Publisher’s version / Version de l'éditeur:
Journal of the American Ceramic Society, 38, 10, pp. 362-366, 1955-10-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Study of efflorescence produced on ceramic wicks by masonry mortars
Ritchie, T.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=e5973637-b0cb-4cef-a72a-c027e279b9be
https://publications-cnrc.canada.ca/fra/voir/objet/?id=e5973637-b0cb-4cef-a72a-c027e279b9be
N21r2
no.
20
c .2
BLDG
STUDY OF EFFLORESCENCE PRODUCED ON CERAMIC WICKS
BY
MASONRY MORTARS
By
T.
RitchieRESEARCH PAPER N O . 20
Division of Building Research, National Research Council,
Ottawa, Ontario, Canada
[Reprinted from The Journal of the A m e r ~ c a n Ceramic Society, 38
[lo]
3 6 2 - 3 6 6 (1955).1P R I C E 10 C E N T S
Study of Efflorescence Produced o n Ceramic Wicks
by
Masonry Mortars
Division of Building Research, National Research Council, Ottawa, Ontario, Canada
Ceramic wicks (Efflorwicks) w e r e partly em- b e d d e d i n cylinders of masonry m o r t a r s of vari- o u s compositions, a n d t h e h a r d e n e d mortars w e r e alternately wetted a n d dried, which pro- duced various a m o u n t s of efflorescence o n t h e wicks. Portland cement i n mortars contributed significantly to efflorescence. T h e alkaline ma- terial of t h e cementing substance of t h e mortars appeared to b e a n important source of t h e
efflorescence.
from the top of wllicli projected the wiclc of fired clay m a - terial, similar in properties t o m a n y building brick.
Efflorescence was formed on m a n y of t h e wicks by alter- nately wetting a n d drying t h e mortar. ,4n arbitrary number (ten) of these wetting and drying cycles was made, after which the wick was broken away from the mortar. I t was dried, weighed, washed i n water, dried, a n d again weighed. The dif- ference in ~ v e i g l ~ t indicated the amount of soluble salts which hadcollected in t h e wiclc. I n this way acornparison wasmade of the amounts of efflorescence produced under the same condi- tions by t h e 1-arious mortars used.
I. Introduction II. Mortar Materials
U
NDER similar conditions of exposure, i t has been found (1) L ; ~ ~on occasion t h a t masonry will develop efflorescence when
Three types of lime were used; two were prepared from
One of Inortar is used but be free of it Or
c,uicl;lime and the other was a llyclrated lime powder. A less affected when mortar of coln~osition is used' cllenlical analysis of these limes before they were made into This situation has been referred t o frecluently in the litera-
putty is given in Table I. Lime A was a high-calcium lilne ture.l The use of cement, particularly, in
from yuicl<lilne. Lime
B
was a hydrated lime made mortars has been found, on occasion, to result in efflorescenceinto putty by soaking in water. I t was used after soaking on the masonry, whereas under the sanle conditions with
for 24 Lime was prepared from cluicklime in ac-
lime mortar there has been none. T h e efflorescence has been cordance the procedure by the manu.
attributed t o alkaline material in the Portland ~ e m e n t . l ( ~ ) ~
facturer. T h e purpose of the investigation described in this paper-
was t o compare the capacity of various common mortar (2) Portland Cement
materials to produce efflorescence on a particular fired clay Portland cement from two sources was used. These ce-
material in contact with them. nlents are designated A and E. An analysis of samples of
A large of 'llortarsl of Of
them, with respect t o some constituents, is presented in Table several types of limes, Portland cements, masonry cements, I .
and sands, was prepared. A standard (for this work) fired
clay material, in the fonn of a wick, was placed in each of the (3) M~~~~~~ cement
freshly mixed mortars, which had been placed to harden in Two masonry cements were used, designated M and S. cylindrical molds. A cylinder of lnortar was thus fonned,
An analysis of these celnents is presented in Table I.
