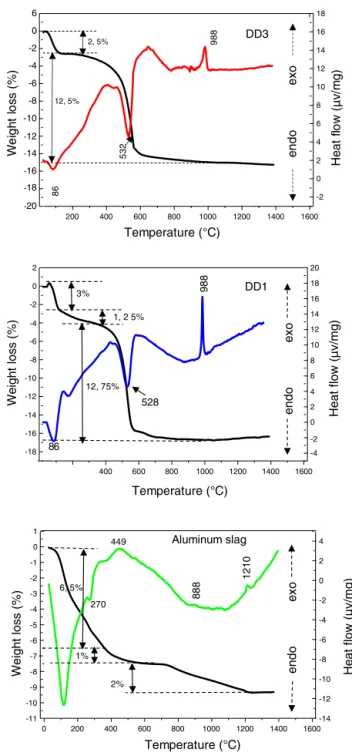
InFig.4,wepresenteddifferentialthermalanalysescurves ofDD1,DD3kaolinandaluminiumslag.TheDTA/TGcurves ofbothkaolinshowthepresenceoftwoendothermicpeaks.
DD1 DD3 10 20 30 40 50 60 70 80 90 100 H H H H H H H K K K K K K K K K K H H 2 Theta (°) Inte n sity (a.u)
Fig.1–XRDspectraoftheusednaturalkaolin,K:kaolinite; H:halloysite. 20 40 60 80 1400°C 1200°C 1000°C 800°C 600°C Raw α α α α α α α α α α α α α δ θ δ δ θ γ γ B G G G Inte n sity (a.u) 2 Theta (°) G B θ
Fig.2–XRDspectraofthealuminiumslagofthethermal treatmentatdifferenttemperatures(␣:alphaalumina;␥:
gammaalumina;:thetaalumina;␦:deltaalumina;B: boehmite;G:gibbsite).
Thefirstoneisveryintense,hasamaximumlocatedat86◦C, it isduetothe departureofthesurface water.Thesecond oneappearedat532◦C.Itisrelatedtothedepartureof con-stitution water (dehydration ofkaolin toform metakaolin) andassociatedtoamasslossof(12.5%forDD3and12.75% forDD1),between400◦Cand 600◦C.Furthermore,theDTA curvespresentanexothermicpeakoflowintensityat988◦C; correspondingtothemullitecrystallizationformedfromthe kaolinitetransformation.
TheDTAcurveofthealuminiumslagshowsan endother-micpeakofaverymarkedintensity towards119◦C,linked to moisture water. Endothermic peak at 270◦C introduces thedehydrationofthetri-hydrateoxidetomono-hydroxide (boehmite). We noticed a mass loss of 6.5%, with these transformations.Therefore,inadditiontotheseendothermic peaks,theTDAcurvepresentsotherexothermiceffects.We attributedthefirstexothermicpeak,whichappearsbetween 400◦Cand600◦Ccentredat449◦C,toarapidtransition reac-tiontriggeredbythebeginningofchangesintheslagcrystal structure,whichisaccompaniedbyalossmassof2%.Raw aluminiumslagisgloballyamorphouswiththepresenceof gibbsiteandboehmite(Fig.2).Thegibbsite–boehmitephase transformationtakesplacearound270◦C(seeDTAcurvein
Fig.4).Between400◦C and550◦C,boehmiteistransformed intogammaalumina.Thistransitionaluminathenleadsto aluminadeltaandthetabetween800◦Cand1200◦C.Above 1200◦C,theformationofthestablealpha-aluminaphasetakes place.Thesetransformationsofhydratedaluminaphasesto transitionsaluminaandthento␣-aluminahavebeendetailed inourpreviousworks[20–24].
WerepresentedinFig. 5the evolutionofthe crystalline structureforthetwokaolinusingXRD.From600◦Cto800◦C, thestructureisamorphousforthetwokaolinsDD1andDD3.
Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018),
Fig.3–Microstructureofkaolinandaluminiumslagused; (a)DD1,(b)DD3and(c)aluminiumslag.
Inthecaseofthesekaolins,theformationofmullitebeginsat 1000◦C.At1200◦C,thepeaksoflowintensitiescorrespondto thegerminationofthemulliteforDD1.
