UNIVERSITE DE SHERBROOKE
Faculte de Genie
Departement de Genie Chimique
SURFACE MODIFICATION OF POWDERS USING
DIELECTRIC BARRIER DISCHARGES
MODIFICATION DE SURFACE DES POUDRES EN
UTILISANT DES DECHARGES A BARRIERE
DIELECTRIQUE
Soumis pour l'obtention du doctorat en sciences appliquees
Speciality : genie chimique
D. GRAVELLE J. JUREWICZ U. KOGELSCHATZ M. BOULOS
COMPOSITION DU JURY
Professeur a l'universite de Sherbrooke Rapporteur Professeur a l'universite de Sherbrooke Examinateur Retraite de la compagnie ABB Corporate Research Examinateur
Professeur a l'universite de Sherbrooke Directeur de these
Christine NESSIM
1*1
Library and
Archives Canada
Published Heritage
Branch
395 Wellington Street Ottawa ON K1A0N4 CanadaBibliotheque et
Archives Canada
Direction du
Patrimoine de I'edition
395, rue Wellington Ottawa ON K1A0N4 CanadaYour file Votre reference ISBN: 978-0-494-42688-3 Our file Notre reference ISBN: 978-0-494-42688-3
NOTICE:
The author has granted a
non-exclusive license allowing Library
and Archives Canada to reproduce,
publish, archive, preserve, conserve,
communicate to the public by
telecommunication or on the Internet,
loan, distribute and sell theses
worldwide, for commercial or
non-commercial purposes, in microform,
paper, electronic and/or any other
formats.
AVIS:
L'auteur a accorde une licence non exclusive
permettant a la Bibliotheque et Archives
Canada de reproduire, publier, archiver,
sauvegarder, conserver, transmettre au public
par telecommunication ou par Plntemet, prefer,
distribuer et vendre des theses partout dans
le monde, a des fins commerciales ou autres,
sur support microforme, papier, electronique
et/ou autres formats.
The author retains copyright
ownership and moral rights in
this thesis. Neither the thesis
nor substantial extracts from it
may be printed or otherwise
reproduced without the author's
permission.
L'auteur conserve la propriete du droit d'auteur
et des droits moraux qui protege cette these.
Ni la these ni des extraits substantiels de
celle-ci ne doivent etre imprimes ou autrement
reproduits sans son autorisation.
In compliance with the Canadian
Privacy Act some supporting
forms may have been removed
from this thesis.
Conformement a la loi canadienne
sur la protection de la vie privee,
quelques formulaires secondaires
ont ete enleves de cette these.
While these forms may be included
in the document page count,
their removal does not represent
any loss of content from the
thesis.
Canada
Bien que ces formulaires
aient inclus dans la pagination,
il n'y aura aucun contenu manquant.
R e s u m e
Le traitement de surface des poudres par plasma hors-equilibre est un outil rapide et indispensable au developpement de nouvelles applications pour les poudres traitees. Dans un domaine tel que la synthase des poudres pyrophoriques metalliques, ou l'ignition est instantanee en cas de non passivation et de contact avec l'air, un traitement par un plasma hors-equilibre permet de modifier les proprietes de surface des poudres afin d'obtenir des poudres moins reactives a basse temperature tout en gardant leur caracteristique energetique a haute temperature. Autre domaine, comme le changement de la mouillabilite des poudres est tres recherche. L'objectif de ce travail est de developper des nouvelles techniques pour le traitement de surface des poudres micro ou nanometriques en utilisant les techniques de plasma hors-equilibre. L'avantage de cette approche est de pouvoir modifier les proprietes de surface de ses dites poudres sans affecter les proprietes globales comme par exemple la structure cristalline, taille des poudres et composition chimiques. En fait, les decharges a barriere dielectrique operant a pression atmospherique sont ideales pour traiter la surface des poudres sans changer leur structure car ils produisent des electrons energetiques pour exciter les especes moleculaires et atomiques a basse temperature. Aussi, l'operation a pression atmospherique per-met l'integration de l'unite de traitement de surface avec l'unite de synthese en evitant l'utilisation des systemes de vide assez dispendieux et difficile a maintenir. Dans ce travail, des experiences sur la deposition ainsi que sur la fonctionnalisa-tion ont ete menes en utilisant deux designs de torche a barriere dielectrique. Pour la deposition, des precurseurs tels que Pethylene, butadiene, pyrrole et acetylene
ont ete utilise pour former des couches organiques sur des poudres micro ou nanometriques. D'autres precurseurs a base de silicium comme le tetraethyloxysil-icates ou l'hexamethyldisiloxane ont ete utilises pour former des depots de nature inorganiques (SiOx) ou organiques (SiOxCyHz) sur des poudres metalliques ou
d'oxydes. Pour les tests de fonctionnalisation, la surface a ete modifiee par des decharges, d'helium-air, helium-oxygene et helium-azote. Dans les experiences de deposition, les effets de temps de residence, de la densite de puissance, de l'injection en mode pulse ou dans la region apres plasma ont ete etudie. Quelques tests ont ete realises pour etablir l'effet de la temperature des gaz et des poudres sur le depot. Quand a la fonctionnalisation, l'effet de la densite de puissance, des types de gaz et de temps de residence ont ete examines.
Pour les depots realises en utilisant des precurseurs organiques ou a base de sili-cium, l'utilisation de mode pulse ainsi que l'injection dans la region apres plasma ont forme des depots dense. Dans la plupart des autres depots effectues dans la decharge, des poudres ont ete formees. Le faite de chauffer les poudres avant leur injection a augmente leur dispersion. Finalement, la fonctionnalisation des poudres de polymeres ont permis leur mouillabilite pour un debit assez eleve (20g/min). Le vieillissement des poudres n'ont pas depasse le 25% pour une periode de 60 jours dependamment de leur methode d'entreposage. II ressort done de ce travail que les decharges a barriere dielectriques sont capables de modifier les surfaces des poudres selon les conditions et le design appliques.
M o t s - C l e f s : Decharge a barriere dielectrique, modification de surface des poudres, deposition organique, deposition inorganique, fonctionnalisation.
Abstract
Powder surface treatment using nonequilibrium plasma is a rapid and indispensable tool for the development of new applications for the treated powders. In a domain like the synthesis of pyrophiric metallic nanoparticles where ignition is instantaneous when the non passivated powders come in contact with air, a nonequilibrium plasma surface treatment permits to modify the properties of the surface in order to protect the powders at low temperature and keep their energetic property at high temperatures. Other domains like changing the wettability of the surface is lately much needed. The objective of this work is to develop a new surface powder treatment technique for macro or nanopowders using a nonequilibrium plasma source. The advantage of this approach is to modify the surface of powders without affecting their general properties like their crystalline structure, powder size and chemical composition.
In fact, the dielectric barrier discharges operating at atmospheric pressure are ideal for treating powders surfaces without their damages as they produce energetic electrons to excite molecular and atomic species at fairly low temperatures. Moreover, dielectric barrier discharges operating at atmospheric pressure permit integration in the same unit of powder production avoiding vacuum which is expensive and difficult to maintain.
In this work, deposition and functionalization experiments were carried out using two different designs of dielectric barrier discharge torches. For the deposition, precursors like ethylene, butadiene, pyrrole and acetylene were used to form a polymer like coating on micro or nanoparticles. Other silicon based precursors like tetraethyloxysilicate and hexamethyl dis-iloxane were use to form either an inorganic like coating (SiOx) or an organic one (SiOxCyHz).
For the functionalization tests, the surface of polymer powders was modified using, helium-air, helium-oxygen and helium-nitrogen discharges. In the experiments, the effect of the residence
time, power density, intermittent mode operation and the injection in the afterglow region were investigated. Some tests were realized to study the effect of gas and powders heating prior to deposition. On the other hand, in the functionalization experiments the effect of the power density, the gas type and the residence time were examined.
For the deposit realised using either organic or silicon based precursors, intermittent mode operation and afterglow injection formed a dense deposit. The other coatings formed in the discharge resulted in powder formation. Heating the particles increased their dispersion. Finally, the functionalization experiments increased the wettability of the polymer powder at fairly high feed rate (20g/min). Aging of the powders did not exceed 25% after 60 days depending on their storage environment. As a conclusion dielectric barrier discharges were able to change the powder surface depending on the condition and the design used.