(4)
SandThree sands were used, two of which were local sands
Received March 1 , 1955. employed for masonry work in the area. These two are desig-
This paper is a contribution from the Division of Building
Research, National Research Council(Report S o 6 5 ) , anclispub- A and L. The other designated a
lishecl with the approval of the Director pure silica sand of uniform particle size, is used as a standard The author is assistant research oficer, Division of Bullcliiig sand i n some cement tests.2 S a n d L conformed in particle-
Research, National Research Council. size gradation t o the requirements of a standard specification
1 (a) Great Britain Department of Scientific and Industrial Re-
search; Report of Building Research Board ,vitll Report of Di. for mortar sands3 whereas sand A did not, being slightly too
rector of Building Research, 1927. coarse. No cllemical analysis of the sands was made.
( b ) . F 0 . Anderegg, H. C Peffer, P. R Judy, a~icl Lee Huber,
"Indlana Limestolie: I, Efflorescence and Staining," Pzcrdzc~ Ill. Fired Clay Wick
Un?z~. Eng ExPt Stn B;c!I , No. 3 3 , 84,pp ( 1 9 2 8 ) .
(c) R. K Robertson, Efflorescence, J Can Cern?)z Snr , 13, A wicli of a fired clay material was placed in each mortar as 40-43 ( 1 9 4 4 ) ; Crram Abstr , 23 [ 9 ] 151 (1934) a means of collecting efflorescence. For this purpose Efflor- wiclcs were used, obtained from the Re- search Department of the New York State College of Ceramics. Alfred University,
Table I. Analysis of Mortar Materials
(%)
P o r t l a n d Portland Masonry hlasonry
1 ~ n 1 e Llme L ~ m e cement cement cement cement -- --
C o m p o n e n t r\ B C A Ii: hl S -. -- Na?O KzO
so4
C1 COa MgO CaO*
Kone. j S o t determined.:
As SO?.362
"Standard Specifications for Masonry Cement," A.S.T.M. Designation C 91-49;
A S.T.M. Book of Standards, Part 3 , 1949.
3 "Standard Specificatiolls for Aggregate for
Masonry Mortar," Designation C 1 4 4 3 4 ; A S.T.M. Rook of Standards, Part 3,
<*&-.
October
1955
Eflorescence Produced o n Cerwm Alfred, New York, where they have been developed for studies of the tendencies of various masonry materials to produce efflorescence.* These wicks had been made by pressing ground shale into thin slabs, which were fired t o a tempera- ture corresponding to t h a t attained in the firing of many structural clay products. This developed a red color in the material, and other properties similar t o many fired clay building brick.The wiclcs used were about 3 in. long, 2 in. wide, and 3/16 in.
tliicl;, orle end being sernicirc~dar in shape, the other rectan- gular. The form of the wiclts is shown in Fig. 1.
IV. Preparation and Treatment of Mortar Samples The mortar materials were proportioned on a volun~e basis. T h e an~ouilt of dry sand in each mortar nlix was 0.05 CLI. ft. The amounts of the other materials were measured by weight. the weight-to-~olu~ne relationship having been detemlined previously.
The mortar ingredients were mixed in a s ~ n a l l mechanical mixer. The cementing materials were made into a slurry with water and to this was added the sand, and additional water as required to produce a p l a ~ t i c mixture. Ordinary t a p water was used. Mixing continued for a t least 5 minutes. The flow of the nlortar was adjusted by t h e addition of water so that, when used, the mortar flow was between 100 and
125y0.2
, Molds designed for the preparation of s ~ n a l l cylinders of concrete for compressi.ve strength tests5 were used to form the sa~nples of mortar. These molds were 4 in. high and 2 in. ill dia~neter. For this work, however, a wooden plug was placed in the lower part of the mold, so t h a t the cylinder of mortar obtained from the mold was 21/2 in. high.
The mortar was placed in the ~ n o l d immediately after mix- i ~ ~ g . A wick, wetted in distilled water to improve its contact with the mortar, was placed t o a depth of 1 in. in the mortar.
