Publisher’s version / Version de l'éditeur:
Cement and Concrete Research, 3, November 6, pp. 729-750, 1973-11-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/0008-8846(73)90008-2
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Hydration characteristics of monocalcium aluminate at a low
water-solid ration
Ramachandran, V. S.; Feldman, R. F.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=17f2734f-a383-4d96-9fe7-d60faae5366a https://publications-cnrc.canada.ca/fra/voir/objet/?id=17f2734f-a383-4d96-9fe7-d60faae5366a
CEMENT and CONCRETE RESEARCH. Vol
.
3, pp. 729-750, 1973. Pergamon Press, I n c . P r i n t e d i n t h e U n i t e d States.HYDRATION CHARACTERISTICS O F MONOCALCIUM ALUMMATE AT A LOW WATER/SOLID RATIO
by
V. S. Ramachandran and R. F. Feldman
R e s e a r c h Officers, Materials Section, Division of Building Research National R e s e a r c h Council of Canada
(Communicated by F. D. ~ a m s s )
ABSTRACT: The hydration c h a r a c t e r i s t i c s of calcium monoaluminate w e r e studied using an effective water/aluminate r a t i o of 0. 15 a t 20" o r 80°C, f r o m a few minutes to two months. T h e m a t e - r i a l hydrated a t 80°C shows a l a r g e shrinkage while a t the lower t e m p e r a t u r e a continuous expansion occurs. The p r o - duct a t 80°C shows a much higher strength than that hydrated at 20°C. The main initial hydration products a r e 2Ca0, AA203, 8H20 and alumina gel. Microcracks a r e developed in t h e products hydrated a t 20°C while at the higher t e m p e r a t u r e a v e r y compact m a s s r e s u l t s . The data indicate that i t i s possible t o obtain a durable high alumina cement by using a low water/cement r a t i o and hydrating a t higher temperatures, and under t h e s e conditions C3AH6
-
C3AHB bond i s favoured. L e s c a r a c t e r i s t i q u e s d'hydratation de lgaluminate monocalcique ont BtB Btudiees 'a l'aide d'un rapport eau-aluminate effectif d e 0. 15 'a 20" ou3
80°C s u r une periode variant d e quelques minutes'a
deux mois. Le m a t e r i a u hydrate 'a 80°C subit un r e t r a i t considerable tandis qug'a une t e m p e r a t u r e plus b a s s e il s e produit une dilatation continue. La r e s i s t a n c e du produit hydrate 'a 80°C e s t beaucoup plus grande qul'a 20°C. Les principaux produits dlhydratation initiaux sont 2Ca0, AA20,,8H20 et l e gel d'alumine. Des m i c r o f i s s u r e s apparaissent dans l e s produits h y d r a t e s
3
20°C, tandis qul'a l a t e m p e r a t u r e plus &levee l e r e s u l t a t e s t une m a s s e tr'es compacte. Les donnees indiquent qu'on peut obtenir un ciment alumineux durable en utilisant un faible rapport eau-ciment et en effectu- ant lthydratation d e s t e m p e r a t u r e s plus elevkes, et que dans de t e l l e s conditions une liaison CaAH,-
C3AH6 e s t facilitee.7 3 0 V o l . 3 , N o . 6
CALCIUM ALUMINATE, WATER CEMENT R A T I O , HYDRATION
Introduction
High a l u m i n a c e m e n t , a l s o known a s aluminous c e m e n t o r calcium a l u m i
-
n a t e c e m e n t develops a v e r y high s t r e n g t h within 24 h r of c u r i n g in w a t e r . It h a s a good r e s i s t a n c e to s u l f a t e a t t a c k and c a n b e used in r e f r a c t o r y c o n c r e t e . T h i s cement i s a l s o used a s a n expanding o r s t r e s s i n g c e m e n t in combination with gypsum and o t h e r constituents.
T h e high a l u m i n a c e m e n t c o m p r i s e s s e v e r a l p h a s e s v i z . , CA, CA,, Cl,A7, CaAS, C G , C,F and C,AF*, Much attention h a s been d i r e c t e d t o a study of t h e hydration c h a r a c t e r i s t i c s of CA. T h e hydration p r o d u c t s of CA c o n s i s t of CAH,,, CaAH,, C3AHB and AH, ( g e l o r c r y s t a l l i n e ) , t h e r e l a t i v e p r o p o r t i o n s of which depend on t h e h y d r a t i o n conditions and c u r i n g p e r i o d . At lower t e m - p e r a t u r e s CAH,,, CaAHs and AH, g e l a r e p r e f e r e n t i a l l y formed and t h e s e a r e converted to C3AH6 and gibbsite a t h i g h e r t e m p e r a t u r e s , a c c o r d i n g t o t h e following s c h e m e (1).
CAH,,
1 2 1
-
35OCC A C2AH3
+
AH,T h e 'conversion' o r ' i n v e r s i o n ' t h a t o c c u r s a s a consequence of t h e t r a n s f o r - m a t i o n of t h e hexagonal p h a s e s , CAHlo o r C2AH, into t h e cubic p h a s e (C,AH6) i s known t o b e accompanied by a l o s s in s t r e n g t h of t h e h a r d e n e d a l u m i n a c em ent
.
T h e c o n v e r s i o n r e a c t i o n s m a y b e d e s c r i b e d a c c o r d i n g to t h e following equations.
3CAHlo C3AH6 -F 2AH3
+
18H 3CaAHe+2C3AHe+ AH,+
9HT h e c o n v e r s i o n of CAHlo t o C3AHe r e s u l t s in a volume d e c r e a s e t o about 50
p e r c e n t w h e r e a s t h a t of CaAHe r e s u l t s in a d e c r e a s e t o about 65 p e r cent of t h e o r i g i n a l volume of t h e r e a c t a n t s .
V o l . 3, N o . 6 73 1 CALCIUM ALUMINATE, WATER CEMENT R A T I O , HYDRATION
S e v e r a l t h e o r i e s have been proposed to explain the l o s s in strength in high alumina cement. They a r e s u m m a r i z e d below.
As a l r e a d y stated the conversion of t h e hexagonal t o t h e cubic phase r e s u l t s in a substantial d e c r e a s e in the solid volume. In t h e hardened c o n c r e t e this m a y b e accompanied by t h e formation of p o r e s and hence lower s t r e n g t h . This i s essentially t h e explanation f o r s t r e n g t h l o s s advanced by Robson ( 2 ) , Neville ( 3 ) , Lafuma ( 4 ) , Cottin ( 5 ) , Midgley (6), and Mehta ( 7 ) . T h e porosity m a y a l s o i n c r e a s e due to t h e evaporation of water that i s formed in t h e con- v e r s i o n reaction.
The e a r l i e r t h e o r y that t h e oxidation of f e r r o u s i r o n in t h e high alumina cement i s responsible for t h e r e t r o g r e s s i o n in s t r e n g t h s h a s been conclusive- l y disproved by Lea and o t h e r s (8, 9, 10).
