HAL Id: hal-02608126
https://hal.inrae.fr/hal-02608126
Submitted on 16 May 2020
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Effects of herbivory pressure by wild ungulates on
diversity herbaceous of forest understory vegetation
Laura Chevaux
To cite this version:
Laura Chevaux. Effects of herbivory pressure by wild ungulates on diversity herbaceous of forest understory vegetation. Environmental Sciences. 2018. �hal-02608126�
Université de Bourgogne
Franche-Comté.
Master 2 Biologie, Ecologie et Evolution,
parcours Dynamique et Conservation de
la Biodiversité.
Academic year 2017-2018
Effects of herbivory pressure by wild ungulates on
diversity herbaceous of forest understory vegetation.
Master's Degree report
Laura Chevaux
Supervised by:
Yoan Paillet, Research engineer
Anders Mårell, Researcher in Ecology
Christophe Baltzinger, Researcher in Ecology
Agnès Rocquencourt
, Study engineer
Acknowledgements
Tout d’abord, je tiens à remercier mes encadrants pour l’ensemble de ce stage. Je remercie Yoan Paillet pour sa confiance, pour la liberté qu’il m’a laissée et pour l’ensemble de ses relectures. Je suis très reconnaissante envers Anders Mårell pour son écoute et ses précieux conseils, et de me donner la chance de pouvoir continuer. Je tiens également à remercier Christophe Baltzinger pour son enthousiasme et ses idées, et Agnès Rocquencourt pour son soutien et sa pédagogie. Je remercie également Aurélie Barboiron de l’ONCFS pour l’ensemble des données de chasse, et Hilaire Martin de m’avoir aidé à traiter ces données.
Je tiens à remercier toutes les personnes, permanents, doctorants, et stagiaires, qui ont fait cette ambiance si particulière dans le domaine. Sans vous, l’ambiance dans le Loiret aurait été si morne. Je remercie particulièrement mes co-bureaux ; Camille, Barthélémy, Maïté et tant d’autres. Je remercie cordialement Romain, Arthur, Michell, Guilhem, Maître Nicolas, pour tous ces bons moments à la Ferme. Je remercie également Manon pour sa folie, sa gentillesse et ses réflexions si intéressantes. Enfin, je tiens à remercie Jeanne qui a toujours été à l’écoute et de bons conseils. Merci à toi d’avoir permis à beaucoup de garder patience et confiance.
Table of content
Introduction ... 1
Materials and methods ... 4
1- Study area ... 4
2- Stand structure description ... 5
3- Estimates of ungulates populations ... 6
4- Flora data ... 6
5- Statistical analyses ... 8
Results ... 10
1- Direct effects of management and altitude on stand structure and herbivory ... 10
2- Alpha diversity: ... 11
3- Gamma diversity: ... 15
Discussion ... 17
1- Competition for resources at the expense of understory vegetation ... 17
2- Facilitation of understory vegetation diversity by ungulates ... 19
3- Conclusion ... 21 Bibliography ... A
List of figures
Fig. 1 Distribution of sites in France. ... 4
Fig. 2 Schematic representation of forest cover strata. ... 5
Fig. 3 Schematic representation of the two scales studied (alpha and gamma) ... 8
Fig. 4 Simplified hypothesis diagram (Metamodel). ... 9
Fig. 5 Example of alpha scale results obtained with SEM. ... 10
Fig. 6 Plots of variations in total species richness, abundance, Shannon index and inverse of Simpson index as a function of browsing (B in %) and canopy cover (%). ... 13
List of tables
Tab. 1 Number of plots according to management and altitude. ... 4Tab. 2 Summary of variables used for alpha and gamma diversities. ... 7
Tab. 3 Results of all SEM on alpha scale, i.e. the plot scale. ... 14
1
Introduction
Ecosystem structure constitute a major driver of communities and involve biotic (intra- and interspecies) and abiotic interactions. Disturbances mediate the effects and intensity of these interactions. White and Pickett (1985) define disturbance as any relatively discrete event over time that disrupts the ecosystem, community or population structure and alters resources, substrate availability or the physical environment. Disturbances affect individuals to ecosystems and landscape differently (Pickett, Kolasa, Armesto, & Collins, 1989). In temperate forests, management and herbivory pressures are two major ecological disturbances that can modify ecosystem structure, with varying degrees of intensity (Côté, Rooney, Tremblay, Dussault, & Waller, 2004; Franklin et al., 2002; Kirby, Webster, & Antczak, 1991). These disturbances have competitive and facilitating effects on understory vegetation. For example, herbivores-resource interactions can be altered in ecosystems influenced by human activity, as forest managers can simultaneously alter consumer populations and forest structure through direct and indirect mechanisms (Faeth, Marussich, Shochat, & Warren, 2005).
