Publisher’s version / Version de l'éditeur:
Macromolecules, 24, 11, pp. 3093-3097, 1991-05-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/ma00011a011
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
A new approach to modeling the cure kinetics of epoxy/amine
thermosetting resins. 1. Mathematical development
Cole, K. C.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=2a02b958-336d-4f37-9e2f-75941d4ef198 https://publications-cnrc.canada.ca/fra/voir/objet/?id=2a02b958-336d-4f37-9e2f-75941d4ef1981991,24, 3093-3097
A New Approach to Modeling the
Cure
Kinetics of
Epoxy Amine
Thermosetting
Resins.
1.Mathematical Development
K.
C.ColeIndustrialMaterialsResearchInstitute, NationalResearchCouncil ofCanada, 75boulevarddeMortagne, Boucherville, Quebec,CanadaJ4B6Y4
ReceivedJuly31, 1990;RevisedManuscriptReceivedNovember9,1990
ABSTRACT: The Horiemodel for describing thecure kineticsofepoxy aminesystems isextendedtoexplicitly includetheetherificationreaction, whichbecomessignificantwhen thereisan excess ofepoxywithrespect
to amine and whenthecure isperformed at higher temperatures. Asolution to thekineticequations is derivedthatmakesitpossibletodeterminetherelationshipbetweenthedegreeofconversionaandthe rate
ofconversionda/dt. Differentpossible mechanismsforthe etherification reactionare considered. The
modelcan beusedtocalculatethe amountsof differentgroups formedinthe reaction andhence provide
informationconcerning thenetwork structure.
Introduction
The widespreaduse ofepoxy-basedcomposites in the
aerospaceindustry,coupledwiththe growthof computer-aideddesign andmanufacturing,hasresultedinincreased interestin modeling theprocessing ofthese materials.1 The object is to optimize the processing parameters in order to consistently obtain high-quality parts and to minimize the experimental work required todesigna cure
cycleforanynew partsthatmaybeintroduced.
With
agoodmodel,
it
is possibleto predicthowthesystemwill
behaveduringcure and whatitsfinal conditionwill
be.Oneofthe mostimportantcomponentsofsucha model
isan accuratedescriptionofthecure kinetics. However,
the chemistry involvedinthe epoxycuringprocess israther complex, andinspiteoftheextensive researchthathas
been done over the years
it
isstill
not completely understood. As a result,it
isvirtually
impossible todescribe
it
rigorously, andexistingmodels alwaysinvolve certain assumptions and approximations.Manycommercialcomposite systems consistof amine-curedepoxy resins. Theepoxyamine reactionproduces hydroxylgroups,whichhavetwoeffects: (1)theycatalyze the reactionthatproducesthem and(2)theythemselves
can react withepoxy rings to form ether linkages. The
progressofthecuring reactionisdescribedquantitatively in termsofthefractionaldegreeofconversionofepoxide groups,usuallydesignateda. Tomodelthe kinetics
it
isnecessarytoderivean equationexpressing
da/dt,
therate ofchangeofa withtime,as afunction ofa andthetem-perature T. In 1970, Horieetal.,2 makinguse of mech-anisms proposedby earlierworkers, developedanequation
todescribethe kineticsofthereactionbetween epoxy and primaryamine. If
it
isassumedthatthe secondaryamine groups formed in the reaction show thesame degree ofreactivity toward epoxy groups as the primary amine groups
initially
present, theirequation simplifies toda/dt
=(Kx + K2a)(l
-a)(B
-a) (1)
where
K\
isarate constantforthe reactioncatalyzedbygroups
initially
present in theresin,Kzisarateconstant for the reaction catalyzed by newly formed hydroxyl groups,andBistheinitial ratio ofamine N-H bondsto epoxide rings. Thusthisequationtakesintoaccountthe autocatalytic nature ofthe epoxyamine reaction, but itdoesnot allowforthepossibility ofother reactionsthat produce ethergroups (“etherification”). It was foundto fit experimental data well at lowlevelsofconversion, up toa = 0.5or so.2,3 The deviationsobserved beyondthis
pointwere attributedtotheonsetof diffusioncontrolas a resultofgelation ofthesystem.
