HAL Id: hal-02410979
https://hal.archives-ouvertes.fr/hal-02410979
Submitted on 21 Sep 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Distributed under a Creative Commons Attribution| 4.0 International License
Challenges, advances, and promises
Pierre Echaubard, James Rudge, Thierry Lefèvre
To cite this version:
Pierre Echaubard, James Rudge, Thierry Lefèvre. Evolutionary perspectives on human infectious
diseases: Challenges, advances, and promises. Evolutionary Applications, Blackwell, 2018, 11 (4),
pp.383-393. �10.1111/eva.12586�. �hal-02410979�
Evolutionary Applications. 2018;11:383–393. wileyonlinelibrary.com/journal/eva
|
383 DOI: 10.1111/eva.12586E D I T O R I A L
Evolutionary perspectives on human infectious diseases:
Challenges, advances, and promises
1 | INTRODUCTION
Modern biomedicine has contributed remarkably to the reduction of infectious diseases worldwide, including the eradication of smallpox and the control of common childhood diseases (e.g., polio, measles, rubella) that once claimed millions of lives and caused suffering of tens of millions. This has been made possible through improved di-agnostics, surveillance, therapeutics, vaccines, and an associated health system infrastructure. These achievements can be largely credited to advances in biomedical sciences and their application in the 20th century.
The spectacular advances in the biomedical sciences were in turn a consequence of the emergence and widespread acceptance of the biomedical paradigm, in which the discovery of microbes and establishment of ‘germ theory’ had played a central role. By the 1960s, a sense began to prevail that infectious diseases had been conquered, or were at least conquerable in the case of malaria, den-gue, and other vector- borne diseases (VBD) that appeared to be “in retreat.”
Yet, by the 1980s, with the appearance of the HIV/AIDS pan-demic and followed by new antimicrobial- resistant strains of bacterial pathogens, confidence began to erode. Major setbacks also increasingly became apparent in the efforts to control VBDs particularly with the resurgence of malaria and dengue worldwide (WHO, 2016). An increasing number of historically localized or otherwise geographically confined VBDs began to spread and even jump continents, with the arrival of West Nile virus and Zika virus in the Americas being the most prominent of many examples. Such pathogens, identified decades ago in their native habitat and hosts as part of natural history studies, were long considered to be of relatively little public health concern or apparent impact (Lederberg, Shope, & Oaks, 1992; Packard, 2007).
Human population growth and anthropogenic environmental changes, accelerating with an unprecedented intensity and scale particularly since the mid- 1900s, are increasingly recognized as underlying much of the emerging infectious diseases (EID) prob-lem (Myers & Patz, 2009; Myers et al., 2013; Whitmee et al., 2015). These disease emergence drivers emblematic of the era of modern
development can be seen as consequences of modern medical and hygiene interventions introduced a century earlier. Together, they radically modified (and continue to alter) landscapes and ecosys-tems worldwide producing what has become a continual state of social, ecological, and evolutionary imbalance (McNeill, 1976). Among the environmental changes, global climate change has be-come widely accepted among experts as a significant contributor to these imbalances that will increasingly influence infectious dis-eases transmission, yet in ways that are difficult to predict (Altizer, Ostfeld, Johnson, Kutz, & Harvell, 2013; Lafferty, 2009; McMichael & Wilcox, 2009).
The complexity of factors and processes underlying infectious diseases and their transmission go well beyond the scope and an-alytic resolution of biomedicine (and conventional biomedical training), requiring a complementary framing approach capable of acknowledging and assessing cross- scale influences, context dependency, and the constant ‘arms race’ between co- evolving organisms (Van Valen, 1973). Evolutionary biology provides these scientific foundations to help refine our understanding of not only the meaning of health and disease (Stearns & Koella, 2008) from the standpoint of adaptation (or maladaptation), but also improve our understanding of the mechanisms underlying infectious disease transmission dynamics within social- ecological systems (Horwitz & Wilcox, 2005; Wilcox & Echaubard, 2016), context- dependent virulence and more effective treatment and control strategies (Echaubard, Sripa, Mallory, & Wilcox, 2016; Nesse, 2008; Restif, 2009). As such, evolutionary biology as a framing approach and methodological toolkit is a needed component of integrated dis-ease control and prevention (Allegranzi et al., 2017) and sustainable health development aligning with the recently adopted sustain-able development goals (SDGs), as part of the 2030 Agenda for Sustainable Development (Carroll et al., 2014).
This special issue is an attempt to present an up- to- date appraisal of the challenges, current advances, and promising research avenues where evolutionary principles and their ecological corollaries can be applied in research as a basis for human infectious disease in-terventions. Accordingly, the contributions published in this special issue together present an illustration of the diverse benefits of com-bining biomedical and public health perspectives with evolutionary
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
causation in the context of infectious diseases for major infectious agents (Table 1).
2 | THEMES OF THE SPECIAL ISSUE
A main challenge of mainstreaming evolution into medicine and public health lies in the often very pragmatic and urgent nature of these fields (e.g., outbreak mitigation; Nesse & Stearns, 2008) and the relatively time- consuming nature of framing questions, interven-tions, and policies following evolutionary principles, which imply a long- term preventive vision rather than a short- term curative do-main of action. However, policy- oriented research and intervention informed by evolutionary biology have the potential to not only ef-fectively mitigate urgent crises but also anticipate, minimize, and respond to the evolution of unwanted epidemiological traits (e.g., antimicrobial resistance). Accordingly, the articles compiled for this special issue present a selected panel of evolutionary applications for infectious disease control, together providing relevant avenues for future research and sustainable disease prevention strategies.
The articles published in this special issue are organized based on their underlying evolutionary innovation and public health implica-tions and not necessarily on the disease study systems the authors are using to address their research questions. A range of disease systems, from macroparasites to microparasites (sensu Anderson & May, 1981), are examined through modeling, experimental and observational research designs as well as describing novel framing approaches.
As an opening paper, Kosoy and Kosoy (2017) explore the mul-tiple dimensions and related complexity of host–pathogens interac-tions revealed by the novel genetic and genomic data, along with extensive environmental parameters being acquired using newly de-veloped computational tools. They highlight the need for flexibility in studying natural systems of zoonotic pathogens with respect to how we choose perspectives within a continuum between unrestricted diversity of related parameters and well- defined roles played by in-fectious agents, potential and actual animal hosts, and environmen-tal variables. They proposed a model of investigation that requires a dynamic shift of perspectives along the simplicity–complexity (‘sim-plexity’) dimension emphasizing the difficulty to accommodate dual representations of both the subjective nature of investigations of zoonotic pathogens and much more objectively derived information, for example, coded in the genetic structure of DNA or in observing the morphology or behavior of bacteria. This also speaks directly to the issue of “problem framing” alluded to in this introduction where tools and perceptions emerging from biomedical sciences do not necessarily accommodate evolutionary- based predictions or public health implications.
Arguably the most widespread recognition of the importance of evolutionary biology for public health and medicine is in the con-text of the emergence of resistance to antimicrobials and insecti-cides. Sternberg and Thomas (2017) explore the overlaps between understanding and managing insecticide resistance in agriculture
and in public health with the aim to identify best practices in resis-tance mitigation strategies in the context of vector- borne disease interventions. They argue that the success of insecticide resistance management strategies is strongly dependent on the biological spe-cifics of each system and that the biological, operational, and reg-ulatory differences between agriculture and public health limit the wholesale transfer of knowledge and practices from one system to the other. Nonetheless, the authors argue that there are some valuable insights from agriculture that could assist in advancing the existing Global Plan for Insecticide Resistance Management (IRM). Accordingly, the authors suggest that for IRM strategies to succeed in public health, there needs to be a shift away from choosing vector control tools or strategies based on direct cost, toward factoring in the benefit of preserving susceptibility.