The overall effect of forest management on forest biodiversity is not yet fully assessed (Siitonen, 2001). However, forest management focused on wood productivity still threatens the survival of many species that depend on natural forest habitats (Bengtsson, 2000). Several factors are identified to improve biodiversity in managed forests, including changes in forest practices to achieve greater naturalness (Bauhus, Puettmann, & Messier, 2009; Reimoser, 2003), notably by setting aside forest reserves where management is abandoned. Forest management, by modifying stand structure, directly affects the amount of light available for understory vegetation and the level of tolerance to damage by ungulates (Reimoser, 2003), which in turn modifies the abundance, richness or composition of understory vegetation. Forest management can hence be seen as a tool to redirect light fluxes towards different strata. Species richness over a wide range of taxa is lower in managed forests compared to unmanaged forests in Europe (Burrascano, Lombardi, & Marchetti, 2008; Horvat, Biurrun, & García-Mijangos, 2017). Moreover, the increase in time since the forest management abandonment increases the overall species richness but its effect may be different depending on the taxon considered (Paillet et al., 2010).
2
On the top of that, wild ungulates, through food webs, also play a considerable role on understory vegetation communities. Since the beginning of the 20th century, population dynamics, and in particular the increase in the abundance of wild ungulates, has been widely studied (Apollonio et al., 2017; Côté et al., 2004; Fuller & Gill, 2001). In temperate forests in the northern hemisphere, some effects of ungulate populations are considered harmful where the population density is sometimes considered too high (Gill, 1992a, 1992b). However, assessing all the effects of wild ungulates on their habitat is therefore a key issue that is often overlooked in the study of forest ecosystems. Among the different behaviours of wild ungulates, some have indirect facilitating effects (e.g. zoochory) or direct competitive effects (rubbing, herbivory) on forest ecosystems (Gill & Beardall, 2001). Herbivory is the behaviour that most seriously alters vegetation, from the part of the plant consumed to all levels of diversity such as community assemblages (Takada, Asada, & Miyashita, 2001; Tanentzap et al., 2009). The quantity and quality of food determine the probability of a plot being browsed by wild herbivores, but depending on the spatial scale considered, the limiting factors are different (Ohse, Seele, Holzwarth, & Wirth, 2017):
(i) At a local scale, i.e. at the plot scale, herbivory pressure by wild ungulates decreases the cover of the understory vegetation (Boulanger et al., 2018; Meier, Stöhr, Walde, & Tasser, 2017). The effects of browsing are species dependent: some plant species increase in abundance while others decrease (Kirby, 2001). In the presence of wild ungulates, species richness in the herbaceous stratum increases while it decreases in the shrub stratum. Indeed, ungulates, by controlling the shrub layer, indirectly increase the richness of understory vegetation by increasing the light reaching the ground (Boulanger et al., 2018). Consequently, plant richness and abundance are higher in managed forests (Kaufmann, Hauck, & Leuschner, 2017).
(ii) At the landscape scale, i.e. at the forest management scale, plant species composition and the quantity of seedlings could be limiting for the establishment of ungulate populations, whereas at the plot scale, the plant species richness would be more important. Indeed, a high diversity of plant species in a plot could increase the probability of ungulates requiring a diversified diet (Augustine & Mcnaughton, 1998).
Therefore, forest management and herbivory pressure can be considered as interactive disturbances (Meier et al., 2017). Much research has focused particularly on the effects of ungulates on tree diversity, but less attention has been paid to understory vegetation where the
3
vast majority (>75%) of vascular species occur (Roberts & Gilliam, 1995). Moreover, at the stand level, concerns about the potential impacts of intensive forestry practices on understory vegetation diversity are widespread (Hartley, 2002; Roberts, 2002), particularly because the contributions of understory vegetation to overall species diversity are significant. In addition, understory vegetation influences energy flows, provides habitat for animals, and partially controls the forest microclimate and plays an important role in nutrient cycling (Arivin Rivaie, 2014; Gilliam, 2007; Poirier, Coyea, Angers, & Munson, 2016). However, the joint effects of ungulates and forest management abandonment on the understory vegetation have rarely been studied within a causal framework.
In this study, we analysed the cascading effects of forest management and herbivory on forest structure and resource availability that could in turn affect the biodiversity of understory vegetation (vascular plants): (i) We hypothesized that increasing browsing by ungulates would
result in a decrease in the relative abundance of some species. In addition, disturbances related to forest management and herbivory would increase the species richness by altering the abundance of understory vegetation, particularly by increasing the abundance of forest species.