Many aerospace materials contain aromatic amines, which require higher curing temperatures thanaliphatic
ones. Furthermore, thereisoftenasignificantexcess of
epoxywithrespecttoamine. Underthese circumstances, theetherificationreactionbecomesmore importantand theHorie approachis lessvalid. Attemptstoincludethe etherificationreaction complicate the mathematics
con-siderably,so an exact solutionofthekinetic equationshas
not been obtained. In order to model cases where the Horie equation is inadequate, Kamal and co-workers developedthe following semiempirical modification:4
da/dt
=(Kx + K2am)(1
-a)n (2)
Theintroductionofthevariable exponentsmandnusually
makes
it
possibleto obtainagoodfit
to experimentaldata,andthisequationhasfound widesuccessful application forbothepoxyand polyestersystems. However, it does
notexplicitlytakeintoaccounttheetherification reaction,
so
it
does not provide a clear description ofthe curing process andits chemistry, whichisimportant for under-standing the networkformationprocess. Theexponentsm andnare oftenfound tobetemperature-dependent,so
thedependencymustbedeterminedover thewhole range oftemperaturesofinterest.
Recently therehave beenafewattempts to include the etherificationreactionintheanalysis.6"10 Whilethesehave
notmadethe assumptionthattheprimaryandsecondary aminegroups showthesame reactivity,theyhave made certain otherassumptionsand approximationsinorder to perform theanalysis. Forinstance, Zukasetal.6assumed that, like the epoxide-amine reaction, the epoxide-hy-droxylreaction involves two rate constants (correspond-ing to“uncatalyzed”andhydroxy-catalyzedreactions)and that their ratio is the same as for the epoxide-amine
reaction. Inorderto obtainan acceptable
fit
totheirdata, theyhad tointroduce semiempirical modificationssuchas letting some ofthe apparent reaction orders deviate
from1 or even varywiththedegreeofcure a. Riccardi
and Williams7·8 usedasimilarmechanismbutadifferent
mathematical treatment tostudyadifferentsystem. They
obtained a good fit to their data but some ofthe rate
constants,includingthosefor etherification,were
difficult
to determinewithgoodaccuracy becausethe datawere
not sufficientlysensitive to them. Other workers have assumedasimpleepoxide-hydroxyl reactionasthe ether-ification mechanism.9·10 Chern and Poehlein, in their 0024-9297/91/2224-3093$02.50/0 Published 1991by the American Chemical Society
3094 Cole Macromolecules, Vol. 24,No.11, 1991 R’ I R I R‘ R 1 I 1 nh2 + 1 CH—CH —> 1 1 NH—CH-—CH— OH Primary
Amine Epoxide Senary Hydroxyl
R R‘ I I R 1 R R· 1 1 R 1 1 1 HO-CH-CH-NH + CH-—CH —> H0-CH-CH-N-CH-CH-0H
V
2 2 Secondary EpoxideAmine TertiaryAmine Hydroxyl
R* R I I R R‘ I I R R | 1 1 NH-CH-CH-OH + 1 1 CH—CH —>
NH-V
CH—CH-O-CH 1 — CH—OHHydroxyl Epoxide Ether Hydroxyl
R 1 R I n CH—CH -> — 1 -CH—CH —0-Epoxide Ether n
Figure1. Mainreactionsinvolvedinthecure ofepoxy resins
with primaryaminecuringagents.
