PHARMACOEPIDEMIOLOGY AND PRESCRIPTION
Patient- and physician-related risk factors for hyperkalaemia
in potassium-increasing drug
–drug interactions
Emmanuel Eschmann&Patrick E. Beeler&
Vladimir Kaplan&Markus Schneemann&
Gregor Zünd&Jürg Blaser
Received: 2 August 2013 / Accepted: 30 September 2013 / Published online: 23 October 2013 # Springer-Verlag Berlin Heidelberg 2013
Abstract
Purpose Hyperkalaemia due to potassium-increasing drug– drug interactions (DDIs) is a clinically important adverse drug event. The purpose of this study was to identify patient- and physician-related risk factors for the development of hyperkalaemia.
Methods The risk for adult patients hospitalised in the University Hospital Zurich between 1 December 2009 and 31 December 2011 of developing hyperkalaemia was correlated with patient characteristics, number, type and duration of potassium-increasing DDIs and frequency of serum potassium monitoring.
Results The 76,467 patients included in this study were prescribed 8,413 potentially severe potassium-increasing DDIs. Patient-related characteristics associated with the development of hyperkalaemia were pulmonary allograft [relative risk (RR) 5.1; p <0.0001), impaired renal function
(RR 2.7; p <0.0001), diabetes mellitus (RR 1.6; p =0.002) and female gender (RR 1.5; p =0.007). Risk factors associated with medication were number of concurrently administered potassium-increasing drugs (RR 3.3 per additional drug; p < 0.0001) and longer duration of the DDI (RR 4.9 for duration ≥6 days; p <0.0001). Physician-related factors associated with the development of hyperkalaemia were undetermined or elevated serum potassium level before treatment initiation (RR 2.2; p <0.001) and infrequent monitoring of serum potassium during a DDI (interval >48 h: RR 1.6; p <0.01). Conclusion Strategies for reducing the risk of hyperkalaemia during potassium-increasing DDIs should consider both patient- and physician-related risk factors.
Keywords Drug–drug interactions . Hyperkalaemia .
Monitoring . Potassium . Risk factors
Introduction
Although drug–drug interactions (DDIs) are often preventable, they constitute an important cause of adverse drug events
leading to increased morbidity and mortality [1–3].
Potassium-increasing DDIs are among the most common DDIs,
occurring in up to 10 % of hospitalised patients [4,5]. Such
DDIs may induce hyperkalaemia and life-threatening cardiac
arrhythmias [6].
It is a challenge for physicians to identify potentially harmful potassium-increasing DDIs due to polypharmacy and the increasing number of therapeutic agents. Computerized physician order entry (CPOE) technology in combination with clinical decision support systems (CDSS) may help the prescribing physician(s) to detect DDIs and alerts them to a potential problem. However, these systems are often based on DDI knowledge databases which do not take patient characteristics into account, resulting in a low specificity of
Electronic supplementary material The online version of this article (doi:10.1007/s00228-013-1597-2) contains supplementary material, which is available to authorized users.
E. Eschmann (*)
:
P. E. Beeler:
J. BlaserResearch Centre for Medical Informatics, Directorate of Research and Education, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland
e-mail: emmanuel.eschmann@usz.ch V. Kaplan
Division of Internal Medicine, Kreisspital für das Freiamt Muri, Spitalstrasse 144, 5630 Muri, Switzerland
M. Schneemann
Division of Internal Medicine, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland
G. Zünd
Directorate of Research and Education, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland
alerts, overalerting and alert fatigue [7–10]. In order to avoid alert fatigue the University Hospital Zurich has implemented on-demand DDI checks into the electronic
health record [11].
Several published studies have analysed the risk factors for developing hyperkalaemia during potassium-increasing DDIs, with a focus on patient-related risk factors, such as demographic parameters and comorbidities, and on medication, such as number and types of drugs involved
[12–18]. One study considered the effect of potassium
monitoring during potassium-increasing DDIs in outpatients
[19]. To our knowledge, no study has as yet investigated the
effect of physician-related risk factors, such as missing serum potassium measurement at the start of therapy and periodicity of serum potassium monitoring during drug therapy in hospitalised patients.
The purpose of this study was to identify patient- and physician-related risk factors for the development of hyperkalaemia. Such information may provide a foundation for the implementation of strategies for reducing the risk of hyperkalaemia during potassium-increasing DDIs without overalerting.