011 each .vvick a deep scratch had been lllade 1 in. from the end, so t h a t when the wick was embedded in the hardened mortar the projecting part of it could be easily broken off at t l ~ e mortar surface. The mortars were left in the molds for 2-1- hours, after which they were removed and stored in the laboratory air for a t least a week before beginning the tests.
T o subject the mortar sanlples to wetting and drying, each sample was placed in a s~llall jar (capacity, about i oz.) and distilled water nras added t o a le\-el just below the top surface of the ~ n o r t a r . T l ~ e wick protruded through a hole in the nletal cover of the jar; the opening between the wick and the cover was reduced i11 size by placing thin glass plates on the cover against the wicf. Each sample was placed in water in tllis way for 24 Ilours. Then it was rernoved from the jar and allowed t o dry in the laboratory air for about 'i hours. I t was placed in the orifiinal water again for wetting, withmore water added t o bring its level just below the top surface of the mor- tar. The cover was replaced and the sample stood in thc water for about 17 Ilours, when the drying procedure was carried out again.
In each 24 hours, therefore, the samples were being wetted for about 17 hours and for about 7 hours were being dried. During the wetting part of this procedure, moisture e-i~apo- rated from the wick. While drying, however, the whole sam- ple was exposed t o the laboratory air, with the wick probaljly drying out more co~npletely than the mortar in the time al- lotted.
*
C. R. Amberg and L. Washburn, "\Vick for Testing Efflores- rellce Tendencies of Materials," Ant. Ceranz. Soc. Bfill., 2 5 [ I ] 7-9 (1946)."Standard Method of Making and Curing Concrete Colnpres- sion and Flexure Test Specimens in the Laboratory," A.S.T.M. Designation C 192-49; A.S.T.M. Book of Standards, Part 3, 1949.
i c W i c k s b y Masonry ,n/lortars 363
Fig. 1. Equipment used i n preparation and treatment of samples.
The equipment used in preparing the samples is shown in Fig. 1. T h e central mold space contains one of the wooden inserts and a nort tar cylinder with wick attached. The type of jar in which t h e samples were wetted and one of the wicks are also sho~vn.
Each mortar sample was wetted ten times a n d dried ten times, after which it was stored for a t least a week in the labo- ratory air. Photographs were then taken of t h e sanlples, and each wick was carefully broken away from t h e mortar in which it had been embedded, a t the scratch made previously in the wick.
Each wick broken off in this way was dried in an oven for -18 hours a t about 100°C., cooled, and weighed. I t was then placed in 50 cc. of distilled water for 24 hours, after which this water was replaced by another 50 cc. iifter one hour the water was replaced again by t h e same amount, and this pro- cedure was continued until each wiclc had been washed in 230 cc. of water. T h e total washinqs from each wick mere re- tained for analysis.
After this washing treatment, the wicks were dried for 48 hours a t about 100°C., cooled, and weighed. T h e difference in the weighings, before and after washings, was taken to represent the amount of soluble lnaterial in t h e wicks. A similar washing treatment, made on several unused wiclcs t o determine the soluble lnaterial present, showed this to be a very slight amount, but it was subsequently taken into ac- count in determinations of the a~nounts of soluble material in thc wicks l~rolcen from mortar samples.
V. Mortar Compositions
The mortars studied consisted of various combinations of the cementing materials and sands, and of various proportions by volume of cementing material t o sand.
The lnaterials used in the mortars have been described. The mortars made fro111 then1 are designated b y letter and number. The mortars composed of Portland cement, lime, and sand are listed in Table 11, which shows, for each mortar, the types of materials used and their proportions. The mortars composed of the masonry cements and sand are listed in Table 111.
VI. Comparison of Amounts of Efflorescence After t h e wetting and drying procedure, the mortars were dried in the laboratory air for a n additional week or more and then photographed. T h e wetting and drying treatment had formed extensive deposits of salts on some wicks; others appeared free of the salts, or slightly marked by them.
