HAL Id: hal-02441466
https://hal.archives-ouvertes.fr/hal-02441466
Submitted on 15 Jan 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Target cell-specific modulation of neuronal activity by
astrocytes
A Kozlov, M Angulo, E. Audinat, S. Charpak
To cite this version:
A Kozlov, M Angulo, E. Audinat, S. Charpak. Target cell-specific modulation of neuronal activity
by astrocytes. Proceedings of the National Academy of Sciences of the United States of America ,
National Academy of Sciences, 2006, �10.1073/pnas.0603741103�. �hal-02441466�
Target cell-specific modulation of neuronal activity
by astrocytes
A. S. Kozlov*†, M. C. Angulo*, E. Audinat, and S. Charpak
Laboratory of Neurophysiology, Institut National de la Sante´ et de la Recherche Me´dicale U603 and Centre National de la Recherche Scientifique FRE2500, Ecole Supe´rieure de Physique et de Chimie Industrielles, and Universite´ Paris Descartes, 75006 Paris, France
Communicated by A. James Hudspeth, The Rockefeller University, New York, NY, May 8, 2006 (received for review April 4, 2006) Interaction between astrocytes and neurons enriches the behavior
of brain circuits. By releasing glutamate and ATP, astrocytes can directly excite neurons and modulate synaptic transmission. In the rat olfactory bulb, we demonstrate that the release of GABA by astrocytes causes long-lasting and synchronous inhibition of mitral and granule cells. In addition, astrocytes release glutamate, leading to a selective activation of granule-cell NMDA receptors. Thus, by releasing excitatory and inhibitory neurotransmitters, astrocytes exert a complex modulatory control on the olfactory network. glutamate兩 GABA 兩 inhibition 兩 olfactory bulb 兩 synchronization
A
comprehensive description of brain circuits must include interactions between neurons and glial cells. Studies in different preparations have shown that glial cells communicate with neurons, respond to their activity, and affect neuronal behavior by releasing various neuroactive substances (for review, see refs. 1 and 2). Astrocytes, the predominant type of glial cells in the central nervous system, form a highly organized multi-cellular syncytium extending throughout the gray matter (3, 4). Calcium imaging in acute brain slices and in vivo has demon-strated that astrocytes can generate various patterns of activity, either local and spatially restricted to processes of individual cells or coordinated between adjacent cells and propagating across the brain tissue (5–12). Modeling indicates that coupling of astrocytic and neuronal activities may give rise to membrane potential instability and oscillations (13). Recently, excessive astrocytic release of excitatory neurotransmitters was shown to lead to hyperexcitability and seizures (14).Glutamate release by astrocytes has been well documented in both cultures and acute brain slices (for review see refs. 1 and 2). In hippocampal slices, astrocytes release glutamate onto pyra-midal neurons to evoke phasic and tonic currents mediated by NMDA receptors (15–18). In contrast, little is known regarding the role of astrocytes in neuronal inhibition. One study reported a potentiation of synaptic inhibition of the CA1 pyramidal neurons in the rat hippocampus, manifested as an increased frequency of inhibitory postsynaptic currents (19). This form of neuronal inhibition was proposed to depend on astrocytic cal-cium signaling and on activation of GABAergic interneurons, presumably by astrocytic glutamate. Another mechanism of neuronal inhibition was reported in the supraoptic nucleus: In response to a decrease of extracellular osmolarity within a physiological range, astrocytes were shown to release taurine and, thus, to activate neuronal glycine receptors (20).
The mammalian olfactory bulb is a valuable system to explore the diversity of neuroglial interactions because it contains many different types of neurons and astrocytes and because most of its neuronal circuits are characterized in detail and their function is well understood (for a review, see ref. 21). In this study, by using acute slices of the rat olfactory bulb, we demonstrate direct and selective excitation and inhibition of neurons by astrocytes. By releasing GABA, astrocytes evoke hyperpolarizing inhibitory currents in mitral and granule cells. These currents may occur synchronously in adjacent neurons and can block neuronal firing for several hundred milliseconds. In addition, astrocytes release
glutamate that activates NMDA receptors of granule cells. This target cell-specific modulation of neuronal activity adds to the complexity of neuro–glial interactions.