ThemullitephasebecomesimportantinthecaseofDD3 kaolinbeyond1200◦C.Alsothat, startingfrom 1200◦C,the excessofsilicaleadstothecrystallizationofthecristobalite. At1500◦C,wenoticedforbothkaolins,theincreasesofthe peaksintensitiescausedbytheincreaseofmullitequantity. Ontheotherhand,atthistemperature,thepeakofthe cristo-balitedisappearsbecauseofitstransformationintovitreous phase.
TheDTA/TGcurvesofMDD1andMDD3mixtures(Fig.6) showtwoendothermicpeaks.Thefirstoneisveryintense, hasamaximumlocatedat100◦C,itiscausedbythedeparture ofthesurfacewater. Thislastisassociatedtoa massloss between20◦Cand400◦C.Thesecondoneappearedat537◦C forMDD3andat532◦CforMDD1.Itcorrespondstothe
depar-Temperature (°C) 400 600 800 1000 1200 1400 1600 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 2 Weigh t lo s s (%) -4 -2 0 2 4 6 8 10 12 14 16 18 20 3% 1, 2 5% 12, 75% 86 528 988 Heat f low (µ v/ m g) DD1 e nd o e xo 0 200 400 600 800 1000 1200 1400 1600 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 1 Temperature (°C) -14 -12 -10 -8 -6 -4 -2 0 2 4 Weigh t lo ss (% ) 2% 6, 5% 270 449 He at f low (µ v/ m g) end o e xo Aluminum slag 888 1210 1% H ea t f low ( µ v /m g) Temperature (°C) 200 400 600 800 1000 1200 1400 1600 -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 6 -2 0 2 4 6 8 10 12 14 16 18 2, 5% 12, 5% DD3 We ig ht l o ss (% ) 86 53 2 98 8 e nd o e xo
Fig.4–Differentialthermalanalysisandthermo
gravimetricbehaviourofkaolinDD3,DD1andaluminium slag.
tureofconstitutionwater(dehydrationofkaolin),associated with a low mass loss between 375◦C and 600◦C. Besides, thesetwoendothermicpeaks,theDTAcurvepresentsother exothermiceffectsoflowintensities,whicharenotassociated withanylossofmass.AccordingtoGononetal.[15],thefirst exothermicpeakinneighbourhoodof1000◦Ccorrespondsto thecrystallizationofthemullitefrommetakaolin.Thesecond lessintenseexothermicpeak,observedintheneighbourhood of1194◦CfortheMDD3and1220◦CfortheMDD1,is associ-atedtotheformationofthesecondarymullitebyreactionof thealuminaresultingfromaluminiumslagwiththeexcess ofsilicaofthekaolin[20,25].Fortheclay;thephase,which
Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018), 10 20 30 40 50 60 70 80 90 100 M M M MM M M MM M M Cr 1500°C Brut 600°C 800°C 1000°C 1200°C 1400°C DD1 2Theta (°) In te ns it y (a. u ) 10 20 30 40 50 60 70 80 90 100 M M M M M M M M M M 1500°C 1400°C 1200°C 1000°C 800°C 600°C Brut DD3 Cr In te ns it y (a. u ) 2 Theta (°)
Fig.5–XRDofthekaolintreatedatdifferenttemperatures. M:mullite;Cr:cristobalite.
crystallized from 850◦C can melt [26]. Its melting occurs between1100◦Cand1300◦C[27].Increasingironcontentinto thevarious startingkaolinsleads tothe secondarymullite formationtowardslowertemperatures[27].
Fig.7representsthe XRDdiagrams ofMDD1and MDD3 mixturesfiredatdifferenttemperatures.
At 1000◦C, the beginning of the crystallization of the primarymulliteformedfrom thekaolin.Atthe same tem-perature, we observeda peak relating to the formation of ␥-alumina at 2Â=65◦. The XRD curves of samples fired at 1100◦C and at 1200◦C show the same formed crystalline phases,namelythealumina␥andtheprimarymullite.We noticedthenon-appearanceofthecristobalitepeak,formed atthistemperatureinthecaseofthekaolinDD3alone.We explainedthis,bytheformationofthesecondarymullitedue tothereactionofthe␥-aluminafromtheslagwiththeexcess ofsilicafromthekaolinbeforethecrystallizationofthelatter intocristobalite.