Focusing on Malaria, Huijben and Paaijmans (2017) analyze the evolutionary consequences of the way antimalarial drugs and insecticide- based interventions are currently implemented, which is leading to resistance and may ultimately lead to control failure. The authors describe how evolutionary principles can be applied to extend the lifespan of current and novel interventions highlighting in particular how understanding fitness costs arising from express-ing, utilizexpress-ing, and maintaining molecular or metabolic pathways of resistance will be essential to mitigate resistance evolution. They continue arguing that similar to insecticide resistance management strategies, large heterogeneity in drug exposure can be created in space (host mosaic) or time (drug rotation) or by deploying different compounds simultaneously (mixed treatment) and that better resis-tance management is achieved if drugs can be combined that select for alternative allelic versions of the target locus.
Glunt et al. (2017) further discuss the challenges of insecti-cide resistance in the context of malaria, with a specific focus on pyrethroids impregnated long- lasting insecticidal bed nets (LLINs) and their efficacy in preventing malaria. About 1 billion LLINs, a major vector control tool, have been distributed in Africa in the last 10 years. During the same period of time resistance to pyrethroids in malaria mosquito vectors has increased significantly. Using a trans-mission model, the authors show that when LLIN- related lethal and sublethal effects were accrued over mosquito lifetimes, they greatly reduced the impact of resistance on malaria transmission potential under conditions of high net coverage. However if coverage falls, the epidemiological impact is far more pronounced. Similarly, if the in-tensity of resistance intensifies, the loss of malaria control increases nonlinearly. The authors argue that their findings help explain why insecticide resistance has not yet led to wide- scale failure of LLINs, as high distribution coverage is generally in place in most African endemic countries, but reinforce the call for alternative control tools and informed resistance management strategies.
While parasites can evolve classical resistance mechanisms (e.g., efflux pumps), it is also possible that changes in life- history traits could help parasites evade the effects of treatment. Birget, Greischar, Reece, and Mideo (2017) investigate how the life history of malaria parasites is governed by an intrinsic resource allocation problem where specialized stages are required for transmission, but
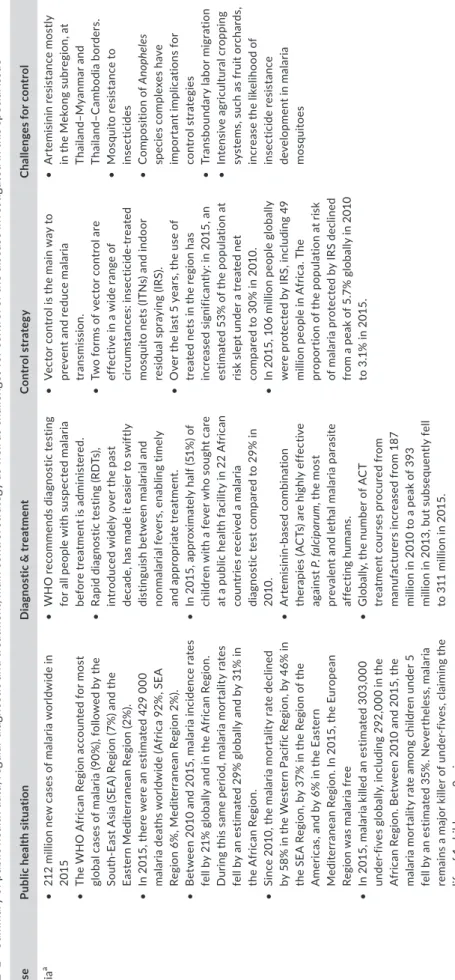
T A B LE 1 Su m m ar y o f p ub lic h ea lth d at a, r eg ul ar d ia gn os tic a nd t re at m en t, m ai n c on tr ol s tr at eg y a s w el l a s c ha lle ng es f or c on tr ol o f t he d is ea se i nv es tig at ed i n t hi s s pe ci al i ss ue D is ea se Pu bl ic h ea lth si tu at io n D ia gn os tic & t re at m en t C on tr ol s tr at eg y C ha llen ge s f or c on tr ol Ma lar ia a • 21 2 m ill io n n ew c as es o f m al ar ia w or ld w id e i n 201 5 • Th e W H O A fr ic an R eg io n a cc ou nt ed f or m os t gl ob al c as es o f m al ar ia ( 90 % ), f ol lo w ed b y t he So ut h-Ea st A si a ( SE A ) R eg io n ( 7% ) a nd t he Ea st er n M ed ite rr an ea n R eg io n ( 2% ). • In 2 01 5, t he re w er e a n e st im at ed 4 29 0 00 m al ar ia d ea th s w or ld w id e ( A fr ic a 9 2% , S EA Re gi on 6 % , M ed ite rr an ea n R eg io n 2 % ). • B et w ee n 2 01 0 a nd 2 01 5, m al ar ia i nc id en ce r at es fe ll b y 2 1% g lo ba lly a nd i n t he A fr ic an R eg io n. D ur in g t hi s s am e p er io d, m al ar ia m or ta lit y r at es fe ll b y a n e st im at ed 2 9% g lo ba lly a nd b y 3 1% i n th e A fr ic an R eg io n. • Si nc e 2 01 0, t he m al ar ia m or ta lit y r at e d ec lin ed by 5 8% i n t he W es te rn P ac ifi c R eg io n, b y 4 6% i n th e S EA R eg io n, b y 3 7% i n t he R eg io n o f t he A m er ic as , a nd b y 6 % i n t he E as te rn M ed ite rr an ea n R eg io n. I n 2 01 5, t he E ur op ea n Re gi on w as m al ar ia f re e • In 2 01 5, m al ar ia k ill ed a n e st im at ed 3 03 ,0 00 un de r-fiv es g lo ba lly , i nc lu di ng 2 92 ,0 00 i n t he A fr ic an R eg io n. B et w ee n 2 01 0 a nd 2 01 5, t he m al ar ia m or ta lit y r at e a m on g c hi ld re n u nd er 5 fe ll b y a n e st im at ed 3 5% . N ev er th el es s, m al ar ia re m ai ns a m aj or k ill er o f u nd er -f iv es , c la im in g t he lif e o f 1 c hi ld e ve ry 2 m in . • W H O r ec om m en ds d ia gn os tic t es tin g fo r a ll p eo pl e w ith s us pe ct ed m al ar ia bef or e t re at m en t i s a dm in is te re d. • Ra pi d d ia gn os tic t es tin g ( RD Ts ), in tr od uc ed w id el y o ve r t he p as t de ca de , h as m ad e i t e as ie r t o s w ift ly di st ing ui sh b et w ee n mal ar ial a nd no nm al ar ia l f ev er s, en abl in g t ime ly and a ppr opri at e t rea tm en t. • In 2 01 5, a pp ro xi m at el y h al f ( 51 % ) o f ch ild re n w ith a f ev er w ho s ou gh t c ar e at a p ub lic h ea lth f ac ili ty i n 2 2 A fr ic an co un tr ie s r ec ei ve d a m al ar ia di ag no st ic t es t c om pa re d t o 2 9% i n 20 10 . • A rt emi sin in -b as ed c om bina tio n th er ap ie s ( A C Ts ) a re h ig hl y e ff ec tiv e ag ai ns t P . f al cip ar um , t he m os t pr ev al en t a nd l et ha l m al ar ia p ar as ite af fe ct ing h uma ns . • G lo ba lly , t he n um be r o f A C T tre at m en t c ou rs es p ro cu re d f ro m m an uf ac tu re rs i nc re as ed f ro m 1 87 m ill io n i n 2 01 0 t o a p ea k o f 3 93 m ill io n i n 2 01 3, b ut s ub se qu en tly f el l to 3 11 m ill io n i n 2 01 5. • Ve ct or c on tr ol i s t he m ai n w ay t o pr ev en t a nd r ed uc e m al ar ia tr an sm is si on . • Tw o f or m s o f v ec to r c on tr ol a re ef fe ct iv e i n a w id e r an ge o f cir cu m st an ce s: in se ct ic id e-tr ea te d m os