364
Journal of T h e American Ceramic Society-Ritchie
Vol.
38,No. 10
position of these mortars is shown in Tables I1 and 111.
The amount of material soluble in water in each wick was determined, in the manner previously described. I n Figs. 7 through 11 the weight of soluble material washed from each wick has been plotted against the composition of the mortar with which it was used.
VII. Analysis of Washing Solutions
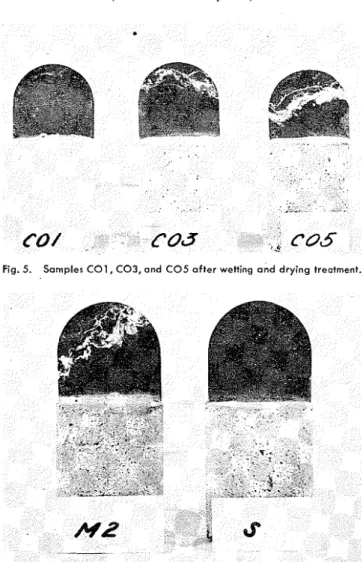
The solutions obtained from the washings of one series of wicks (mortars A1 through AG), and from other wicks, were analyzed with re- spect t o some constituents. The re- sults of this analysis, which have Fig. 2. Samples A1, A2, A3, A4, A5, and A 6 after wetting and drying treatment.
been expressed as parts per million of the solution, are shown in Table IV.
Photographs of some of the mortars, which illustrate these VIII. Discussion
differences, are shown in Figs. 2 through 6. (Figure G is of The capacity of mortars composed of many different in- mortars of the masonry cements and sand; the others are of gredients to produce efflorescence under certain conditions the lime, Portland cement, and sand mortars.) The corn- has been studied. Ceramic wicks, developed and employed
( T e x t c o n t i n ~ l e d o n
p.
366)Fig. 3. Samples A O I , A 0 3 , and A 0 5 after wetting and drying treatment. Fig. 5. Samples C 0 1 , C 0 3 , and C 0 5 after wetting and drying treatment.
October 1033
lZtfloresce~z~e
P r o d ~ ~ ~ e d
on Ceram
Table II. Materials Used a n d Their Proportions in the Portland Cement, Lime, a n d Sand Mortars
Type of mortar Proportions by vol. ol rnater~al mortar materials
_-._--___ _-A__---
Mortar Portland Portland
designation L11ne cenlent Sand L ~ m c cement Sand
AL6 ( * ) A L ( O ) 1 . 0 3 . 0 ALlA A (") L 1 . 0 (:$) 2 . 0 AL2 A A A L 0 . 8 0 . 2 2 . 0 AL3A A A L 0 . 6 0 . 4 2 . 0 AL4A A A L 0 . 4 0 . 6 2 . 0 AL5A A A I, 0 . 2 0 . 8 2 . 0 ALGA (") A L ( " 1 . 0 2 . 0 A L l B 1 . 0 (*) 4 . 0 AL2 B A A
kC)
I, 0 . 8 0 . 2 4 . 0 AL3 B A A L 0 . 6 0.4 4 . 0 AL4B A A L 0 . 4 0 . 6 4 . 0 AL5B A A L 0 . 2 0 . 8 4 . 0 AL6B (*) A I, ( * . 1 . 0 4 . 0 A 0 1 A(:*I
0 1 . 0 (") 3 . 0 A 0 3 A A 0 0 . 6 0 . 4 3 . 0 A 0 5 A A 0 0 . 2 0 . 8 3 . 0 B1 B ) A 1 . 0 (") 3 . 0 B3 B A A 0 . 6 0 . 4 3 . 0 B 5 B A A 0 . 2 0 . 8 3 . 0 B 0 1 B (") 0 1 . 0 (*) 3 . 0 B 0 3 B A 0 0 . 6 0 . 4 3 . 0 B 0 5 B A 0 0 . 2 0 . 8 3 . 0 E6(*I
E A(*I
1 0 3 . 0 EL6("1
I3 L ( 1 0 3 . 0 E 0 6( 9
0(9
- 1 0 3 . 0 Nonc present.Table Ill. Materials Used a n d Their Proportions in the M a - sonry Cement and S a n d Mortars
Type of mortar Proportions by vol. of material mortar materials
,--- -_.A___
i\Iortar Masonry bfasonry
desiznation
.