K e y w o r d : Dielectric barrier discharge, surface modification of powders, organic coating,
Acknowledgement
First I would like to express my profound gratitude to my thesis supervisor Dr. M a
-her B o u l o s . I thank him that he accepted to direct my work and for giving me the opportunity
to realize my experimental work in industry. His confidence and his support allowed me to finish this work.
I would like to express my sincerest gratitude to Dr. Ulrich K o g e l s c h a t z for his advices and recommendations.
My thanks go to the Jury member, Dr. J e r z y Jurewicz and Dr. D e n i s Gravelle who accepted to evaluate this work. Also to P e t e r Lanigan for his language and technical revision of the manuscript.
Next, I would like to thank the entire Tekna Plasma System Inc. group for the cheer-ful and nice environment at work. Particular thanks to A l e x a n d r e A u g e r who technically helped in the experimental part, J a c q u e s B e d a r d and S e b a s t i e n T h e l l e n d for the techni-cal drawing, J e a n Francois B e r u b e for his support in the characterization techniques and
T h o m a s Labrot for his help with the F T I R analysis.
I would like also to thank the group at the university of Sherbrooke who helped me, espe-cially R e a l D u b u c the glass blower, Francis B a r r e t t e the technician at the C R E P E group,
Irene Levesque for her assistance with the microscope photos and other characterization
techniques, Sonia Blais for her help with the XPS, S t e p h a n e G u t i e r r e z for his valuable discussions with the TGA, Charles B e r t r a n d for his help with the transmission electron microscope, A l a i n D e s p o n t s for his time with the F T I R technique and S y l v i e Lebrun for her usual assistance with the administration papers.
I would like to thank from all my heart my parents a n d m y sisters for all their help during those years of studies. Finally I would like to address a special thanks to my husband
Contents
1 I n t r o d u c t i o n 1
2 N o n e q u i l i b r i u m P l a s m a and D i e l e c t r i c Barrier D i s c har g e ( D B D ) 3
2.1 Nonequilibrium plasma 3
2.1.1 Low pressure plasma 4
2.1.2 Atmospheric-pressure plasmas 7
2.1.2.1 Corona discharges 8
2.1.2.2 Atmospheric pressure plasma jets (APPJs) 9
2.1.2.3 Microhollow cathode (MHC) and capillary plasma electrode
(CPE) discharges 1.0
2.1.2.4 Dielectric barrier discharges (DBD) 10
2.1.3 Comparison between the atmospheric pressure plasma sources 11
2.2 Dielectric barrier discharge 15
2.2.1 DBD configurations 15
2.2.2 Microdischarges properties 16 2.2.3 Diffuse glow dielectric barrier discharge 21
C O N T E N T S
2.3 Applications of DBDs 30
2.3.1 Ozone generation 30
2.3.2 Pollution control 32
2.3.3 Ultraviolet excimer lamps 32
2.3.4 Mercury-free fluorescent lamp 34
2.3.5 Plasma display panels 34
2.3.6 Surface treatment 35
2.4 Particle charging in the electrostatic precipitator 37
2.4.1 Mechanism of the processes involved in electrostatic precipitation . . . . 39
2.4.2 Particle charging phenomena 40
2.4.3 Effect of particles material 41
2.4.4 What to expect in our particle coating process? 42
3 S t a t e of Art of Particle Surface T r e a t m e n t in N o n e q u i l i b r i u m P l a s m a 44
3.1 Low pressure PECVD 45
3.2 Atmospheric-pressure PECVD 47
3.2.1 An example of indium and tin oxide deposition 49
3.2.2 Examples of silicon dioxide deposition 49
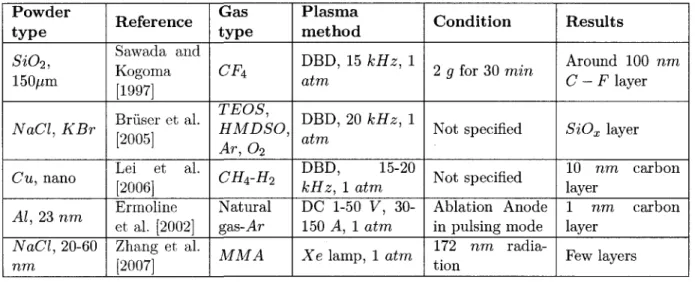
3.2.3 Other examples 51
3.3 Atmospheric-pressure glow discharge coating in DBDs 54
3.4 Powder coating 55
3.4.1 Low pressure powder coating 56
C O N T E N T S
3.4.1.2 Powders surface modified within the afterglow region 59
3.4.2 Atmospheric pressure powder coating 61
3.4.2.1 DBD examples 61
3.4.2.2 Other examples 84
3.5 Surface functionalization of polymer using non-thermal plasmas 65
3.5.1 Surface functionalization of polymer sheets 65
3.5.2 Surface functionalization of polymer powders 68
3.6 Particle charging 69
3.7 Summary of particle coating in nonequilibrium plasmas 73
4 D B D Torch D e s i g n and Electrical Characteristics 76
4.1 Flow through DBD torch design 76
4.1.1 Coaxial torch geometry 77
4.1.2 Shell electrode torch design 79
4.1.3 Coaxial versus shell electrode configurations 81
4.1.4 Specifications of the power supply 82
4.1.5 General procedure for torch start up 83
4.2 Electrical characteristics of the torch 84
4.2.1 Basic principles 85
4.2.2 Electrical set up 88
4.2.3 Energy balance 89 4.2.4 Electrical results for the coaxial design 90
C O N T E N T S
4.3 Estimate of average gas temperature I'll
4.3.1 Temperature estimation using tracer powders 112
4.3.2 Temperature estimation using an equation developed for ozone generator 11.5
4.3.3 Temperature measurements using emission spectroscopy 115
4.3.4 General remarks 118
4.4 Particle charging measurements 118
4.5 Summary of the torch characterization investigations 120
5 P o w d e r T r e a t m e n t U s i n g a F l o w T h r o u g h D B D Torch 121
5.1 Configurations used for powder treatment 1.21
5.2 Powder feeders techniques 124
5.2.1 Powder feeder for fumed silica particles 1
5.2.2 Powder feeder for fine aluminum particles 125
5.2.3 Powder feeder for macro particles 128
5.2.4 Powder feeder for polymer particles 129
5.2.5 Fluidised bed for polymer powders 129
5.3 Powder characterization techniques 130
5.3.1 Scanning electron microscopy and dispersive elemental analysis 130
5.3.2 Transmission electron microscopy 130
5.3.3 X-ray Photoelectron Spectroscopy 132
5.3.4 Fourier transform infrared spectroscopy 132 5.3.5 Measurements of water penetration into the powder beds to evaluate the
C O N T E N T S
5.3.6 Water contact angle measurements 136
5.3.7 Soxhlet extraction method 138
5.3.8 Thermal Gravimetric Analysis 1.40
5.3.9 Specific Surface Area 141
5.3.10 Molecular Weight 142
5.3.11 Other Methods 143
5.3.11.1 X-ray diffraction pattern 145
5.3.11.2 Particle size distribution using laser method 145
5.3.11.3 Molecular weight of the organic coating 146
5.3.11.4 Mass spectrometry 149
5.3.11.5 Atomic force microscopy 149
6 P o l y m e r C o a t i n g of P o w d e r s 151
6.1 Organic coating experiments 151
6.1.1 Butadiene and ethylene coating using both torch configurations 152
6.1.2 Acetylene coating performed on micron size metallic particles 152
6.1.2.1 Effect of metallic micron particles on the discharge in both
torch configurations 154
6.1.2.2 Operating conditions tried with the acetylene precursor . . . . 155
6.1.3 Organic coatings using acetylene, ethylene, butadiene and pyrrole
pre-cursors on fumed silica particles 156
6.2 Results of the organic coatings 158
C O N T E N T S
6.3.1 Effect of residence time on the deposited film with both torch
configu-rations 153
6.3.2 Effect of power density on deposited films with both torch configurations 167
6.3.3 Effect of the intermittent mode on deposited films with both torch
con-figurations 168
6.3.4 Difference between the coaxial configuration and the shell torches . . . . 173
6.4 Results of acetylene coating on micron size metallic particles 174
6.4.1 Effect of the intermittent mode on the deposited film using acetylene
monomer and the coaxial design 174
6.4.2 Effect of the afterglow powder injection on the deposited film using
acety-lene precursor and the coaxial design 179
6.4.3 Further characteristics of the deposited films on metallic aluminum
par-ticles using acetylene as a precursor 183
6.4.4 Summary of acetylene induced deposition on metallic aluminum particles 187
6.5 Results of acetylene, ethylene, butadiene and pyrrole induced coatings on the