Thefiring ofMDD1 and MDD3 mixtures at1300◦C and 1400◦Cleadstotheaccelerationoftheformationofthe sec-ondarymullite.At1500◦C,practicallyalmostcompletemullite formationofmixtures.Thepresentimpuritiesinthe precur-sors(slagandkaolin)withonepartofthesilicaformaglassy
200 400 600 800 1000 1200 1400 1600 -24 -22 -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 2 Temperature (°C) -12 -10 -8 -6 -4 -2 0 2 4 6 Heat f low ( µv /m g) 53 7 We ig ht lo ss ( %) 119 4 15% 4, 5% 4, 5% MDD3 99 3 e nd o e xo 10 0 200 400 600 800 1000 1200 1400 19% 6% 2% 5% Temperature (°C) 53 2 99 8 122 0 MDD1 e nd o e xo -12 -8 -6 -4 -2 0 2 4 6 -30 -25 -20 -15 -10 -5 0 5 10 0 Hea t f low (µ v/ m g) We ig h t lo ss (% ) -10 1600
Fig.6– DTA/TGofmixturesMDD1andMDD3.
1000°C 1300°C 1200°C 1100°C 1400°C 1500°C M M M M M M M M M M MDD3 In tens it y (a. u ) 2 Theta(°) 10 20 30 40 50 60 70 80 Raw M M M M M M 90 100 M MMM M γ 10 20 30 40 50 60 70 80 90 100 M M M M M M M M M M M M M M M M M MDD1 Raw 1000°C 1500°C 1300°C 1200°C 1100°C 1400°C 2 Theta (°) In tens it y (a. u ) γ
Fig.7–DiffractogramsofMDD1andMDD3mixturesat differentstagesofthethermaltreatment(M:mullite;␥: gammaalumina).
Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018), 1000 1100 1200 1300 1400 1500 20 30 40 50 60 70 80 90 Rat e of cry s ta lli nit y ( %) Temperature (°C) MDD1 MDD3
Fig.8–VariationofthecrystallinityrateofMDD1and MDD3mixturesasafunctionoftemperaturetreatment.
120 0 1300 1400 1500 3, 08 3, 09 3, 10 3, 11 3, 12 3, 13 3, 14 3, 15 3, 16 3, 17 3, 18 3, 19 3, 20 Temperature (°C) MDD3 MDD1 Ab so lut e de ns it y ( g /c m 3)
Fig.9–EvolutionoftheabsolutedensityofMDD1and MDD3mixturesafunctionofthetemperature.
phase.ThedifferencebetweentheDRXspectraofthemixtures MDD1andMDD3residesinthequantityofmulliteformed.
Accordingtothecalculationoftheintensitiesofrays char-acterizingthemullite(Fig.8),thecrystallizationrateofthe mullitephaseincreaseswiththeincreaseinthetemperature oftheheattreatment.
Thisratereachespractically94%forthemixtureMDD1and 89%forthe mixtureMDD3forsamplesfiring during2hat 1500◦C.Weexplainedthisdifferencebetweenbothmixtures bythehighrateofSiO2inDD1kaolin.
ThequantityoftheformedmulliteisimportantinMDD1 mixture,ofthefactthatthehighcontentofsilicainitsraw material(DD1).Thisnecessitytoanadditionofaluminium slagismoreimportant.
Wemeasuredabsolutedensitiesofmixtures(slag+kaolin), curedbetween 1200◦C and 1600◦C,byheliumpycnometry. WegatheredtheresultsinFig.9.Morethefiringtemperature increase,theabsolutedensitiesofmixturesalsoincrease.At 1600◦C,theyalmostreachthetheoreticalvalueofthemullite density(3.17g/cm3).
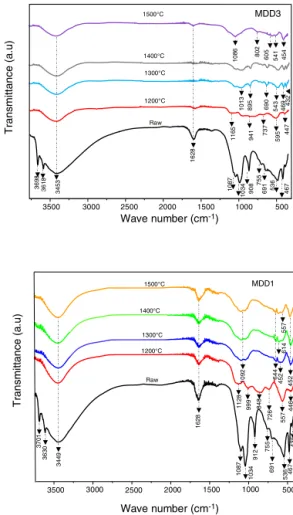
Fig. 10 shows FTIR ofMDD1 and MDD3 mixturescured atdifferenttemperatures. Inits naturalstate; intherange 4000–3000cm−1 thespectraare characterizedbytwobands locatedat3701cm−1and3630cm−1,andawidebandcentred at3449cm−1forMDD1,andforMDD3bandsarerespectively locatedat3698cm−1,3618cm−1and3453cm−1,thesebands areduetostretchingvibrationhydroxyls.