cement Sand cement Sandlit 1lick.s
b yMasonry iVIortars
B65x 0 3 Z 0.30 u 0 0.25 W >
2.;;
0.20I f
W 0.15 m-
5
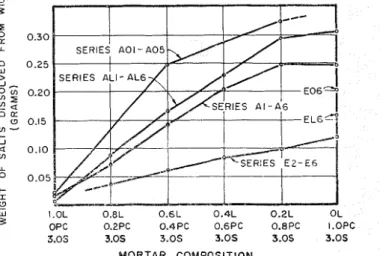
a 0.10 m u O 0.05 t- I (1 - - w I.0L 0.8L 0.6L 0.4L 0.2L OL 3 OPC 0.2PC 0.4PC 0.6PC 0.8PC I.OPC 3.05 3.0s 3.05 3.0s 3.0s 3.0s MORTAR COMPOSITIONFig. 7. Comparison o f amounts o f salls dissolved from wicks used with mortars o f lime A and various sands and Portland cements. (1) indicates lime, (PC) Portland cement, and (S) sand. Proportions are b y volume
- - w 1.OL 0.8L 0.6L 0.4L 0.2L OL 3 OPC 0.2PC 0.4PC 0.6PC 0.8PC I.0PC 3.0s 3.0s 3.0s 3.0s 3.0s 3.0s MORTAL? COMPOSITICN
Fig. 8. Comparison o f amounts o f salts dissolved from wicks used with mortars o f lime B, Portland cement, and various sands. (1) indicates lime, (PC) Portland cement, and (S) sand. Proportions are by volume.
MORTAR COMPOSITION
Fig. 9. Comparison of amounts o f salts dissolved from wicks used with mortars o f lime C, Portland cement, and various sands. (1) indicates lime, (PC) Portland cement, a n d (S) sand. Proportions a r e by volume.
366
J o~srnal
of T h e A nzerica~c Ceramic Society--Ri
fchie
Vol.
38,No. 10
Table IV. Analysis of Washing Solutions"'
- ---
Mortar
Solut1o11 -- -
.
- - -constltue~lt A1 A2 A3 A 4 A5 AG E3 EL6 EOG MO ALL M2
--
NalO 5 0 88 5 160 265 280 250 91 122 105 196 248 248
K20 6 0 55 0 105 150 210 220 140 253 365 3319 229 161
SO4 4 2 4 3 6 2 3 0 3 2 1 2 5 4 8 0 6 22 49 07 142 84 56
C1 <2
<
2 <2<
2<
2<
2 Sonc None Sonc Sonc Sane Sonecod
-18 3 194 7 294 0 528 0 572 0 478 0 204 26'7 438 360 477 474iMg0 2 9 None None Sone None ti 5 Sone None Sane None None Sone
CaO 19 6 4 2 4 2 4 2 4 2 4 . 2 Sone None Sone Xone Xone None
-
* Expressed as parts per inillion of the solution
elsewhere for similar studies, were used with nlortar sainples which were subjected t o cycles of wetting and drying t o pro- duce efflorescence on the wicks. The amounts of such salt deposits have been colnpared visually and h y the loss i11 weight of the wicks from a washing treatiilent
For all the Portland cement, lime, and sand ~ n o r t a r s studied, the amount of efflorescence tended to increase as the propor-
W
3 I . 0 L 0.8L 0.6L 0.4L 0.2 L O L
OPC 0.2PC 0.4PC 0.GPC 0.8PC I.0PC
MORTAR COMPOSITION
Fig. 10. Comparison o f amounts o f salts dissolved from wicks, in relation to changes in mortar composition a n d ratio o f cementing m a t e r i a l to sand.