Lehman and L e e r s (1 1) a s c r i b e the higher s t r e n g t h s in t h e alumina cement to CAH,, having a high s u r f a c e a r e a . Conversion, producing t h e cubic f o r m with a lower s u r f a c e a r e a i s thought t o b e r e s p o n s i b l e for low s t r e n g t h s . In a r e c e n t paper Mehta (12) h a s affirmed that a poor adhesive capacity of t h e cubic p h a s e and low specific a r e a a r e additional f a c t o r s contributing to a l o s s in strength. It i s a l s o possible that the conversion of the flat plates with o v e r - lapping o r interlocking s y s t e m to i c o s i t e t r a h e d r o n o r cubic morphology dis
-
locates t h e bonds.Wells and C a r l s o n (13a) failed to o b s e r v e substantial d e c r e a s e in t h e s u r - f a c e a r e a during conversion and hence attribute t h e f a l l in s t r e n g t h to t h e for
-
mation of m a c r o c r y s t a l l i n e alumina h y d r a t e f r o m t h e m i c r o c r y s t a l l i n e a l u - mina hydrate. This is not in accord with t h e opinion of Lehman and L e e r s (11) who believe that c r y s t a l l i n e alumina h y d r a t e i n c r e a s e s the strength in high alumina c e m e n t s .R6vay found that during conversion reaction t h e s u r f a c e a r e a i s actually i n - c r e a s e d due to the formation of alumina gel (13b).
Ueda (14a) did not find a d i r e c t relationship between t h e p o r o s i t y and s t r e n g t h in high alumina cement. He h a s attributed t h e l o s s in strength to a d e c r e a s e in the combined w a t e r in t h e converted product and a l s o to the i n - c r e a s e in t h e quantity of water filling the s m a l l p o r e s .
C A L C I U M A L U M I N A T E , W A T E R C E M E N T R A T I O , H Y D R A T I O N
Vol.
3,
No.
6
T h e d e t e r i o r a t i n g influence of CO,, t h r o u g h t h e f o r m a t i o n of CaCO,
+
AH,+
H f r o m C3AH6 h a s b e e n p r o p o s e d b y T a l a b e r f o r a l u m i n a c e m e n t s (14b).It h a s now b e e n d i s c o v e r e d t h a t c o n v e r t e d a l u m i n a c e m e n t c a n b e s t r o n g and of low p e r m e a b i l i t y if p r e p a r e d a t a low w a t e r / c e m e n t r a t i o . Robson and o t h e r s (1 5, 16) m a i n t a i n that a t a low w a t e r / c e m ent r a t i o t h e w a t e r r e l e a s e d d u r i n g t h e ' c o n v e r s i o n ' r e a c t s with t h e a n h y d r o u s c e m e n t k e r n e l not utilized i n t h e i n i t i a l h y d r a t i o n r e a c t i o n , and f i l l s t h e p o r e s and thus p r e v e n t s s t r e n g t h l o s s e s . S t i g l i t z (17) explains t h a t t h e s t r e n g t h l o s s i s m a i n l y c a u s e d by i n - c r e a s e d p o r o s i t y d u e t o t h e e v a p o r a t i o n of w a t e r r e l e a s e d d u r i n g c o n v e r s i o n . At low w a t e r / c e m e n t r a t i o s t h i s w a t e r c a n bind with t h e unhydrated CA. A c c o r d i n g t o Midgley (18) t h e m a j o r f a c t o r t h a t p r e v e n t s l o s s i n s t r e n g t h i s t h e g r a i n s i z e of t h e c o n v e r t e d m i n e r a l s v i z . , C,AH, and AH,. At low w a t e r / c e m e n t r a t i o s t h e low p o r o s i t y i s m a i n t a i n e d b y t h e packing of t h e s m a l l c r y s - t a l l i t e s w h i l e a t h i g h e r w a t e r / c e m e n t r a t i o s t h e p o r o s i t y i s i n c r e a s e d b y t h e c r y s t a l s i z e . F r o m t h e f o r e g o i n g s u r v e y i t c a n b e concluded that t h e s t r e n g t h l o s s in a n o r m a l l y c u r e d high a l u m i n a c e m e n t i s a s c r i b e d t o t h e c o n v e r s i o n r e a c t i o n ; t h e exact m e c h a n i s m h o w e v e r , i s not c l e a r . It i s a l s o i m p l i c i t in t h e l i t e r a - t u r e t h a t t h e cubic h y d r a t e (C3AH6) d o e s not h a v e a binding c a p a c i t y . F o r
example, t h e s t r e n g t h s of t h e a l u m i n a t e h y d r a t e s a r e thought t o b e in t h e d e - c r e a s i n g o r d e r , CAH,,>C2AHe>C,AH6(19). But t h i s i s not in c o n s o n a n c e with t h e s t r e n g t h r e s u l t s obtained a t low w a t e r / c e m e n t r a t i o s even a f t e r c o n v e r s i o n
(16, 20).
E a r l i e r w o r k (21) on t h e h y d r a t i o n of C3A i n d i c a t e d t h a t t h e c o n v e r s i o n of C,A t o C3AHB u n d e r c e r t a i n conditions, in f a c t , e n h a n c e s t h e s t r e n g t h . It a p p e a r s t h a t t h e f o r m a t i o n of C3AH6 p e r s e should not b e c o n s t r u e d a s d e t r i - m e n t a l t o s t r e n g t h . In o r d e r t o e x a m i n e t h e a p p l i c a b i l i t y of t h e above findings t o t h e h y d r a t i o n and s t r e n g t h development in high a l u m i n a c e m e n t s , CA w a s h y d r a t e d a t a low w a t e r / c e m e n t r a t i o a t 2 0 ° C and 80°C f o r d i f f e r e n t p e r i o d s . T h e w a t e r / s o l i d r a t i o w a s c h o s e n a t 0. 1 5 t o e m p h a s i z e i t s s i g n i f i c a n t effect on t h e m e c h a n i s m of h y d r a t i o n and s t r e n g t h development. T h e r e s u l t s m a y b e a d v a n t a g e o u s l y extended t o p r a c t i c a l m i x e s h a v i n g low w a t e r / s o l i d r a t i o s .
V o l . 3, No. 6
733
CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATION
c h a r a c t e r i s t i c s and other properties such a s microhardness, porosity, m o r - phology and dimensional changes.
Experimental
Materials
The calcium monoaluminate used for this investigation was supplied by Tetratech International, San Diego. The sample contained 35. 2 p e r cent, CaO, 64. 3 p e r cent A& 0, with f r e e lime l e s s than 0. 2 per cent. The x - r a y analysis of this m a t e r i a l showed all the lines typical of CA. The surface a r e a was 0. 62m2/g. No t h e r m a l effects w e r e observed in the differential scanning calorimeter (DSC) or thermogravimetric analysis (TGA).
Calcium monoaluminate powder was compacted at a load of 75, 000 lb into discs 1. 25 in. in nominal diameter and 0. 05 in. thick. The effective water: aluminate ratio for hydration was calculated to be 0. 15. TBe compacts were hydrated in duplicates at a t e m p e r a t u r e of 20°C or at 80°C for 10 min, 30 min,
1 h r , 2 h r , 5 h r , 10 h r , 1 day, 2 days, 5 days, 10 days, 21 days and 60 days. At the end of each period the sample was washed with alcohol and evacuated f o r 24 h r using liquid a i r t r a p . The sample was ground to approximately 100 m e s h s i z e for t h e r m a l and x - r a y analysis, fractured for microscopic exam- ination and cut t o a rectangular shape for length change m e a s u r e m e n t s .