3500 Wave number (cm-1) 36 98 36 18 10 87 10 34 908 75 5 69 1 53 6 46 7 1200°C 11 65 94 1 59 5 44 7 73 7
Transmittance (a.u) Raw
1300°C 89 5 10 13 69 0 432 46 9 54 3 1400°C 10 86 80 2 45 4 54 1 60 5 1500°C 34 53 16 28 3000 2500 2000 1500 1000 500 MDD3 Wave number (cm-1) T ran smi tt an ce ( a .u) 1200°C 112 8 84 8 99 9 72 6 55 7 44 6 61 4 1400°C Raw 370 1 108 7 363 0 162 8 103 4 75 5 91 2 69 1 46 7 53 6 43 5 344 9 1500°C 55 7 1300°C 45 2 64 4 45 2 10 92 3500 3000 2500 2000 1500 1000 500 MDD1
Fig.10–FTIRspectraofmixturestreatedatdifferent temperatures.
Weassignedthebandslocatedat3701cm−1and3698cm−1 to the hydroxyls ofthe edges ofleaves. Bands situatedat 3630cm−1and3618cm−1are forthe hydroxylsofsurfaceof thelayeroctahedralininteractionwithbasicoxygen ofthe tetrahedraladjacentlayer.Finally,thebandsat3449cm−1and 3453cm−1arecausedbytheinternalhydroxylslinkedtothe aluminium.
Thebandsthatappearedat1087cm−1and1034cm−1was assigned toSi O Sistretchingvibration. Weattributedthe bandsat912cm−1and908cm−1forMDD1andMDD3 respec-tivelytovibrationsoflinksAl OH.
Si O AlVI [12] and Si O AlIV stretching vibrations are
respectivelycharacterizedbybandsat536cm−1and755cm−1. WeobservedthebandassociatedwithAl O Alstretching forthetwomixturesat691cm−1[12].Ontheotherhand,the deformationoflinksSi Oischaracterizedbythepresenceof bandsat467cm−1and435cm−1.
ForathermaltreatmentofMDD1at1200◦C,bandslocated respectivelyat1087cm−1and1134cm−1aretransformedata broaderbandconstitutedbytwobands:thefirstoneiscentred at1128cm−1andthesecondtowards999cm−1.
The bands located towards 755cm−1 and 691cm−1 are transformedintwowidebands.Thefirstoneindicates the formationofspinelSi Alandthesecondindicatesthe crys-tallizationofAl2O3[10].
Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018),
Inaddition,boththecharacteristicbandscorrespondingto Si Ostretchingvibrationaretransformedtothesingleband ofweak intensity centredat 446cm−1, which explainsthe decreaseofthequantityofSiO2.
Theappearanceofawidebandintheregion920–770cm−1 centredtowards848cm−1assignedtoAl OHstretching vibra-tion, is attributed to the crystallization of the secondary mullite[13].
On the other hand, the spectrum of mixture MDD3 at 1200◦Cshowsthe appearanceofawideband intheregion 1200–600cm−1centredrespectivelyat1165,941and737cm−1, compared with the spectrum of MDD1. This wide band explainsthetrainingofamoreimportantquantityofmullite. Foratreatmentat1300◦C,thebandcorrespondingtothe trainingofthemullitehasbecomewider.Thiswidebandis composedoftwobandscentredrespectivelytowards1104and 1028cm−1,whatcanbeexplainedbythetrainingofaddition allinksofSi O Alincrystalsofmullite.
Also, the wide band located at 848cm−1 for the MDD1 mixtureis replacedby awide shoulder, and the spectrum ofmixtureMDD3show the appearanceoftwonew bands, onelocatedat895cm−1andtheotherat690cm−1,andaless intenseshoulderthanthatobservedintheMDD1spectrum. Thethermaltreatmentat1500◦Cleadstotheincreaseofthe intensityofthebandlocatedat1013cm−1.
The SEM results reveal that the morphology of micro-structuresofMDD1andMDD3doesnotpresentasignificant differencebetweenbothmixturesafterfiredat1400◦Cduring 2h(Figs.11and12).