fumed silica particles 188
6.5.1 Effect of residence time on deposited films of different monomers on the
fumed silica particles 188
6.5.2 Effect of power density on the deposited film with the use of different
monomers 192
6.5.3 Effect of the intermittent mode on deposited film using different monomersl97
6.5.4 Effect of the argon addition and the heating of the particles and the
gases prior to injection in the shell torch 198
6.5.5 Further characteristics of the deposited film on the fumed silica particles,
C O N T E N T S
6.5.6 Summary of the different monomers deposited on the fumed silica particles204
7 Silicon B a s e d C o a t i n g o n P o w d e r s 206
7.1 Inorganic coating experiments 206
7.1.1 Inorganic coatings of TEOS on macro aluminum particles using the
coaxial design 207
7.1.2 HMDSO coating on nano aluminum and nano silica particles, using the
shell design torch 208
7.1.2.1 Effect of nano aluminum particles on the shell electrode discharge208
7.1.2.2 Difference between nano oxide and nano metallic powders . . . 208
7.1.2.3 Tests conditions of the HMDSO on nano oxide and metallic
particles 211 7.2 Results of the inorganic coatings 211
7.3 Results of the TEOS on macro aluminum particles 212
7.3.1 Effect of the afterglow injection on the deposited film using TEOS as
monomer 212
7.3.2 Summary of the TEOS deposited on micron size aluminum particles . . 21.3 7.4 Results of the HMDSO on nano aluminum and silica particles, using the shell
design 2.1.4
7.4.1 Effects of the power density and the residence time on the deposits
ob-tained with the HMDSO 214
7.4.2 Effect of 02 : ( 02 + Ar) and HMDSO : 02 ratios on the deposits
obtained with the HMDSO 21.8
7.4.3 Further characteristics of the deposited films on the nano aluminum
C O N T E N T S
7.4.4 Summary of the HMDSO induced deposition on nano aluminum and
silica particles 228
8 Surface Functionalization E x p e r i m e n t s and R e s u l t s 229
8.1 Surface functionalization of polymer particles 229
8.2 Aging of surface treated polymer powders 231
8.3 Results of surface functionalization 231
8.3.1 Effect of the discharge gap and the power density on the surface
func-tionalization 232
8.3.2 Effect of the processing gas on the surface wettability 234
8.3.3 Effect of the treatment time on the surface wettability 235
8.3.4 Scaling up and treating up to 100 g/min 235
8.3.5 Aging effect on the processed powders in different environments 236
8.3.6 Summary of the U H M W P E functionalization experiments 237
C o n c l u s i o n s 2 3 8
List of Figures
2.1 Direct current discharge characteristics (Druyvesteyn and Pennning [1.940]) . . 5
2.2 Direct current discharge voltage distributions (Parsons [1970]) 6
2.3 Plane to plane corona discharge electrode 8
2.4 Cross-sectional view of the atmospheric pressure plasma jet (Babayan et al. [1998]) 9
2.5 An example of a DBD configuration (Kogelschatz [2003]) 1.0
2.6 Siemens' historical ozone discharge tube of 1857 (Kogelschatz [2003]) 15
2.7 Microdischarges of a plate ozonizer of 6 cm x 6 cm size within 20 ms exposure
time (Kogelschatz [2003]) 1.6
2.8 (a) (b) (c) (d) and (e) are different dielectric barrier discharge configurations
(Fanelli [2005]) 17
2.9 Sketch of a microdischarge and a simple equivalent circuit (Kogelschatz [2003]) 18
2.10 Starting Phase of a Microdischarge (1 bar 20% C02 / 80% H2) (a) an
elec-tron avalanche propagates towards the anode (b) Reverse elecelec-tron propagation
towards the cathode, Egli et al. [1998] 19
2.11 Cathode layer formation (a) Just before the peak of the total current (b) Peak
current, Egli et al. [1998] 20
2.12 Local field collapse in the area defined by surface discharge, Egli et al. [1998] . 20
LIST O F F I G U R E S
2.14 Diffuse DBD in a helium air mixture (Laroussi 1999, taken from Dr. Kogelschatz
presentation in ISPC17) 22
2.15 Paschen breakdown curves in different gases, Vollrath and Thomer [1967] . . . 24
2.16 The three consecutive stages of streamer breakdown, Fanelli [2005] 25
2.17 Configuration of discharge tubes in an ozone generator, Kogelschatz [2003] . . . 31
2.18 Sealed cylindrical and planar excimer lamp configurations, Kogelschatz et al.
[2000] taken from Dr. Kogelschatz presentation in ISPC 17 33
2.19 Schematic of fluorescent lamps based on xenon excimers, Kogelschatz [2003]
taken from Dr. Kogelschatz presentation in ISPC 17 34
2.20 Electrode and complete plasma display configuration, the observer is on the
front pannel (Kogelschatz [2003]) 35
2.21 Electrostatic precipitator principle (taken from Dr. Kogelschatz's presentation
i n t h e I S P C 1 7 ) 39
3.1 Growth rate of a — C : H films by PECVD as a function of ionization potential
of the precursor gas, Robertson [2002] 46
3.2 Overview of infrared absorption spectra of amorphous hydrogenated carbon. The negative bias voltage of the cathode on which the substrates were placed controls the character of the films and is noted with each spectrum along with the rf power driving the plasma. The topmost spectrum is that of tetrahedral amorphous carbon deposited from a plasma beam source under conditions which
yield the highest sp3 content, Ristein et al. [1998] 48
3.3 Experimental set up of the atmospheric R F barrier torch plasma jet used
by Churpita et al. [2003] for plasma deposition of thin oxide films 50
3.4 Experimental set up of the atmospheric pressure PECVD apparatus developed
L I S T O F F I G U R E S
3.5 Schematic diagram of the A P G D reactors used by Sawada and Kogoma [1997]
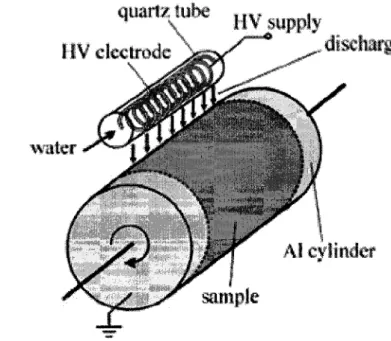
for the plasma treatment of silica particles 62
3.6 Set up used to treat sheets of U H M W P E in an atmospheric pressure DBD,
Bor-cia et al. [2005] and BorBor-cia et al. [2004c] (36
3.7 Set up used to treat powders of LDPE in a low pressure R F plasma, Bretagnol
et al. [2004] 69
3.8 Charged droplet collected on a positively charged substrate, Fridman et al. [2005] 72
4.1 (a) Design of the coaxial DBD torch working at atmospheric pressure (b) Photo
of the 1 mm torch in operation 78
4.2 (a) Technical 3D drawing of the shell electrode configuration (b) Photo of the
24 mm shell electrode in operation 80 4.3 Power density as a function of the applied voltage for both coaxial and shell
designs 82
4.4 Symbolic presentation of microdischarge activity and corresponding
voltage-charge Lissajous figure, Kogelschatz [2003] 85
4.5 (a) Sketch of the coaxial design (b) Sketch of the shell design 88
4.6 Shell design showing overal less heat transfer to the cooling circuit compared
with the coaxial one 91
4.7 Typical voltage signal from the power supply 91
4.8 ((a) Homogeneous plasma for the shell design in pure helium (b) Filamentary
plasma for the shell design in a mixture of helium, argon and oxygen 93
4.9 (a) Voltage and current traces for a homogeneous discharge with helium at 20 kHz and 5 kV for the shell configuration(b) Voltage and current traces for microdischarges with helium at 20 kHz and 7 kV for the coaxial configuration . 94
LIST O F F I G U R E S
4.10 Filamentary discharge of the coaxial configuration with pure helium and an
applied voltage of 6 kV 95
4.11 (a) Lissajous figure for the coaxial design with helium at 30 1/min, 6.4 kV and 60 W (b) Lissajous for the coaxial design with helium at 30 1/min acetylene at
1 1/min 11 kV and 420 W 95
4.12 (a) Voltage and current traces for one shell electrode with helium at 30 1/min, 5 kV and 30 W (b) Voltage and current traces for one shell electrode with helium
at 30 1/min, 9 kV and 130 W 97