Methods
Differential thermograms of the samples w e r e obtained by a Differential Scanning Calorimeter (DSC) supplied a s a module to Du Pont 900 t h e r m a l analyser. This unit utilizes chromal-constantan f o r differential t e m p e r a t u r e measurement. The reference m a t e r i a l was ignited
a
-
AJ2O, and the heating r a t e was maintained at 1 o 0 c / m i n . The differential temperature was regis-
tered at a sensitivity of 0. 02 mv/in. Thermograms w e r e obtained in a i r . Both the sample and the reference m a t e r i a l w e r e contained in aluminum foil cups. In each experiment 40 m g of the sample was weighed into the aluminum cup.
Thermogravimetric analysis (TGA) of the samples was c a r r i e d out using a sensitive Cahn Balance at a heating r a t e of 1 o 0 c / m i n . All the runs were c a r r i e d out in a continuous vacuum.
7 3 4 V o l . 3 , No. 6 CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATION
T h e x - r a y powder photographs w e r e obtained with P h i l i p s C a m e r a u s i n g Cu K s o u r c e . T h e r e l a t i v e i n t e n s i t i e s of t h e l i n e s w e r e obtained b y d e n s i -
a
t o m e t e r t r a c e s of t h e powder p h o t o g r a p h s . T h e f r a c t u r e d s u r f a c e s of t h e s p e c i m e n s w e r e examined b y a s c a n n i n g e l e c t r o n m i c r o s c o p e supplied b y C a m b r i d g e Co. S a m p l e s cut t o a r e c t a n g u a l a r s h a p e a p p r o x i m a t e l y 1 i n . b y 0. 25 in. w e r e exposed t o w a t e r a t 2 0 ° C o r 8 0 ° C continuously f o r p e r i o d s of up t o 7 days in a c e l l and t h e length change w a s m e a s u r e d p e r i o d i c a l l y by a modifiedT u c k e r m a n gauge e x t e n s o m e t e r . T h e d e t a i l s of t h i s method a r e d e s c r i b e d e l s e w h e r e (22).
T h e V i c k e r s h a r d n e s s v a l u e of t h e unhydrated and t h e h y d r a t e d c o m p a c t s was d e t e r m i n e d with a L e i t z m i n i l o a d h a r d n e s s t e s t e r . E a c h value r e p r e - s ents an a v e r a g e of 10 d e t e r m i n a t i o n s c a r r i e d out on both s i d e s of t h e c o m
-
p a c t .T h e Aminco-Winslaw p o r o s i m e t e r w a s u s e d . t o d e t e r m i n e t h e p o r e s i z e d i s t r i b u t i o n and p o r o s i t y of both t h e u n h y d r a t e d and h y d r a t e d s a m p l e s . T h e i n s t r u m e n t m e a s u r e s p o r e s i z e d i a m e t e r i n t h e r a n g e 0 . 1 m m t o 0 . 012 p
.
T h e t o t a l p o r o s i t y w a s a l s o d e t e r m i n e d b y v a c u u m - s a t u r a t i n g t h e s p e c i m e n s with m e t h a n o l .A conduction c a l o r i m e t e r with s i x c h a m b e r s w a s u s e d t o follow t h e r a t e of h e a t development d u r i n g t h e h y d r a t i o n of CA. T h e s e n s i t i v i t y of t h e c a l o r i - m e t e r w a s 20
~ v / w .
S u r f a c e a r e a w a s obtained withN2
a s t h e a d s o r b a t e by a N u m i n c o - O r r s u r f a c e a r e a-
p o r e volume a n a l y s e r . R e s u l t s and D i s c u s s i o n D i f f e r e n t i a l Scanning C a l o r i m e t e r R e p r e s e n t a t i v e d i f f e r e n t i a l s c a n n i n g c u r v e s of CA h y d r a t e d a t 20" o r 80°C f o r d i f f e r e n t p e r i o d s of up t o 60 days a r e shown in F i g u r e s 1 and 2. At 20°C h y d r a t i o n p r o c e e d s slowly i n t h e f i r s t few h o u r s . At 10 h r h y d r a t i o n i s i n d i - c a t e d b y a n e n d o t h e r m a l v a l l e y with a p e a k a t about 1 0 0 ° C . A s m a l l endo- t h e r m a l doublet in t h e r a n g e of 175" t o 2 2 5 ° C i s a l s o r e g i s t e r e d . At 1 day aVol. 3, No. 6
7 3 5
CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATION
' I ' I ' I ' I '
*
1-
a::
\
'
+
T E M P E R A T U R E , O C FIG. 1 T h e r m o g r a m s of C A Hydrated t o Different P e r i o d s a t 20°C a t a w a t e r / s o l i d Ratio of 0. 15 5 M I N 1 0 M I N 3 0 M I N 1 HR 2 HRS 5 HRS 1 0 HRS 1 D A Y 2 D A Y S 5 D A Y S 1 0 D A Y Sa
2 0 D A Y S 6 0 D A Y S 0 1 0 0 200 300 400 500 600 T E M P E R A T U R E , "C FIG. 2 T h e r m o g r a m s of C A Hydrated a t 80°C t o Different P e r i o d s a t a w a t e r / s o l i d Ratio of 0. 15736 V o l . 3, N o . 6
CALCIUM ALUMINATE, WATER CEMENT R A T I O , HYDRATION
s h a r p endothermal p e a k a p p e a r s a t about 175°C and i n c r e a s e s in intensity a s the hydration p r o g r e s s e s . Also an endotherm of s m a l l magnitude appearing
a t about 260°C a t 1 day grows in intensity. An additional endothermal p e a k a p p e a r s a t about 300°C a t 5 days and t h i s a l s o i n c r e a s e s in intensity up t o 60 days. T h e l a r g e endotherm appearing f r o m t h e v e r y e a r l y period with a peak around 100°C m a y be attributed t o r e m o v a l of w a t e r f r o m t h e alumina gel. This endotherm i s followed by another a t about 125°C (not a p p a r e n t in s o m e i n s t a n c e s ) and m a y b e a s c r i b e d t o t h e p r e s e n c e of C,AH8. An unmistakable endotherm appearing at about 175°C i s due t o t h e p r e s e n c e of CAH,,. T h e dual peaks o c c u r r i n g in the t e m p e r a t u r e r a n g e 200" to 325" C r e p r e s e n t dehydration r e a c t i o n s in gibbsit e and C3AH6. Gibbsite i s f o r m e d f r o m t h e c r y s t a l l i z a t i o n of alumina gel and C3AH6 i s t h e conversion product of CAH,, and C,AH,.
T h e t h e r m a l behaviour of CA hydrated a t 80°C i s significantly different f r o m t h a t hydrated a t 20°C ( F i g u r e 2). A detectable amount of hydration i s evident even at 10 m i n . By 30 m i n l a r g e endothermal effects a p p e a r a t about 100°, 145", 210°, and 280°C. T h e f i r s t effect i s caused by alumina gel and i s p r a c t i c a l l y absent a f t e r 2 days of hydration. T h e endotherm effect a t about 150°C i s p r e s e n t in a l l s a m p l e s up t o 1 day and i s a t t r i b u t a b l e t o t h e p r e - s e n c e of C,AH,. T h e endothermal effect a t 280°C which i n c r e a s e s in i n t e n s i - ty with hydration i s mainly caused by C3AHB and gibbsite. T h e i n i t i a l down- w a r d slope of t h i s c u r v e s u g g e s t s the possibility of an additional endotherm.