At gamma level, i.e. at the forest level, there would be no difference in richness or species diversity between managed and unmanaged forests. Furthermore, there is no difference according to the forest management abandonment (Dieler et al., 2017). (ii) We hypothesized
that disturbances induced by forest management increase diversity at landscape scale, i.e. the gamma diversity of understory vegetation. (iii) We hypothesized that management could mitigate the effects of herbivory, for example clearcutting and artificial reforestation would
reduce a forest's predisposition to damage, such as reduced diversity caused by ungulates (Reimoser & Gossow, 1996). On the contrary, in the presence of an overabundance of ungulates, the effects of browsing on tree species composition appear to be greater than the effects of hardwood forest management (Schulze et al., 2014). The extent of vegetation change is proportional to the abundance of ungulates, and tends towards changing community assemblages and landscape homogenization (Boulanger et al., 2018). (iv) We hypothesized that
the joint effects of forest management and ungulates on the understory vegetation appear to depend on ungulate density. Forest management would have greater effects on the understory vegetation than herbivory. In contrast, when there is an overabundance of ungulates, forest management would have less effects on the diversity of the understory vegetation than herbivory.
4
Materials and methods
1- Study area
We collected all the data within the framework of the national project "Gestion forestière, Naturalité et Biodiversité" (www.gnb.irstea.fr), from 2008 to 2014, the aim was to study the effect of forest management abandonment on stand structure and biodiversity compartments.
The sampling design comprised 212 plots distributed over 15 sites; 9 lowland (elevation ≤ 800 m) and 6 mountain forest sites (elevation > 800 m) (Fig. 1). We sampled mixed oak-beech-hornbeam forests at the lowland sites and beech-fir-spruce forests in the mountains. The following three criteria were used to select the sites: (1) dominant tree species in the stands studied were all indigenous species, (2) the presence of a forest reserve for which forest management has been abandoned for at least 20 years and (3) site characteristics (homogeneous areas in terms of chemical, physical factors and soil type, suitable for a certain set of species) were similar between the managed and unmanaged stands. All managed plots were selected within a 5-km radius buffer of the reserve boundaries.
Fig. 1 Distribution of sites in France; in red the lowland forest sites and in blue the mountain forest sites.
Tab. 1 Number of plots according to management and altitude.
5
Among the 212 plots, 108 plots are in managed and 104 in unmanaged forests i.e. in forest reserves (Tab. 1). In this study, unmanaged areas are management abandonment by setting aside forest reserves. From a forest management point of view, these areas are considered as forest reserves, but they are not strict reserves for ungulates because they are regularly hunted.
2- Stand structure description
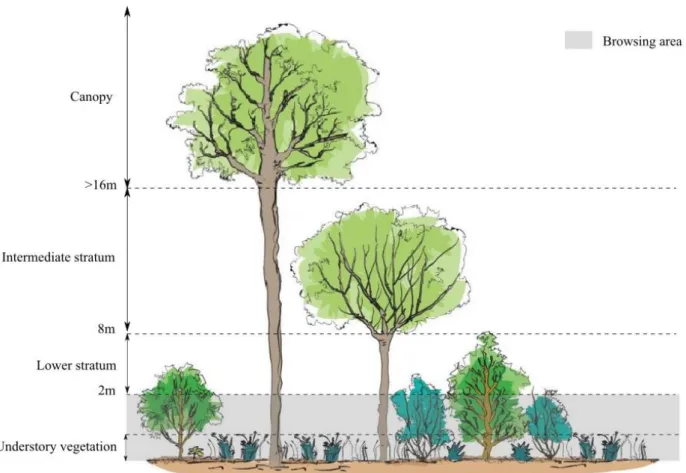
Based on stand structure description (Paillet et al., 2015), we calculated the Stand Density Index (SDI) as a competition index between trees at the plot level. SDI is defined as the number of trees per plot (= per unit area), and the Quadratic Mean Diameter based on the diameter of the tree at breast height (1m30, DBH). This index is a relative measure of stand density and can be used as a measure of stocking density (Reineke, 1933). We visually assessed forest cover (%) by stratum: canopy (height > 16m), intermediate stratum (8m<height <16m) and lower stratum (2m<height<8m). The total forest cover sums the covers of these three strata and can thus exceed 100% (Fig. 2). We showed during data exploration that basal area and SDI were highly correlated, so we only use SDI for the following.
Fig. 2 Schematic representation of forest cover strata : We analysed the response of understory vegetation. Below 2m, ungulates effect is measured by a browsing index. The three upper strata are used to study forest cover: canopy (height > 16m), intermediate stratum (8m<height <16m) and lower stratum (2m<height<8m).