4.13 Voltage and current traces for one shell electrode with helium at 30 1/min, 11
kV and 160 W 98
4.14 (a) Voltage and current for one shell at 6 kV and 50 W (b) Voltage and current
for two consecutive shells at 6 kV and 80 W 99
4.15 (a) Voltage and current figure for two separated shells at 6 kV and 80 W (b)
Voltage and current for three shells at 6 kV and 110 W 100
4.16 (a) Voltage and current for one shell at 9 kV and 130 W (b) Voltage and current
for two consecutive shells at 9 kV and 190 W 101
4.17 (a) Voltage and current for two separated shells at 9 kV and 190 W (b) Voltage
and current for three shells at 9 kV and 270 W 102
4.18 (a) Voltage and current for one shell at 11 kV and 160 W (b) Voltage and current
for two consecutive shells at 11 kV and 290 W 103 4.19 (a) Voltage and current for two separated shells at 11 kV and 290 W (b) Voltage
and current for three shells at 11 kV and 370 W 104
4.20 (a) Voltage and current for one shell at 5 kV and 30 W (b) Voltage and current
for two consecutive shells at 5 kV and 50 W 105
4.21 (a) Voltage and current for two separated shells at 5 kV and 50 W (b) Voltage
L I S T O F F I G U R E S
4.22 (a) Lissajous figures obtained with one shell electrodes at 5 kV with pure helium (b) Lissajous figures obtained with Two separated shells at 5 kV with pure helium 107
4.23 (a) Torch used to investigate the new Lissajous figures with an internal
diam-eter of 24 mm (b) Torch in operation 108
4.24 Exact dimensions of the torch used to investigate the new Lissajous figures
with an internal diameter of 24 mm 108
4.25 (a) Current-voltage diagram of the non-cooled torch used to investigate the new Lissajous figures performed at pure helium 5 kV and 20 W(b) The
correspond-ing charge-voltage diagram of the non-cooled torch 109
4.26 (a) Condensation of the ferrocene precursor at 8 kV and 200 W with higher color intensity at the first shell compared to the lower ones (b) Zoom on the condensation of the ferrocene precursor with higher intensity at the first shell
compared to the lower ones 114
4.27 No condensation of the ferrocene precursor is seen in the discharge zone at 10
kV and 370 W 115
4.28 Axial distribution of the rotational temperature, lonascut-Nedelcescu et al. [2007] 117
4.29 (a) Lissajous figure for the shell electrode at 5 kV and 20 W in pure helium just before powder addition (b) Lissajous figure few second after nano-aluminum
powder addition (c) Lissajous figure 5 minutes after the powder addition . . . . 1.19
4.30 (a) Lissajous figure for the shell electrode at 5 kV and 20 W in pure helium just before powder addition (b) Lissajous figure few second after
macro-aluminum powder addition 120
5.1 (a) Block diagram of the steps followed for powder coating in case of the coaxial geometry (b) Block diagram of the steps followed for powder coating in case of
L I S T O F F I G U R E S
5.2 Block diagram of the steps followed for polymer functionalization in case of the
shell geometry with upward directed gas flow 124
5.3 (a) Final setup used for the fumed silica feeding (PF'400—Prototype) (b) Sketch
drawing of the PF400-prototype 126
5.4 (a) Powder feeder PF300 (b) Cross section figure of the upper cylinder of the
PF300 127
5.5 (a) Vibrating feeder photo (b) Technical drawing showing the powder circular
path followed 128
5.6 (a) PFLinear-Vibration feeder photo (b) Sketch drawing of the feeder 129
5.7 (a) High speed gas probe connected to the reactor bottom to allow powder recirculation in the discharge zone (b) The setup used for powder recirculation
inside the shell electrode design 131
5.8 Illustration of the diffuse reflectance spectroscopy technique, Keckll [2001] . . . 133
5.9 (a) Quartz tube inserted in the metal mesh on the top of the filter paper (b)
Illustration of how to hang the powder bed on the balance 137
5.10 (a) Quartz tube hung on the balance (b) Balance tared with the quartz tube . 137
5.11 (a) and (b) Photos of the final set up 138
5.12 Soxhlet apparatus with a 500 ml round flask at the bottom, a place to put the
sample in the middle and a condensation part on the top 139
5.13 Change in the specific surface area with the thickness of the added deposit . . 143
5.14 Ubbelohde viscometer used to estimate the molecular weight of the polyethylene
powders (dimensions in millimeters) 144 5.15 XRD patterns showing no essentially difference between the powders, before
L I S T O F F I G U R E S
5.16 XRD patterns of the deposit on its own done in case of acetylene monomer
injection 146
5.17 Particle size distribution of aluminum particles coated with acetylene 147
5.18 Difference between conventional polymers and plasma polymers 147
5.19 Form and color of the polymer formed after acetylene monomer injection . . . . 148
5.20 AFM photo after 15 minutes of silicon wafer exposure to acetylene injection in
the plasma 150
6.1 (a) Charging and formation of brihgt spots in the coaxial design after macro alu-minum particles addition (b) Lichtenberg dust figure prooving particle charging. 154
6.2 (a) Discharge in the shell configuration just before macro aluminum particles addition (b) The discharge look once the macro aluminum particles were injected. 155
6.3 Amount of carbon deposited as a function of the residence time with both torch configurations in case of butadiene (Coaxial design: V=8kV, Pd—2 W/cm3,
<t>Si02= 2-10 g/min, ^c4if6=0.02 slpm, Shell design: V=9kV, Pd=2 W/cm3,
4>Si02= 3-10 g/min, ^ C4H6= 0 . 0 2 slpm) 159
6.4 Amount of carbon deposited as a function of the residence time with both torch configurations in case of ethylene (Coaxial design: V=10kV, P<2=3 W/cm3,
4>Si02= 1 g/min, C/>C2H2=0.02 slpm, Shell design: V=12kV, Pd=3 W/cm3,
§Si02=- 2 g/min, (/>c2ff2=0.02 slpm) 159
6.5 (a) Effect of the residence time on oxygen XPS atomic concentration and hy-drophobicity for the coaxial design with butadiene monomer (b) Effect of the residence time on oxygen XPS atomic concentration and hydrophobicity for the
L I S T O F F I G U R E S
6.6 (a) Effect of the residence time on oxygen XPS atomic concentration and hy-drophobicity for the coaxial design with ethylene monomer (b) Effect of the residence time on oxygen XPS atomic concentration and hydrophobicity for the
shell design with ethylene monomer 162
6.7 F T I R spectra of butadiene deposition using both torch configurations (Coaxial design: V=7kV, Pd=2 W/cm3, t = 0 . 7 s, shell design: V=9kV, Pd=2 W/cms) . 163
6.8 F T I R spectra of ethylene deposition using both torch configurations (Coaxial
design: V=10kV, Pd=4 W/cm3, shell design: V=12kV, Pd=3 W/cm3) 164
6.9 (a) (b) (c) and (d) TEM of the original silica powders 165
6.10 (a) and (b) TEM of the butadiene coated sample using the coaxial design at
t = 0 . 7 s 166 6.11 (a) and (b) T E M of the ethylene coated sample using the coaxial design at t = 0 . 7 s!66
6.12 Amount of carbon deposited as a function of the power density with both torch configurations in case of butadiene (Coaxial design: t=0.4 s, 4>si02= 2 g/min,
</>c4#6=0.02 slpm, Shell design: t=0.4 s, 4>si02= 4 g/min, <^c4#6=0.02 slpm) . 167
6.13 (a) Effect of the power density on oxygen XPS atomic concentration and hy-drophobicity for the coaxial design with butadiene monomer (b) Effect of the power density on oxygen XPS atomic concentration and hydrophobicity for the
shell design with butadiene monomer 169
6.14 (a) F T I R spectra of butadiene deposition using the coaxial configuration as a function of the power density ( t = 0 . 4 s)(b) F T I R spectra of butadiene deposited using the shell configuration as a function of the power density ( t—0.4 s) . . . 170