In f a c t a t 2 days a c l e a r endotherrn e m e r g e s a t about 225°C which m a y b e due to CAH1,; a maximum r a t e of f o r m a t i o n of this compound s e e m s t o occur between 2 and 5 days. P o s s i b l y gibbsite a l s o contributes t o t h i s endothermal effect. T h e resolution of t h e l a r g e endothermal effect into two effects a t about 300" and 340°C a t 5 days c o n f i r m s the f o r m a t i o n of gibbsite and C3AH6. The endothermal p e a k a t about 500°C r e p r e s e n t s t h e typical stepwise dehy- dration effect of C3AH,.
T h e r m o g r a m s suggest that c o m p a r e d with hydration a t 20°C that a t 8 0 ° C p r o c e e d s a t a much f a s t e r r a t e . T h e d e g r e e and r a t e of conversion of t h e hexagonal p h a s e s and alumina gel t o t h e cubic and gibbsite p h a s e s r e s p e c t i v e - ly a r e a l s o enhanced a t higher t e m p e r a t u r e . In addition it i s p o s s i b l e that c o n v e r s i o n t o C,AH, and gibbsite p h a s e s o c c u r s d i r e c t l y on the s u r f a c e of CA
V o l . 3 , No. 6
CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATION
p a r t i c l e s . After an initial f a s t r e - action, f u r t h e r hydration, e s p e c i a l - l y a f t e r about 5 days, tends t o b e slow a t 8 0 ° C . T h e r e i s s t i l l a l a r g e amount of unhydrated CA even a t 60 days. T h e r a t e of formation and the conversion effect a r e not only depen- dent on the t e m p e r a t u r e of h y d r a - tion but a l s o on the initial w a t e r / solid r a t i o . At room t e m p e r a t u r e a higher water/solid r a t i o of 0. 5
p r o m o t e s f a s t e r conversion and c r y s t a l l i z a t i o n r e a c t i o n s c o m p a r e d with CA hydrated a t a lower/solid r a t i o ( F i g u r e 3). X - r a y diffraction R a t e s of hydration w e r e c o m - p a r e d using d e n s i t o m e t e r t r a c i n g s of the x - r a y f i l m s f o r CA compacts hydrated t o different p e r i o d s . T h e T E M P E R A T U R E , "C t r a c i n g s w e r e not intended f o r e v a l - FIG. 3
uating, quantitatively, the h y d r a - T h e r m o g r a m s of CA Hydrated a t 20°C a t a ~ a t e r / S o l i d Ratio of 0. 5 tion reaction but w e r e a guide t o
t h e new products that a r e f o r m e d . As t h e peaks w e r e l e s s distinct a t higher d values, the f i l m s w e r e used f o r c o m p a r i s o n p u r p o s e s . T a b l e I shows s o m e of the m o r e prominent l i n e s by which v a r i o s s p h a s e s w e r e recognized.
The unhydrated CA shows a l l t h e s t a n d a r d d values. At 20°C up to 10 h r t h e r e i s no indication of any hydrated product though DSC shows formation of s o m e hydration product. At 1 day faint lines a p p e a r a t 10. 5 and 5 . 2 0 due t o C,AH,. At 5 days the existence of CAH,, i s indicated. T h e CAH,, lines b e - c o m e m o r e intense a t 60 days and t h e r e i s a l s o a s m a l l amount of C,AHG. Gibbsite could not be detected with c e r t a i n t y . At 60 days c o n s i d e r a b l e amounts of unhydrated CA a r e s t i l l p r e s e n t .
V o l . 3 , N o . 6
CALCIUM ALUMINATE, WATER CEMENT R A T I O , HYDRATION
T A B L E I P h a s e 0 d v a l u e s (A) G i b b s i t e 4. 85, 4. 32-4. 37, 2. 42-2. 45, 2. 39. T h e c h a r a c t e r i s t i c s of h y d r a t i o n of CA a t 8 0 ° C a r e d i f f e r e n t f r o m t h o s e of CA h y d r a t e d a t 2 0 ° C . CA i s c o n s u m e d m o r e r a p i d l y a t e a r l y t i m e s . At 60 m i n u t e s t h e r e i s a n indication of t h e f o r m a t i o n of C2AHB but no evidence of CAH,,. C3AH, i s p o s s i b l y f o r m e d a t t h i s s t a g e . At 5 days l i n e s due t o C3AH, b e c o m e c l e a r e r and CAHlobegins t o f o r m . Gibbsite i s a l s o detected a t t h i s p e r i o d . At 10 days i n c r e a s e d a m o u n t s of CAH,, a r e f o r m e d . At 21 days and 60 days t h e p h a s e s p r e s e n t a r e CA, CAH,,, C2AH8, C3AH6 and gibbsite. T h e r e s u l t s a r e g e n e r a l l y in a c c o r d with t h o s e of DSC. C o m p a r e d with x - r a y DSC a p p e a r s t o b e a m o r e s e n s i t i v e method f o r identification and e s t i m a t i o n of h y d r a t i o n p r o d u c t s of CA.
T h e r m o g r a v i m e t r i c a n a l y s i s
TGA was c a r r i e d out i n a continuous vacuum and t h e t e m p e r a t u r e w a s m e a - s u r e d b y a t h e r m o c o u p l e p l a c e d n e a r t h e s a m p l e . TGA showed inflections c o r r e s p o n d i n g t o t h e d i f f e r e n t i a l c u r v e s obtained b y DSC but t h e t e m p e r a t u r e s w e r e d i s p l a c e d .
CA h y d r a t e d f o r 5 m i n a t 8 0 ° C showed a t o t a l weight l o s s of about 1 . 8 p e r c e n t a t 500°C and i s a s s o c i a t e d with a b r o a d e n d o t h e r m a l v a l l e y in t h e DSC c u r v e of t h i s s a m p l e ( F i g u r e 2). At 10 m i n t h e hydration s e e m s t o h a v e p r o - c e e d e d t o a g r e a t e r extent a s indicated b y a weight l o s s of 9 . 98 p e r c e n t . At 30 m i n TGA c l e a r l y shows two inflections and DSC a l s o exhibits two endo- t h e r m s a t t h i s p e r i o d . T h e f i r s t inflection c o r r e s p o n d s t o a weight l o s s of
6 . 97 p e r cent and t h e s e c o n d , t o about 6. 6 p e r c e n t . At 5 h r s TGA exhibits f o u r s t e p s in weight l o s s e s c o r r e s p o n d i n g t o f o u r e n d o t h e r m a l e f f e c t s . At t h i s p e r i o d t h e t o t a l weight l o s s i s 20. 76 p e r c e n t . At 1 day t h e t o t a l weight
V o l . 3, N o . 6 739 CALCIUM ALUMINATE, WATER CEMENT R A T I O , HYDRATION
l o s s i s 18. 8 4 p e r c e n t . At 2 d a y s only v e r y l i t t l e l o s s i n weight o c c u r s up t o 200°C and DSC a l s o shows a m u c h r e d u c e d p e a k below 2 0 0 ° C . At 5 d a y s t h e t o t a l weight l o s s i s 28. 22 p e r c e n t , a n i n c r e a s e of 7. 86 p e r c e n t f r o m 2
d a y s . Most of t h i s i n c r e a s e a r i s e s f r o m t h e f i r s t inflection c o r r e s p o n d i n g t o CAH,,, Between 2 and 5 days t h e weight l o s s c u r v e i n d i c a t e s t h a t t h e amount of C,AHG h a s not i n c r e a s e d .