Weobservedtheexistencesofbigcrystalsofmullitewith arandomdistribution.Theprimarymullitehasalong nee-dleshape.Theglassymatrixsurroundedthesmallestcrystals
ofsecondarymullitewhicharenucleatedinthetransitional liquid bydissolution ofthealumina[16]. Thegrainsofthe secondarymullitepresentadifferentstructure(acicular)and grainssizesmallerthanthatoftheprimarymullite.The struc-tureofthesampleMDD3showsthepresenceofmanypores withbiggerdimensions.
Theneedle-likemullitegrainsobservedforsampleMDD3 treatedat1400◦CislargerthanthatobservedforMDD1 sin-tered atthesametemperature, thisdifferenceiscausedby thehighironcontentinMDD3.AccordingtoLecomte[28],the heattreatmentat1400◦C,performedoniron-enriched sam-ples giverise tothe formation oflarge needle-like mullite grains,widelyseparatedfromeachotherbyaglassyphase. Also,thepresenceofironoxide(Fe2O3)impuritiescontributes
toreducetheliquidphaseformationtemperature.This pro-motesanincreaseinthealuminadissolutionrate(Al2O3)in
theliquidphase,inwhichAlionsreactinhigherproportions withSiions[29].Accordingtotheliterature[29,30]intheclay mineralsormixturesofclayminerals,thealkalis,ironoxide (Fe2O3)andotherimpuritiesalterthecompositionoftheliquid
anditsperformancedirectlyinfluencestherecrystallization mechanisms.So,highalkalineoxidecontentfavourscrystal growth[30].
Fortheiron-richkaolinicmaterials,atabout1400◦C,the interactionsbetweenthedifferentphasesinequilibriumlead totheformationofgreatamountofglassyphase.Theiron compoundspresentinthesamplesmayleadtotheformation ofeutecticliquidsbetween1300and1400◦C[28].Indeed,this greatamountofglassyphaseisalsorelatedtoother impuri-ties.ThepresenceofNa2O3andK2Opromotestheformation
of vitreousphase at a low melting point during the firing
[31].
Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018),
Fig.12–MicrostructuresobtainedbySEMformixturesfiredat1500◦Cduring2h.(c)MDD3,(d)MDD1,P:primarymullite,S: secondarymullite.
Thefiringat1500◦Cleadstoastructurewithabimodal morphology.Thesizeoftheprimarymullitecrystalsisbigger, comparedtothatobtainedat1400◦C.Athightemperature,we observedagrainsgrowthsizeofthemullitecrystalsandthe formationofmoresecondarymullitecrystals[16].
Weknowthatmullitegrainsgrowthmechanismforhigh temperature in clays is a dissolution-precipitation process
[32].During heat treatment, dissolutionrate ofAl2O3 in to
liquidphaseincreases,whichpromotesthemullitecrystals growth[33].
At1500◦C,the mullitegrains sizeof crystalsformed in MDD1mixtureisgreaterthanthatformedatMDD3one.We explainedthisobservationbytheratioAl2O3/SiO2,whichis
equalfor1.45toMDD1and1.22forMDD3.
Conclusion
Theobtainedresultsshowthe possibilityofusing the alu-minium slag asan addition (in replacementof ␣-alumina) fortheelaborationofthemullitefromkaolin.Thecurvesof differentialthermalanalysisandthermogravimetricanalysis (DTA/TG)showthatthereareseveralphasetransformations duringfiring. Themicrostructuralanalysis ofsamples sin-teredat1500◦Cshowsthe presenceoftheprimarymullite in the form of lengthened needles and small crystals of secondary mullite, which are nucleated in the transient liquid.Thetransformationofthemetakaoliniteformedtothe primarymullite,whilethesecondarymulliteresultsfromthe reactionofthealuminaofslagandfromtheexcessofsilica ofthekaolin.Throughthisstudy,byreactivefiringadense
mullite fromnatural kaolinand aluminiumslag,this isan industrialobtainedwaste.
r
e
f
e
r
e
n
c
e
s
[1]T.Huang,M.N.Rahaman,T.I.Mah,A.Triplicane,A. Parthasarathay,Anisotropicgraingrowthand
microstructuralevolutionofdensemulliteabove1550◦C,J. Am.Ceram.Soc.83(1)(2000)204–210.