L I S T O F F I G U R E S
6.15 Amount of carbon deposited as a function of the intermittent mode time with both torch configurations and both monomers (Coaxial design-Butadiene: V=10kV, t = 0.3 s, 4>si02= 2 g/min, </>c4i/6 =0.02 slpm, Shell design-Butadiene:
V=9kV, t = 0.4 s, 4>si02= 4 g/min, <I>CAH&~^-^ slpm, Coaxial design-Ethylene:
V=10kV, t = 0.3 s, 4>si02= 1 g/min, ^c, 2H2 =0-01 slpm, Shell design-Ethylene:
V=10kV, t = 0.3 s, (j>si02= 3 g/min, (f>c2H2=0.02 slpm) 1.71
6.16 (a) F T I R spectra of butadiene deposited using both torch configurations as a function of the intermittent mode variation (t=0.3 s) (b) F T I R spectra of ethy-lene deposited using both torch configurations as a function of the intermittent
mode variation (t=0.3 s) 172
6.17 Amount of carbon deposited as a function of the intermittent mode time in case of acetylene monomer and the coaxial design (V=10kV, t = 0.3 s, (f>Ai— 2 g/min,
4>C2H2—0-01 slpm) 174
6.18 Effect of the intermittent mode duration on oxygen XPS atomic concentration
and hydrophobicity for the coaxial design with acetylene monomer 175
6.19 F T I R spectra of acetylene deposited on micron size aluminum particles using the coaxial design at different intermittent modes (V=10kV, t = 0.3 s, 4>AI= 2
g/min, </>c2#2=0.01 slpm) 176
6.20 SEM of the original Valimet H10 powder 177
6.21 (a) and (b) SEM of coated particles with no pulsing (c) and (d) SEM of coated
particles with long pulsing duration of 0.9 s On and 1 s Off 178
6.22 (a) SEM of coated particles at 0.2 s discharge On (b) SEM of coated particles
at 0.3 s discharge On 179 6.23 Amount of carbon deposited when injecting the powders in the afterglow region
using the acetylene as a monomer and the coaxial design (V=8kV, 4>AI— 0.2
L I S T O F F I G U R E S
6.24 F T I R spectra of acetylene deposition on micron size aluminum particles using the coaxial design at different afterglow injection positions(V=8kV, (f>Ai= 0.2
g/min, </>C2H2=:0.01 slpm) 181
6.25 (a) and (b) SEM of coated particles in the afterglow region at 1.5 mm (c) and
(d) SEM of coated particles in the afterglow region at 20 mm 182
6.26 a) and (b) SEM of coated particles in the afterglow region with application of
intermittent mode 183
6.27 Mapping on the sample injected in the afterglow region at 1.5 mm with the application of the intermittent mode, the red color corresponds to the Al and
the white to the carbon coat 183
6.28 TGA of polymer coated samples using acetylene as precursor 185
6.29 (a) and (b) Morphology of the yellow polymer deposit from acetylene monomer 186
6.30 F T I R spectra of the yellow polymer deposit from acetylene monomer 187 6.31 Amount of carbon deposited as a function of the residence time with different
monomers on fumed silica particles (In the case of ethylene: V=12kV, Pd=3 W/cm3, <j>si02 = 2 g/min, (/>c2#2=0.02 slpm, other monomers: V—9kV, Pd=2
W/cm3, <j>si02= 3-10 g/min, </>monOmer=0.02 slpm) 188
6.32 Effect of residence time on the oxygen XPS atomic concentration and
hydropho-bicity for different monomers 189
6.33 FTIR spectra of different monomers deposited at 0.7 s residence time (In case of ethylene: V=12kV, -Pd=3 W/cm3, <j>si02 = 2 g/min, (/>C2H2—0.02 slpm, other
monomers: V=9kV, Pd=2 W/cm3, 4>Si02— 3-10 g/min, <pmonomer=0-02 slpm) . 190
6.34 TGA of the butadiene and ethylene coated samples at 0.7 s residence time . . . 191 6.35 (a) and (b) T E M of the butadiene coated sample using the shell design at t = 0 . 7 si.92
L I S T O F F I G U R E S
6.37 (a) and (b) TEM of the pyrrole coated sample using the shell design at t = 0 . 7 s 193
6.38 (a) and (b) TEM of the acetylene coated sample using the shell design at t=0.7 s i 9 4
6.39 Amount of carbon deposited as a function of the power density with different
monomers used (t=0.4 s, 4>si02= 4 g/min) 194
6.40 Effect of the power density on oxygen XPS atomic concentration and
hydropho-bicity for ethylene, acetylene and butadiene monomer 195
6.41 (a) F T I R spectra of ethylene deposited as a function of the power density ( t = 0 . 4 s)(b) F T I R spectra of acetylene deposited as a function of the power
density ( t = 0 . 4 s) 196
6.42 Amount of carbon deposited as a function of the intermittent mode time with different monomers (In case of acetylene and butadiene: V=9kV, t = 0.4 s, 4>Si02= 4 g/rnin, (f>monomer=0.02 slpm, ethylene: V=10kV, t = 0.3 s, (f>si02= 3
g/min, ( / > C2H2= 0 . 0 2 slpm) 197
6.43 (a) F T I R spectra of butadiene deposited as a function of the intermittent mode variation (t=0.4 s) (b) F T I R spectra of ethylene deposited as a function of the
intermittent mode variation (t=0.3 s) 199
6.44 F T I R spectra of acetylene deposited as a function of the intermittent mode
variation (t=0.4 s) 200
6.45 Comparison between the amount of carbon deposited before and after argon addition with different monomers (In case of acetylene, pyrrole and butadiene: V=9kV, t = 0.4 s, (psi02= 4 g/min, </>mOnomer=0.02 slpm, ethylene: V=10kV,
t = 0 . 3 s, 4>si02= 3 g/min, (j>c2H2=0-02 slpm) 200
6.46 (a) and (b) TEM of the butadiene monomer deposited on the powders without any gas or powder heating (V=9kV, t = 0.4 s, 4>si02= 4 g/min, ^ C4H6= 0 . 0 2 slpm)201
L I S T O F F I G U R E S
6.47 (a), (b), (c) and (d) T E M of the butadiene monomer deposited on the powders after gas and powder heating at about 82°C (V=9kV, t = 0.4 s, 4>Si02— 4 g/min,
cf>CiH6=0-02 slpm) 202
6.48 (a), (b) and (c) T E M of the ethylene monomer deposited on the powders after gas and powder heating at about 82°C (V=10kV, t = 0 . 3 s, 4>sio2= 3 g/min,
</>c2#2=0-02 s lPm) 203
7.1 (a) Discharge in the shell configuration just before nano aluminum particles addition (b) The discharge look after 5 minutes of powder injection at 1 g/min 209
7.2 (a) No powder accumulation at the wall after injection of nano aluminum par-ticles (b) Powder accumulation on the wall of the shell electrode after a few
minutes of nano oxide powders injection 210
7.3 Amount of silicon and oxygen deposited when injecting the powders in the af-terglow region, using the TEOS as a monomer and the coaxial design (V=10kV,
0SiO2= 1 g/min, (pTEOS=0.001 slpm) 213
7.4 F T I R spectra of TEOS, deposited using the coaxial design on micron size alu-minum particles after injection either into the main discharge or into the
after-glow region 214
7.5 ((a) SEM of the sample injected in the main discharge (b) SEM of sample
injected in the afterglow 215
7.6 Amounts of silicon deposited as a function of the power density with both pow-der types in the case of the HMDSO (t=1.7 s, <J>AI= 1 g/min, <J){HMDSO/O2)—
0.04) 216
7.7 Effect of the change in power density on the powder hydrophobicity for both powder types in case of the HMDSO (t=1.7 s, <f>Powder= 1 g/min, 4>{HMDSO/02) =
LIST O F F I G U R E S
7.8 Effect of the change in residence time on the amount of silicon deposited on nano-aluminum particles with HMDSO (Pd=2.5 W/cm3, 4>AI= 1 g/min,
<t>(HMDSO/02)= °-0 4) 2 1 7
7.9 (a) F T I R spectra of HMDSO deposited on nano-aluminum particles as a func-tion of the power density ( t = 1 . 7 s)(b) F T I R spectra of HMDSO deposited on
fumed silica particles as a function of the power density ( t=1.6 s) 219
7.10 F T I R spectra of HMDSO deposited on nano-aluminum particles as a function
of the residence time (P<2=2.5 W/cm3) 220
7.11 (a) Effect of the argon amount on the amount of silicon deposited in case of the HMDSO precursor (t=1.7 s, 4>AI— 1 g/min, <J>{HMDSO/O2)— 0.04)(b) Effect
of the HMDSO amount on the amount of silicon deposited (t=1.7 s, 4>AI— 1
g/min, 4>(02/{02+Ar))"= 0-1) 221.