Results
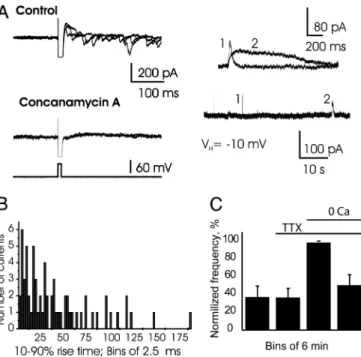
Slow GABAergic Inhibition of Mitral Cells.Prolonged intracellular recording from mitral cells revealed the spontaneous occurrence of rare, long-lasting inhibitory events. These slow hyperpolar-izations, which could block the discharge of mitral cells (Fig. 1A), had a mean rise time of 47.2⫾ 4.2 ms (range: 7.6–165 ms; n ⫽ 73 events; here and throughout the text, see figure legends for additional statistics). Corresponding slow outward currents (SOCs) were detected in voltage-clamp mode at a holding membrane potential of⫺50 mV, with an equilibrium potential for chloride ions (ECl⫺) of ⫺78 mV. Their reversal potential
shifted with ECl⫺as expected for currents carried by chloride
ions (Fig. 1B; nⴝ 7 cells). SOCs were sensitive neither to 10M strychnine nor to 50M (1,2,5,6-tetrahydropyridin-4-yl) meth-ylphosphinic acid (TPMPA), antagonists of glycine and GABAC
receptors, respectively (n ⫽ 4 for each drug). In particular, normalized frequency of SOCs was 98.4⫾ 2.3% in control and 71⫾ 17% in 50 M TPMPA (P ⫽ 0.13 with ANOVA; for a detailed description of all data analysis, see Supporting Text, which is published as supporting information on the PNAS web site), which rules out the involvement of GABACreceptors. In
contrast, the currents were completely blocked by a specific competitive antagonist of GABAAreceptors gabazine (11M;
n ⫽ 6; data not shown), and by a noncompetitive antagonist
picrotoxin (Fig. 1C; n⫽ 7). Note that at a low concentration (2 M), gabazine similarly blocked synaptic currents and SOCs (89 ⫾ 9% and 92 ⫾ 9% inhibition; P ⫽ 0.01 and 0.006, respectively; n⫽ 3). When used at 40M, bicuculline, another competitive antagonist of GABAA receptors, completely
blocked synaptic outward currents but only partially blocked spontaneous slow outward currents (Fig. 1D; n⫽ 5 cells). A complete block of spontaneous SOCs required higher concen-trations of bicuculline (ⱖ100M). These results indicate that SOCs differ from classical GABAergic synaptic currents and correspond to uncharacterized events involving GABAA
recep-tors. In addition, all three antagonists of GABAAreceptors also
blocked a tonic outward current (shown for picrotoxin in Fig. 1C), as previously reported in the cerebellum and hippocampus (for review, see ref. 22).
SOCs Occur in the Absence of Neuronal Vesicular Release.To further investigate the origin of SOCs, we found it necessary to establish a precise criterion enabling us to distinguish them from synaptic currents. To eliminate the latter, we preincubated the slices for
Conflict of interest statement: No conflicts declared.
Abbreviations: SOC, slow outward current; SIC, slow inward current; TTX, tetrodotoxin. *A.S.K. and M.C.A. contributed equally to this work.
†To whom correspondence should be sent at the present address: Laboratory of Sensory
Neuroscience, The Rockefeller University, 1230 York Avenue, New York, NY 10021. E-mail: akozlov@rockefeller.edu.
several hours with concanamycin A or bafilomycin A1 (2M and 4M, respectively), both blockers of vesicular H⫹-ATPases. The abolition of the H⫹gradient is known to prevent the vesicular accumulation of GABA and, thus, GABAergic synaptic trans-mission (23, 24). In the absence of spontaneous and evoked synaptic GABAergic currents, SOCs still were present (Fig. 2A;
n⫽ 17 cells and 10 slices). At a holding potential of –10 mV,
SOCs 10–90% rise time spanned a large range (1.3–189 ms) with a mean of 41.2⫾ 4.9 ms (n ⫽ 66 SOCs) and a distribution (Fig. 2B) such that 86.4% of SOCs had a rise time ⬎5 ms. The amplitude of SOCs was similar in slices treated with bafilomycin A1 (100.3 ⫾ 18.3 pA; n ⫽ 76) and in same-day control slices (94.9⫾ 7.8 pA, n ⫽ 49; n ⫽ 6 cells for each condition, P ⫽ 0.79 with two-tailed Student t test). Because some SOCs had a rise time compatible with synaptic currents, we used a rise time of 5 ms as a threshold to separate SOCs from synaptic currents in all recordings performed in the absence of vesicular H⫹-ATPase blockers. Using this criterion, we detected SOCs in 49 of 77 mitral cells with a mean frequency of 0.95⫾ 0.16 per minute, a mean amplitude of 266⫾ 26 pA (range 28–1,700 pA), and a
mean decay time of 350⫾ 25 ms (range 10–2,144 ms). In contrast to synaptic currents, SOCs therefore are rare events that have variable amplitudes and slow kinetics and that do not involve vesicular release from nerve terminals.