Methods
Setting
The University Hospital Zurich is a publicly owned, tertiary care academic medical centre with 850 beds and 35,000 hospital admissions per year. We included all in-patients from 1 December 2009 to 31 December 2011 except for patients on dialysis and those hospitalized exclusively in intensive care units.
The local research ethics committee approved the analyses, and patient consent was waived.
Drug–drug interactions and potassium-increasing drugs Drug–drug interactions were identified using the knowledge base galdat/hospINDEX (distributed by e-mediat AG, Berne, Switzerland—derived from ABDATA Pharma-Daten Service, Werbe- und Vertriebsgesellschaft Deutscher Apotheker, Eschborn, Germany), which tiers DDIs by severity (severity
level 1: recommended activity ‘contraindicated’; 2:
‘contraindicated as precaution‘,3: ‘monitoring or adaptation
required’, 4: ‘monitoring or adaption in case of risk factors’, 5:
‘monitoring as a precaution’, 6: ‘no activity required’) [20].
DDIs with severity levels 1 to 3 were considered to be severe. Potassium-increasing drugs (PIDs) were defined as drugs involved in severe potassium-increasing DDIs according to the knowledge base galdat/hospINDEX. Thus, PIDs included angiotensin-converting enzyme inhibitors (ACE inhibitors),
angiotensin antagonists (angiotensin-receptor blockers), direct renin inhibitors, immunosuppressive agents (calcineurin inhibitors), potassium-sparing diuretics (aldosterone-receptor antagonists and epithelial sodium channel blockers), potassium supplements and trimethoprim (ingredient of cotrimoxazole), irrespective of whether these drugs were ordered as a monotherapy or participated in a DDI.
Serum potassium measurements
Serum potassium levels were categorized into four groups: (1)
hypokalaemia: serum potassium ≤3.2 mEq/l [21]; (2)
reference range: ≥3.3 mEq/l to ≤4.5 mEq/l [22]; (3)
moderately increased: ≥4.6 mEq/l to ≤5.4 mEq/l; (4)
hyperkalaemia:≥5.5 mEq/l [12].
The following parameters, explored for their association with hyperkalaemia, need to be defined:
(1) Estimated glomerular filtration rate (GFR) was
calculated using the Cockcroft–Gault equation [23] with
the first serum creatinine level measured during DDI±
1 day. The kidney dysfunction categories ‘normal’,
‘mild’ and ‘moderate to severe’ were assigned to a
GFR of≥90 ml/min, 60 ml/min ≤ GFR < 90 ml/min
and GFR <60 ml/min, respectively [24].
(2) Blood transfusions were identified by ICD-9 (International Classification of Diseases, World Health Organization, Geneva, Switzerland) treatment code 99.0.
(3) Comorbidities were identified using ICD-10 discharge diagnosis codes E10, E11, E12, E13, and E14 for diabetes mellitus, code I10 for arterial hypertension, code I50 for heart failure, codes I50.11 and I50.12 for NYHA class I/II, codes I50.13 and I50.14 for NYHA class III/IV and code Z94.2 for pulmonary allograft.
Statistical analyses
Data analyses and statistical tests were performed using the R language and environment for statistical computing, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria). p levels of ≤0.05 were considered to be statistically significant. The two-tailed chi-square test was used to analyse categorical data of 2 × 2 contingency tables. p levels for multiple comparisons were adjusted using the Holm
correction [25]. Binomial confidence intervals were calculated
using the Clopper–Pearson method [26].
Results
We analysed data of 76,467 inpatients. Mean duration of hospitalisation for all patients and for patients with
characteristics are listed in the Electronic Supplementary
Material (ESM)1.
A total of 1,543,578 drugs were prescribed, resulting in 20.1 drugs per patient on average. PIDs accounted for 5.0 % of all drug prescriptions. Potassium supplements were the most frequently ordered PIDs (30 % of all PID prescriptions), followed by immunosuppressive agents (21 %), ACE inhibitors (20 %), angiotensin antagonists (14 %), trimethoprim (9 %), potassium-sparing diuretics (6 %) and direct renin inhibitors (0.3 %).
Drug–drug interactions
We identified 197,351 potential DDIs (2.6 per patient) in the included patients, of which 90,811 were classified as severe (1.2 per patient). The 8,413 potassium-increasing DDIs constituted the largest part of level-1 DDIs (972 of 1,402 DDIs) and also played an important role in level-3 DDIs (7, 441 of 84,093 DDIs).