analysis,9 determined only the rate constant for the reactionbetween epoxide and primaryamine;allothers
were expressed as a fixed multiple of thisone based on
data taken fromtheliteratureforsimilarsystems. Chiao10
also useddatafromothersystemsin order tofixtheratio ofcertainpairsofrateconstants. Althoughagood
fit
andreasonableresultswere obtained,thetransferofsuchdata fromone system to anotherissubject to question,given
that some of the rate constants are dependent on the
amountof catalytic impurities in theparticularsystem. In this paper, an alternative approach to solving the
kineticequationsisdeveloped,in which the Horie treat-ment is extended to include theetherification reaction. Althoughasimpleequation relating
da/dt
toacannotbeobtained,
it will
beshownthat itispossibletoindirectly determinethe exactrelationshipbetweenthe two. Dif-ferentpossible mechanisms fortheetherification reactionare considered,either involvingor not involving hydroxyl
andtertiary aminegroups. Model Development
Epoxy Reactions. The cure ofepoxyaminesystems
has beenreviewed by Barton11 andRozenberg.12 For a
primaryaminecuringagent,themain reactionsthatoccur are illustratedin Figure1. Theirrelative importancehas
beenthe subject ofmuch research,butthesituationisfar from clearly understood. Thefirstreaction, whichoccurs
quite readily,isbetweenanepoxidering(E) andaprimary
aminegroup(PA)to producealinkcontainingasecondary
amine group (SA) and a hydroxyl group (OH). The
secondaryamine group formedin thisreactioncan react
further to give a tertiary amine group (TA) and a new
hydroxyl group. As mentioned above, both of these reactions have beenshown to be catalyzedby hydroxyl groups. Anotherreactionthatcan occur isthatbetween
an epoxide ringand ahydroxylgroup to form an ether
link
anda new hydroxylgroup,whichisthenavailablefor furtherreaction. The epoxide-hydroxyl reactionis gen-erallyslowerthan the epoxide-amine reaction andbecomesimportantonlywhenthecure is performed at high
tem-peraturesor whenthereisan excess ofepoxywithrespect
to amine.11 Thesituation regarding the reactionbetween
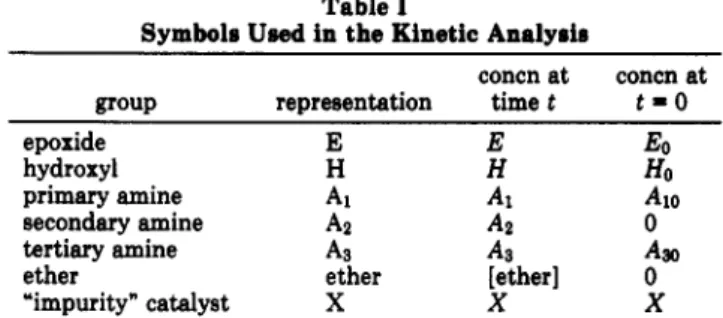
Table I
Symbols Usedin theKinetic Analysis
group representation concn at time t concn at t-0 epoxide E E So hydroxyl H H Ho
primaryamine Ai A, A10
secondary amine A2 0
tertiaryamine As A3 Aao
ether ether [ether] 0
“impurity”catalyst X X X
epoxide and secondary amine is less clear. There is evidence that the secondary aminegroups react at the
same rateastheprimaryones,butthereis alsoevidence that they reactsignificantlymore slowly.12-13
Evenif no reactive amineispresent,epoxy resins
will
polymerize on theirown if heated toa sufficientlyhigh temperature. This isattributedto a“homopolymeriza-tion"reaction,which maybeinitiatedbyimpuritygroups present in the resin or by (nonreactive) tertiary amine groups. Itisgenerallyconsideredtobe
difficult
toachieveunless specific catalystssuchasborontrifluoridecomplexes are present.
Kinetic
Equations. The resin system may becon-sideredas a collectionofepoxide groups, aminegroups, hydroxylgroups,ethergroups, andcatalysts. Thevarious
speciesand theirconcentrations maybe representedby
thesymbols showninTable I. Weassume thatthe
un-reacted resincontains onlyepoxide,primaryandtertiary amine,hydroxyl,and catalyst(otherthanhydroxyl)groups. Theprimaryaminegroups come from the curingagent.
Tertiaryamine groupsmaybepresentaspartoftheepoxy
molecule,so an
initial
concentrationterm Agoisincludedtoallowfortheirpresence; suchisthecase,forexample, in the well-known commercial productTGDDM (bis[4-(diglycidylamino)phenyl]methane). The “unknown" cat-alyst
X
representsimpurities thatmaybepresentinthe resin; the concentration is taken to be constant. The proposedreactionscheme is as follows:*1 E+ Ax (+
)
— A2+ (+H) k\ E+Aj
(+X)-*· A2+ (+X) *2 E+ A2(+H)^A3
+H(+
H) *'2 E+ A2(+X)— A3+ (+ X) *3 E+ mH+ nA3-»· ether+ mH+ n A3The first equation describesthe reaction between an
epoxide group and a primary amine group to form a
secondaryamine group anda new hydroxylgroup.