The frequency of SOCs was unchanged when the neuronal network activity was disrupted by 1M tetrodotoxin (Fig. 2C). However, SOCs were sensitive to the extracellular Ca2⫹ con-centration: Bath application of calcium-free solution evoked SOCs or transiently enhanced their mean frequency (Fig. 2C;
n ⫽ 6 cells). Because calcium-free solution triggers several
mechanisms in astrocytes (16, 25–27) and these cells have the ability to release GABA in culture (28–30), we investigated whether SOCs result from the astrocytic release of GABA.
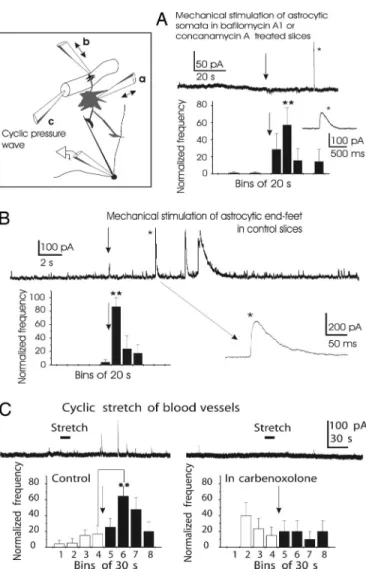
Mechanical Stimulation of Astrocytes Evokes SOCs. Mechanical stimulation is largely used to study glial cells in culture and acute brain slices, and it has been shown to cause astrocytic Ca2⫹waves and the release of glutamate and ATP (6, 31). We therefore tested the effects of three types of mechanical stimulation. Fig. 3A shows that in slices preincubated with blockers of the vesicular H⫹-ATPase and in the presence of tetrodotoxin (TTX), mechanical stimulation of individual astrocyte somata located in the external plexiform layer and in the vicinity (⬍200M) of the recorded neuron evoked SOCs (n⫽ 8 cells). Note that the delay could reach tens of seconds. Mechanical stimulation of mitral cell somata had no effect (n⫽ 4 cells and 6 stimulations; data not shown). We found that mechanical stimulation of astrocyte end-feet covering small blood vessels (32) was very effective. For these experiments, we chose mitral cells with almost no spontaneous SOCs. Pressure application onto blood vessels reproducibly triggered
Fig. 1. Slow GABAergic inhibition of mitral cells of the rat olfactory bulb. (A)
The spontaneous activity of a mitral cell monitored under current-clamp conditions is sporadically inhibited by slow hyperpolarizing events (asterisks,
Upper; n⫽ 73 slow hyperpolarizations for 20 cells) that are detected as SOCs of variable kinetics in voltage-clamp recordings (ECl⫺⫽ ⫺78 mV; Lower; same
cell). (B) SOCs reverse at the Cl⫺equilibrium potential ECl⫺⫽ ⫺50 mV (n ⫽ 7
cells). (C Top and Middle) Bicuculline (40M) completely blocks fast synaptic outward currents but only partially blocks SOCs (asterisks). The frequency of SOCs in the presence of bicuculline is decreased by 85.8⫾ 4.7% (n ⫽ 5 cells; P ⫽ 0.03). (C Bottom) Picrotoxin (100M; n ⫽ 7 cells) blocks all SOCs and a tonic inhibitory current (VH⫽ ⫺10 mV). (D) Time-to-peak distribution of all outward
currents recorded at VH⫽ ⫺10 mV (n ⫽ 5 cells). In control condition (Upper),
most events correspond to fast, synaptic GABAergic currents. Enlargement of the histogram shows the rare SOCs (double white arrowhead). In the presence of 40M bicuculline (Lower), synaptic currents are abolished, whereas some SOCs persist.
Fig. 2. SOCs have a nonneuronal origin. (A) SOCs persist when vesicular
release from neurons is blocked. (A Left) In a control slice (Upper), depolar-izing voltage steps (from⫺70 to ⫺10 mV; duration 10 ms) evoke dendroden-dritic inhibition in a mitral cell (intracellular solution with ECl⫺⫽ 0 mV).
Preincubation of the slice (Lower) with the vesicular H⫹-ATPase inhibitor concanamycin A (2M; 3–5 h) abolishes dendrodendritic inhibition. (A Right) SOCs of variable kinetics persist (n⫽ 17 cells and 10 slices in 7 rats). SOCs were recorded by using intracellular solution with ECl⫺⫽ ⫺50 mV. (B) A 10–90% rise
time distribution of SOCs recorded in slices preincubated with 4M bafilo-mycin A1 or 2M concanamycin A. (C) SOCs are independent of neuronal network activity (1M tetrodotoxin). Calcium-free solution (Ca2⫹, 0 mM;
Mg2⫹, 3 mM) triggers or transiently increases SOCs frequency.