TGA c u r v e s f o r CA h y d r a t e d a t 2 0 ° C show inflections t h a t could b e c o r - r e l a t e d with t h e e n d o t h e r m a l p e a k s i n DSC. T h e ignition l o s s e s f o r s a m p l e s h y d r a t e d f o r 1 h r , 10 h r , 24 h r , and 5 days a r e r e s p e c t i v e l y 1 . 34, 2. 49,
18. 96 and 22. 83 p e r cent and t h e c o r r e s p o n d i n g v a l u e s f o r s a m p l e s h y d r a t e d a t 8 0 ° C a r e 17. 38, 21. 13, 18. 8 4 and 28. 22 p e r c e n t . T h e m a g n i t u d e of t h e s e v a l u e s does not r e p r e s e n t t h e d e g r e e of h y d r a t i o n but i t d o e s show t h a t t h e h y d r a t i o n s e q u e n c e a t 20°C i s d i f f e r e n t f r o m t h a t a t 8 0 ° C . Conduction c a l o r i m e t r y T h e r a t e s of h e a t development of t h e c o m p a c t s exposed t o w a t e r w e r e d e t e r m i n e d a t 2 5 ° C o r 7 2 ° C by t h e conduction c a l o r i m e t r i c c u r v e s ( F i g u r e 4). Though t h e c u r v e s i n d i c a t e t h e r e l a t i v e r a t e s of h e a t development f o r h y d r a - tion t h e y a r e not d i r e c t l y c o m p a r a b l e t o t h e d i f f e r e n t i a l t h e r m o g r a m s . At TIME, HR FIG. 4 Conduction C a l o r i m e t r i c C u r v e s of CA H y d r a t e d a t 2 5 ° C o r 7 2 ° C
7 4 0
Vol.
3,
No.
6CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATION
25" C an initial peak r e s u l t s immediately on contact with water due to heat of wetting and possibly some s u r f a c e hydration. At about 1 day hydration seems to commence at a f a s t r a t e with maximum intensity in the interval between 28 and 31 h r . The r a t e of heat evolution d e c r e a s e s steadily thereafter and hydrati& proceeds slowly after 2 days. DSC curves show that considerable hydration takes place at 1 day, further hydration and conversion progressing
slowly ( F i g u r e 1). The hydration at 72°C s e e m s to proceed f a s t e r , a s evi- denced by an intense heat effect with a peak appearing e a r l i e r than 10 min. The major heat development occurs within 30 min. In the differential t h e r m o - g r a m s considerable reaction s e e m s to occur between 10 and 30 min ( F i g u r e 2 ) .
As already shown hydration in compacts i s c a r r i e d out at an effective water/solid r a t i o of about 0 . 1 5 and it i s expected that hydration and conver-
sion reactions should occur at a m o r e rapid r a t e at a higher water/solid ratio. This can be demonstrated by comparing the c a l o r i m e t r i c curves of CA at a water/solid r a t i o of 0. 15 or 0. 5 ( F i g u r e 4). The maximum r a t e of hydration i s found t o proceed e a r l i e r (12 and 20 h r ) and the total heat devel- oped i s a l s o of g r e a t e r magnitude at a higher water/solid ratio in the f i r s t few days. The total heat developed i s also m o r e than that registered f o r the compact hydrated at 72" C ( F i g u r e 4). The total amount of heat evolved for compacts hydrated at 72°C (for the f i r s t 30 min), 25" C (in about 2 days) and at 25°C a t a water/solid ratio of 0. 5 (1 day) a r e , respectively, 56. 0, 39. 0 and 72.6 ~ a l / ~ m .
Microstructure
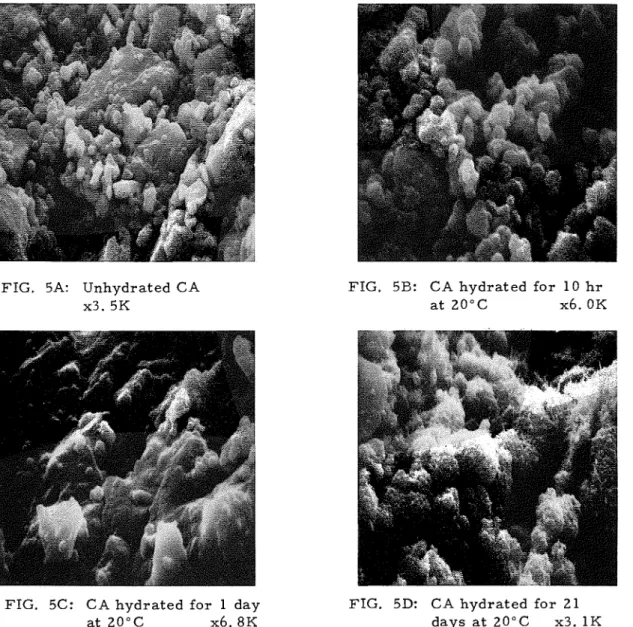
The unhydrated CA consists of p a r t i c l e s of indefinite morphology of v a r i - ous s i z e s ( F i g u r e 5A). P r a c t i c a l l y no hydration products a r e detected up t o 10 h r ( F i g u r e 5B). At 1 day t h e r e i s evidence of hydration on the s u r f a c e of the p a r t i c l e s ( F i g u r e 5C). The hydrated sample i s a l s o characterized by extensive microcracking. The c r a c k s m a y have either developed during hydration o r during the preparation for the microscopic examination. It i s m o r e likely that they would have developed during hydration a s the c r a c k s seem to pass through the unhydrated CA p a r t i c l e s ; the micrograph of un- hydrated CA does not show any c r a c k s . In some instances hydrated products
V o l . 3, N o . 6 7 4 1 C A L C I U M ALUMINATE, WATER CEMENT R A T I O , HYDRATION
FIG. 5A: Unhydrated CA x3. 5K
FIG. 5B: CA h y d r a t e d f o r 10 h r a t 2 0 ° C x6. OK
FIG. 5C: C A h y d r a t e d f o r 1 day FIG. 5D: CA h y d r a t e d f o r 2 1 a t 2 0 ° C x 6 . 8 K d a y s a t 20°C x3. 1K
tinuous expansion a s t h e hydration p r o g r e s s e s and t h i s m a y h a v e caused c r a c k development. A l a r g e amount of f i b r o u s m a t e r i a l i s s e e n in t h e s a m p l e hydrated f o r 21 days ( F i g u r e 5D) indicating t h e e x i s t e n c e of CAH,,
.