It
iscatalyzed by hydroxylgroups, H. These participate in thereactionbutremainunchanged,sotheyare shownin parentheses. The second equation describes the same
reactionbutcatalyzedbygroupsX
initially
present in the system. Thethirdandfourthequationsare similar,exceptthat they refer to the reactions between epoxide and secondaryaminegroups toformtertiaryamine and hy-droxylgroups. Thefirstfour equationsare equivalent to
thoseusedbyHorie.2 Thefifth isnew and representsthe
etherification reaction. In the following treatment, we use the term etherificationtorefer toboth the epoxide-hydroxylreaction andthe homopolymerization reaction. Inbothcases,the netresultisthesame: an epoxidering istransformedintoanetherlinkage andthereis no change
Macromolecules, Vol. 24, No. 11, 1991 Modeling the CureKineticsofEpoxyResins.
Table II
Coefficients in Equation30Correspondingto Different PossibleMechanisms for theEtherification Reaction (Equation8)
term m n Ci c2 c3 c4
E 0 0 0 0 1 -1
EH 1 0 0 0 Y-R -(Y+1)
Em 2 0 -( +1) 0 (Y-fi)2
-(Y+l)2
EA$ 0 1 -(«+ 1) 0 Z+R2 ~(Z+1)
3
1 1(R+l)(R-
Y-l) -V=(fl+1) (Z+ R*)(Y-R) -(Z+1MY+1) inthenumberofhydroxylgroups. However,inthefirstcase onehydroxylgroupisdestroyed and anotherisformed. Inthesecond case,hydroxylgroups donot participate. To
cover thedifferentpossibilities, the equationiswrittenin
a general form with coefficient “m” representing the
number of hydroxylgroupsinvolved(aseither reactantor
catalyst) and“n"the numberof tertiaryamine groups(as
catalyst). Bothmandncouldbezero. (These exponents m andn shouldnotbeconfusedwiththose usedineq 2.)
Thekineticequationsfortheabovereactionschemeare as follows:
dE/dt
= -k1HEA1 -k’1XEA1 -k2HEA2 -k'2XEA2-k3EHmA3n (3)dAJdt
= -k1HEAl-k\XEA1
(4) dA2/dt=+klHEAl
+ k'1XEAl -k2HEA2-k'2XEA2 (5) dAg/df = +k2HEA2 + k'2XEA2 (6)dH/dt
= +k1HEAl +k\XEAl
+ k2HEA2 + k'2XEA2 (7) d[ether]/di
= +k3EHmA3n (8)The term ineqs3and8arisesfrom the etherification reaction.
It
is assumedto befirst
orderwithrespectto epoxide concentration, butthe exponents m and n may be0,1,or 2,dependingon theotherspeciesparticipatinginthe reaction. By manipulation ofeqs3-8,
it
shouldbepossibletosolvefortheconcentrationofallsix components as a function of time. The concentration of catalytic impurities,
X,
is unknown but is assumed to remain constantthroughoutthecure. Thus the quantitiesk\X
and k'zX could be replaced by constants
k'\
andk"i,
respectively. This is the same formulation that wouldresult
if
the reactionwere assumedtobeuncatalyzed,so asfarasthe mathematical developmentis concerned,itdoesnot matter whether the reactionisconsideredtobe
uncatalyzed, X-catalyzed,or both.
Solution of
theEquations. Thereare three materialbalance constraints inherent in these equations. First, thelossofone
N-H
bondalwaysresults inthe formation ofoneO-H
bond,sothetotalnumberofN-H
bondsplus O-H bonds is constant. Thus2Aj + A2+ = constant =
2A10+ H0 so that
A2=
2A10+
H0-2A1-H
(9)Second,thetotal numberofnitrogenatomsisconstant,
so that
Aj
+ A2+ A3= constant= A10+ A30 and A3— Ago+ A10-Aj
-A2Substituting forA2 fromeq 9 gives
A3=
Ago+ A1
-A10+
H
-H0 (10)
Third, thetotalnumberofoxygen atomsisconstant, so
E+H+ [ether] = constant= E0+ H0 and [ether] = E0+
H0-E
- H (11)Onsubstitutingeqs9and10intoeqs3,4, and7we are
left withthe following three equations
dE/dt
+dH/dt
= -k3EHm(,A30+A1 + -A10-H0)n (12)dAJdt
+dH/dt
= (k'2X+k^I){2Aw
+H0-2A1-H)E
(13)dAJdt
=-(k\X
+ k1H)EA1 (14) Equations12-14involve only three unknowns(£,A\,and H) and may besolvedforthese as a functionoft. Theremaining three unknowns(A2, A3, and [ether]) maybe
obtainedfromthesebymeans ofeqs9-11. Tosolveeqs
12-14,
it
ishelpfultomakethe following transformation todimensionless variables: = 1-Jr
or E = E0(1-a)
(15) 2A, +A2H-H0
0= 1--= or H = Ho+ 2Alo0 (16) ¿ ^ 10 7= 1- T^" orAj