qu ito n et s ( IT N s) a nd i nd oo r re si du al sp ra yi ng (IR S) . • O ve r t he l as t 5 y ea rs , t he u se o f tr ea te d n et s i n t he r eg io n h as in cr ea se d s ig ni fic an tly : i n 2 01 5, a n es tim at ed 5 3% o f t he p op ul at io n a t ris k s le pt u nd er a t re at ed n et co m pa re d t o 3 0% i n 2 01 0. • In 2 01 5, 1 06 m ill io n p eo pl e g lo ba lly w er e p ro te ct ed b y I RS , i nc lu di ng 4 9 m ill io n p eo pl e i n A fr ic a. T he pr op or tio n o f t he p op ul at io n a t r is k of m al ar ia p ro te ct ed b y I RS d ec lin ed fr om a p ea k o f 5 .7 % g lo ba lly i n 2 01 0 to 3 .1 % i n 2 01 5. • A rt em is in in r es is ta nc e m os tly in t he M ek on g s ub re gi on , a t Th ai lan d– M yanm ar an d Tha ila nd –C am bo di a b or de rs . • M os qu ito r es is ta nc e t o in se ct ic id es • C om po si tio n o f A nop he le s sp ec ie s c om pl ex es h av e im por tan t i m pl ic at ion s f or co nt ro l s tr ate gi es • Tr an sb oun dar y l ab or m ig ra tion • In te ns iv e a gr ic ult ur al cr op pin g sys te m s, s uch a s f rui t o rcha rd s, in cr ea se t he l ik el ih oo d o f in se ct ic id e r es is ta nc e de ve lo pmen t i n m al ar ia m os qu ito es (Co nt in ue s)
D is ea se Pu bl ic h ea lth si tu at io n D ia gn os tic & t re at m en t C on tr ol s tr at eg y C ha llen ge s f or c on tr ol D en gu e b • O ne r ec en t e st im at e i nd ic at es 3 90 m ill io n den gu e i nf ec tio ns p er y ea r ( 95 % c re di bl e i nt er va l 28 4– 52 8 m illio n) , o f w hi ch 9 6 m illio n ( 67 –1 36 m illio n) m an ife st c lin ic all y • Th e p re va le nc e o f d en gu e i s e st im at ed a t 3 .9 bi lli on p eo pl e, i n 1 28 c ou nt rie s, a t r is k o f in fe ct io n w ith den gu e v iru se s • Th e n um be r o f c as es r ep or te d i nc re as ed f ro m 2. 2 m ill io n i n 2 01 0 t o 3 .2 m ill io n i n 2 01 5. • B ef or e 1 97 0, o nl y 9 c ou nt rie s h ad e xp er ie nc ed se ver e den gu e ep idem ic s. T he d is ea se is no w en de m ic i n m or e t ha n 1 00 c ou nt rie s i n t he W H O re gi on s o f A fr ic a, t he A m er ic as , t he E as te rn Me di ter ra ne an , SE A , a nd th e W es ter n P ac ifi c. • C as es a cr os s t he A m er ic as , S ou th -E as t A si a, a nd W es te rn P ac ifi c e xc ee de d 1 .2 m ill io n i n 2 00 8 an d o ve r 3 .2 m ill io n i n 2 01 5. R ec en tly , t he nu m be r o f r ep or te d c as es h as c on tin ue d t o in cr ea se . I n 2 01 5, 2 .3 5 m ill io n c as es o f d en gu e w er e r ep or te d i n t he A m er ic as a lo ne , o f w hi ch 10 ,2 00 c as es w er e d ia gn os ed a s s ev er e d en gu e ca us in g 1 181 d ea th s. • A n e st im at ed 5 00 ,0 00 p eo pl e w ith s ev er e den gu e r eq ui re ho sp ita liz at io n e ac h y ea r, a nd ab ou t 2 .5 % o f t ho se a ff ec te d d ie . • D en gu e f ev er i s a s ev er e, f lu -li ke ill ne ss t ha t a ff ec ts i nf an ts , y ou ng ch ild re n, a nd a dult s • D en gu e s ho ul d b e s us pe ct ed w he n a hi gh f ev er i s a cc om pa ni ed b y s ev er e he ad ac he , p ai n b eh in d t he e ye s, m us cl e a nd j oi nt p ai ns , n au se a, vo mi tin g, s w ol le n gla nd s o r r as h. • Se ver e den gu e i s a p ot en tia lly de ad ly co m pl ic at io n d ue t o p la sm a l ea ki ng , flui d a ccu m ula tio n, s ev er e b le edin g, o r or ga n im pa irm en t. • Th er e i s n o s pe ci fic t re at m en t f or den gu e f ev er . M ai nt en anc e o f t he pa tie nt ’s b od y f lu id v ol um e i s c rit ic al to s ev er e den gu e c ar e. • In l at e 2 01 5 a nd e ar ly 2 01 6, t he f irs t den gu e v ac ci ne , D en gv ax ia (C YD -TD V ) b y S an of i P as te ur , w as re gi st er ed i n s ev er al c ou nt rie s f or u se in i nd iv id ua ls 9 –4 5 ye ar s o f a ge l iv in g in e nd emic a re as . • W H O r ec om m en ds t ha t c ou nt rie s sho uld c on si der in tr od uc tio n o f t he de ng ue v ac ci ne C YD -T D V o nl y i n ge og ra phi ca l s et tin gs w he re epi de mi ol og ic al d at a in dic at e a h ig h bu rden o f d is ea se . A t p re se nt , t he m ai n m et ho d t o c on tr ol or p re ve nt t he t ra ns m is si on o f de ng ue v iru s i s t o c om ba t v ec to r m os qu ito es th ro ug h: • Pr ev en tin g m os qu ito es f ro m ac ce ss in g eg g-la yin g ha bi ta ts b y en vi ro nmen ta l m an ag emen t a nd m od ifi ca tio n • D is po si ng o f s ol id w as te a nd co ve rin g, e m pt yi ng , a nd c le an in g o f do me st ic w at er s to ra ge c on ta in er s on a w ee kl y b as is • A pp ly in g a ppr opri at e i ns ec tic id es to w at er s to ra ge o ut do or c on ta in er s an d en vi ro nmen ta l m an ag emen t • U si ng o f p er so na l ho us eho ld pr ot ec tio n s uc h a s w in do w s cr ee ns , lo ng -s le ev ed c lo th es , in se ct ic id e-tr ea te d m at er ia ls , c oi ls , a nd vap or iz er s; • Im pr ov in g c om m un ity p ar tic ip at io n an d m ob ili za tio n f or s us ta in ed ve ct or c on tr ol • In cr ea sin g p op ula tio n m ov emen t, g lo ba liz at io n o f tr ad e a nd u rb an iz at io n w ith ou t ad eq ua te m ea su re s t o p re ve nt ve ct or b re edin g • Ru bb er p la nt at io ns e xp ans io n • M os qu ito v ec to r a nd p at ho ge n adap ta tion (Co nt in ue s) T A B LE 1 (Co nti nue d)