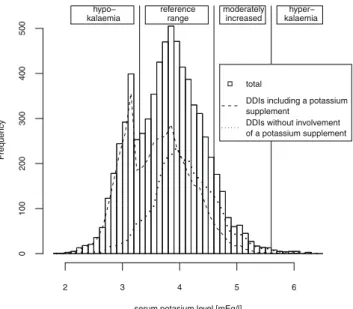
In 17 % of the potassium-increasing DDIs, treatment had been started in the absence of a serum potassium measurement within the preceding 48 h. Moreover, 60 potassium-increasing DDIs (0.7 %) were initiated despite the presence of hyperkalaemia. The histogram of the last serum potassium levels measured within 48 h prior to a
potassium-increasing DDI shows a bimodal distribution (Fig.1), with
one peak in the hypokalaemic range and another one in the reference range. Most of the therapies that were started with the patient in the hypokalaemic range included a potassium supplement, suggesting that these therapies were purposely prescribed to correct the low potassium level.
The frequency of daily serum potassium level measurements decreased from 72 % on the day before the DDI to 59 % on the first day of the DDI and remained between 46 and 53 % during
the next 2 weeks (the frequencies per day are shown in ESM2).
The intervals between serum level measurements during potassium-increasing DDIs showed a wide range (median interval 24 h) with 84.8 % of the serum level measurements
embedded in intervals of ≤48 h. Thus, 15.2 % of the
monitoring intervals exceeded 48 h (the frequency distribution
of serum potassium monitoring intervals is presented in ESM3).
Similar monitoring frequencies were observed in surgical and non-surgical specialties, with the median monitoring period in both groups being 1.0 days. In surgical specialties the monitoring intervals exceeded 48 and 96 h in 14.3 and 2.3 % of DDIs, respectively; in non-surgical specialties the respective numbers were 15.6 and 2.5 %.
Hyperkalaemia
The occurrences of hyperkalaemia were analysed after the exclusion of DDIs started in the presence of hyperkalaemia (60 DDIs in 28 patients), leaving 8,353 potassium-increasing
DDIs in 5,609 patients for further analysis. Hyperkalaemia was observed during 161 DDIs involving 78 patients. A further increased serum potassium level of >6.0 mEq/l occurred during 31 DDIs observed in 15 patients.
Two physician-related risk factors for hyperkalaemia were the consideration of the initial serum potassium level and the serum potassium monitoring frequency. First, the initiation of a potassium-increasing DDI with either an undetermined or an elevated (>4.5 mEq/l) serum potassium level (2,252 DDIs, 71 with hyperkalaemia during DDI) was associated with an increased relative risk (RR) of 2.2 of developing hyperkalaemia, compared to starting a DDI at normokalaemic or hypokalaemic levels (6,101 DDIs, 90 with hyperkalaemia during DDI; chi-square test p <0.001). Second, monitoring intervals exceeding 48 h during the interaction period were associated with a higher risk of hyperkalaemia: a new hyperkalaemia event was detected in 1.00 % (36 out of 3,635) of the serum potassium levels measured more than 48 h after the preceding measurement, compared to 0.61 % (122 out of 20,092) when levels were measured within 48 h (chi-square test: p <0.01). Three medication factors were associated with the development of hyperkalaemia:
(1) The higher the number of PIDs concurrently administered,
the higher the risk for hyperkalaemia (Table 1). Starting
with a RR of 1 for patients without PIDs, the RR increased to 3.9 for PID monotherapies (chi-square test: p <0.001) and 5.7 for therapies with two concurrent PIDs (p =0.040), peaking at 18.1 for three or more concurrent PIDs (p < 0.001). The triple PID combination of immunosuppressive agents + trimethoprim + ACE inhibitor achieved the
serum potasium level [mEq/l]
Frequency 2 3 4 5 6 0 100 200 300 400 500 hypo− kalaemia reference range moderately increased hyper− kalaemia total
DDIs including a potassium supplement
DDIs without involvement of a potassium supplement
Fig. 1 Last serum potassium level measured within 48 h prior to initiation of potassium-increasing drug–drug interactions (DDIs) with and without potassium supplements
highest RR of 29. Nevertheless, the absolute risk was low, with hyperkalaemia occurrences of 0.7, 1.0 and 3.3 % for patients treated with one, two or more than two PIDs, respectively.