6
3- Estimates of ungulates populations
3.1. Browsing pressure - alpha level
At the plot scale, we assessed the browsing pressure using the modified Aldous method (Ballon, 1992). To account for the presence of red deer in the study area, the maximum height of the surveys was 2m in its presence and 1m20 in its absence. We recorded plant cover (dij) and
browsing rate (aij) (proportion of shoots consumed by ungulates) for woody and semi-woody
species at three circular subplots (400m²) per study plot. We estimated it respectively by cover class and by species i browsing class on plot j (in %). We replaced the class values by their respective exact class medians for statistical analyses. We calculated the average plot browsing (B in %) by weighting the browsing rate of each species on the subplot by its plant cover,
according to the formula :
𝐵 =
∑𝑒𝑖=1𝑎𝑖𝑗.𝑑𝑖𝑗 ∑𝑒𝑖=1𝑑𝑖𝑗
We used the mean value for all three subplots in the subsequent analyses as the estimated browsing pressure at the plots level.
3.2. Hunting data statistics – gamma level
At the forest scale, on all the sites studied, we used hunting statistics for ungulate species present in the forests studied, recorded by Office National de la Chasse et de la Faune Sauvage, as an estimate of herbivore density at the forest scale. Hunting statistics are an approximation of herbivory pressure, but they are spatially homogeneous and reveal differences in ungulate species abundances (Boulanger et al., 2018). The data of hunting seasons corresponds to the years of study on the plots, or the seasons closest to the year of study. On all studied sites, five species of wild ungulates were present: mainly red deer (Cervus elaphus), roe deer (Capreolus
capreolus), wild boar (Sus scrofa), and occasionally sika deer (Cervus nippon) and fallow deer
(Dama dama). For red deer, we collected data at the territorial management unit level. We recorded data for the other species (wild boar, roe deer, fallow deer, sika deer) at the municipality level where plots are present but also in neighbouring towns.
4- Flora data
We recorded the relative abundance of all plant species exhaustively on the whole plot of 1000m², according to the Braun-Blanquet method (1952) over the height from 0 to 2m corresponding to the herbaceous and the low shrub strata. Two experienced botanists conducted
7
the surveys during a controlled census time of 35min (+/-5min). The relative abundance corresponds to percentage cover classes. In the following, we used the class median as an abundance estimate for each species. Herbaceous, woody and semi-woody species were recorded in the field but we used only herbaceous species as a response variable for biodiversity index calculations to avoid correlation/bias between Aldous estimations and understory biodiversity. Julve attributes characteristic phytosociological values to the French vascular flora (Julve, 2007). We extracted these values from the Baseflor floristic database of the Catminat project. We used understory vegetation preferences for environmental type to classify them into three categories: forest species, intermediate species and open habitat species (Julve, 2007). We considered that species with a value lower than 13, in the determination key established by Julve, were open habitat species. For example, these different habitat types include crops, heathlands, wastelands, and lawns. For forest species, we considered species with a value of 16 to be the only corresponding category. This category includes forest vegetation, woods and tree groves. Finally, we decided that plants with a value between 13 and 16 would be intermediate species. These habitats include heterogeneous habitats such as wastelands and edges.
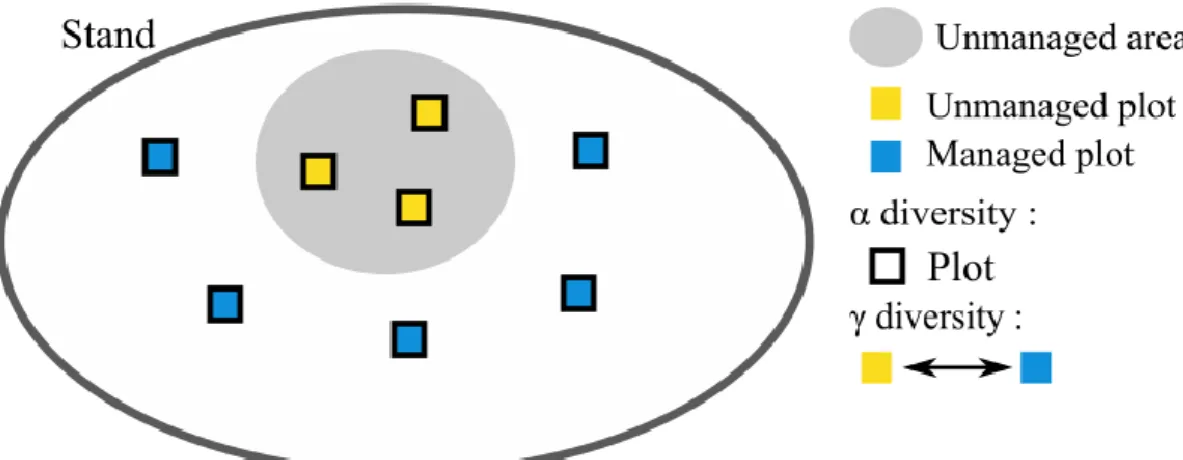
We studied two different spatial scales (Fig. 3):
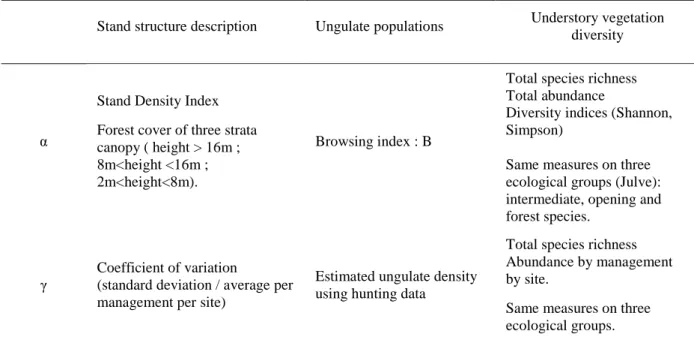
o We calculated alpha diversity indices corresponding to the plot scale: total species richness, total abundance and two indices of diversity (Shannon, Simpson) on understory vegetation. We calculated these indices for the three different ecological groups: intermediate, open-habitat and forest species (Tab. 2).