[2]M.Sardy,A.Arib,K.Abbassi,R.Moussa,M.Gomina, Elaborationandcharacterizationofmulliterefractory productsfromMoroccanandalusite,NewJ.GlassCeram.2 (2012)121–125.
[3]I.A.Aksay,D.M.Dabbs,M.Sarikaya,Mulliteforstructural, electronic,andopticalapplications,J.Am.Ceram.Soc.74 (10)(1991)2341–2721.
[4]H.-J.Kleebe,F.Siegelin,T.Straubinger,G.Ziegler,Conversion ofAl2O3–SiO2powdermixturesto3:2mullitefollowingthe stableormetastablephasediagram,J.Eur.Ceram.Soc.21 (2001)2521–2533.
[5]H.Rhanim,C.Olagnon,G.Fantozzi,R.Torrecillas, Experimentalcharacterisationofhightemperaturecreep resistanceofmullite,Ceram.Int.23(6)(1997)497–507.
[6]M.Hamidouche,N.Bouaouadja,C.Olagnon,G.Fantozzi, Thermalshockbehaviourofmulliteceramic,Ceram.Int.29 (2003)599–609.
[7]T.Abdezadeh,Formationofmullitefromprecursorpowders: sintering,microstructureandmechanicalproperties,Mater. Sci.Eng.A355(1–2)(2003)56–61.
[8]L.B.Kong,T.S.Zhang,Y.Z.Chen,J.Ma,F.Boey,H.Huang, Microstructuralcompositemullitederivedfromoxidesviaa high-energyballmillingprocess,Ceram.Int.30(2004) 1313–1317.
Pleasecitethisarticleinpressas:F.Chargui,etal.,Mullitefabricationfromnaturalkaolinandaluminiumslag,Bol.Soc.Esp.Cerám.Vidr.(2018),
[9]H.-J.Kleebe,F.Siegelin,T.Straubinger,G.Ziegler,Conversion ofAl2O3–SiO2powdermixturesto3:2mullitefollowingthe stableormetastablephasediagram,J.Eur.Ceram.Soc.21 (2001)2521–25337.
[10]J.Roy,S.Maitra,Synthesisandcharacterizationof sol–gel-derivedchemicalmullite,J.Ceram.Sci.Technol.5 (01)(2014)57–62.
[11]R.Septawendar,B.S.Purwasasmita,L.Nurdiwijayanto,A newsynthesisrouteofnanomullitebycalciningpulp precursors,J.Aust.Ceram.Soc.47(1)(2011)42–47.
[12]M.A.Sainz,F.J.Serrano,J.M.Amigob,J.Bastida,A.Caballero, XRDmicrostructuralanalysisofmullitesobtainedfrom kaoliniteandaluminamixture,J.Eur.Ceram.Soc.20(2000) 403–412.
[13]L.Andrini,M.R.Gauna,M.S.Conconi,G.Suarez,F.G.Requejo, E.F.Agleitti,N.M.Rendtorff,Extendedandlocalstructural descriptionofakaoliniticclay,itsfiredceramicsand intermediates:anXRDandXANESanalysis,J.Appl.ClaySci. 124–125(2016)39–45.
[14]Y.-F.Chena,M.-C.Wang,M.H.Hona,Phasetransformation andgrowthofmulliteinkaolinceramics,J.Eur.Ceram.Soc. 24(2004)2389–2397.
[15]M.Gonon,G.Fantozzi,H.Osmani,M.Hamidouche,M.A. Madjoubi,N.Bouaouadja,K.Loucif,Etudedela
transformationdetroisnuancesdekaolinenfonctiondela température,Silic.Ind.Cera.Sci.Tech.65(11–12)(2000) 119–124.
[16]T.J.Mackenzie,J.D.Schurcker,H.Schneider,J.Mcmanus,S. Wimperis,Phaseevolutioninmechanicallytreatedmixtures ofkaoliniteandaluminahydrates(gibbsiteandboehmite),J. Eur.Ceram.Soc.20(2000)413–421.
[17]Y.F.Chen,M.C.Wang,M.H.Hon,Secondarymulliteformation inkaolin–Al2O3ceramics,J.Mater.Res.18(2003)1355.
[18]M.Ohashi,Y.Iida,Hotforgingofmullite-basedceramics preparedfromkaolin-alumina,J.Am.Ceram.Soc.83(7) (2000)1825–1827.