(2) The duration of potassium-increasing DDIs was
significantly associated with hyperkalaemia (Table2).
(3) A high number of concurrently administered potassium-decreasing drugs were associated with a higher risk of
hyperkalaemia (Table2). Blood transfusions showed a
non-significant trend towards an increased risk of hyperkalaemia.
Five out of seven electronically available patient-related characteristics predicted the development of hyperkalaemia in
a univariate analysis (Table2): (1) female gender, (2) renal
impairment, (3) diabetes mellitus, (4) pulmonary allograft and (5) serum potassium level exceeding the normokalaemic range before onset of potassium-increasing DDI.
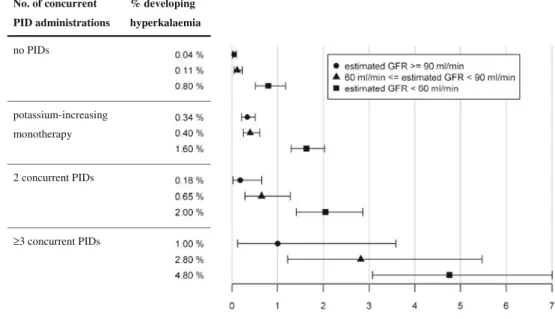
The effect of renal impairment on the risk of hyperkalaemia was analysed in greater detail as a function of the number of
concurrently administered PIDs (Fig.2). The risk significantly
increased for patients with moderate to severe kidney impairment compared to patients with mild kidney impairment. Hyperkalaemia observed during potassium-increasing DDIs was associated with a significantly increased risk of transfer
to the intensive care unit (ICU) or death (Table3). The risk of
death within 2 days after detecting hyperkalaemia was 5.3-fold higher than that for non-hyperkalaemic patients also treated with concurrent PIDs. The likelihood of being transferred to the ICU was 1.9-fold higher for hyperkalaemic patients than for non-hyperkalaemic ones. Furthermore, the length of hospitalisation increased significantly for patients with hyperkalaemia during potassium-increasing DDIs.
Discussion
A number of patient- and physician-related factors have been analysed to identify characteristics associated with the development of hyperkalaemia during potassium-increasing DDIs.
The results of this study demonstrate that physician-related factors affect the risk for developing hyperkalaemia in hospitalised patients. First, less frequent monitoring of serum potassium during DDI was associated with an increased risk of hyperkalaemia, likely because the rapid development of hyperkalaemia may be missed with longer monitoring
intervals [27] and subsequent therapy adjustments may be
delayed. Among our patient cohort, the frequency of serum potassium level measurements decreased after the first day of
DDI (as shown in ESM2), despite the finding that the risk of
hyperkalaemia increased with a longer DDI duration (Table2).
Secondly, the lack of serum level monitoring at onset of
potassium-increasing DDIs correlated with an increased risk
for developing hyperkalaemia (Table2). This association has
been reported in a previous study on outpatients [19].
Medication also affected the risk for hyperkalaemia. As expected, the risk for hyperkalaemia increased with the number of concurrently administered PIDs. Surprisingly, the risk of hyperkalaemia also rose with a growing number of concurrent potassium-decreasing drugs. A possible explanation for this finding might be that potassium-decreasing drugs were used to correct higher serum potassium
Table 1 Occurrences of hyperkalaemia as a function of concurrent administration of potassium-increasing drugs
Therapy Hyperkalaemia
No. of concurrent PID administrations and PIDs involved in concurrent administrations Total no. of patientsa No. of patients Per total No PID administration 30,723 56 0.2 % PID monotherapyb 17,010 122 0.7 %
Concurrent administration of 2 PIDs 4,221e 44 1.0 %
ACE inhibitor or angiotensin antagonist + potassium supplement
2,311 24 1.0 %