Stand structure description Ungulate populations Understory vegetation diversity
α
Stand Density Index Forest cover of three strata canopy ( height > 16m ; 8m<height <16m ; 2m<height<8m).
Browsing index : B
Total species richness Total abundance
Diversity indices (Shannon, Simpson)
Same measures on three ecological groups (Julve): intermediate, opening and forest species.
γ
Coefficient of variation
(standard deviation / average per management per site)
Estimated ungulate density using hunting data
Total species richness Abundance by management by site.
Same measures on three ecological groups.
8
o We calculated gamma diversity based on the comparison between all plots of each management modality (managed vs. unmanaged) in each site. For this scale, we use total species richness and abundance as well as each ecological group for each management modality (Tab. 2).
We calculated two diversity indices on the alpha scale. The Shannon-Weaver index gives more weight to rare species : 𝐻 = − ∑𝑠𝑠=1𝑝𝑠ln 𝑝𝑠 with 𝑝𝑖 =
𝑁𝑖
𝑁 ; Ni is the abundance for species i
(Marcon, 2016). In addition, we calculate an index giving more weight to dominant species; the Simpson index: D= 1 − ∑𝑠𝑠=1(𝑝𝑖)² (Marcon, 2016).
5- Statistical analyses
We used structural equation modelling analyses (SEM) to assess the effects of forest management, stand structure and ungulate populations on herbaceous richness, abundance and diversity within a causal framework. Piecewise structural equation modelling represents a method for statistically evaluating a network of dependence relationships through the analysis of covariances (Lefcheck, 2016). Structural equation models are probabilistic models that use several variables that can be predictors or responses in the same causal network. These models are often represented by path diagrams, with directional relationships between the observed variables. In the package piecewiseSEM (Jon, Byrnes, & Grace, 2018), the path diagram consists in a set of linear (structured) equations, which are then evaluated individually
Fig. 3 Schematic representation of the two scales studied (alpha and gamma). Unmanaged plots are located in the forest reserve (yellow). The managed plots are located within a radius of 5km around the reserve. Alpha diversity is calculated within each plot while gamma diversity is calculated across all unmanaged plots and all managed plots for a given site.
9
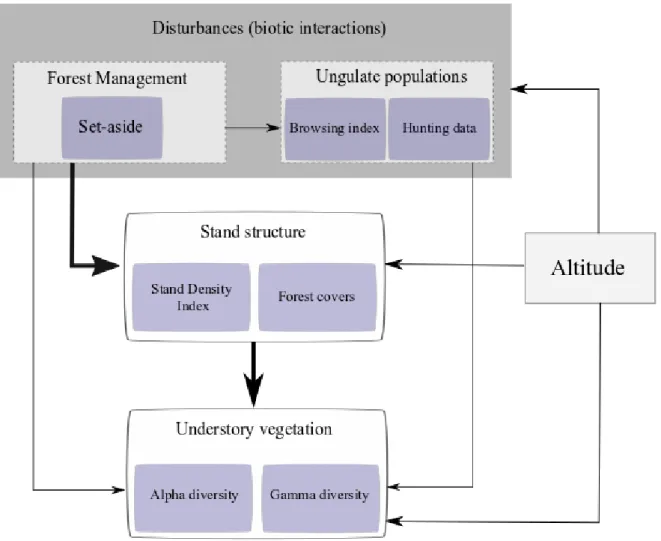
(Lefcheck, 2016). Each SEM corresponds to a final response variable (Fig. 4), so 15 SEMs were performed for the alpha scale and 6 for the gamma scale. For each model, the site was considered as random effect and altitude as continue co-variable. When stand structure variables (SDI and three forest covers) and browsing index were response variables we used generalized linear mixed-effects models (GLMM) with a Gamma error distribution and log-link for continuous positive data for alpha scale. For these same variables at gamma scale, we used GLMM with negative binomial distribution to account for overdispersion in count data. For richness and hunting data, we used GLMM with a Poisson error distribution for count data. Finally, we used linear mixed effects models for abundance and different diversity indices.