D is ea se Pu bl ic h ea lth si tu at io n D ia gn os tic & t re at m en t C on tr ol s tr at eg y C ha llen ge s f or c on tr ol In flu en za (z oo no tic ) • H um an s c an b e i nf ec te d w ith a vi an , s w in e, a nd ot her z oo no tic in flu en za v iru se s, s uc h a s a vi an in flu en za v iru s s ub ty pe s A (H 5N 1) , A (H 7N 9) , a nd A (H 9N 2) a nd s w in e i nf lu en za v iru s s ub ty pe s A (H 1N 1), A (H 1N 2), a nd A (H 3N 2) . • Th e m aj or ity o f h um an c as es o f i nf lu en za A (H 5N 1) a nd A (H 7N 9) v iru s i nf ec tio n h av e b ee n as so ci at ed w ith d ire ct o r i nd ire ct c on ta ct w ith in fe ct ed l iv e o r d ea d p ou ltr y. C on tr ol lin g t he di se as e i n t he a ni m al s ou rc e i s c rit ic al t o de cr ea se r is k t o h um an s. • A qu at ic b ird s a re t he p rim ar y n at ur al r es er vo ir fo r m os t s ub ty pe s o f i nf lu en za A v iru se s. M os t ca us e a sy m pt om at ic o r mi ld in fe ct io n in bir ds , w he re t he r an ge o f s ym pt om s d ep en ds o n t he vi ru s p ro per tie s. • V iru se s t ha t c au se s ev er e d is ea se i n p ou ltr y a nd re su lt i n h ig h d ea th r at es a re c al le d h ig hl y pa th og en ic a vi an i nf lu en za ( H PA I). V iru se s t ha t ca us e m ild d is ea se i n p ou ltr y a re c al le d lo w -p at ho gen ic a vi an in flu en za (L PA I). • A vi an , s w in e, a nd o th er z oo no tic in flu enz a v irus in fe ct io ns in h um an s m ay c au se d is ea se r an gi ng f ro m m ild up pe r r es pi ra to ry t ra ct i nf ec tio n (fe ve r a nd c ou gh ), e ar ly s pu tu m pr od uc tio n, a nd r ap id p ro gr es si on t o se ve re p ne um on ia , s ep si s w ith s ho ck , ac ut e r es pi ra to ry d is tr es s s yn dr om e an d e ve n d ea th . • La bo ra to ry t es ts a re r eq ui re d t o di agn os e h uma n i nf ec tio n w ith zo ono tic in flu en za . • Ra pi d in flu enz a di ag no st ic te st s (R ID Ts ) h av e l ow er s en si tiv ity co m pa re d t o P C R a nd t he ir r el ia bi lit y de pe nd s l ar ge ly o n t he c on di tio ns un de r w hi ch t he y a re u se d. • Ev idenc e s ug ge st s t ha t s ome a nt iv ira l dr ug s, n ot ab ly n eu ra mi ni da se in hib itor (o se lta mi vir , z ana mi vir ), c an re du ce t he d ur at io n o f v ira l r ep lic at io n an d i m pr ov e p ro sp ec ts o f s ur vi va l; ho w ev er , o ng oi ng c lin ic al s tu di es a re ne ed ed . • In s us pe ct ed a nd c on fir m ed c as es , ne ur amin id as e i nh ibi to rs s ho ul d b e pr es cr ib ed a s s oo n a s p os si bl e t o m ax im iz e t her ap eu tic b en ef its . • In flu en za v iru se s, w ith t he v as t si le nt r es er vo ir i n a qu at ic b ird s, a re im po ss ib le to e ra dic at e. • To m in im ize p ub lic h ea lth r is k, qu al ity s ur ve ill an ce i n b ot h a ni m al an d hum an p op ul at ion s, th or ou gh in ve st ig at io n o f e ve ry h um an in fe ct io n a nd r is k-ba se d p an de m ic pl an ni ng a re e ss en tia l. • A pa rt f ro m a nt iv ira l t re at m en t, t he pu bl ic h ea lth m an ag emen t inc lu de s per so na l p ro te ct iv e m ea su re s lik e r eg ula r ha nd w as hin g, go od r es pi ra to ry h yg ie ne a nd e ar ly se lf-i so la tio n o f t ho se f ee lin g un w el l, f ev er is h a nd h av in g o th er sy m pt om s o f i nf lu en za. • Pre -e xp os ure o r p os te xp os ure pr op hy la xi s w ith a nt iv ira ls i s po ss ibl e bu t dep en ds o n s ev er al fa ct or s, for e xam ple, in di vi du al fa ct or s, t yp e o f e xp os ur e, a nd r is k as so ci at ed w ith th e ex po su re. • In ter na tio na l m ov emen t o f st ra ins • G en et ic re as so rt men ts • Emer genc e o f o se lta m ivi r re si st an ce h as b ee n r ep or te d. • M os t r ec en t A (H 5) a nd A (H 7N 9) v iru se s a re re si st -an t t o a dam an tan e an tiv ira l dr ug s ( e. g. , a m an ta di ne a nd rim an ta di ne ) a nd a re t he re fo re no t r ec om men de d f or m on ot he rap y. (Co nt in ue s) T A B LE 1 (Co nti nue d)
D is ea se Pu bl ic h ea lth si tu at io n D ia gn os tic & t re at m en t C on tr ol s tr at eg y C ha llen ge s f or c on tr ol C ha gas d is eas e c • C ha ga s d is ea se , a ls o k no w n a s A m er ic an tr yp an os om ia si s, i s a p ot en tia lly l ife -t hr ea te ni ng ill ne ss c au se d b y t he p ro to zo an p ar a-si te T ry pa no so m a c ru zi ( T. c ru zi ). • A bo ut 6 m ill io n t o 7 m ill io n p eo pl e w or ld w id e ar e e st im at ed t o b e i nf ec te d w ith T ry pa ns os om a cr uzi . • C ha ga s d is ea se i s f ou nd m ai nl y i n e nd em ic a re as of 2 1 L at in A m er ic an c ou nt rie s, w he re i t i s m os tly v ec to r-bo rn e t ra ns m itt ed t o h um an s b y co nt ac t w ith f ec es o r u rin e o f t ria to m in e b ug s • C ha ga s d is ea se o cc ur s p rin ci pa lly i n t he co nt in en ta l p ar t o f L at in A m er ic a a nd n ot i n t he C ar ib be an i sl es . I n t he p as t d ec ad es , h ow ev er , i t ha s b ee n i nc re as in gl y d et ec te d i n t he U ni te d St at es o f A m er ic a, C an ad a, a nd m an y E ur op ea n an d s om e W es te rn P ac ifi c c ou nt rie s. T hi s i s d ue m ai nl y t o p op ul at io n m ob ili ty b et w ee n L at in A m er ic a a nd t he r es t o f t he w or ld . • C ha ga s d is ea se c an b e t re at ed w ith be nz ni daz ol e a nd a ls o n ifu rt im ox . • B ot h m ed ic in es a re a lm os t 1 00 % ef fe ct iv e i n c ur in g t he d is ea se i f g iv en so on a ft er i nf ec tio n a t t he o ns et o f th e a cu te p ha se i nc lu di ng t he c as es o f co ng en ita l t ra ns m is si on . T he e ff ic ac y of b ot h d im in is he s, h ow ev er , t he lo ng er a p er so n h as b ee n i nf ec te d. • Th e p ot en tia l b en ef its o f m ed ic at io n in p re ve nt in g o r d el ay in g t he de ve lo pmen t o f Ch ag as d is ea se sh ou ld b e w ei gh ed a ga in st t he l on g du ra tio n o f t re at m en t ( up t o 2 m on th s) an d p os si bl e a dv er se r ea ct io ns (o cc ur rin g i n u p t o 4 0% o f t re at ed pat ie nt s) . • Th e c os t o f t re at m en t f or C ha ga s di se as e r em ai ns s ub st an tia l. I n C ol om bi a a lon e, th e annua l c os t o f m ed ic al c ar e f or a ll p at ie nt s w ith t he di se as e w as e st im at ed t o b e a bo ut U S 26 7 m ill io n i n 2 00 8. S pr ay in g in se ct ic id e t o c on tr ol v ec to rs w ou ld co st n ear ly U S 5 m ill ion annua lly — le ss th an 2 % o f t he m ed ic al c ar e c os t. • Th er e i s n o v ac ci ne f or C ha ga s di se ase . • Ve ct or c on tr ol i s t he m