Kozlov et al. PNAS 兩 June 27, 2006 兩 vol. 103 兩 no. 26 兩 10059
SOCs (Fig. 3B; n ⫽ 5 cells). Because these two types of mechanical stimulation may excite passing dendrites and ax-ons, we investigated the effect of a third type of mechanical stimulus: A broken quartz micropipette was introduced into a blood vessel (see Methods), and cyclic pressure waves were applied through the pipette. Such a stimulus should mechan-ically stretch endothelial and smooth muscle cells and astro-cyte end-feet (32). Mechanical stretch of the blood vessels from the inside reproducibly (two stimulations per vessel) evoked SOCs (Fig. 3C Left). Because signal propagation along the gliovascular interface critically depends on functional gap junctions (32), blocking the latter could affect the effect of this particular type of stimulation. Indeed, application of 100M carbenoxolone suppressed the responses to cyclic pressure
waves applied in the vessel lumen (Fig. 3C Right, same cells and vessels as in Fig. 3C Left). In contrast, carbenoxolone did not affect the frequency of spontaneous SOCs: 0.66 ⫾ 0.3 SOCs per minute in control versus 0.45 ⫾ 0.16 SOCs per minute in carbenoxolone (n⫽ 5 cells; P ⫽ 0.45 with ANOVA). This result indicates that GABA is not released through carbenoxolone-sensitive channels.
Overall, the results obtained with the different types of mechanical stimulation support the hypothesis that GABA is released from astrocytes to evoke mitral cell SOCs. In view of the possible mechanisms underlying GABA release, we tested and excluded several of them. Nevertheless, we observed that the frequency of SOCs was sensitive to extracellular osmolarity, suggesting that volume-regulated anion channels may be in-volved, although pharmacological tools did not allow us to conclude (Supporting Text; see also Figs. 6 and 7, which are published as supporting information on the PNAS web site).
SOCs Occur Synchronously in Adjacent Mitral Cells.When recording from pairs of mitral cells, we noticed that despite the low frequency of SOCs (see above), in some pairs, a large fraction of these currents occurred in both cells within a narrow time period of tens of milliseconds. This observation raised the question of a possible synchronizing role for SOCs. Because the majority of mechanisms supporting synchronized mitral cell firing are linked to the glomerular organization, we documented the glomerular affiliation of recorded cells in all pairs (Fig. 4 A and B). Some paired recordings were done in a calcium-free medium (with 1M TTX) to increase SOC frequency.
Fig. 3. Mechanical stimulation of astrocytes evokes SOCs. The inset
illus-trates three types of mechanical stimuli. (A) In slices preincubated with bafilo-mycin A1 or concanabafilo-mycin A, mechanical stimulation of single astrocytes (arrow) evokes SOCs (in the presence of 1M TTX). Note the delay. The histogram is based on recordings from 8 cells and 16 astrocyte stimulations. Mechanical stimulation of mitral cell somata does not cause SOCs (n⫽ 4 cells and 6 stimulations; data not shown). (B) Mechanical stimulation of astrocyte end-feet covering small blood vessels evokes SOCs. The blood vessels were located in the external plexiform layer at⬍200M from the recorded mitral cell. The histogram is based on recordings from 5 cells and 10 vessel stimula-tions. (C Left) Cyclic pressure waves applied inside blood vessels also evoke SOCs. Blood-vessel stretch (two stimulations per vessel) reproducibly evokes SOCs (five blood vessels and five neurons). (C Right) Carbenoxolone (100M) blocks the frequency enhancement in response to the stretch of blood vessels (same blood vessels and neurons as in C Left).
Fig. 4. SOCs occur synchronously in mitral cells, independently of the
glomerular organization. (A) Paired recordings from two mitral cells whose apical dendrites project to the same glomerulus. Recordings were performed with extracellular solution containing 1M TTX and 0 mM Ca2⫹. Note that not
all currents are synchronized (VH⫽ ⫺10 mV; ECl⫺⫽ ⫺50 mV). (B) Paired
recordings from two mitral cells whose apical dendrites project to two differ-ent glomeruli. Recordings were performed in control solution (VH⫽ ⫺10 mV;
ECl⫺⫽ ⫺50 mV). (C) Distribution of SOC interevent intervals between two
simultaneously recorded mitral cells (18 pairs). For each cell, the onset of every current was measured, and its delay with all currents recorded in the second cell was calculated. The histogram of interevent intervals was constructed with 5-ms bins. Only the region of the histogram corresponding to the ⫾200-ms intervals is shown, because only one peak was present and centered at 0 ms during the 5-min period of data acquisition. (D) Synchronization is not due to electrical coupling through gap junctions. Two mitral cells recorded at different holding potentials and with different electrochemical gradients for Cl⫺presented synchronized SOCs of opposite polarity, excluding the involve-ment of electrical coupling.