= A10(l -7) (17)Thequantityaisthe well-knownextentofconversion,as
defined in termsof thefraction ofepoxidegroups reacted. The variable0 isthefractionofthe
N-H
bondsthathave reactedwithepoxide, and sincethelossofeachN-H
bond resultsinformationofone O-Hbond,it
isalsoameasureofthenumberof hydroxylgroupsproducedby the reaction. Finally,7 isa measure ofthenumber of primaryamine groups reacted. Whent = 0(unreactedresin),a =
ß- y
= 0,and whenthereactioniscompletea = ß= y= 1.The
evolutionofthesethree variables with time completely describesthe curingprocess, sincetheycorrespondto the reactionofepoxidewith primaryamine only (7),primary plus secondary amine (0), and primary amine plus secondaryamine plus hydroxyl (a). We alsodefine the followingconstants: B= 2Aio/£q= amine-to-epoxide ratio
in unreactedresin; Y=
Hq/2Aiq=
Ho/BEo- measure of hydroxylcontent in unreactedresin; Z=
A30/A10= 2A30/
BEo - measure of tertiary amine content in unreacted resin. Ontransformation, eqs12-14 become
=
k3(BE0)m+n(.Y +
d)m((l/2)Z-
(1/2)
+3096 Cole Macromolecules, Vol.24,No. 11, 1991
^-||*
=WzXE,+
WoE,
+^02 ß\
X(7-0)(l-«)
(19)d7/dt
=\k\XE0
+ kxH0E0+ kxE2B0\ X(l-7)(l-«)
(20)It
isimpossible tosolvetheseequationswithoutmakingsome simplifications.
Horie etal.madethe assumptionthatthereactivity of thesecondaryamine groups as comparedto that ofthe
primaryamine groupsisthesame forboth the X-catalyzed
andthe hydroxyl-catalyzedreactions. In otherwords k2/k1 -
k'2/k\
= r (21)and we obtain
da
_ 3( +ß (
+ ß2)"ß
(
+2ß)(1-ß)
Thisequation maybesolvedexactly,usingthemethodof integration bypartialfractions, togivea as a function of ß. For integralvalues ofm and n withm + 2n < 3, the
term ontherightmaybeexpandedinthe following form:
[Tl
+ + Kx +2ß
+W?]
wherethecoefficientsT¡dependon theparticularvalues
ofm andn. Suchan expressionis easilyintegrated and
thesolution to eq 29maybe written in the form
With
thisassumption,dividingeq 20intoeq 19leadstoThismaybesolved(withthe
initial
condition7= 0whenß =
0) togive
^
=+1^ ^(1-7)-(1-7)
(23)2(1
~r)
Thisestablishestherelationbetweenßand7, and makes itpossibleto eliminateßfromeqs18-20,reducing them to two equations in two unknowns. Unfortunately, an
exactsolution is
still
impossible,andtoproceed furtherit
isnecessaryto assume thatr =x/z· This impliesthat thesecondaryamine
N-H
bonds reactwiththeepoxide ringsasreadilyasdotheprimaryamineN-H
bonds.Sinceasecondaryamine grouphasonlyone
N-H
bond, whereas aprimaryaminegrouphastwo, theprobabilityofreactionis halved. As already mentioned, there is conflicting experimentalevidenceon thispoint,withcases wherethe assumptionhasbeenfound tobevalid andothers where it hasnot.12,13 Whenit is valid,eq 23 simplifies to
d=
l-(l-7)1/2
or 7 =0(2-0)
(24)Oncombiningeqs 19and 20and usingthis relationship to eliminate7, we obtain
d0/dt
= (Kx +2ß)(1
-0)(1 -a) (25) where Kx=(l/2)E0(k\X
+ kxH0) and K2=(1/2)E0\
(26)It
shouldbenotedthatKi
combinestheeffectsofcatalysis byallgroupsinitially
present,both unknown(X)and hy-droxyl(Ho). Although not specificallyconsidered here,if anyuncatalyzedepoxide-amine reactionwere presentitwouldalsobeincludedin
K\
andeq 25wouldstill
apply. Thesecond rate constant Kzcorresponds to catalysisby onlythose hydroxyl groupsformed inthe reaction.Oneliminating7 fromeq 18, we obtain
^- &
=3( +ß) (
+ß2 - )
(27)where
K3 =
k32~n(BE0)m+n (28)
Wenow haveasetoftwo equations (eqs 25and27) in
two unknowns (aandß). It is still impossible to obtain exact solutionsfora and/3as a function oft. However, if we divideeq 27by eq 25,the (1
-a)term cancels out
a =
ß
++ß [^ß
+C/
+ C3In(1 + RTlfi) + C4In(1-0)]
(30)whereR = Kx/BKz. Theinitial condition a = 0when
ß
= 0hasbeenapplied. Expressionsfor the coefficientsC\,
Cz, C3, and C4 are given in Table II for five possible
combinations ofm and n.