os t e ff ec tiv e m et ho d o f p re ve nt io n i n L at in A m er ic a. B lo od s cr ee ni ng i s ne ce ss ar y t o p re ve nt i nf ec tio n thr ou gh tr an sf us ion an d or gan tr an sp lan ta tion . • Th e c on tr ol t ar ge ts a re e lim in at io n of t he t ra ns m is si on a nd e ar ly he al th ca re a cc es s f or t he i nf ec te d an d ill p op ul tio n. • D ep en di ng o n t he g eo gr ap hi ca l ar ea , W H O r ec om m en ds t he fo llo w in g a pp ro ac he s t o p re ve nt io n an d c on tr ol : o S pr ay in g o f h ou se s a nd su rr ou nd ing a re as w ith re si du al in se ct ic id es o H ou se imp ro ve m en ts a nd h ou se cl ea nl in es s t o p re ve nt v ec to r in fe st at io n; o B ed net s • Ps ych o-so ci al cha lle ng es s uch as s tig m a r el at ed t o p ov er ty an d e m ot io na l f ea rs o f b ei ng ju dg ed l ea di ng t o l ow r ep or tin g an d s cre eni ng • In cr ea sin g in se ct ic id e re si st an ce o f T ria to m in e ve ct or s an d r ap id re col on is a-tio n o f h ou se ho ld s b y v ec to rs af te r sp ra yi ng • D ia gn os is (Co nt in ue s) T A B LE 1 (Co nti nue d)
D is ea se Pu bl ic h ea lth si tu at io n D ia gn os tic & t re at m en t C on tr ol s tr at eg y C ha llen ge s f or c on tr ol Sc hi st os om ia si s d • Sc hi st os om ia si s i s a n a cu te a nd c hr on ic p ar as iti c di se as e c au se d b y b lo od f lu ke s ( tr em at od e w or m s) o f t he g en us S ch is to so m a. • Es tim at es s ho w t ha t a t l ea st 2 06 .4 m ill io n p eo pl e re qu ire d p re ve nt iv e t re at m en t i n 20 16 . • Sc hi st os om ia si s t ra ns m is si on h as b ee n r ep or te d fr om 78 c ou nt rie s. H ow ev er , p re ve nt iv e ch em ot her ap y f or s ch is to so m ia si s, wh er e p eo pl e an d c om mun iti es ar e t ar ge te d f or lar ge -s ca le tr ea tm en t, i s o nl y r eq ui re d i n 5 2 e nd em ic co un tr ie s w ith m od er at e- to-hi gh tr an smi ss io n. • Sc hi st os om ia si s i s p re va le nt i n t ro pi ca l a nd su bt ro pi ca l a re as , e sp ec ia lly i n p oo r c om m un iti es w ith ou t a cc es s t o s af e d rin ki ng w at er a nd ad eq ua te s an ita tio n. I t i s e st im at ed t ha t a t l ea st 91 .4 % o f t ho se r eq ui rin g t re at m en t f or sc hi st os om ia si s l iv e i n A fr ic a. • Th er e a re 2 m aj or f or m s o f s ch is to so m ia si s— in -te st in al a nd u ro ge ni ta l— ca us ed b y 5 m ai n sp ec ie s o f b lo od flu ke. • Sc hi st os om ia si s m os tly a ff ec ts p oo r a nd r ur al co m m un iti es , p ar ticula rly a gr icult ur al a nd fi sh in g po pu la tio ns . W omen d oi ng d ome st ic c ho re s i n in fe st ed w at er , s uc h a s w as hi ng c lo th es , a re a ls o at r is k a nd c an d ev el op f em al e g en ita l sc hi st os om ia si s. • Sc hi st os om ia si s i s d ia gn os ed th ro ug h th e d et ec tio n o f p ar as ite e gg s i n s to ol or u rin e s pe ci m en s. A nt ib od ie s a nd /o r an tig en s d et ec te d i n b lo od o r u rin e sa m pl es a re a ls o i nd ic at io ns o f inf ec tio n. • Fo r u rog enit al s chi st os om ia si s, a fil tr at io n te ch ni qu e us in g ny lo n, pa pe r, or p ol yc ar bo na te f ilt er s i s t he st an dar d d ia gn os tic te chn iqu e. C hi ld re n w ith S . h aem at ob iu m a lm os t al w ays ha ve micr os co pic b lo od in th eir ur in e, w hi ch c an b e d et ec te d b y ch emic al re ag en t s tr ips . • Th e e gg s o f i nt es tin al s ch is to so m ia si s ca n b e d et ec te d i n f ec al s pe ci m en s th ro ug h a t ec hn iq ue u si ng m et hy le ne bl ue -s ta in ed c el lo ph an e s oa ke d i n gl yc er in o r g la ss s lid es , k no w n a s t he K at o-K at z te ch ni qu e. • Th e c on tr ol o f s ch is to so m ia si s i s ba se d o n l ar ge -s ca le t re at m en t o f at -r is k p op ul at io n g ro up s, a cc es s t o sa fe w at er , i m pr ov ed s an ita tio n, hy gi en e e du ca tio n, a nd s na il con tr ol . • Th e W H O s tr at eg y f or s ch is to so -m ia si s c on tr ol f oc us es o n r ed uc in g di se as e t hr ou gh p er io di c, t ar ge te d tr eat m ent w ith p ra zi qu ante l t hr ou gh th e l ar ge -s ca le tr ea tmen t ( pr ev en -tiv e c he m ot he ra py ) o f a ff ec te d po pu la tio ns . • It i nv ol ve s r eg ul ar t re at m en t o f a ll at -r is k g ro up s. I n a f ew c ou nt rie s, w he re t he re i s l ow t ra ns m is si on , t he in te rr up tio n o f t he t ra ns m is si on o f th e d is ea se s ho ul d b e a im ed f or . • En vi ro nmen ta l de gr ad at io n • D am s a nd l ar ge -s ca le i rr ig at io n pr oj ec t t ha t c on tr ib ut e t o s na il inte rm ed iate h os t p ro lif er at io n • A ni m al r es er vo irs • Po te nt ia l f or p ra zi qu ante l re si st an ce aW H O (2 016 ). bBha tt e t a l. ( 20 13 ); B ra dy e t a l. ( 20 12 ). cTa rle to n, R ei th in ge r, U rb in a, K itr on , a nd G ür tle r ( 20 07 ). dW or ld H ea lth O rg an iz at io n & U N IC EF ( 20 04 ). T A B LE 1 (Co nti nue d)
producing these stages comes at the cost of producing fewer of the forms required for within- host survival. The underlying rationale is that drug treatment, by design, alters the probability of within- host survival and so should alter the costs and benefits of investing in transmission. The authors use a within- host model of malaria infec-tion to predict optimal patterns of investment in transmission in the face of different drug treatment regimens and determine the extent to which alternative patterns of investment can buffer the fitness loss due to drugs. This work emphasizes that in addition to classical resistance mechanisms, drug treatment generates selection for al-tered parasite life history. It also suggests that understanding how any shifts in life history will alter the efficacy of drugs, as well as any limitations on such shifts, is important for evaluating and predicting the consequences of drug treatment.