All statistical analyses were performed in the R 3.5.0 statistical platform (R Core Team, 2018). Diversity indices were calculated using the « Vegan package » (Oksanen et al., 2018). All
Fig. 4 Simplified hypothesis diagram (Metamodel) : we hypothesized that the most important disturbance effect is due forest management which modifies stand structure, measured by SDI and forest cover at the alpha scale, and measured by variation coefficients of these variables at gamma scale. We used the browsing index to study ungulate populations at alpha scale. At the gamma scale, we used hunting data to study ungulate populations by site management.
10
models were processed with the « lme4 package » (Bates et al., 2015) and piecewise SEM analyses were processed with the « piecewiseSEM package » (Jon et al., 2018).
Results
In total, we did 21 SEMs: 15 for the alpha scale (Tab. 3) and 6 for the gamma scale (Tab. 4). All these SEMs were validated (Fisher d-separation test, p>0.05) without using any alternative path (Fig. 5). Botanists identified 142 herbaceous species of understory vegetation; 45 forest species, 97 open habitat species but no herbaceous species considered as intermediate species according to Julve's classification.
1- Direct effects of management and altitude on stand structure and
herbivory
1.1. Altitude effects
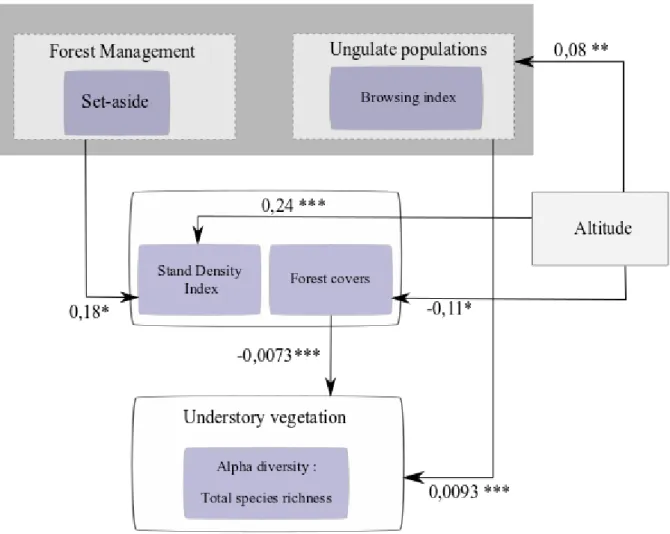
Fig. 5 Example of alpha scale results obtained with SEM. Here the answer variable is the total species richness of understory vegetation. Arrows and estimate values represent only significant results.
11
First, the effects of altitude were the strongest significant effects at the alpha scale (scaled estimates = 0,20). Altitude had a strong significant negative effect on stand density (p<0.01), and a smaller negative effect on the low stratum (2 to 8m) (p<0.01). In addition, altitude also had a strong negative effect on ungulate density (p=0.043). Second, at the gamma scale, altitude only had a significant strong effect on ungulate density. Indeed, the increase in altitude had a negative effect on ungulate density (p=0.020). Altitude had no effect on stand structure diversity.
1.2.Management effects
At the alpha scale, management abandonment by setting aside forest reserves had a significant effect on stand diversity (p<0,01), measured by SDI, and on lower forest stratum (2m to 8m) (p>0,01). However, setting aside forest reserves had no effect on canopy cover (p=0,904) or on intermediate cover (p=0,184). In addition, setting aside forest reserves had no effect on browsing pressure (p=0,146).
At the gamma scale, management abandonment had no effect on stand structure diversity. Indeed, setting aside forest reserves had no effect on the coefficient variation of stand density (p=0,695). Moreover, setting aside forest reserves also had no effect on the coefficient variation of canopy cover (p=0,423), the intermediate stratum (p=0,4225) and the lower stratum (p=0,1753). As on the alpha scale, setting aside forest reserves had no effect on ungulate density (p=0,956).