ACE inhibitor or angiotensin antagonist + potassium-sparing diuretic 824 9 1.1 % Trimethoprimc+ PIDs 418 3 0.7 % Potassium-sparing diuretic + potassium supplement 380 6 1.6 %
Immunosuppressive agent + PID 353 2 0.6 %
Concurrent administration of 3 or more PIDsd
1,028 34 3.3 %
ACE inhibitor or angiotensin antagonist + potassium-sparing diuretic + potassium supplement
380 10 2.6 %
Immunosuppressive agent + trimethoprimcplus PID
359 19 5.3 %
ACE inhibitor or angiotensin antagonist + potassium supplement + trimethoprimc
245 2 0.8 %
PIDs, Potassium-increasing drugs; ACE, angiotensin converting enzyme
a
Patients with at least one serum potassium level measurement within 48 h prior to onset of therapy, with the last value not being hyperkalaemic (no time restriction for patients with no PID administration)
b
Involved the PIDs (in decreasing order of monotherapy leading to h y p e r k a l a e m i a ) : p o t a s s i u m s u p p l e m e n t s , t r i m e t h o p r i m , immunosuppressive agents (calcineurin inhibitors), ACE inhibitors, angiotensin antagonists, potassium-sparing diuretics and direct renin inhibitors
c
Trimethoprim is part of the combination antibiotic cotrimoxazole (sulfomethoxazole and trimethoprim) used as therapy or prophylaxis for certain infections
dConcurrent DDIs with <100 patients exposed were not considered eTotal of patients exposed– sum of patients exposed, as the same patient
might have been considered for two different potassium-increasing DDI groups if they were not overlapping
Ta b le 2 Development o f hyperkalaemia durin g potass ium-increasing D DIs as a functio n o f selected p arameters Group Characteristic Property T otal no. of potas sium-incr ea sing DD Is No. o f p otass ium-incr ea sing DD Is wi th hyperkalaemia P ercen tage of DD Is w ith h yperkalaemia Rel ati ve ri sk (95 % CI ) Ch i-square test O v er al l 8,353 161 1.9 Demographics Age (years ) (in rounded-of f quartiles ) <57 2 ,147 54 2.5 1 .00 0 .0984 ≥ 5 7 , < 66 2,076 32 1.5 0 .62 (0.40 –0.95 ) ≥ 6 6 , < 75 1,983 39 2.0 0 .79 (0. 5 2– 1.19) ≥ 7 5 2,147 36 1.7 0 .67 (0. 4 4– 1.02) Gender M ale 5 ,041 80 1.6 1 .00 0 .0067* Female 3,312 81 2.5 1 .53 (1. 1 3– 2.07) T h er apy S eve ri ty le ve l o f potassium-increasing D DI a 1 9 6 8 20 2.1 1 .00 0 .8341 3 7 ,385 141 1.9 0 .92 (0. 5 8– 1.47) Duration of potassium-increasing D DI (days) (in rounded-of f quartiles) <1 1,938 14 0.7 1 .00 < 0.000 1* ≥ 1 , <3 2,506 29 1.2 1 .60 (0. 8 5– 3.01) ≥ 3 , <6 1,856 46 2.5 3 .37 (1. 8 6– 6.1 1 ) ≥ 6 2 ,053 72 3.5 4 .72 (2. 6 7– 8.35) Number of concurrent pot assi um-d ec re asi n g drugs b 0 2 ,092 38 1.8 1 .00 0 .0027* 1 4 ,150 64 1.5 0 .85 (0. 5 7– 1.27) ≥ 2 2 ,1 1 1 59 2.8 1 .52 (1. 0 2– 2.28) Re cent b lood tra n sfu sion c No 8,000 148 1.9 1 .00 0 .0243 Y es 3 5 3 13 3.7 1 .96 (1. 1 2– 3.41) Comorbi d itie s R en al fu ncti on (e st imate d G F R) (i n m l/min) ≥ 9 0 2,429 29 1.2 1 .00 < 0.000 1* ≥ 6 0 to < 0 2,313 38 1.6 1 .37 (0. 8 5– 2.21) <60 2 ,727 87 3.2 2 .62 (1.73-3.98) Unknown d 88 4 7 0.8 0 .67 (0. 2 9– 1.51) Diabetes mellitus N o 6 ,231 103 1.7 1 .00 0 .0024* Y es 2 ,122 58 2.7 1 .64 (1. 1 9– 2.25) Primary arterial hypertensi on No 3,855 71 1.8 1 .00 0 .6545 Y es 4 ,498 90 2.0