While evolutionary principles can help design more sustain-able insecticide and antimicrobial resistance mitigation strategies, evaluating the risk of emergence and transmission of vector- borne diseases also requires knowledge of the genetic and environmental contributions to pathogen transmission traits. In their perspective article, Lefevre et al. (2017) discuss how the associations between malaria parasites transmission traits and their related trade- offs and constraints could have important implications for understanding the evolution of parasite transmission and how so doing could inform disease control. For instance, they argue, frontline vector- borne disease prevention tools such as insecticide- treated bednets and in-door residual spraying rely on reducing mosquito contact rates with human hosts and reducing vector survival. Reduced vector survival has the benefits of decreasing mosquito abundance, the number of bites a mosquito can take over the course of its lifetime, and the probability that mosquitoes survive past the parasite’s development time. These effects likely shape the selective environment for para-sites within the vector. However, whether parapara-sites can respond to interventions by evolving shorter EIPs or other heritable extended phenotypes that lengthen mosquito survival or change vector be-havior merit further investigation.
As in the case of malaria described by Lefevre et al. (2017), life- history trait evolution theory and its attributes can help better un-derstand the adaptive potential of triatomines—the vector of Chagas disease. The review by Flores- Ferrer, Marcou, Waleckx, Dumonteil, and Gourbière (2017) suggests that current knowledge of the deter-minants of high diversity and low virulence of the Trypanosoma cruzi parasite remains too limiting to design evolution- proof strategies, while such attributes may be part of the future of Chagas disease control after the 2020 WHO’s target of regional elimination of intra-domiciliary transmission has been reached. The authors argue that the eco- epidemiological relationships that build- up the selective pressures at work have been assiduously studied over the last cen-tury, so that, combined with concepts and modeling inspired from life- history evolution, a good evolutionary understanding could be rapidly gained. Although more specific information will surely be needed, the authors suggest that an effective research strategy would be to integrate data into the conceptual and theoretical frame-work of evolutionary ecology and life- history evolution to provide
the quantitative backgrounds necessary to understand and possibly anticipate adaptive responses to public health interventions.
Public health interventions targeting helminth diseases often rely on mass drug administration to reduce human morbidity and mortality. Considering the frequency of such interventions and the strength of the selective pressure they impose, the emergence and spread of drug resistance is a concern. In the case of schistosomiasis, although hotspots of reduced efficacy of the drug praziquantel have been reported, resistance is not widespread. However, parasite pop-ulations often exhibit considerable genetic variability in their natural tolerance, or acquired resistance, to drugs which, as Viana, Faust, Haydon, Webster, and Lamberton (2017) emphasize, is related to the fitness costs associated with such resistance compared to suscepti-ble lines. Using Bayesian state- space models (SSMs) fitted to data from an in vivo laboratory system, the authors tested the hypothesis that the spread of resistant Schistosoma mansoni may be limited by life- history costs not present in susceptible counterparts. S. man soni parasites from a praziquantel- susceptible (S), a praziquantel- resistant (R) or a mixed line of originally resistant and susceptible parasites (RS) were exposed to a range of praziquantel doses. Results showed that parasite adult worm survival and fecundity in the mu-rine host decreased across all lines, including R, with increasing drug pressure. The authors also observed trade- offs between adult sur-vival and fecundity in all untreated lines, and these remained strong in S with praziquantel pressure. In contrast, trade- offs between adult survival and fecundity were lost under praziquantel pressure in R. Additionally the authors showed that life- history traits within the molluscan intermediate host were complex, but trade- offs were demonstrated between parasite establishment and cercarial output. These results have theoretical and applied implications and appli-cations for future schistosomiasis control programs and for other host–parasite treatment programs in general.
Complementing the work by Viana et al., 2017; Borlase, Webster, and Rudge (2017) present the case example of haematobium group Schistosoma spp. hybrids in West Africa, a system involving multiple interacting parasites and multiple definitive hosts, in a region where zoonotic reservoirs of schistosomiasis were not previously consid-ered to be of importance. The authors consider how existing math-ematical model frameworks for schistosome transmission could be expanded and adapted to zoonotic hybrid systems, exploring how such model frameworks can utilize molecular and epidemiological data, as well as the complexities and challenges this presents. The authors also highlight the opportunities and value such mathematical models could bring to this and a similar multihost, multiparasite sys-tems, including informing priorities for data collection, diagnostics and laboratory studies and exploring the impact that hybridizations may have on control measures, as well the impact that evolutionary pressures including control measures may have on driving the emer-gence and spread of parasite hybrids.
Evolutionary- based mathematical modeling is also increasingly used to enhance both understanding and design of integrated inter-vention in the context of microparasites such as Dengue. Lourenço et al. (2017) review and analyze the biological and epidemiological
background of dengue, together with the major achievements of computational approaches including highlighting critical knowl-edge gaps and research underachievements that call for an urgent renewed focus. The authors argue that possible advancements based on new processing strategies, including real- time computa-tional analysis of genetic data, phylodynamic modeling frameworks, within- host model frameworks and GPU accelerated computing al-ready implemented for other pathogens, are alal-ready at reach of the Dengue research community. These new approaches are expected to make a significant contribution to our understanding of the evo-lutionary ecology and immunology of the dengue virus and support the design of novel integrated control strategies adaptable to other microparasite systems such as avian influenza affecting domestic animals and humans alike.
Influenza pandemics represent a significant threat to global public health. Four major pandemics have been recorded since the 1900s, occurring in 1918, 1957, 1968, and 2009 when influenza A vi-ruses with genes from animal sources adapted to the human popula-tion, a process known as antigenic shift. The H1N1/2009 pandemic virus emerged from swine that contained gene segments ultimately derived from previously circulating human and avian viruses, high-lighting a key role of segmental reassortment of genes from multiple hosts for host adaption and pandemic emergence. In this context, Joseph, Vijaykrishna, Smith, and Su (2017) used a relaxed molec-ular clock model to test whether the European avian- like swine (EA- swine) influenza virus originated through the introduction of a single avian ancestor as an entire genome, followed by an analysis of host- specific selection pressures among different gene segments. The results indicate independent introduction of gene segments via transmission of avian viruses into swine followed by reassort-ment events that occurred at least 1–4 years prior to the EA- swine outbreak. All EA- swine gene segments exhibited greater selection pressure than avian viruses, reflecting both adaptive pressures and relaxed selective constraints that are associated with host switching. Key amino acid mutations in the viral surface proteins (H1 and N1) that play a role in adaptation to new hosts were also observed sug-gesting adaptive changes in viral genomes following the transmis-sion of avian influenza viruses to swine and the early establishment of the EA- swine lineage.