2- Alpha diversity:
2.1. Overall diversity
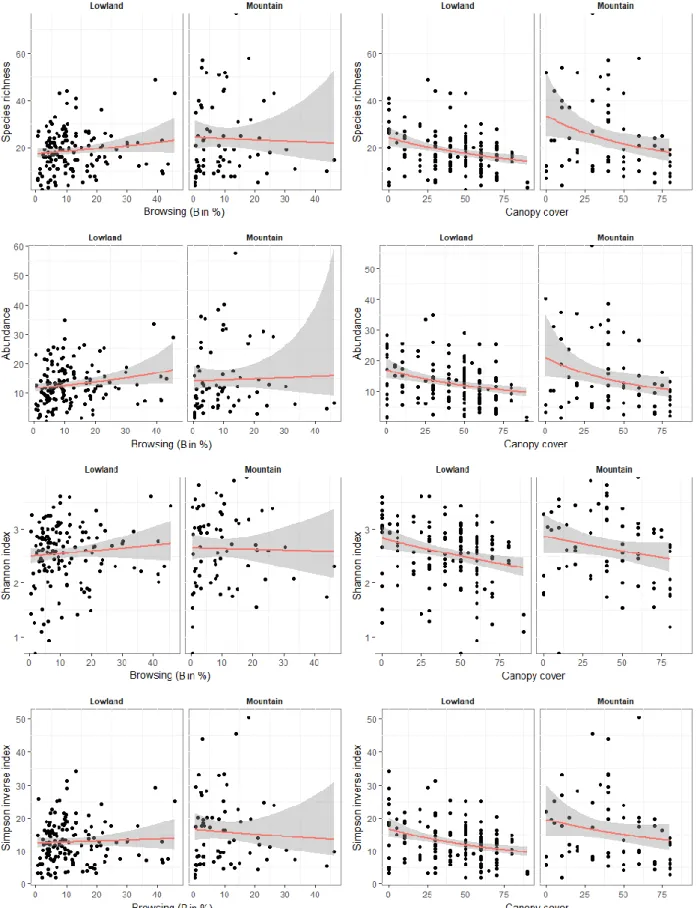
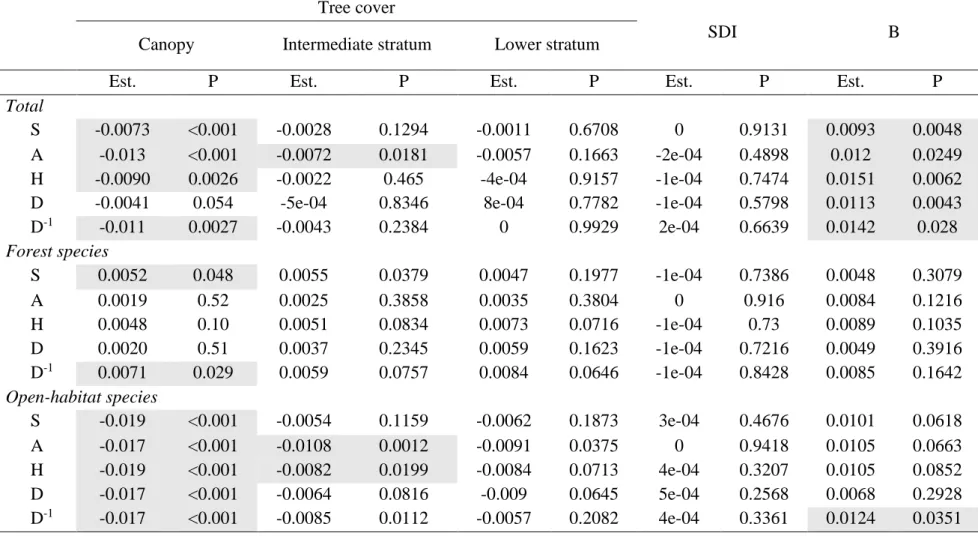
Canopy cover and browsing pressure had opposite effects of similar magnitudes on the herbaceous understory diversity. Indeed, stand structure variables, mainly canopy cover, have a negative effect on understory vegetation diversity while browsing pressure had a positive effect (Tab. 3). Diversity indices (Shannon, Simpson inverse) for all species and total species richness were positively impacted by browsing pressure and negatively canopy cover (Fig. 6). However, Simpson diversity index was only significantly impacted by browsing (Tab. 3). Other stand structure variables did not appear to have any effect on species richness.
Like other diversity indices, abundance was negatively impacted by canopy cover and positively impacted by browsing pressure. However, the intermediate stratum had also a
12
significant negative effect on total abundance, although the magnitude of intermediate stratum effect is about half as strong as the canopy effect (Tab. 3). The magnitude of the canopy cover and browsing pressure effects were almost identical (standardized estimate value).