7.12 (a) F T I R spectra of HMDSO deposited on nano-aluminum particles as a func-tion of the amount of argon ( t = 1 . 7 s)(b) F T I R spectra of HMDSO deposited on nano-aluminum particles as a function of the amount of precursor ( t=1.6 s) 222
7.13 (a) and (b) Raw metallic nano powders of aluminum 223
7.14 (a)(b) (c) and (d) are the TEM of the HMDSO coated sample with 02/(02 +
Ar)=0.1, Pd = 2.5W/cm3, t = 1.7 s and HMDSO/02 = 0.04 224
7.15 (a) and (b) TEM of the powder deposited on the wall during the deposition
experiments performed with HMDSO 225
7.16 TGA of nano aluminum particles coated with HMDSO 227
8.1 (a) and (b) Filamentary nature of the discharge in the case of the surface
LIST O F F I G U R E S
8.2 The contact angle measurements and the corresponding amount of oxygen added on the suface as a function of the power density with both torch configurations (Coaxial design (1mm): t=0.06 s, <t>UHMWPE= 2 g/min, </>o2=0.1 slpm, Shell
design: t=0.06 5, <J>UHMWPE— 3 g/min, (/>o2=0.2 slpm) 232
8.3 Relationship between the molecular weight of the polyethylene and the intrinsic
viscocity 233
8.4 Heating different molecular weight of the P E powders to determine their
resis-tance to melting 234
8.5 Contact angle measurements and the corresponding amount of oxygen added on the suface as a function of the mole fraction of oxygen (t=0.06 s, 4>UHMWPE=
3 g/min, Pd=1.5 W/cm3) 235
8.6 The contact angle measurements and the corresponding amount of oxygen added on the suface as a function of the residence time (Pd—1-5 W/cm3, <f>UHMWPE=
3 g/min, <po2— 0-1 slpm) 236
8.7 Aging effect for two samples stored in different environments for 60 days (Pd=1.5
List of Tables
2.1 Advantages and Disadvantages of the different nonequilibrium plasma discharges 14 2.2 Typical properties of microdischarges in an air DBD of 1 mm gap and 1 bar,
Ko-gelschatz et al. [2000] 18
2.3 Differences between microdischarges and glow discharges generated at
atmo-spheric pressure 29
2.4 Typical operation conditions during DBD plasma treatment, Becker et al. [2005b] 38
3.1 Summary of nonequilibrium plasma particle coating at low pressure 74
3.2 Summary of nonequilibrium plasma particle coating at atmospheric pressure . . 75
4.1 Coaxial versus shell electrode geometry 83
4.2 Characteristics of the power supply with the torches 83
4.3 Comparison between capacitance calculated theoretically and from the lissajous 89
4.4 Examples of the calculation of energy dissipated in the water circuit for the
coaxial design 90
4.5 Data for estimating the average temperature using polymer powders 113 4.6 Calculation of the average temperature due to the discharge heating in the
L I S T O F T A B L E S
5.1 Infrared absorption band assignments for hydrocarbon films, Fanelli [2005]
and F T I R [2007] 134
5.2 Infrared absorption band assignments for inorganic and organic silicon films, FTIR
[2007] and Alexandrov et al. [2005b] and Moravej and Hicks [2005] 135
6.1 Monomer characteristics 152
6.2 Test conditions for butadiene and ethylene coatings using the coaxial and the
shell configurations (i/eedmg or feeding time is 5 min.) 153
6.3 Test conditions for acetylene coating on macro aluminum particle using the
coaxial configuration (tfee<nng is 5 min.) 156
6.4 Monomer characteristics used for the coating of fumed silica 1.57
6.5 Test conditions for different monomers producing coatings on fumed silica
pow-ders using the the shell configuration {tfeeding is 5 min.) .1.57
6.6 Amounts of carbon before and after toluene extraction 204
7.1 organometallic monomer characteristics 207
7.2 Tests condition used to coat the macro aluminum particles with TEOS 208
7.3 Test conditions of HMDSO coating on nano aluminum and fumed silica powders
using the shell configuration (tfeecnng is 5 min.) 211
7.4 Amount of carbon before and after toluene extraction in case of the HMDSO . 227
7.5 Thickness of the formed deposit based, on the BET using HMDSO precursor . 228
8.1 Test conditions for the surface functionalization of U H M W P E powders {tfee.dmg
is 15 min.) 231 8.2 The intrinsic viscocity measurements at different power densities 234
Chapter 1
Introduction
Nanoparticles have the characteristic of high specific surface area. Controlling the surface properties of nanoparticles offers vast opportunity for the use for a wide range of applica-tions. In radio frequency thermal plasmas where the production of nano particles has been successfully demonstrated, reliable and reproducible ways capable of coating particles in flight immediately after their synthesis, along with high productivities are urgently needed. The choosing of polymer-like materials to form thin coatings is a good solution as they conserve the high specific area of the nanoparticles. Also, such polymers have excellent optical prop-erties and are highly flexible. Coating nano particles is also very important in the case of pyrophoric nanoparticles where ignition is instanteneous when non passivated powders come into contact with air. Some of these pyrophoric metallic particles are used as propellants, in this case, polymer like coating materials are ideal as they protect the powders at low temper-ature. They are decomposed also at high temperature, so they keep their energetic properties and can be safely stored and handled at low temperatures (Ermoline et al. [2002]).
In addition, the wettability and functionality of the surface can be tailored for certain applications by the use of the appropriate macromolecules or certain chemical groups, such as hydroxyl, carboxyl or amino groups. Changing the wettability of the powders can also enhance their processing. This is especially the case when mixing with materials where different hydrophilicity properties are required. So, one can change the powders characteristics, from hydrophilic to hydrophobic and vice versa.
Dielectric Barrier Discharges (DBD), operating at atmospheric pressure, are excellent candidates for powder modification technology as they are ideal in surface treatment processes due to the presence of energetic electrons capable to excite atomic and molecular species as well as to break chemical bonds at fairly low temperature. This allows for the treatment of the surfaces without changing the bulk properties and also avoids any polymer decomposition taking place in coating processes. DBDs operating at atmospheric pressure, have the further advantage that they permit integration within the same unit of powder production, thereby avoiding both vacuum and low pressure intermediate treatment steps, which are both expensive and difficult to maintain. Another advantage of DBDs is that they can be scaled up to large installations without too much efforts. Typical examples of the industrial use of DBDs are found in ozone generators and plasma display panels (Kogelschatz [1995], Kogelschatz [2003]). In this present work, it is proposed to employ different designs of dielectric barrier discharge torches operating at atmospheric pressure. The applications investigated here include deposition of a thin organic and inorganic films on powders and surface functionalization of polymer powders to change their hydrophilicity.
In order to describe this work, one starts by presenting the nonequilibrium plasma pro-cesses with a more extended description of dielectric barrier discharges. The different modes of operation in the DBD, namely the microdischarge and homogeneous diffuse discharge modes, the different applications and the important phenomenon of particle charging are also pre-sented. The following chapter is dedicated to a literature survey covering the different dis-charge plasmas used for the coating and functionalization applications, especially with respect to powder treatments. The torch designs used in the present investigation, along with their electrical characteristics, are presented, followed by a chapter dedicated to powder feeders and the characterization techniques. The last three chapters cover various investigations performed for powder surface treatment with the objective of either coating the powder with a thin film or the surface modification of the treated powder without the addition of an external coating material.