Complementing the work by Joseph et al., 2017; Grear, Hall, Dusek, and Ip (2017) investigated mechanisms of intercontinental highly pathogenic avian influenza virus (HPAIV) spread through wild bird reservoirs possibly related to the North America outbreak in 2014. This introduction resulted in several reassortment events with North American (NA) lineage low- pathogenic avian influenza viruses and the reassortant EA/NA H5N2 that went on to cause one of the largest HPAIV poultry outbreaks in North America. In their research article, the authors used a time- rooted phylodynamic model that explicitly incorporated viral population dynamics with evolutionary dynamics to estimate the basic reproductive number (R0) and viral migration among host types in domestic and wild birds, as well as between the EA H5N8 and EA/NA H5N2 in wild birds. While the authors did not find evidence to support the hypothesis
that transmission of novel HPAIVs in wild birds was restricted by mechanisms associated with highly pathogenic phenotypes or that the HPAIV poultry outbreak was self- sustaining and required viral input from wild birds, the model estimates of the transmission pa-rameters suggested that the HPAIV outbreak met or exceeded the threshold for persistence in wild birds (R0 > 1) and poultry (R0 ≈ 1). Overall, the results of this work suggest that this novel HPAIV and reassortments did not encounter any transmission barriers sufficient to prevent persistence when introduced to wild or domestic birds and highlight the relevance of phylodynamic methods to test hy-potheses about geographical spread of AIVs in wild birds, multiyear evolutionary processes of AIVs in reservoir hosts and relative fitness of highly pathogenic versus low- pathogenic AIVs in wild birds for more integrated surveillance systems.
3 | CLOSING REMARKS: EVOLUTION,
ADAPTIVE MANAGEMENT, AND
SUSTAINABLE HEALTH DEVELOPMENT
While disease control and prevention have achieved great successes, the paradigm within which it has been developed, including our un-derstanding of host–parasite relationships, infection, and disease, as well as best management practices, arguably is not enough. A major shortcoming is that the solutions it provides, often grounded in the curative domain, are not lasting. Evolutionary thinking applied to medical and public health problems promises to substitute the short- sighted use of drugs with sustainable solutions: If we cannot eradicate infections by frontal assault, we may be able to keep them at bay durably provided we can understand the fundamental nature of host–parasite relationships.
The evolutionary perspective asks ultimate questions, which are about why mechanisms or epidemiological phenotypes are the way they are (i.e., how has this mechanism given a selective advantage? what is the evolutionary history of this mechanism?). Most medical research and perspectives focus on proximal questions, questions about the mechanisms themselves (i.e., how does the mechanism work, what is the ontogeny of the mechanisms?). The distinction be-tween proximate (mechanistic) and ultimate (evolutionary) explana-tions was emphasized and formalized several decades ago but remain unfamiliar to the medical sciences and public health domain despite its epistemological importance (Nesse, 2013). Both types of expla-nations are necessary, neither substitutes for the other, and they in-form each other (Nesse, 2013; Nesse & Stearns, 2008). Incorporating evolutionary thinking in infectious disease research helps improving our understanding of diseases transmission dynamics, infection pat-terns, and disease manifestation trends by superimposing a context- dependent, systems dynamics prism that appreciates that organisms and their interactions are in constant flux (Levin, 1998). Accordingly, evolutionary biology can help identify new relevant questions such as the ones addressed in this special issue, and doing so, can help inform more integrated disease control interventions as well as more adaptive health management strategies.
Adaptive health management, standing on strong evolutionary foundations, has the potential to address two fundamental errors underpinning most current public health interventions targeting in-fectious diseases. The first error is often the implicit assumption that pathogens or parasite responses to human intervention and/or that human–parasite/pathogens interactions are linear, predictable, and controllable. The second error is the assumption that pathogens and parasites can be treated independently of the social- ecological sys-tem within which they evolve (Horwitz & Wilcox, 2005). By explicitly avoiding these errors, evolutionary- based adaptive health manage-ment could help strengthen the representation of the operational concept of resilience in public health and restore the health systems capacity to buffer infectious and noninfectious challenges, learn, and develop—as a framework for understanding how to sustain and enhance adaptive capacity in a complex world of rapid transforma-tions (Folke et al., 2002).
ACKNOWLEDGEMENTS
The authors wish to thank Serge Morand and Bruce A. Wilcox for their comments and suggestions on earlier drafts.
Pierre Echaubard1,2
James W. Rudge3,4
Thierry Lefevre5,6
1Global Health Asia Institute, Faculty of Public Health Mahidol
University, Bangkok, Thailand
2Department of Biology, Laurentian University, Sudbury, ON, Canada
3Department of Global Health and Development, London School of
Hygiene and Tropical Medicine, London, UK
4Faculty of Public Health, Mahidol University, Bangkok, Thailand
5Institut de Recherche en Sciences de la Santé (IRSS), Bobo Dioulasso,
Burkina Faso
6MIVEGEC, IRD, CNRS, University. Montpellier, Montpellier, France
REFERENCES
Allegranzi, B., Kilpatrick, C., Storr, J., Kelley, E., Park, B. J., & Donaldson, L. (2017). Global infection prevention and control priorities 2018–22: A call for action. The Lancet Global Health, 5, e1178–e1180. https://doi. org/10.1016/S2214-109X(17)30427-8
Altizer, S., Ostfeld, R. S., Johnson, P. T. J., Kutz, S., & Harvell, C. D. (2013). Climate change and infectious diseases: from evidence to a predic-tive framework. Science, 341, 514–519. https://doi.org/10.1126/ science.1239401
Anderson, R. M., & May, R. M. (1981). The population dynamics of micro-parasites and their invertebrate hosts. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 291, 451–524. https://doi.org/10.1098/rstb.1981.0005
Bhatt, S., Gething, P. W., Brady, O. J., Messina, J. P., Farlow, A. W., Moyes, C. L., … Hay, S. I. (2013). The global distribution and burden of den-gue. Nature, 496, 504–507.
Birget, P. L. G., Greischar, M. A., Reece, S. E., & Mideo, N. (2017). Altered life history strategies protect malaria parasites against drugs. Evolutionary Application, 11, 442–455. https://doi.org/10.1111/eva.12516 Borlase, A., Webster, J. P., & Rudge, J. W. (2017). Opportunities and
chal-lenges for modelling epidemiological and evolutionary dynamics in a
multihost, multiparasite system: Zoonotic hybrid schistosomiasis in West Africa. Evolutionary Application, 11, 501–515. https://doi.org/ 10.1111/eva.12529
Brady, O. J., Gething, P. W., Bhatt, S., Messina, J. P., Brownstein, J. S., Hoen, A. G., … Hay, S. I. (2012). Refining the global spatial limits of dengue virus transmission by evidence- based consensus. PLoS Neglected Tropical Diseases, 6, e1760.