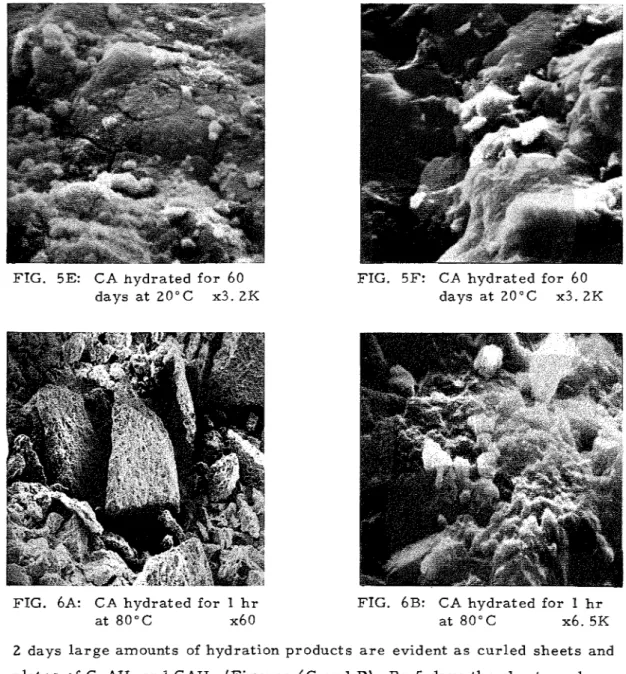
At 60 d a y s t h e s a m p l e shows s o m e unhydrated a m o r p h o u s , p l a t y and f i b r o u s m a t e - r i a l with no evidence of cubic C3AH6 a s typified by t h e two m i c r o g r a p h s ( F i g u r e s 5 E and F ) .T h e m i c r o s t r u c t u r e f e a t u r e s of t h e h y d r a t e d p r o d u c t a t 8 0 ° C a r e d i f f e r - ent f r o m t h a t f o r m e d a t 20°C ( F i g u r e 6). Even a t 1 h r t h e r e i s a n indication of t h e hydration p r o d u c t s c o v e r i n g t h e CA p a r t i c l e s ( F i g u r e s 6A and B). By
742 V o l . 3 , No. 6
CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATTON
FIG. 5E: CA hydrated f o r 60 days a t 20°C x3. 2K
FIG. 5F: CA hydrated for 60 L
days a t 20°C x3. 2K
FIG. 6A: CA hydrated for 1 h r FIG. 6B: CA hydrated for 1 h r a t 8 0 ° C x6 0 a t 8 0 ° C x6. 5K
2 days l a r g e amounts of hydration p r o d u c t s a r e evident a s c u r l e d s h e e t s and p l a t e s of C,AH, and CAH,, ( F i g u r e s 6C and D). By 5 days t h e s h e e t s and p l a t e s h a v e d i s a p p e a r e d and a d e n s e s t r u c t u r e i s f o r m e d (Figu're 6E). At 60 days t h e s u r f a c e p r e s e n t s a ' v i t r i f i e d ' a p p e a r a n c e with occasional m a s s e s , p r o b a b l y of C,AHG ( F i g u r e s 6 F and G). T h e r e was no indication of c r a c k development in a n y of t h e s a m p l e s h y d r a t e d a t 8 0 ° C .
Leneth changes
Length change m e a s u r e m e n t s in compacted bodies h a v e been shown t o p r o - vide i m p o r t a n t data on t h e m e c h a n i s m of hydration (21 t o 26). In this study
V o l . 3 , No. 6
CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATION
F I G . 6C: C A hydrated for 2 days F I G . 6D: C A hydrated for 2 days at 8 0 ° C x l . 4K at 8 0 ° C x 3 . 5 K
F I G . 6E: C A hydrated for 5 days F I G . 6 F : C A hydrated for 6 0 days at 8 0 ° C x 3 . 3K at 8 0 ° C x 0 . 7 K
F I G . C A hydrated for 60 days at 8 0 ° C x l . 5K
744
Vol.
3,
No.
6
C A L C I U M ALUMINATE, WATER C E M E N T RATIO, HYDRATION
a n a t t e m p t h a s b e e n m a d e t o u n d e r s t a n d t h e h y d r a t i o n c h a r a c t e r i s t i c s of CA b a s e d on t h e length changes o c c u r r i n g a t 2 0 ° C o r 8 0 ° C , up t o a p e r i o d of 5 d a y s . T h e r e s u l t s a r e plotted in F i g u r e s 7 and 8. At 2 0 ° C c o n s i d e r a b l e e x - p a n s i o n o c c u r s within a n h o u r and i t continues a t a s l o w e r r a t e t h e r e a f t e r . At 4hr, d u r i n g which about 50 p e r c e n t of t h e t o t a l expansion o c c u r s , h y
-
d r a t i o n h a s not p r o g r e s s e d t o a n y significant extent ( F i g u r e 1). S i n c e t h e p a r t i c l e s a r e in i n t i m a t e c o n t a c t with e a c h o t h e r t h e i n i t i a l p r o d u c t s f o r m e d a t t h e i n t e r p a r t i c l e s u r f a c e s tend t o p u s h e a c h o t h e r . T h e expansion m a y a l s o b e c o n t r i b u t e d t o i n i t i a l l y b y a d e c r e a s e in t h e s u r f a c e e n e r g y . A s t h e h y d r a t i o n p r o g r e s s e s t h e hexagonal a l u m i n a t e h y d r a t e s f o r m e d c r e a t e s o m eexpansion and t h e a l u m i n a g e l i s a c c o m m o d a t e d in t h e p o r e s . At 5 days v e r y a
s l i g h t c o n v e r s i o n h a s t a k e n p l a c e ( F i g u r e 1). At 8 0 ° C a n a l m o s t i m m e d i a t e expansion i s followed b y l a r g e s h r i n k a g e ( F i g u r e s 7 and 8). T h i s t y p e of b e h a v i o u r h a s not b e e n o b s e r v e d b e f o r e d u r i n g h y d r a t i o n of c o m p a c t e d b o d i e s . T h e s m a l l i n i t i a l expansion i s s i m i l a r t o t h a t o b s e r v e d f o r t h e s a m p l e c u r e d a t 2 0 ° C . T h e l a r g e amount of s h r i n k - a g e o c c u r r i n g within t h e f i r s t 30 m i n i s a l s o r e f l e c t e d in t h e c o n s i d e r a b l e h y - TIME. H R FIG. 7
Length Changes i n C o m p a c t s of Monocalcium A l u m i n a t e H y d r a t e d a t 2 0 ° C o r 8 0 ° C i n t h e F i r s t F e w H o u r s
Vol.
3,
No.