Of 74 pairs of mitral cells recorded for 500 s, 18 pairs were selected for the quantification of synchronization by using the following criterion: Occurrence of one SOC in both cells with an interevent interval⬍1 s (see Methods for a detailed description of the statistical analysis). To quantify the synchrony, we mea-sured the time intervals between each SOC in one cell and all other SOCs in the second cell. The distribution of SOC intervals (Fig. 4C) reveals that 26% of SOCs (100 of 379 SOCs) occurred within a narrow time window of 50 ms (⫾ 25 ms). Because the average rate of SOC occurrence for the 36 cells (18 pairs) was 1.26 per min, the probability that during a 500-s recording two SOCs occur by chance, within a time window of 50 ms, equals 0.001. The probability of observing, by chance, 100 of 379 SOCs within 50 ms, is⬇10⫺103; this low probability implies that SOCs are synchronized. Because synchronized SOCs of opposite po-larities were recorded when the membrane potential of the two cells was maintained at different values (note that ECl⫺differed
in the two cells), SOC synchronization cannot be due to electrical coupling between mitral cells (Fig. 4D). Interestingly, synchro-nized SOCs were observed in adjacent mitral cells irrespective of whether their apical dendrites contacted the same (n⫽ 2 pairs) or different (n⫽ 6 pairs) glomeruli, or even when one apical dendrite was cut before reaching a glomerulus (n⫽ 2 pairs). In particular, the time-interval distribution obtained for the six pairs of mitral cells that projected their dendrites to different glomeruli showed a clear peak centered at zero (data not shown). Therefore, astrocytes elicit synchronous currents in mitral cells independently of the glomerular organization.
Target Cell-Specific Modulation of Neuronal Activity by Astrocytes.
Stimulation of astrocytes with low-calcium solution or mechan-ically recently has been shown to evoke in hippocampal pyra-midal neurons slow inward currents (SICs), mediated by NMDA receptors (15, 16). Because mitral cells express functional glu-tamate receptors on the somatodendritic membrane (15), it is noteworthy that we observed only slow GABAergic and not glutamatergic currents in mitral cells upon stimulation of astro-cytes. To test the hypothesis that astrocytes selectively release GABA or glutamate according to the identity of the target neuron, we recorded from granule cells (n⫽ 47) in the presence of 1M TTX and observed both SOCs and SICs. Of 47 granule cells, 10 cells showed only SOCs, 27 cells only SICs, and 10 cells both SOCs and SICs. In Fig. 5A, SICs were recorded at a membrane potential at which GABA-mediated currents were outward. SICs were evoked by the same stimulation protocols that evoked SOCs in mitral cells: by applying cyclic stretch to blood vessels (Fig. 5B; n ⫽ 4) and by reducing extracellular osmolarity to 265 mOsm (0.24⫾ 0.1 SICs per minute in control versus 1.32⫾ 0.3 SICs per minute in the hypotonic solution; P ⫽ 0.0016 with two-tailed t test; n ⫽ 8 cells). As expected from currents mediated by NMDA receptors activated by glutamate, SICs were observed in the presence of saturating concentration ofD-serine (coagonist of NMDA receptors; 200M) and were completely blocked by 20 M MK-801, a noncompetitive an-tagonist of NMDA receptors (n⫽ 4; data not shown). Like SOCs in mitral cells, SICs and SOCs persisted in granule cells in slices treated with bafilomycin A1 (Fig. 5C; n⫽ 9 cells)
If both glutamate and GABA were released simultaneously at a same site opposed by the two types of receptors, one would expect to observe SOCs and SICs at the same time. Even though we did observe synchronous SICs in 2 of 6 granule cell pairs and synchronous SOCs in 4 of 16 mitral cell-granule cell pairs (Fig. 5D), SOCs in mitral cells and SICs in granule cells never occurred synchronously.
Discussion
In this study, we demonstrate that astrocytes in the rat olfactory bulb release two classical transmitters, GABA and glutamate, to
evoke synchronous currents in mitral and granule cells. Although SICs mediated by NMDA receptors and due to glutamate release from glial cells have been observed in thalamic and hippocampal slices (9, 15, 16, 18, 33), SOCs mediated by GABAA
receptors have not been reported heretofore.