[19]J.Pascual,J.Zapatero,M.C.JimenezdeHaro,I.Varona,A. Justo,J.L.Perez-Rodriguez,P.J.Sanchez-Soto,Porousmullite andmullite-basedcompositesbychemicalprocessingof kaoliniteandaluminummetalwastes,J.Mater.Chem.10 (2000)1409–1414.
[20]H.Belhouchet,M.Hamidouche,N.Bouaouadja,V.Garnier,G. Fantozzi,Crystallizationkineticsof␣-aluminaand
mullite-zirconiainboehmiteandzirconmixture,J.Mater. Sci.Eng.A3(12)(2013)814–819.
[21]H.Belhouchet,M.Hamidouche,N.Bouaouadja,V.Garnier,G. Fantozzi,Elaborationandcharacterizationof
mullite-zirconiacompositesfromgibbsite,boehmiteand zircon,J.Ceram.Silik.53(3)(2009)205–210.
[22]H.Belhouchet,M.Hamidouche,R.Torrecillas,G.Fantozzi, Thenon-isothermalkineticsofmulliteformationin boehmite–zirconmixtures,J.Therm.Anal.Calorim.116 (2014)795–803.
[23]H.Belhouchet,H.Makri,M.Hamidouche,N.Bouaouadja,V. Garnier,G.Fantozzi,Elaborationandcharacterizationof multiphasecompositesobtainedbyreactionsinteringof boehmiteandzircon,J.Aust.Ceram.Soc.50(2)(2014) 135–146.
[24]S.Lamouri,M.Hamidouche,N.Bouaouadja,H.Belhouchet,V. Garnier,G.Fantozzi,J.F.Trelkat,Controlofthe␥-aluminato ␣-aluminaphasetransformationforanoptimizedalumina densification,Bol.Soc.Esp.Cerám.Vidrio56(2)(2017) 47–54.
[25]V.Viswabaskaran,F.D.Gnanam,M.Balasubramanian,Effect ofMgO,Y2O3andboehmiteadditivesonthesintering behaviorofmulliteformedfromkaolinite-reactivealumina, J.Mater.Process.Technol.142(2003)275–281.
[26]P.G.Wherllyson,V.J.Silva,R.R.Menezes,G.A.Neves,H.L.Lira, L.N.L.Santana,Microstructural,physicalandmechanical behaviorofpastescontainingclaysandaluminawaste,J. Appl.ClaySci.137(2017)259–265.
[27]C.J.McConville,W.E.Lee,Microstructuraldevelopmenton firingilliteandsmectiteclayscomparedwiththatin kaolinite,J.Am.Ceram.Soc.88(2005)2267–2276.
[28]G.Lecomte-Nana,J.P.Bonnet,N.Soro,Influenceofironon theoccurrenceofprimarymulliteinkaolinbasedmaterials: asemi-quantitativeX-raydiffractionstudy,J.Eur.Ceram. Soc.33(2013)669–677.
[29]V.J.Silva,M.F.Silva,W.P.Gonc¸alves,R.R.deMenezes,G.A. Neves,H.L.Lira,L.N.L.Santana,Porousmulliteblockswith compositionscontainingkaolinandaluminawaste,Ceram. Int.42(2016)15471–15478.
[30]S.Deniel,N.T.Doyen,C.Dublanche-Tixier,D.Chateigner,P. Blanchart,Processingandcharacterizationoftextured mulliteceramicsfromphyllosilicates,J.Am.Chem.Soc.30 (2010)2427–2434.
[31]A.Arib,A.Sarhiri,R.Moussa,T.Remmal,M.Gomina, Caractéristiquesstructuralesetmécaniquesdecéramiquesà based’argiles:Influencedelasourcedefeldspath,C.R. Chim.10(2007)502–510.
[32]H.P.Fang,M.H.Huang,Z.H.Chen,K.Xu,Y.G.Liu,Y.G.Huang, EffectofLa2O3additivesonthestrengthandmicrostructure ofmulliteceramicsobtainedfromcoalgangueand␥-Al2O3, Ceram.Int.39(2013)6841–6846.
[33]X.Xu,X.Lao,J.Wu,Y.Zhang,X.Xu,K.Li,Microstructural evolution,phasetransformations,andvariationsinphysical propertiesofcoalserieskaolinpowdercompactduring firing,Appl.ClaySci.115(2015)76–86.