(L) indicates lime, (PC) Portland cement. Proportions a r e b y volume o f lime; ratios b y volume o f cementing material to sand.
MORTAR COMPOSITION
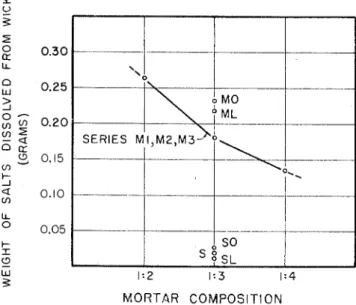
Fig. 1 1 Comparison o f amounts o f salts dissolved from wicks used with mortars o f masonry cements M a n d S a n d various sands for different
ratios b y volume o f cementing m a t e r i a l to sand.
tion of I'ortland cenierit in the liiortar increased (Figs. 7 through 10).
Under t h e sailie conditions, lllortars containing one type of Portland celllent produced greater ainounts of efflorescence than those of another (Fig. 7, inortar series A1 through A6 and E2 through EG). T h e lesser amounts of efflorescence were produced by mortars in which the Portland cement con- tained less alkaline material (Na?O and R 2 0 ; see Table I). Similarly, mortars of the two lnasonry cements diflered in the anlount of efflorescence produced, under the same conditions, as is shown in Fig. 11. I n this case, the lesser amounts of efflorescellce were produced by the ~ilortars of the masonry cement which contained less alkaline inaterial (Na?O and K 2 0 ; see Table I).
For those mortars whicli ga.\.e relatively high alliounts of efflorescence, t h e type of sand wed appeared t o exert con- siderable influence on the amount of efflorescence which de- veloped on t h e wick. Figures 7, S, 9, and 11 show that, other conditions being the saine, t h e lnortars of s a n d 0, the silica sand of uiliforln particle size, produced t h e greatest anlou~lts of efflorescence, and niortars of sand A t h e least. This is probably due t o differences in the ease of water move- ment through the inortar samples, resulting frolii differences in
particle-size characteristics of tlie various sands.
T h e analysis of the ivashing solutions obtained fro111 one series of lllortars ( A 1 tl~rougli A(;, Tahle I V ) indicated for that series that. sodim11 and putassiulri carboilate predomi- nated in the washings of the wicl;s used with liiortars contain- ing Portlalid celllent, and calciuni carl~onate in the washings of the wicl; used wit11 the lime mid s a ~ i d mortar. Sulfate material was present iii relatively appreciable (pantities in the washings wlleii Portlarld c e ~ n e ~ i t was used i n these mortars. An increase in the proportiou of cenienting material to \and in the rnortars producecl a greater amount of efflores- cence, as show11 in Figs 10 and I I .
IX. Conclusions
Marked differelices iri a ~ n o u n t s of effloresce~lce occurred uil ceralnic wiclts partially embedded in mortar samples which were subjected to a certain wetting and drying pro- cedure. Sixty nort tars were studied, composed of three limes, two Portland ceinents, two rnasonry cements, and three sands. T h e capacity of the cementing materials t o contrib- ute to efflorescence appeared t o be related t o the alkaline rnaterial present (expressed ill this paper as N a 2 0 a n d K20). The cementing niaterials relatii-ely low In alkaline content produced mortars which ga\,e relatively s ~ n a l l amounts of eflorescence on the wicIts; conversely, the cementing nia- terials of relatively high alkaline content produced mortars which gave relatively large amounts of efflorescence on the ivicks
Acknowledgments
In this study the author was assisted by J.-M. Billy of the Ui- vision of Building Research, National Research Council. Except that for Portlar~d cernc~it E, the chemical analyses given in this paper were niade by E C Gooclhue and P. Tymchuk, Division of Applied Chcinistry, Natior~al Rescarch Council.