We propose that both GABA and glutamate, transmitters mediating SOCs and SICs, respectively, are released by astro-cytes and not by neurons. Recent studies have shown that optical (16) and mechanical (15) stimulation of astrocytes evoke SICs. Mechanical stimulation, in particular, is used largely to study glial cells in culture and in acute brain slices. Direct stimulation of individual glial cells and minimal activation of neurons, which is often an issue with less discriminate pharmacological stimuli, are the main advantages of this method. Because any particular type of mechanical stimulation may suffer from its own specific drawbacks, we used three different types of mechanical stimu-lation. A potential problem with direct stimulation of individual astrocytes is that a patch pipette penetrating the tissue will encounter glial and neuronal processes that cannot be discrim-inated under infrared video microscopy. Nevertheless, when the pipette is held at a distance of⬇1m from the target for tens of seconds before the application of a gentle pressure, the stimulation is restricted to a small volume of neuropil and the
Fig. 5. Glutamate- and GABA-mediated slow currents in granule cells. (A)
Spontaneous slow currents mediated by glutamate in granule cells. At⫺30 mV, SICs occur. Note that at this potential, GABAergic currents would be outward (see C and D). (B) Cyclic stretch of blood vessels (n⫽ 4) evokes SICs in the presence of 1M TTX. The currents were blocked by 100 M D-AP5 (data not shown). (C) Both SOCs and SICs can be observed in some granule cells. The slice had been preincubated with 4M bafilomycin A, and the extracellular osmolarity was reduced to 265 mOsm to evoke the currents (1M TTX). (D) Some SOCs (ECl⫺⫽ ⫺50 mV) occur simultaneously in adjacent mitral and
granule cells, as assessed by paired recordings. In these experiments, transient bath application of 265 mOsm extracellular solution was used to enhance SOC frequency.
Kozlov et al. PNAS 兩 June 27, 2006 兩 vol. 103 兩 no. 26 兩 10061
target and may be assumed to stimulate the target preferentially. Our second method of mechanical stimulation, the application of pressure to the vessel outer wall, is designed to activate astrocyte end-feet that cover⬎90% of the surface of small blood vessels. This approach very efficiently triggered SOCs in the absence of synaptic transmission (blocked by bafilomycin A1), supporting the involvement of astrocytes in the generation of SOCs. Furthermore, vascular distension from the inside, a potent means of evoking SOCs, is very unlikely to stimulate neurons directly (it also was efficient in the presence of TTX). Indeed, when stretching blood vessels, we observed no activation of mitral cell firing. In addition, direct mechanical stimulation of neurons did not evoke SOCs; likewise, SICs were not evoked by mechanical stimulation of neurons in the hippocampus (15). Because all three types of mechanical stimulation led to the same result, activation of SOCs, we conclude that GABA is released from astrocytes.
Our data raise the question of the source of GABA released by astrocytes. Although several studies have suggested that GABA or GABA-like substances can be secreted from glial cells in culture (28, 30, 34, 35), it is generally assumed that glial cells do not contain the enzymes necessary for the synthesis of GABA and that, if present, GABA is taken up from the extracellular medium by GABA transporters. Our results suggest that GABA is most likely the transmitter involved, even though other transmitters might substitute (36). Taurine would be a possible candidate because it is abundant in the olfactory bulb (37, 38) and induces a slow, ‘‘nondesensitizing’’ inhibition of mitral cells during a prolonged bath application (39). The same study reported, however, that taurine does not affect granule cells. In contrast, we observed SOCs in 40% of granule cells. Moreover, the requirement of high concentrations of bicuculline to block the SOCs and the absence of activation of glycine receptors present on mitral cells argue against the involvement of taurine. Finally, the kinetics of SOCs is slower in the presence of nipecotic acid, a blocker of GABA transporters (Supporting
Text). We thus propose that the transmitter responsible for SOCs
is GABA.
Do SOCs and SICs involve two types of astrocytes that would release either GABA or glutamate? Alternatively, can a single astrocyte release both GABA and glutamate, possibly at the same site, with an effect on neurons depending on the type of neuronal receptors that face the release site? In the glomerular layer, astrocytes respect the glomerular organization (40), surrounding a single glomerulus and extending processes that wrap and isolate bundles of dendrites with dendrodendritic synapses (41). Such compartmentalization should favor neu-ronal synchronization because of spillover of a transmitter, whether released by neurons or by glial cells. In the external plexiform layer (40), an important feature of astrocytes is the regular spacing of their cell bodies and the spatially limited overlap of their processes. The presence of such astrocytic functional domains (42) suggests that it is likely that two different types of astrocytes are responsible for SOCs and SICs. Indeed, SICs were not observed in mitral cells, whereas their dendrites are covered by functional glutamate receptors (43), and synchronous SOCs, but not synchronous SOCs and SICs, were detected in paired recordings of mitral and granule cells. However, we cannot rule out the possibility that a single astrocyte releases GABA and glutamate separately at differ-ent and spatially distant release sites.
SOCs obviously differ from classical GABAergic synaptic currents. They occur at a low frequency, display slow and variable kinetics, and require high concentrations of a compet-itive antagonist, bicuculline, to be blocked. These properties could result from an astrocytic ensheathment of dendrites that creates a barrier for diffusion and clearance of GABA, from the release kinetics, or from the involvement of particular subtypes
of GABAAreceptors. Because the synaptic sites and dendritic
shafts of mitral cells are wrapped together but synaptic currents and SOCs have different sensitivities to bicuculline, different GABAAreceptor subtypes may mediate synaptic currents and
SOCs, as it is the case for the tonic GABAergic current (for review, see ref. 22). However, gabazine had similar effects on synaptic currents and SOCs, in contrast to its differential action observed on synaptic and tonic currents in the hippocampus (22). It has been demonstrated that several intra- and interglomeru-lar mechanisms support synchronization of mitral cell activity, a firing behavior important in the processing of odors (44, 45). We propose that an additional type of mitral cell synchronization, mediated by astrocytes, operates in the olfactory bulb. It occurs at a very low frequency, its time course is slow, it does not respect the glomerular organization, and its physiological or patholog-ical relevance remains to be established in vivo.