Ta b le 2 (continued ) Group Characteristic Property T otal no. of potas sium-incr ea sing DD Is No. o f p otass ium-incr ea sing DD Is wi th hyperkalaemia P ercen tage of DD Is w ith h yperkalaemia Rel ati ve ri sk (95 % CI ) Ch i-square test 1.08 (0. 8 0– 1.48) lung trans p lant No 7,757 1 1 6 1 .5 1.00 <0.000 1* Y es 5 9 6 45 7.6 4 .76 (3. 4 1– 6.66) Progression parameters Last seru m p o tass ium before onset of DDI e Hypokalaemic 1,390 21 1.5 1 .03 (0. 6 3– 1.67) <0.000 1* Normokalaemic 4,71 1 6 9 1 .5 1.00 >4.5 mEq/l 8 1 5 36 4.4 2 .93 (1. 9 7– 4.36) Unknown d 1,437 35 2.4 1 .65 (1. 1 0– 2.46) Department Sur g ical specia lties 3,408 63 1.8 1 .00 0 .7232 Non-sur g ic al spe cia lties 4,945 98 2.0 1 .07 (0. 7 8– 1.46) *Si gnif ica nt using the H o lm co rr ec tion for mul tipl e tes ting (w ith α = 0 .05, n = 12) DD I, D rug –drug interaction; GFR , gl omer ular fil tra tion rate ; CI, conf idenc e inte rva l a C o -ad m inistration o f potassium-s paring diuretics + potassium supplem en ts is the o nly severity level 1 (“ contraindicated ”) p otass ium-increasing D DI lis ted in the used knowledge database. A ll other potassium-increasing D DIs inc lude d in this study ar e class ifi ed as se v er ity le vel 3 DDI s (“ monitoring or adaptation required ”) b P rescriptions of kaliuretic d iuretics coexisting at onset of potas sium-increasing DDIs c Administered between th e d ay prior to the DDI and the end of DDI d N o t consi d er ed in chi -squa re te st eMaximum o f 4 8 h before onset of DDI
levels. Thirdly, the level of severity of the involved DDIs was not predictive for the likelihood of developing hyperkalaemia. This might be due to the frequent usage of the contraindicated level-1 potassium-increasing DDI (i.e. the combination of potassium-sparing diuretics with potassium supplements) to
correct a hypokalaemia (Fig.1) [10] including a high degree
of physician awareness [28]. The agreement on whether these
DDIs should indeed be labelled contraindicated is low [29,30].
Finally, the results of our study confirmed that specific patient-related characteristics are associated with the
development of hyperkalaemia. These include moderately or
severely impaired renal function (Fig.2), diabetes mellitus [6,
31], pulmonary allograft (which was associated with the highest
risk mainly due to PID combinations of immunosuppressive
agents, trimethoprim and ACE inhibitor) [32, 33], female
gender (confirming another analysis of gender as a predictor
of drug-induced hyperkalaemia [34]) and elevated serum
potassium level (>4.5 mEq/l) at onset of the DDI.
In contrast to other studies, we were unable to establish an association between hyperkalaemia during potassium-increasing DDIs and age or hospitalisation in surgical versus
non-surgical specialties [12].
There are limitations to our analysis. Dosing of PID prescriptions was not considered. Only DDIs between two specific drugs were explored. PIDs not listed in the DDI knowledge base, such as nonsteroidal anti-inflammatory drugs or heparin, were not taken into account.
The association between the presence of hyperkalaemia during potassium-increasing DDIs and transfer to the ICU or death within 2 days was considered to be statistically significant. However, the positive correlation does not imply causation, since the relationship is influenced by comorbidities and therapies. Overall, the risk of developing hyperkalaemia was low, occurring only in one of 50 potassium-increasing
DDIs in our study. This is in line with other reports [28].
Despite infrequent occurrences, hyperkalaemias must be detected in order to avoid severe complications. Clinical decision support systems may help the prescribing physicians to adjust the therapy early. However, unspecific alerts will lead to alert fatigue
[7–9]. The results of our study improve our understanding of
factors associated with hyperkalaemia [12] and add
physician-related factors, which may help to design more specific alerts.