Carroll, S. P., Jørgensen, P. S., Kinnison, M. T., Bergstrom, C. T., Denison, R. F., Gluckman, P., … Tabashnik, B. E. (2014). Applying evolutionary biology to address global challenges. Science, 346, 1245993. https:// doi.org/10.1126/science.1245993
Echaubard, P., Sripa, B., Mallory, F. F., & Wilcox, B. A. (2016). The role of evolutionary biology in research and control of liver flukes in Southeast Asia. Infection, Genetics and Evolution, 43, 381–397. https://doi.org/10.1016/j.meegid.2016.05.019
Flores-Ferrer, A., Marcou, O., Waleckx, E., Dumonteil, E., & Gourbière, S. (2017). Evolutionary ecology of Chagas disease; what do we know and what do we need?. Evolutionary Application, 11, 470–487. https:// doi.org/10.1111/eva.12582
Folke, C., Carpenter, S., Elmqvist, T., Gunderson, L., Holling, C. S., & Walker, B. (2002). Resilience and sustainable development: Building adaptive capacity in a world of transformations. AMBIO Journal of the Human Environment, 31, 437–440. https://doi. org/10.1579/0044-7447-31.5.437
Glunt, K. D., Coetzee, M., Huijben, S., Koffi, A. A., Lynch, P. A., N’Guessan, R., … Thomas, M. B. (2017). Empirical and theoret-ical investigation into the potential impacts of insecticide re-sistance on the effectiveness of insecticide- treated bed nets. Evolutionary Application, 11, 431–441. https://doi.org/10.1111/ eva.12574
Grear, D. A., Hall, J. S., Dusek, R. J., & Ip, H. S. (2017). Inferring epide-miologic dynamics from viral evolution: 2014–2015 Eurasian/North American highly pathogenic avian influenza viruses exceed transmis-sion threshold, R0 = 1, in wild birds and poultry in North America. Evolutionary Application, 11, 547–557. https://doi.org/10.1111/eva.12 576
Horwitz, P., & Wilcox, B. A. (2005). Parasites, ecosystems and sustain-ability: An ecological and complex systems perspective. International Journal for Parasitology, 35, 725–732. https://doi.org/10.1016/j. ijpara.2005.03.002
Huijben, S., & Paaijmans, K. P. (2017). Putting evolution in elimination: Winning our ongoing battle with evolving malaria mosquitoes and parasites. Evolutionary Application, 11, 415–430. https://doi.org/ 10.1111/eva.12530
Joseph, U., Vijaykrishna, D., Smith, G. J. D., & Su, Y. C. F. (2017). Adaptive evolution during the establishment of European avian- like H1N1 influenza A virus in swine. Evolutionary Applications, 11, 534–546. https://doi.org/10.1111/eva.12536n/a–n/a.
Kosoy, M., & Kosoy, R. (2017). Complexity and biosemiotics in evolution-ary ecology of zoonotic infectious agents. Evolutionevolution-ary Applications, 11, 394–403. https://doi.org/10.1111/eva.12503
Lafferty, K. D. (2009). The ecology of climate change and infec-tious diseases. Ecology, 90, 888–900. https://doi.org/10.1890/ 08-0079.1
Lederberg, J., Shope, R. E., & Oaks, S. C. (1992). Emerging Infections: Microbial Threats to Health in the United States. Washington: National Academies Press.
Lefevre, T., Ohm, J., Dabiré, K. R., Cohuet, A., Choisy, M., Thomas, M. B., & Cator, L. (2017). Transmission traits of malaria parasites within the mosquito: Genetic variation, phenotypic plasticity, and conse-quences for control. Evolutionary Application, 11, 456–469. https:// doi.org/10.1111/eva.12571
Levin, S. A. (1998). Ecosystems and the biosphere as Complex Adaptive Systems. Ecosystems, 1, 431–436. https://doi.org/10.1007/ s100219900037
Lourenço, J., Tennant, W., Faria, N. R., Walker, A., Gupta, S., & Recker, M. (2017). Challenges in dengue research: A computational perspective. Evolutionary Application, 11, 516–533. https://doi.org/10.1111/eva. 12554
McMichael, A. J., & Wilcox, B. A. (2009). Climate Change, Human Health, and Integrative Research: A Transformative Imperative. EcoHealth, 6, 163–164. https://doi.org/10.1007/s10393-009-0262-9
McNeill, W. H. (1976). Plagues and peoples. New York: Anchor Press. Myers, S. S., Gaffikin, L., Golden, C. D., Ostfeld, R. S., Redford, H.,
Ricketts, K. H. T., … Osofsky, S. A. (2013). Human health impacts of ecosystem alteration. Proceedings of the National Academy of Sciences of the United States of America, 110, 18753–18760. https://doi. org/10.1073/pnas.1218656110
Myers, S. S., & Patz, J. A. (2009). Emerging Threats to Human Health from Global Environmental Change. Annual Review of Environment and Resources, 34, 223–252. https://doi.org/10.1146/annurev. environ.033108.102650
Nesse, R. (2008). What evolutionary biology offers public health. Bulletin of the World Health Organization, 86, 83. https://doi.org/10.2471/ BLT.00.00000
Nesse, R. M. (2013). Tinbergen’s four questions, organized: A response to Bateson and Laland. Trends in Ecology & Evolution, 28, 681–682. https://doi.org/10.1016/j.tree.2013.10.008
Nesse, R. M., & Stearns, S. C. (2008). The great opportunity: Evolutionary applications to medicine and public health: Evolutionary applications to medicine and public health. Evolutionary Applications, 1, 28–48. https://doi.org/10.1111/j.1752-4571.2007.00006.x
Packard, R. M. (2007). The making of a tropical disease: A short history of malaria. Baltimore, MD, USA: JHU Press.
Restif, O. (2009). Evolutionary epidemiology 20 years on: Challenges and prospects. Infection, Genetics and Evolution, 9, 108–123. https://doi. org/10.1016/j.meegid.2008.09.007
Stearns, S. C., & Koella, J. C. (2008). Evolution in Health and Disease, 2nd edn. Oxford , New York: Oxford University Press.
Sternberg, E. D., & Thomas, M. B. (2017). Insights from agriculture for the management of insecticide resistance in disease vectors. Evolutionary Application, 11, 404–414. https://doi.org/10.1111/eva.12501 Tarleton, R. L., Reithinger, R., Urbina, J. A., Kitron, U., & Gürtler, R. E.
(2007). The challenges of Chagas disease— grim outlook or glimmer of hope? PLoS Medicine, 4, e332.
Valen, V. (1973). A new evolutionary law. Evolutionary Theory, 1, 1–30. Viana, M., Faust, C. L., Haydon, D. T., Webster, J. P., & Lamberton, P. H.
L. (2017). The effects of subcurative praziquantel treatment on life- history traits and trade- offs in drug- resistant Schistosoma mansoni. Evol utionary Application, 11, 488–500. https://doi.org/10.1111/eva.12558 Whitmee, S., Haines, A., Beyrer, C., Boltz, F., Capon, A. G., de Dias, B. F.,
… Yach, D. (2015). Safeguarding human health in the Anthropocene epoch: Report of The Rockefeller Foundation- Lancet Commission on planetary health. The Lancet, 386, 1973–2028. https://doi. org/10.1016/S0140-6736(15)60901-1
WHO. (2016). World malaria report: 2016.
Wilcox, B. A., & Echaubard, P. (2016). Balancing biomedical and ecologi-cal perspectives in research framing of liver fluke and cholangiocarci-noma in NE Thailand. Parasitology International, 66, 372–377. https:// doi.org/10.1016/j.parint.2016.10.002
World Health Organization & UNICEF. (2004). Prevention and control of schistosomiasis and soiltransmitted helminthiasis. Geneva: World Health Organization.