Methods
Slice Preparation and Electrophysiology.Horizontal slices (300–400 m) from olfactory bulbs of 2- to 5-week-old Wistar rats were cut in ice-cold extracellular solution (for composition of all solutions used in the study, see Supporting Text) and incubated either at room temperature or at 34°C. Recordings were done with borosilicate patch pipettes with a resistance of 2–7 M⍀. Data were acquired by using Axopatch 200A and 200B amplifiers and
P-CLAMP8software (Axon Instruments, Union City, CA). Analog
signals recorded in voltage clamp and current clamp modes were low-pass filtered with a four-pole Bessel filter at 2 kHz and 5 kHz, and sampled, respectively, at 10 kHz and 20 kHz. Series resistance was not compensated, and leak currents were not subtracted. For recordings with intracellular solutions contain-ing gluconate as the major anion, all potentials were corrected for a⫺10 mV junction potential.
Slice preincubation in the presence of the vesicular H⫹ -ATPase inhibitors bafilomycin A1 or concanamycin A was carried out at 34°C for 3–5 h. Control slices were taken from the same animals and incubated at 34°C in the absence of drug.
Mechanical Stimulation of Astrocytes and Blood Vessels.A patch pipette containing the extracellular solution was positioned near astrocytes and blood vessels located in the external plexiform layer within 100–200M from the recorded neuron. A gentle pressure was transiently (⬍1 s) applied onto the astrocyte membrane or onto the blood vessel wall. Cyclic stretch of blood vessels (arterioles and venules) was performed from the inside as follows. A quartz micropipette with a broken and sharp tip was forced through the vessel wall, and after several minutes, a cyclic pressure wave (2 Hz) was applied for 20 s. The vessel was chosen such that its penetration site was located several hundred micrometers from the recorded neuron, and it or one of its effluents was running near the neuron.
Statistics.To determine the statistical significance of the differ-ence between means, the two-tailed Student t test and the ANOVA test were used; P⬍ 0.05 was considered statistically significant. P⬍ 0.05 and 0.01 are indicated in the figures by single and double asterisks, respectively. Averaged values are reported as mean ⫾ SEM. For more information on data analysis and statistics, see Supporting Text.
We thank M. Hanafi for technical assistance. A. J. Hudspeth made helpful comments on the manuscript. A.S.K. was supported by Interna-tional Brain Research Organization and Ecole Supe´rieure de Physique et de Chimie Industrielles. Support was provided by the Institut National de la Sante´ et de la Recherche Me´dicale, the Ministe`re de l’Education Nationale de la Recherche et de la Technologie, the Centre National de la Recherche Scientifique, and Fondation pour la Recherche Me´dicale Grant ICP20001222128.
1. Auld, D. S. & Robitaille, R. (2003) Neuron 40, 389–400. 2. Volterra, A. & Meldolesi, J. (2005) Nat. Rev. Neurosci. 6, 626–640. 3. Bushong, E. A., Martone, M. E., Jones, Y. Z. & Ellisman, M. H. (2002)
J. Neurosci. 22, 183–192.
4. Ogata, K. & Kosaka, T. (2002) Neuroscience 113, 221–233.
5. Nett, W. J., Oloff, S. H. & McCarthy, K. D. (2002) J. Neurophysiol. 87, 528–537. 6. Newman, E. A. & Zahs, K. R. (1998) J. Neurosci. 18, 4022–4028.
7. Aguado, F., Espinosa-Parrilla, J. F., Carmona, M. A. & Soriano, E. (2002)
J. Neurosci 22, 9430–9444.
8. Pasti, L., Volterra, A., Pozzan, T. & Carmignoto, G. (1997) J. Neurosci. 17, 7817–7830.
9. Parri, H. R., Gould, T. M. & Crunelli, V. (2001) Nat. Neurosci. 4, 803–812. 10. Hirase, H., Qian, L., Bartho, P. & Buzsaki, G. (2004) PLoS Biol. 2, E96. 11. Basarsky, T. A., Duffy, S. N., Andrew, R. D. & MacVicar, B. A. (1998)
J. Neurosci. 18, 7189–7199.
12. Grosche, J., Matyash, V., Moller, T., Verkhratsky, A., Reichenbach, A. & Kettenmann, H. (1999) Nat. Neurosci. 2, 139–143.