Table 3 Correlation of death, transfer to intensive care unit and length of stay with the presence and absence of hyperkalaemia during potassium-increasing drug–drug interactions
Effect Patients with
hyperkalaemia during potassium-increasing DDIs Patients without hyperkalaemia during potassium-increasing DDIs Death* 5/103 (4.9 %)a 50/5,506 (0.9 %)b Transfer to ICU* 9/103 (8.7 %)a 245/5,506 (4.5 %)b Length of hospitalisa-tion (days)** 26.8±26.5c 12.2±13.8c
*Significant increase at p <0.05 (chi-square test); **significant increase at p <0.001 (Mann–Whitney test)
a
Death or transfer to ICU within 2 days of occurrence of hyperkalaemia
b
Death or transfer to ICU during or <2 days after potassium-increasing DDI
c
Mean ± standard deviation
No. of concurrent PID administrations % developing hyperkalaemia no PIDs potassium-increasing monotherapy 2 concurrent PIDs ≥3 concurrent PIDs Fig. 2 Occurrences of
hyperkalaemia as a function of the number of concurrently administered potassium-increasing drugs (PIDs) categorized by the estimated glomerular filtration rate (GFR); inclusion and exclusion criteria based on those of Table1, whereby only patients for whom an GFR estimation was available were considered. /Symbols Mean, Lines encompass a 95 % confidence interval
Conclusions
Strategies for the prevention of hyperkalaemia during treatment with potassium-increasing drugs should consider both patient- and physician-related characteristics. Patient-related factors include impaired renal function, diabetes mellitus and pulmonary allograft. Risk factors associated with medication include a higher number of concurrently administered PIDs and a longer DDI duration. Physician-related factors include undetermined or elevated serum potassium levels before the initiation of treatment and inadequate frequency of potassium monitoring during DDIs. Therefore, the systematic measuring of serum potassium levels before therapies involving potassium-increasing DDIs are prescribed and periodic monitoring are essential for reducing the risk of hyperkalaemia.
Disclosure of conflict of interests None.
References
1. Kohn LT CJ, Donaldson MS (eds) (1999) To err is human. Building a safer health system. Committee on Quality in Health Care, Institute of Medicine, Washington D.C.
2. Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM (2008) Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med 168(17): 1890–1896. doi:10.1001/archinternmed.2008.3
3. Hamilton RA, Briceland LL, Andritz MH (1998) Frequency of hospitalization after exposure to known drug–drug interactions in a Medicaid population. Pharmacotherapy 18(5):1112–1120
4. Sanchez Munoz-Torrero JF, Barquilla P, Velasco R, Fernandez Capitan Mdel C, Pacheco N, Vicente L, Chicon JL, Trejo S, Zamorano J, Lorenzo Hernandez A (2010) Adverse drug reactions in internal medicine units and associated risk factors. Eur J Clin Pharmacol 66(12):1257–1264. doi:10.1007/s00228-010-0866-6
5. Zwart-van Rijkom JE, Uijtendaal EV, ten Berg MJ, van Solinge WW, Egberts AC (2009) Frequency and nature of drug-drug interactions in a Dutch university hospital. Br J Clin Pharmacol 68(2):187–193. doi:
10.1111/j.1365-2125.2009.03443.x
6. Gennari FJ (2002) Disorders of potassium homeostasis. Hypokalemia and hyperkalemia. Crit Care Clin 18(2):273–288 7. van der Sijs H, Aarts J, Vulto A, Berg M (2006) Overriding of drug
safety alerts in computerized physician order entry. J Am Med Inform Assoc 13(2):138–147. doi:10.1197/jamia.M1809
8. Smithburger PL, Buckley MS, Bejian S, Burenheide K, Kane-Gill SL (2011) A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin Drug Saf 10(6): 871–882 doi:10.1517/14740338.2011.583916
9. Abarca J, Colon LR, Wang VS, Malone DC, Murphy JE, Armstrong EP (2006) Evaluation of the performance of drug-drug interaction screening software in community and hospital pharmacies. J Manag Care Pharm 12(5):383–389
10. Oertle M (2012) Frequency and nature of drug-drug interactions in a Swiss primary and secondary acute care hospital. Swiss Med Wkly 142:w13522. doi:10.4414/smw.2012.13522
11. Beeler PE, Eschmann E, Rosen C, Blaser J (2013) Use of an on-demand drug-drug interaction checker by prescribers and consultants:
a retrospective analysis in a swiss teaching hospital. Drug Saf 36(6): 427–434. doi:10.1007/s40264-013-0022-1