Combiningeqs 25and 27gives
da/dt
=[B(KX +
2ß)(
1-0)+ K3(Y+ 0)m(Z + 02)n] X
(l-«)
(31) It isimpossible toinverteq 30 toobtain0 intermsofa.Consequently,eq31cannotbeconvertedintoan analytical expression relating
da/dt
to a. However, the exactrelationshipbetween
da/dt
anda can bedeterminedon a point-by-point basis. Thus, for a given set of rate constants( ,
Kz, K3) anda given valueof0, eq 30may beusedtocalculatethe correspondingvalueofa,followedbyeq31tocalculate
da/dt.
Repeating thisprocedureforasetofvaluesof0rangingfrom0to1 givesan exact set
ofpoints(a,
da/dt),
whichcan beusedtoplotacurve and comparewithexperimentaldata. The rate constantscanthenbevaried
until
thebestmatchisobtained. Different reactionmechanisms fortheetherification reactioncan be investigated by using the appropriate values of the exponents m and n, as given in TableII. It
should benoted that this procedure allows no control over the
particularvaluesofaobtained. If itisdesiredtocalculate thevaluesof
da/dt
corresponding toparticularvaluesofa,thenan alternative procedurecan beusedinwhicheq 30is solved numericallyfor0 andeq 31 is then usedto calculate
da/dt.
Theuse ofthetwovariablesa and0tomodelthecure
makesitpossible tofollowquantitativelythetworeaction paths, epoxy amine and etherification. The relative importanceofthese
will
vary dependingon thetemper-ature. Once thethree rate constantsandtheir temper-ature dependence have beendetermined, thecure can be
numerically modeled for any temperature program of interest byusingeqs 25and 31. If theratio ofhardener to epoxy in theresin ischanged,in principle thiscan be
takenintoaccountby calculating thechangesinBandEo
andmaking the appropriate adjustments to
K\,
Kz,and K3(eqs 26and28). Itshouldbenotedthateq 30,relatinga toß,applies onlyforthecase ofisothermalcure, where
therate constants do not change. Itsmainusefulness lies in analyzing isothermal cure datato determine the rate
constants.
The concentrations ofthe various speciesinvolved in
the reactionsmay also berelated to a andß,bymaking
Macromolecules, Vol. 24,No. 11, 1991
initial
epoxide concentrationEq,these are as follows Epoxide E/E0 = 1 -a (32) Hydroxyl H/E0-B{Y
+ ß) (33) Primaryamine A1/E0= (1/2)B(1 -ß)2 (34) Secondaryamine A2/Eo=B0(l-0)
(35) Tertiaryamine (formedinreaction)AJE0
=(1/2)B02 (36)
Ether
[ether]/£o =
a-B0
(37)Equations25and30-37thus allowacomplete
descrip-tionofthecuringprocess. Furthermore,aseq31issimply adifferentrepresentationofeq3,itmaybebrokendown
as follows into contributions from the three different reactions involved:
PA-E
reaction(da/dt)!
= BIKX +)(
1-a)(l
-ß)2 (38) SA-Ereaction(da/dt)2 =
BiKi
+2ß)(
1-a)(l
-ß)ß (39)
Etherificationreaction
(da/dt)3
= 3( +ß) (
+ß2 - )
(40)It
is sometimes assumed that the reaction can beapproximately dividedintotwostages.