13. Nadkarni, S. & Jung, P. (2003) Phys. Rev. Lett. 91, 268101.
14. Tian, G. F., Azmi, H., Takano, T., Xu, Q., Peng, W., Lin, J., Oberheim, N., Lou, N., Wang, X., Zielke, H. R., et al. (2005) Nat. Med. 11, 973–981.
15. Angulo, M. C., Kozlov, A. S., Charpak, S. & Audinat, E. (2004) J. Neurosci. 24, 6920–6927.
16. Fellin, T., Pascual, O., Gobbo, S., Pozzan, T., Haydon, P. G. & Carmignoto, G. (2004) Neuron 43, 729–743.
17. Cavelier, P., Hamann, M., Rossi, D., Mobbs, P. & Attwell, D. (2005) Prog.
Biophys. Mol. Biol. 87, 3–16.
18. Perea, G. & Araque, A. (2005) J. Neurosci. 25, 2192–2203.
19. Kang, J., Jiang, L., Goldman, S. A. & Nedergaard, M. (1998) Nat. Neurosci. 1, 683–692.
20. Deleuze, C., Duvoid, A. & Hussy, N. (1998) J. Physiol. (London) 507, 463–471. 21. Shepherd, G. M., Chen, W. R. & Greer, C. A. (2004) in The Synaptic
Organization of the Brain, ed. Shepherd, G. M. (Oxford Univ. Press, New York),
pp. 165–203.
22. Semyanov, A., Walker, M. C., Kullmann, D. M. & Silver, R. A. (2004) Trends
Neurosci. 27, 262–269.
23. Zhou, Q., Petersen, C. C. & Nicoll, R. A. (2000) J. Physiol. (London) 525, 195–206.
24. Rossi, D. J., Hamann, M. & Attwell, D. (2003) J. Physiol. (London) 548, 97–110.
25. Zanotti, S. & Charles, A. (1997) J. Neurochem. 69, 594–602.
26. Ye, Z. C., Wyeth, M. S., Baltan-Tekkok, S. & Ransom, B. R. (2003) J. Neurosci.
23,3588–3596.
27. Duan, S., Anderson, C. M., Keung, E. C., Chen, Y., Chen, Y. & Swanson, R. A. (2003) J. Neurosci. 23, 1320–1328.
28. Liu, Q. Y., Schaffner, A. E., Chang, Y. H., Maric, D. & Barker, J. L. (2000)
J. Neurophysiol. 84, 1392–1403.
29. Verderio, C., Bruzzone, S., Zocchi, E., Fedele, E., Schenk, U., De Flora, A. & Matteoli, M. (2001) J. Neurochem. 78, 646–657.
30. Jow, F., Chiu, D., Lim, H. K., Novak, T. & Lin, S. (2004) Neurochem. Int. 45, 273–283.
31. Koizumi, S., Fujishita, K., Tsuda, M., Shigemoto-Mogami, Y. & Inoue, K. (2003) Proc. Natl. Acad. Sci. USA 100, 11023–11028.
32. Simard, M., Arcuino, G., Takano, T., Liu, Q. S. & Nedergaard, M. (2003)
J. Neurosci. 23, 9254–9262.
33. Demarque, M., Represa, A., Becq, H., Khalilov, I., Ben Ari, Y. & Aniksztejn, L. (2002) Neuron 36, 1051–1061.
34. Neal, M. J. & Bowery, N. G. (1979) Brain Res. 167, 337–343.
35. Wu, P. H., Durden, D. A. & Hertz, L. (1979) J. Neurochem. 32, 379–390. 36. Pasantes-Morales, H., Murray, R. A., Sanchez-Olea, R. & Moran, J. (1994)
Am. J. Physiol. 266, C172–C178.
37. Collins, G. G. (1974) Brain Res. 76, 447–459.
38. Didier, A., Ottersen, O. P. & Storm-Mathisen, J. (1994) NeuroReport 6, 145–148.
39. Belluzzi, O., Puopolo, M., Benedusi, M. & Kratskin, I. (2004) Neuroscience 124, 929–944.
40. Bailey, M. S. & Shipley, M. T. (1993) J. Comp. Neurol. 328, 501–526. 41. Kasowski, H. J., Kim, H. & Greer, C. A. (1999) J. Comp. Neurol. 407, 261–274. 42. Nedergaard, M., Ransom, B. & Goldman, S. A. (2003) Trends Neurosci. 26,
523–530.
43. Lowe, G. (2003) J. Neurophysiol. 90, 1737–1746.
44. Stopfer, M., Bhagavan, S., Smith, B. H. & Laurent, G. (1997) Nature 390, 70–74. 45. MacLeod, K., Backer, A. & Laurent, G. (1998) Nature 395, 693–698.
Kozlov et al. PNAS 兩 June 27, 2006 兩 vol. 103 兩 no. 26 兩 10063