6
745
C A L C I U M A L U M I N A T E , W A T E R C E M E N T R A T I O , H Y D R A T I O N
d r a t i o n and i n t e r c o n v e r s i o n t h a t h a s o c c u r r e d a t t h i s p e r i o d ( F i g u r e s 2, 4, 6). T h e c o n t r a c t i o n m a y b e a t t r i b u t e d t o t h e f o r m a t i o n of hexagonal p h a s e s t h a t b r i d g e between p a r t i c l e s ; t h e i r v e r y r a p i d c o n v e r s i o n t o t h e m o r e d e n s e C,AH, p h a s e a t t h e o r i g i n a l s i t e s p r o v i d e s m o t i v e f o r c e f o r t h i s e f f e c t . It i s p o s s i b l e , h o w e v e r , t h a t a d i r e c t f o r m a t i o n of C3AH6 and AH, o c c u r r i n g a te a r l y p e r i o d s on t h e s u r f a c e of CA a l s o involves a b r i d g i n g effect of t h e i n t e r - f a c e s . T h e c o n t r a c t i n g f o r c e m a y b e caused by t r a n s p o r t a t i o n of AH, into t h e p o r e s . A f t e r a d a y t h e amount of C3AH6 f o r m e d d o e s not i n c r e a s e v e r y m u c h but t h e r e i s a g r a d u a l i n c r e a s e in t h e f o r m a t i o n of CAH,,; t h i s i s followed by a s l o w continuous expansion.
P o r o s i t y and m i c r o h a r d n e s s
A t t e m p t s w e r e m a d e t o s t u d y t h e effect of h y d r a t i o n on t h e p h y s i c a l and m e c h a n i c a l behaviour of CA. T h e p o r e s i z e distribution m a y b e evaluated f o r t h e unhydrated and h y d r a t e d CA f r o m F i g u r e 9. T h e unhydrated CA h a s a t o t a l p o r o s i t y of about 28 p e r c e n t of which t h e m a j o r p o r t i o n h a s a d i a m e t e r in t h e r a n g e of 0. 35 t o 0. 1 p . A f t e r 5 d a y s of h y d r a t i o n p o r o s i t y d e c r e a s e s TIME. HR FIG. 8 Length C h a n g e s i n C o m p a c t s of Monocalcium A l u m i n a t e H y d r a t e d a t 2 0 ° C o r 8 0 ° C up t o 5 Days
746 V o l . 3 , N o . 6 CALCIUM ALUMINATE, WATER CEMENT R A T I O , HYDRATION
4 0 I I I 1 I I I I I I I I I I I I I 1
--
- U N H Y D R A T E D C A --
-
-
H Y D R A T E D C A A T 2 0 ° C O R 8 0 ° C F O R 5 D A Y S I L-+,+-: ; ; 21 .O 10.5 7 . 0 4 . 0 3 . 0 2 . 0 1 .5 1 .O 0.5 0 . 4 0.3 0 . 2 0.1 0.05 0.04 0.02 0.01 D I A M E T E R O F P O R E S , p FIG. 9 P o r e S i z e Distribution C u r v e s of CA Hydrated a t 20°C o r 80°Ct o about 1 to 2 p e r cent whether CA i s hydrated a t 20°C o r 8 0 ° C . T h e d e - c r e a s e in p o r o s i t y cannot b e e a s i l y explained just in t e r m s of the absolute volume changes in the hydrated p r o d u c t s . T h e hydration of CA t o CAHloin- volves an enormous i n c r e a s e in t h e volume, w h e r e a s a d e c r e a s e in volume i s expected in t h e conversion of CAHl0 and C&H, t o t h e cubic p h a s e . In addition, the d e g r e e of hydration and i n t e r c o n v e r s i o n , t h e morphology, the possibility of clogging of m i c r o p o r e s by alumina gel and other products, a r e other f a c t o r s that cannot be e a s i l y subjected t o quantitative evaluation. It i s , however, obvious that t h i s i s m a i n l y due t o t h e p o r e s being filled up with the hydration p r o d u c t s . T h e t o t a l p o r o s i t y was a l s o determined b y vacuum s a t u - r a t i o n using methanol t o check if m e r c u r y had p e n e t r a t e d a l l the p o r e s . The methanol method gave a t o t a l p o r o s i t y of 29. 5 p e r cent f o r unhydrated CA and 2. 0 p e r cent f o r products obtained at 5 d a y s . T h i s indicated that m e r c u r y p e n e t r a t e s p r a c t i c a l l y a l l the p o r e s intruded b y methanol.
F o r a p a r t i c u l a r m a t e r i a l , if a l l t h e conditions a r e t h e s a m e , then s t r e n g t h i s known to be a function of p o r o s i t y . It follows that the reduced porosity due t o hydration of CA m u s t r e s u l t in an i n c r e a s e in t h e h a r d n e s s values for CA
V o l . 3 , No. 6 747 CALCIUM ALUMINATE, WATER CEMENT R A T I O , HYDRATION
a t 20" C o r 8 0 ° C . C o m p a r e d with a value of 19. 9 k g / m m 2 for the unhydrated compact of CA s a m p l e , those hydrated at 20" o r 8 0 ° C show h a r d n e s s of 109. 5 and 160. 5 k g / m m 2 r e s p e c t i v e l y . It i s g e n e r a l l y believed that higher t e m p e r a t u r e s a r e d e t r i m e n t a l t o s t r e n g t h development in high alumina c e m e n t , a s t h e s e conditions favour t h e formation of t h e cubic p h a s e . T h e s e r e s u l t s d e m o n s t r a t e that n e i t h e r t h e high t e m p e r a t u r e n o r t h e formation of t h e cubic phase i s d e t r i m e n t a l t o s t r e n g t h development. On t h e other hand, although t h e s a m p l e hydrated a t 20°C h a s about t h e s a m e p o r o s i t y a s that hydrated a t 8OoC, t h e l a t t e r h a s a much higher h a r d n e s s value. At 80°C t h e r e a c t i o n p r o c e e d s v e r y f a s t and enhances hydration of CA t o t h e C3AH6 and AH, p h a s e s . As the p a r t i c l e s of CA in t h e compact l i e v e r y c l o s e t o each o t h e r , d i r e c t bond formation between C&H6 p r o d u c t s i s enhanced. At 20°C however, d i - r e c t bond formation due t o C3AH6 m a y not b e favoured a s C3AH6 and AH, products a r e t r a n s p o r t e d and r e c r y s t a l l i z e d in the p o r e s . T h i s i s evident f r o m t h e m i c r o s c o p i c examination in which only d e n s e s t r u c t u r a t i o n i s evident a t 8 0 ° C ( F i g u r e 6).
At 60 days t h e h a r d n e s s of t h e hydrated product f o r m e d a t 20" and 80°C a r e 73. 9 and 152. 4 k g / m m 2 respectively. T h e f a l l i n s t r e n g t h f o r the s a m p l e hydrated a t 20°C i s m a i n l y due t o t h e conversion of CAH,, and C&H, t o
alumina gel and C&H6. T h i s p r o c e s s involves a s l o w e r conversion of CA t o C+H6 and hence C3AH6
-
C3AHB bonds a r e not promoted t o the s a m e extent. It i s v e r y likely that a t ambient conditions and a t a w a t e r / c e m e n t r a t i o higher than that used i n this investigation a s t i l l p o o r e r s y s t e m would r e s u l t . The s a m p l e s continuously exposed a t 80°C up t o 60 days do not significantly change in s t r e n g t h due t o s t r o n g bonds developed by C+H6 a t e a r l y s t a g e s . It i s expected that for higher s t r e n g t h s , CA m a y b e hydrated a t higher t e m p e r a t u r e s a t a sufficiently low w a t e r / s o l i d r a t i o f o r a few h o u r s only and then exposed t o n o r m a l t e m p e r a t u r e s .T h e s e r e s u l t s s u g g e s t t h a t t h e p r e s e n t t h e o r i e s on s t r e n g t h d e t e r i o r a t i o n in high alumina c e m e n t s should t a k e into account t h e i m p o r t a n t contribution of t h e s t r e n g t h promoting p r o p e r t i e s of t h e cubic and gibbsite p h a s e s , e s p e - c i a l l y when f o r m e d a t low water/solid r a t i o s . It h a s a l s o been observed that t h e i n c r e a s e in the s u r f a c e a r e a p e r s e need not n e c e s s a r i l y m e a n that s t r e n g t h s
748
Vol.