At
the beginning ofthe cure, the amine-epoxide reaction dominates and etherificationisinsignificant. Thus,K$can beset equaltozero. Equation 27thenreducesto =
ß,
andeq 25 reducestoeq1,the Horie equationwithr =1/2. Toward
theendofthecure,the approximationcan bemadethat all the amine groups have reacted and the hydroxyl concentrationis constant. In thiscase
0=1
andeq 31reducesto
da/dt
= K3(Y+ 1)m(Z + 1)"(1 -a) = K'3{1 -a) (41)Inotherwords,attheendofthecure the reaction tends
to become simply first order with respect to epoxide
Modeling the Cure Kinetics ofEpoxy
concentration. This approximate approach has been appliedwith some success to one commercialsystem.14
Conclusion
Anew approach to modeling the cure ofepoxyresins
with primaryamineshasbeen developed.
It
is basedonthe modelproposedby Horie but isextended to include the etherification reaction. The basic assumptions in-herentinthe modelare (i) the epoxide-amine reactions
are hydroxylcatalyzed; (ii) thesecondaryaminegroups have thesame reactivity withrespectto epoxide as the
primaryamine groups; and(iii)the etherification reaction isfirst orderwith respectto epoxideconcentration and mayalsoinvolvehydroxylgroups,tertiaryamine groups,
or both. Themodel makesuse ofnoempirical parameters
andno noninteger reactionorders. Theevolutionofthe reaction isdescribed intermsoftwo variables, a and 0.
The
first
is the usual overall epoxide conversion. The second isa measure oftheextentofthe epoxide-aminereaction. Thus the two differentreaction pathwayscan be followed quantitatively. The kineticequationshave been solvedtoobtainan analytical relationshipbetween
a and0. Althoughan explicitexpression cannotbederived torelate
da/dt
toa, bothcan be expressedintermsof0.Thus the exact relationship between the two can be
calculatedon apoint-by-pointbasisforagivensetofthe three rate constants involved. The best values for the rate constantscan be determined by calculatinga curve
of
da/dt
versus a,comparingitto experimental data,and varying the rate constants until a suitable match isachieved. Oncethe rate constantsare known, the curing
processcan becompletely described, includingthe
evo-lution ofthe differentchemicalspecies as a function of
thedegreeofcure. The effecton thekineticsofachange
inamine/epoxyratiocan alsobepredicted. Compared to
previous models, thisnew approach has the important advantageofallowinganaccuratedescription ofthecuring
processover thewhole rangeofcure,without introducing
empirical parametersor makingapproximationssuchas
separating the reactioninto distinct regimes. Although developed forepoxy curedwith primaryamine,themodel couldbemodified tocover thecaseofsecondaryor mixed
amines. Inthesucceedingpaper, thesuccessfulapplication ofthemodeltoatypicalcommercial productisdescribed.
References andNotes
(1) Roberts, R. W.SAMPE J. 1987,23(5) (Sept/Oct.) 28. (2) Horie,K.; Hiura, H.;Sawada,M.;Mita,I.; Kamoe, H.J.Polym.
Sci.,PartA-l 1970, 8, 1357.
(3) Sourour,S.;Kamal, M.R.Thermochim.Acta 1976,14,41. (4) Kamal, M.R.Polym.Eng. Sci. 1974,14, 231.
(5) Zukas, W.X.;Schneider, N.S.;MacKnight,W.J.Polym.Mater. Sci. Eng. 1983, 49, 588.
(6) Zukas, W.X.; Dunn,D. A.;Gilbert, M.D.Polym. Mater.Sci. Eng. 1987,56, 346.
(7) Riccardi,C. C.;Williams,R. J. J.J.Appl.Polym.Sci. 1986,32, 3445.
(8) Riccardi,C. C.;Williams,R. J.J. Crosslinked Epoxies,Proc. Discuss. Conf.,9th1986, 291.
(9) Chern, C. S.;Poehlein,G.W.Polym.Eng. Sci.1987,27, 788. (10) Chiao, L. Macromolecules1990,23, 1286.
(11) Barton,J.M.Ado.Polym.Sci. 1985, 72,111. (12) Rozenberg, B. A. Ado.Polym. Sci. 1986, 75, 113.
(13) Charlesworth,J.J. Polym. Sci.,Polym. Chem. Ed. 1980, 18, 621.
(14) Lee, W.L;Loos, A. C.;Springer,G.S.J.Compos.Mater.1982, 16, 510.