Chapter 2
Nonequilibrium P l a s m a a n d
Dielectric Barrier Discharge (DBD)
It is important to well understand the properties of the different types of plasma in order to justify the choice of the dielectric barrier discharge to realize this project. Thus, a general classification between the thermal and nonequilibrium plasmas is given. The different nonequi-librium or cold plasma sources are then presented according to their operating pressure either at low pressure or at atmospheric pressure. After comparison of the different available sources, operation at atmospheric pressure and the use of the DBD is justified. A more detailed discus-sion is then dedicated to dielectric barrier discharges including their history and fundamentals. To emphasize the ease of the scaling up of DBDs, their many applications are enumerated along with a brief description. Finally, as our application involves the presence of metallic and non metallic particles in the discharge, a section is dedicated to particle charging, a process used on an industrial scale, the behaviour of fine particles in electrostatic processes.
2.1 Nonequilibrium plasma
Plasmas can generally be divided into two main groups according to the temperature reached by its species (electrons, ions, atoms, molecules,...): Thermal plasmas and nonequilibrium or cold plasmas. In considering the thermal plasma, also referred to as hot plasma, the electron and gas temperatures are in local thermodynamic equilibrium over the range of 104K. In
2.1 N o n e q u i l i b r i u m p l a s m a
nonequilibrium plasmas (cold plasma), the temperature of the electrons can be as high as 10 eV while the gas temperature typically remains much lower. It can even remain close to room temperature ( « 300K). Thus nonequilibrium plasma discharges are ideal for treating thermally sensitive materials, such as polymers and are thus examined here in detail.
Historically, nonequilibrium plasmas were mainly classified as low pressure plasmas (Fra-cassi et al. [1997]). More recently, various sources of nonequilibrium plasmas produced at at-mospheric pressure have been developed. Nonequilibrium plasma can be produced in the form of various types of low pressure glow and rf discharges as well as in corona, barrier and hollow cathode discharges at atmospheric pressure (Becker et al. [2005c]). Interest in atmospheric pressure plasmas is rapidly growing as the disadvantage of vacuum operation are overcome and thus it has become both easier and less expensive to scale up in industrial processes. Plasma users are actually more interested in nonequilibrium plasmas sources operating at at-mospheric pressure so that the particle coating process can be integrated with the synthesis of the ultrafine particles.
One starts with the description of the recently developed discharges at atmospheric pressure and presents a comparison with the dielectric barrier discharges to emphasize the advantage of the DBD in the realisation of this work. The term cold plasma was originally only used for low pressure discharges, recently for ceratin types of atmospheric-pressure discharges also. The principle of electrical breakdown and the main physical properties of each type of discharge are given. Finally, a detailed description of dielectric barrier discharges operating at atmospheric pressure is given.
2 . 1 . 1 L o w p r e s s u r e p l a s m a
The reason that nonequilibrium plasmas used to be assigned only to low pressure is that at pressures lower than 10 torr, the mean free path of the electrons is relatively long being estimated to be of the order of 50 to 100 mm, when compared to atmospheric pressure where it is of the order of 0.1 [ira. Therefore, inter-particle collisions are insufficient to bring about the state of thermal equilibrium at low pressures (Parsons [1970]).
2.1 N o n e q u i l i b r i u m p l a s m a
In applying a high electric field to gases at low pressure four types of discharges can be identified as illustrated in figure 2.1. This illustration has been reproduced many times in the literature. For this reason it may be appropriate to state the parameters employed in the original experiment. It was a discharge in 1 Torr Ne with planar electrodes of 10 cm2 area
and a 50 cm electrode separation.
100
-Current Density (A cm \
Figure 2.1 - Direct current discharge characteristics (Druyvesteyn and Pemming [1940])
the dark discharge or Townsend discharge (from B to D),
the glow discharge (from E to F),
the abnormal glow (from F to H) and
the arc discharge (starting at point K)
The dark discharge, also referred to as the Townsend discharge, is self sustaining and is characterized by the fact that the current density and the charge density in the plasma is so low
2.1 Nonequilibrium plasma
that it has practically no influence on the applied field. In this regime, an exponential growth of the electron density from the cathode to the anode is observed and practically the entire volume is filled with positive ions. At higher current densities the Townsend discharge changes to a glow discharge. Now, space charge fields play an important role and the voltage necessary to sustain the discharge drops to a few hundred volts. Then, there is a region of abnormal glow where the voltage is increasing with the current and finally as the current increases the transition to the arc discharge occurs and the required voltage drops considerably to about 10 V.
Many nonequilibrium plasmas, as used for materials processing operate in the glow discharge region. Glow discharges have also found widespread applications in fluorescent lamps and as a processing medium for surface modification and plasma enhanced chemical vapor deposition (PECVD) (Becker et al. [2005c] and Schutze et al. [1998]). The characteristics of a glow discharge are shown in figure 2.2.
3ZEZ> Potential Drop V < +
1
I Anode Fall ! /l I
DistanceF i g u r e 2.2 - Direct current discharge voltage distributions (Parsons [1970])
T h e p o t e n t i a l d r o p across t h e discharge c a n b e divided i n t o t h r e e d i s t i n c t sections,
namely the cathode fall, the positive column drop and the anode fall. The largest voltage drop occurs in the cathode region and is a function of cathode material and the nature of the
2.1 N o n e q u i l i b r i u m p l a s m a
gas used only. The proportion of the discharge zone occupied by the cathode fall is inversely proportional to the applied pressure. In wider gaps the largest distance is occupied by the positive column. The anode fall is approximately equal to the ionization potential of the gas. The positive column provides large volumes of quasi neutral nonequilibrium plasma (Parsons [1970]).
Low pressure discharges have low breakdown voltages, normally form a uniform glow over a large gas volume and have less tendency to produce arcing. They have high electron and charged species densities and are ideal for the processing of heat sensitive materials. In a weakly ionized gas at low pressure, the electron density ranges between 108-1013 cm~3.
For comparison, a hot plasma generally has electron densities of between 1016-1019 cm~3
(Lieberman and Lichtenberg [1994]). For a typical oxygen low pressure glow discharge, the concentrations of oxygen atoms and metastable oxygen molecules reach about 0.2 and 2 x 1013 cm"3 respectively. On the other hand, an arc discharge has oxygen atom concentrations
about 1015 cm~3 and metastable oxygen molecules up to 1018 cm~3 (Schiitze et al. [1998]).
It is thus instructive to compare atmospheric-pressure plasmas with these criteria, to determine if any of these sources can match the performance of the above described low-pressure discharges.
2 . 1 . 2 A t m o s p h e r i c - p r e s s u r e p l a s m a s
Also, at atmospheric pressure the discharge can be charcterized by the traditional classifica-tion performed at low pressure (figure 2.1). In general, the tendency of high pressure glow discharges is to operate at much higher current densities than those obtained in low pressure glow discharges.
Glow discharges at atmospheric pressure can only operate at high current densities with extremely thin cathode layers. High voltages are required for gas breakdown at 760 Torr and sometimes arcing can occur. Arcing is usually prevented in dielectric barrier and corona discharges as their energy uptake is limited and typically does not exceed 50 W/cm3. In other
2.1 N o n e q u i l i b r i u m p l a s m a
usually used to maintain the nonequilibrium nature of the plasma (Alexandrov et al. [2005b]). Several kinds of stable discharges operating at atmospheric pressure have been developed. Examples are corona, barrier, plasma jet and hollow cathode discharges.
2.1.2.1 C o r o n a discharges
Corona discharges are characterized by highly asymmetric electrode configurations as shown in figure 2.3 and appear as a luminous glow localized within the small space around the electrode tip. The high voltage applied to a small geometry electrode induces an intense field generating a nonequilibrium plasma. The most typical configurations of electrode systems used in practice to generate corona discharges are the pin to plane, the wire to plane or a wire at the axis of a cylinder.
- HV
Electrode
X
Ground
Electrode
Figure 2.3 - Plane to plane corona discharge electrode
The restricted volume of the active corona discharge has limited its applications in the materials processing field. Moreover, at atmospheric pressure arcing can occur above a certain current range.
Compared to low pressure discharges, the density of electrons is lower. Especially within the region between the two electrodes it reaches 106 cm~3 compared to 1013 cm~3 at low
pressures. The density of the metastable oxygen in an oxygen plasma is also lower, reaching 1012 cm~3 versus 1014 cm~3 for densities reported at low pressure. Finally, the breakdown
2.1 N o n e q u i l i b r i u m p l a s m a
voltage is much higher, being of the order of 5 kV for an electrode gap of 13 mm at atmospheric pressure, compared to 0.2 kV at lower pressures (Schutze et al. [1998]).
