SUPPLEMENTARY INFORMATION
Isomerization and aggregation of 2-(2-(2-hydroxy-4-nitrophenyl)hydrazono)-1-phenylbutane-1,3-dione: recent evidences from theory and experiment
Silvia Hristova1, Fadhil S. Kamounah2,3, Aurelien Crochet4, Poul Erik Hansen3, Katharina M.
Fromm4, Daniela Nedeltcheva1, Liudmil Antonov1*
1 Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences,
Acad. G. Bonchev str., bldg. 9, 1113 Sofia, Bulgaria
2 Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100
Copenhagen Ø, Denmark
3 Department of Science and Environment, Roskilde University, DK-4000, Roskilde, Denmark 4 Department of Chemistry, University of Fribourg, Chemin du Musée 9, 1700 Fribourg,
Switzerland
* Corresponding author.
Table S1. Experimental [1] and predicted (B3LYP/6-311+G(2d,p)//M06-2X/TZVP) 1H chemical
shifts (in ppm) of the major and minor forms of 1 and 3 in DMSO-d6.
Heavy atoms (see Scheme 4) 1 major form 1 minor form 3 major form 3 minor form * ** * ** * ** * ** 1 29.27 2.51 29.27 2.57 29.27 2.5 29.27 2.57 3´ 23.80 7.68 23.80 7.7 23.69 7.93 23.68 7.91 5´ 23.75 7.7 23.52 7.83 23.70 7.91 23.47 8.02 6´ 24.24 7.18 23.64 7.76 24.24 7.26 23.65 7.86 2" 23.66 7.9 23.92 7.7 23.67 7.91 23.93 7.72 3" 24.08 7.54 24.16 7.5 24.08 7.55 24.17 7.52 4" 23.92 7.67 23.98 7.64 23.92 7.68 23.98 7.66 NH 17.63 14.14 19.25 11.65 17.65 14.11 19.23 11.58 OH / OCH3 26.71 11.51 26.74 11.5 27.81 4.08 27.83 4
* predicted nuclear shieldings; ** experimentally measured chemical shifts.
Table S2. Linear equations between experimental (Y) and predicted (X) values from Table S1.
Compound Isomer Slope Intercept R2
1* major -1.00 31.64 0.998
minor -0.91 29.24 0.997
3 major -0.99 31.41 0.999
minor -0.90 29.23 0.997
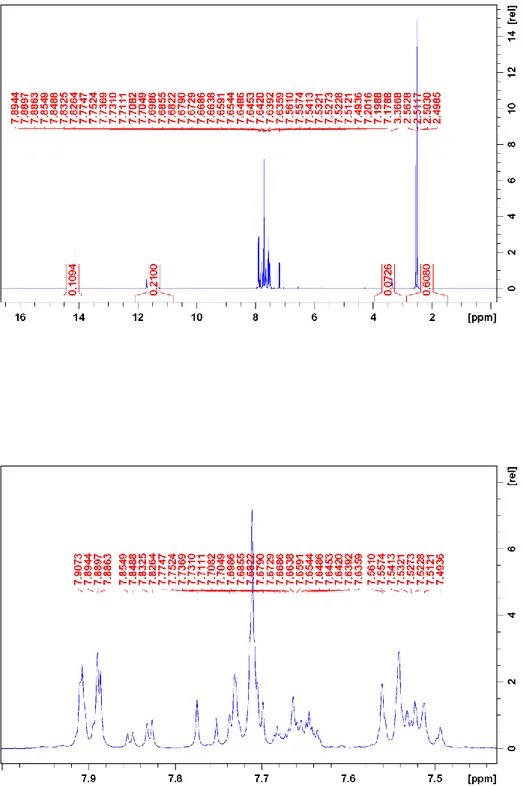
Figure S1. 1H NMR spectrum of 1. The spectrum shows both major and minor species. The
major species has the following assignment: 7.90, H-2”; 7.72, H-5´; 7.70, H-3´; 7.76, H-4”; 7.53, H-3”, 7.20, H-6´; 2.51, CH3 all in ppm. For numbering see Scheme 4.
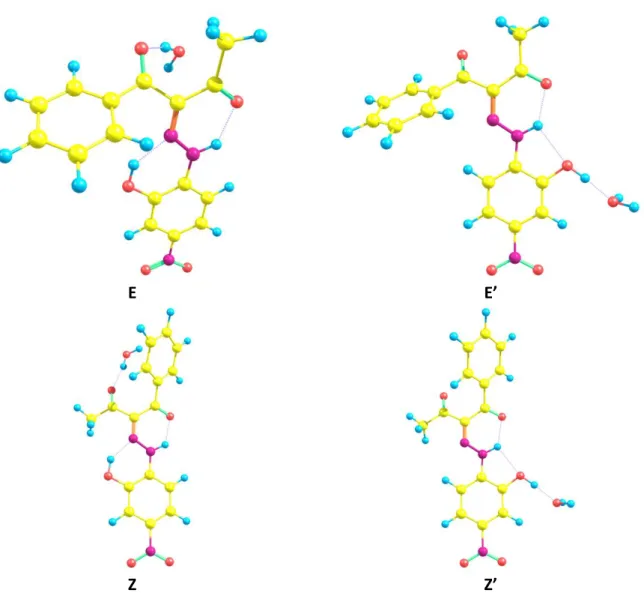
E E’
Z Z’
Figure S3. H-bonds motifs of 1, H-bonds are draw as blue dash lines, Symm.: #1: 1/2+x, 3/2-y, 1/2+z; #2: -1/2+x, 3/2-y, -1/2+z.
Figure S6. Deprotonation as a function of the content of water in DMSO: dry DMSO (black solid like), increasing water content (from dashes to red solid line).
References
[1] S. Hristova, F.S. Kamounah, N. Molla, P.E. Hansen, D. Nedeltcheva, L. Antonov, The possible tautomerism of the potential rotary switch
2-(2-(2-Hydroxy-4-nitrophenyl)hydrazono)-1-phenylbutane-1,3-dione, Dyes and Pigments. 144 (2017) 249– 261. doi:10.1016/j.dyepig.2017.05.021.
[2] A. Lyčka, 15 N NMR study of ( E )- and ( Z )-2-(2-(2-hydroxy-4-nitrophenyl)hydrazono)-1-phenylbutane-1,3-diones. A suitable method for analysis of hydrazone isomers, Dyes and Pigments. 150 (2018) 181–184. doi:10.1016/j.dyepig.2017.10.023.
![Table S1. Experimental [1] and predicted (B3LYP/6-311+G(2d,p)//M06-2X/TZVP) 1 H chemical shifts (in ppm) of the major and minor forms of 1 and 3 in DMSO-d 6](https://thumb-eu.123doks.com/thumbv2/123doknet/14810119.610855/2.892.96.759.170.484/table-experimental-predicted-tzvp-chemical-shifts-major-minor.webp)