12. Uijtendaal EV, Zwart-van Rijkom JE, van Solinge WW, Egberts TC (2011) Frequency of laboratory measurement and hyperkalaemia in hospitalised patients using serum potassium concentration increasing drugs. Eur J Clin Pharmacol 67(9):933–940. doi: 10.1007/s00228-011-1028-1
13. Zopf Y, Rabe C, Neubert A, Janson C, Brune K, Hahn EG, Dormann H (2009) Gender-based differences in drug prescription: relation to adverse drug reactions. Pharmacology 84(6):333–339. doi:10.1159/ 000248311
14. Klein U, Klein M, Sturm H, Rothenbuhler M, Huber R, Stucki P, Gikalov I, Keller M, Hoigne R (1976) The frequency of adverse drug reactions as dependent upon age, sex and duration of hospitalization. Int J Clin Pharmacol Biopharm 13(3):187–195
15. Henz S, Maeder MT, Huber S, Schmid M, Loher M, Fehr T (2008) Influence of drugs and comorbidity on serum potassium in 15 000 consecutive hospital admissions. Nephrol Dial Transplant 23(12): 3939–3945. doi:10.1093/ndt/gfn380
16. Johnson ES, Weinstein JR, Thorp ML, Platt RW, Petrik AF, Yang X, Anderson S, Smith DH (2010) Predicting the risk of hyperkalemia in patients with chronic kidney disease starting lisinopril. Pharmacoepidemiol Drug Saf 19(3):266–272. doi:10.1002/ pds.1923
17. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA (2004) Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 351(6): 543–551. doi:10.1056/NEJMoa040135
18. Muzzarelli S, Maeder MT, Toggweiler S, Rickli H, Nietlispach F, Julius B, Burkard T, Pfisterer ME, Brunner-La Rocca HP (2012) Frequency and predictors of hyperkalemia in patients≥60 years of age with heart failure undergoing intense medical therapy. Am J Cardiol 109(5):693–698. doi:10.1016/j.amjcard.2011.10.027
19. Raebel MA, Ross C, Xu S, Roblin DW, Cheetham C, Blanchette CM, Saylor G, Smith DH (2010) Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 25(4):326–333. doi:10.1007/s11606-009-1228-x
20. Hansten PD, Horn JR, Hazlet TK (2001) ORCA: OpeRational ClassificAtion of drug interactions. J Am Pharm Assoc (Wash) 41(2):161–165
21. Ho JM, Juurlink DN, Cavalcanti RB (2010) Hypokalemia following polyethylene glycol-based bowel preparation for colonoscopy in older hospitalized patients with significant comorbidities. Ann Pharmacother 44(3):466–470. doi:10.1345/aph.1M341
22. Bonini P, Ceriotti F, Keller F, Brauer P, Stolz H, Pascual C, Garcia Beltran L, Vonderschmitt DJ, Pei P (1992) Multicentre evaluation of the Boehringer Mannheim/Hitachi 747 analysis system. Eur J Clin Chem Clin Biochem 30(12):881–899
23. Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
24. K/DOQI (2002) Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39[2 Suppl 1]:S1–S266
25. Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat Theory Appl 6:65–70
26. Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustratied in the case of the binomial. Biometrika 26(4):404– 413. doi:10.1093/biomet/26.4.404
27. Indermitte J, Burkolter S, Drewe J, Krahenbuhl S, Hersberger KE (2007) Risk factors associated with a high velocity of the development of hyperkalaemia in hospitalised patients. Drug Saf 30(1):71–80
28. Ponce SP, Jennings AE, Madias NE, Harrington JT (1985) Drug-induced hyperkalemia. Medicine (Baltimore) 64(6):357–370 29. Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, Van
provided in four drug interaction compendia. J Am Pharm Assoc 44(2):136–141
30. Wang LM, Wong M, Lightwood JM, Cheng CM (2010) Black box warning contraindicated comedications: concordance among three major drug interaction screening programs. Ann Pharmacother 44(1):28–34. doi:10.1345/aph.1M475
31. Uribarri J, Oh MS, Carroll HJ (1990) Hyperkalemia in diabetes mellitus. J Diabet Complications 4(1):3–7
32. Schuurmans MM, Tini GM, Zuercher A, Hofer M, Benden C, Boehler A (2012) Practical approach to emergencies in lung
transplant recipients: how we do it. Respiration 84(2):163–175. doi:
10.1159/000339345
33. Knoop C, Rondelet B, Dumonceaux M, Estenne M (2010) Medical complications of lung transplantation. Rev Pneumol Clin 67(1):28– 49. doi:10.1016/j.pneumo.2010.08.002
34. Ramirez E, Rossignoli T, Campos AJ, Munoz R, Zegarra C, Tong H, Medrano N, Borobia AM, Carcas AJ, Frias J (2012) Drug-induced l i fe- thr eate ning pot assiu m dist urbanc es detected by a pharmacovigilance program from laboratory signals. Eur J Clin Pharmacol 69(1):97–110. doi:10.1007/s00228-012-1303-9