2.1.2.2 A t m o s p h e r i c pressure p l a s m a j e t s ( A P P J s )
This source consists of two concentric metal electrodes through which a mixture of helium, oxygen, and other gases flow. By applying 13.56 MHz R F power to the inner electrode at a voltage of between 100 - 250 V, the gas discharge is ignited at atmospheric pressure. In comparison to the low pressure discharges, the densities of the electron and the charged species are very similar, ranging from 1011 cmrz to 1012 cm~3. Also, in an oxygen plasma,
the densities of the metastable oxygen reach values up to 1016 cm~s, higher than the values
reported at low pressure. For the breakdown voltage an extremely low voltage can be used for ignition, values of not higher than 0.2 kV are used. The only drawback for this source is that the electrodes are made of metal and not dielectric, so arcing can occur easily. Figure 2.4 shows a typical set up for the A P P J (source Babayan et al. [1998]).
H»/OsGffl»lil \
Figure 2.4 - Cross-sectional view of the atmospheric pressure plasma jet (Babayan et al. [1998])
Hubicka et al. [2002] modified this torch by adding a dielectric layer to the bare metal electrodes, to thereby avoid the transition to the arc regime, the authors called it a barrier torch discharge plasma source. Their experiments showed that arcing was prevented and deposition was performed easily on conductive substrates. But the fact that the frequency range is high, the current limitation by the dielectric is limited. To obtain more benefit from the presence of the dielectric, operation in the range of kHz is preferred.
2.1 N o n e q u i l i b r i u m p l a s m a
2.1.2.3 Microhollow c a t h o d e ( M H C ) and capillary p l a s m a e l e c t r o d e ( C P E ) dis-charges
Two discharge types that have been used successfully to generate and maintain atmospheric pressure plasmas in air are the microhollow cathode (MHC) and the capillary plasma electrode (CPE) discharges. Common to both of these discharges is the fact that they are created in spatially confined geometries, whose critical dimensions are in the range of 10 — 500 \im.
A microhollow cathode discharge device consists of a cathode with small hole sizes and an arbitrarily shaped anode, the hole size being in the range of 250 \im. The C P E discharge uses electrodes with perforated dielectric covers.
Measurements conducted in MHC shows that an electron density in the order of 1015
cm"3 is created with higher values for discharges operating in the pulse mode. The gas
temperature in the air at atmospheric pressure was measured and found to be between 1700 and 2000 K for discharge currents of between 4 and 12 mA, and was lower for noble gases (Becker et al. [2005a]). There is a strong continuing research activity on these and similar devices. Scale up may present a problem for some of these devices.
2.1.2.4 Dielectric barrier discharges ( D B D )
Dielectric barrier discharges, also referred to as silent discharges, are self-sustained electrical gas discharges in which at least one of the electrodes is separated from the discharge space by a dielectric layer (figure 2.5)
High Voltage r
fr
AC Generator \ j i
Discharge Gap
TTT
Dielectric Barrier
• Ground Electrode
Figure 2.5 - An example of a DBD configuration (Kogelschatz [2003
2.1 N o n e q u i l i b r i u m p l a s m a
— 100 ns and being distributed in both space and time. These microdischarges are believed to be 100 \xm in diameter. Under certain conditions, depending mainly on the gas type and the electrical characteristics of the discharge, a diffuse DBD discharge can be obtained and no filaments are observed. The homogeneous glow is believed to provide a more homogeneous treatment of the surfaces in the case of the chemical vapor deposition process. On the other hand, the microdischarges DBD are widely used in pollution control and ozone generation and for the surface modification of plastic foils. In both regimes, the dielectric has an important function. It limits the amount of charge and energy imparted to an individual microdischarge and, at the same time, distributes the microdischarges over the entire electrode area. In the homogeneous DBD the dielectric limits the uniform current density. An intact dielectric guarantees that no spark or arc can occur in the discharge gap. Typically, DBDs are generated by sinusoidal voltage waves in the range of several kV. At very high frequencies the current limitation by the dielectric becomes less effective. For this reason DBDs are normally operated between line frequency and about 10 MHz (Kogelschatz [2003]).
Compared to the low pressure discharges, similar charged species densities are obtained giving values between 1012 to 1015 cm~3. The production of ozone is reported to be up to
1018 cm~3 Oz molecules, which is the highest value given by all the other sources operating
either at low or high pressure. This discharge is easy to ignite, reported breakdown voltages are between 5 and 25 kV, and the use of dielectrics prevents arcing.
DBDs have been successfully scaled up in case of ozone generation and plasma display panels.
2 . 1 . 3 C o m p a r i s o n b e t w e e n t h e a t m o s p h e r i c p r e s s u r e p l a s m a s o u r c e s
Generally, from the physics of the different sources developed at atmospheric pressure, one can expect that films obtained from those sources can be very similiar to those obtained at low pressure. However, there are usually some limitations or problems that need to be either overcome or addressed when performing coatings at high pressure (Alexandrov et al. [2005b]:
2.1 N o n e q u i l i b r i u m p l a s m a
• Partial pressures of precursors are about three orders of magnitude higher than in low
pressure systems and this can lead to a significant increase in the rate of homogeneous reactions; in many cases these reactions are responsible for the formation of powders deposited at the same time as the layers that grow heterogeneously on surfaces,.
• the significant higher precursor partial pressures can lead to higher film growth rates compared with low pressure processes, which in turn can cause depletion of an inter-mediate active species in the gas stream over the substrate resulting in poor thickness uniformity in the flow direction,
• mass transport limitations are much more significant than at lower pressure and this can cause poor thickness uniformity as well.
One has to take into account these limitations when developing equipment and choosing the precursors. To meet our objective i.e. the deposition of a polymer like material on particles in flight, one needs a source that:
• Can treat heat sensitive materials by maintaining low gas temperatures,
• operates easily at atmospheric pressure for the scaling up together with the synthesis unit,
• has both high electron and metastable species densities to help in the chemical reactions of the polymerization of the monomer,
• does not lead to arc formation in the presence of conductive particles such as metal particles
From all of the sources presented above, dielectric barrier discharges offer the most attractive route for the coating experiments needed to realize this work. Table 2.1 lists the advantages and disadvantages of each discharge type. One can clearly conclude that DBDs satisfy the conditions needed. The only real drawback is that an operation in the filamentary mode might possibly cause non homogeneous treatment of powders. However, a homogeneous
2.1 Nonequilibrium plasma
glow discharge can be obtained by taking certain precautions. More details on the principle and the conditions required to obtain a homogeneous plasma are discussed in the following section.
![Figure 2.9 - Sketch of a microdischarge and a simple equivalent circuit (Kogelschatz [2003])](https://thumb-eu.123doks.com/thumbv2/123doknet/5422321.126795/52.921.210.675.202.444/figure-sketch-microdischarge-simple-equivalent-circuit-kogelschatz.webp)
![Figure 2.17 - Configuration of discharge tubes in an ozone generator, Kogelschatz [2003]](https://thumb-eu.123doks.com/thumbv2/123doknet/5422321.126795/65.922.204.692.163.391/figure-configuration-of-discharge-tubes-ozone-generator-kogelschatz.webp)

![Figure 2.20 - Electrode and complete plasma display configuration, the observer is on the front pannel (Kogelschatz [2003])](https://thumb-eu.123doks.com/thumbv2/123doknet/5422321.126795/69.921.209.695.507.766/figure-electrode-complete-plasma-display-configuration-observer-kogelschatz.webp)
![Figure 3.1 - Growth rate of a — C : H Rims by PECVD as a function of ionization potential of the precursor gas, Robertson [2002]](https://thumb-eu.123doks.com/thumbv2/123doknet/5422321.126795/80.921.228.677.252.567/figure-growth-pecvd-function-ionization-potential-precursor-robertson.webp)
![Figure 3.4 - Experimental set up of the atmospheric pressure PECVD apparatus developed by Moravej and Hicks [2005]](https://thumb-eu.123doks.com/thumbv2/123doknet/5422321.126795/86.922.192.692.155.467/figure-experimental-atmospheric-pressure-pecvd-apparatus-developed-moravej.webp)