3, No. 6 CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATIONi n c r e a s e . F o r example, the unhydrated CA has a surface a r e a of 0. 6 ~ m ' / ~ , the samples hydrated for 5 days at 20°C o r 80°C yield a surface a r e a of
1. 88m2/g and 1. 5 ~ m ' / ~ respectively but -the strength of the product formed a t 80°C i s much higher than other samples.
Conclusions
This study h a s thrown new light on the hydration c h a r a c t e r i s t i c s of CA and suggests r e a s s e s s m e n t of the popular concepts in the field of high alumina cements. At a low water/solid r a t i o some of the c h a r a c t e r i s t i c s of the hy- drated product formed at 80°C a r e different from that prepared at 20°C. At 80°C a direct bond formation of C,AH, m a y occur on the surface of CA even within 30 min due to rapid conversion of the hexagonal phase; curing at 20°C involves a continuous expansion that at 80°C results in a substantial shrinkage. Strength development occurring a t 80°C in the f i r s t few days does not change during further hydration over s e v e r a l months. A deterioration in strength occurs in the sample hydrated a t 20°C a s the hydration and interconversions occur over longer periods.
Both a t 20°C and 80°C, C a H B and alumina gel and not CAH,, seem to be the main initial hydration products. At 80" C the hydration products do not show any definite morphological c h a r a c t e r i s t i c s of CAH,,, C a H , or C&He Contrary to general opinion enhanced t e m p e r a t u r e actually r e s u l t s in the de
-
velopment of strength provided the water/solid r a t i o i s low. Also the for
-
mation of C&H6 by itself should not n e c e s s a r i l y be construed a s detrimental to strength development; in fact C,AH6 enhances strength. An i n c r e a s e in the surface a r e a need not n e c e s s a r i l y r e s u l t in an i n c r e a s e in strength. S i m i - l a r l y in hydrated CA, identical strengths need not result for products of equal porosity. The present theories seeking to explain strength retentions in high alumina cement produced a t low water/solid r a t i o should a l s o take into account the possible contribution of C&H, phase for strength development.
It appears possible that a durable high alumina concrete can be produced within minutes of curing using a low water/cement r a t i o and higher t e m p e r a - t u r es
.
Vol.
3,No.
6C A L C I U M A L U M I N A T E , W A T E R C E M E N T R A T I O , H Y D R A T I O N
Acknowledgment
T h e a u t h o r s acknowledge with thanks t h e m a j o r e x p e r i m e n t a l contributions of G. M. P o l o m a r k and S . E. Dods and t h e valuable a s s i s t a n c e of P . J.
Lefebvre, E. G. Quinn and R. M y e r s . T h i s p a p e r i s a contribution f r o m t h e Division of Building R e s e a r c h , National R e s e a r c h Council of Canada, and i s published with t h e a p p r o v a l of t h e D i r e c t o r of t h e Division.
R e f e r e n c e s
1. A. C. C . T s e u n g and K. G. C a r r u t h e r s . T r a n s B r i t . C e r a m . Soc. ,
62,
305-3213 1963.2. T . D. Robson. T h e C h e m i s t r y of C e m e n t s ( E d . H. F. W. ~ a ~ l o r ) Academic p r e s s . New York, p. 3-35, 1964.
3. A . M . Neville. P r o c . Inst. Civil E n g . , 10, 185-192, 1958.
-
4. H. Lafuma. Epitoanyag,2 ,
162-167, 1969.5. B. C o t t i n a n d P . Reif. Rev. Mat. d e c o n s t r . , n o 6 6 1 , 293-305, 1971.
-
6. H. G. Midgley. T r a n s . B r i t . C e r a m . Soc. ,66,
161 -187, 1967.I
7. P. K. Mehta. Minerals P r o c e s s i n g , 2, 16 -19, 1964.
-
8. F. M. Lea. J. Soc. Chem. I n d . ,2,
18-21, 1940.9. N. Iltchenko and H. Lafuma. Chim. et I n d . , 38, 438-440, 1937.
-
10. J. S e a i l l e s . 14th Congr. d e Chim. Ind. , vol 11, P a r i s , 56, 1934. 11. H. L e h m a n n a n d K . J . L e e r s . Tonind. Z t g . ,87,
29-41, 1963. 12. P . K. Mehta. J. A m e r . C e r a m . S o c . ,54,
210-212, 1971.13a. L. S. Wells and E. T . C a r l s o n . J. R e s . Nat. B u r . S t d . , 57, 335-353, 1956.
13b. M. RCvay. ( P r i v a t e Communication)
14a. S. Ueda. Rev. Mat. C o n s t r . , No. 654, 55-60, 1970. 14b. J. T a l a b e r . Epitoanyag,
2,
1-
9, 1967.15. T . D. Robson. High Alumina C e m e n t s and C o n c r e t e s , John Wiley and Sons, New York, 1962.
16. P . J . F r e n c h , R . G . J . M o n t g 0 m e r y a n d T . D . Robson. Concrete,
2,
3-8, 1971. 17. P. Stiglitz. Epitoanyag,24,
45-52, 1972. 18. H.G. Midgley and K. P e t t i e r . T r a n s . B r i t . C e r . S o c . ,71,
55-59, 1972. 1 9 . K. M i s h i m a . P r o c . V I n t e r n a t i o n a l Symp. C h e m . C e m . , Tokyo, 1968, P a r t 111, 1969, p. 167-
174.7 5 0
Vol.
3, No. 6 CALCIUM ALUMINATE, WATER CEMENT RATIO, HYDRATION20. R. Alegre. Rev. Mat. C o n s t r . , NO. 630, 101-108, 1968
21. R. F. Feldman and V.S. Ramachandran. J. A m e r . C e r a m . S o c . , 49,
-
268 -273, 1966.22. R. F. Feldman, P. J. S e r e d a and V. S. Ramachandran. Highway Res. Rec. N o . 62, 106-118, 1964.
23. V.S. Ramachandran and R. F. Feldman. J. App. Chem. ( L o n d . ) , 17,
-
328-3323 1967.24. V. S. Ramachandran, P. J. S e r e d a and R. F. Feldman. Nature, 201,
-
288-289, 1964.25. P. J. S e r e d a , R. F: Feldman and V. S. Ramachandran. A m e r . C e r a m . Soc. B u l l . ,
44,
151-155, 1965.26. V.S. Ramachandran, P. J. S e r e d a and R. F. Feldman. Mat. Res. Stand. ,