2.2.Forest species
The ecological plant group of forest specialists responded to few explanatory variables. Forest species richness and the inverse of the Simpson index were positively impacted by stand structure variables (intermediate stratum cover and canopy cover, respectively). Forest species richness was positively explained by intermediate stratum cover. The inverse of the Simpson index calculated on forest species is positively impacted by the canopy cover (Tab. 3).
2.3.Open habitat species
As for all species, the ecological group of open habitat species was significantly negatively impacted by forest cover, mainly the two highest strata (8-16m and above 16m), but only the Simpson inverse index of open species was impacted by browsing pressure.
Open habitat species richness was only negatively impacted by canopy cover (Tab. 3), while open habitat species abundance was negatively impacted by the three forest cover: canopy, intermediate stratum and lower stratum cover. Scale magnitudes of each effect are close to 0,1 (Tab. 3).
Diversity indices calculated on open habitat species were generally negatively impacted by canopy and intermediate stratum cover. Shannon index and Simpson inverse index of open habitat species were negatively impacted by canopy and intermediate stratum cover, whereas Simpson index of open habitat species was negatively impacted by canopy cover (Tab. 3). Moreover, browsing pressure had a positive effect on the Simpson inverse (Tab. 3).
To sum up, at alpha scale, i.e. at the plot scale, forest cover had a stronger effect on the total diversity notably through canopy cover which had a significant effect on the total diversity and on the ecological group of open habitat species. Except for forest species diversity, forest cover variables always had a negative effect. Conversely, browsing pressure had a positive effect on the diversity of all ecological groups. However, these results were not found in the two different ecological groups. Significant effects generally had the same order of magnitude. Moreover, at this scale, Stand Density Index, a stand structure variable, had no significant effect on the diversity of understory vegetation.
13
Fig. 6 Plots of variations in total species richness, abundance, Shannon index and inverse of Simpson index as a function of browsing (B in %) and canopy cover (%). The black dots represent raw values. The red lines are issued of generalised models (see statistical analyses section), the grey ribbons represent the 95% confidence intervals. Each response studied is separated according to the two altitude levels: lowland (elevation<800m) and mountain (elevation>800m).
14
Tab. 3 Results of all SEM at plot scale. P-values (P) and standardized estimates (Est.) are given for the same response variables in each ecological group and for the total, based on each stand structure variable (three forest cover and SDI) and browsing. Grey filling identifies significant effects (p<0.05). SDI = Stand Density Index; B = Browsing index; S = Species richness; A = Abundance; H = Shannon diversity index; D = Simpson diversity index; D-1 = inverse of Simpson diversity index.
Tree cover
SDI B
Canopy Intermediate stratum Lower stratum
Est. P Est. P Est. P Est. P Est. P
Total
S -0.0073 <0.001 -0.0028 0.1294 -0.0011 0.6708 0 0.9131 0.0093 0.0048 A -0.013 <0.001 -0.0072 0.0181 -0.0057 0.1663 -2e-04 0.4898 0.012 0.0249 H -0.0090 0.0026 -0.0022 0.465 -4e-04 0.9157 -1e-04 0.7474 0.0151 0.0062 D -0.0041 0.054 -5e-04 0.8346 8e-04 0.7782 -1e-04 0.5798 0.0113 0.0043 D-1 -0.011 0.0027 -0.0043 0.2384 0 0.9929 2e-04 0.6639 0.0142 0.028 Forest species S 0.0052 0.048 0.0055 0.0379 0.0047 0.1977 -1e-04 0.7386 0.0048 0.3079 A 0.0019 0.52 0.0025 0.3858 0.0035 0.3804 0 0.916 0.0084 0.1216 H 0.0048 0.10 0.0051 0.0834 0.0073 0.0716 -1e-04 0.73 0.0089 0.1035 D 0.0020 0.51 0.0037 0.2345 0.0059 0.1623 -1e-04 0.7216 0.0049 0.3916 D-1 0.0071 0.029 0.0059 0.0757 0.0084 0.0646 -1e-04 0.8428 0.0085 0.1642 Open-habitat species S -0.019 <0.001 -0.0054 0.1159 -0.0062 0.1873 3e-04 0.4676 0.0101 0.0618 A -0.017 <0.001 -0.0108 0.0012 -0.0091 0.0375 0 0.9418 0.0105 0.0663 H -0.019 <0.001 -0.0082 0.0199 -0.0084 0.0713 4e-04 0.3207 0.0105 0.0852 D -0.017 <0.001 -0.0064 0.0816 -0.009 0.0645 5e-04 0.2568 0.0068 0.2928 D-1 -0.017 <0.001 -0.0085 0.0112 -0.0057 0.2082 4e-04 0.3361 0.0124 0.0351
15
3- Gamma diversity:
3.1. On all ecological groups
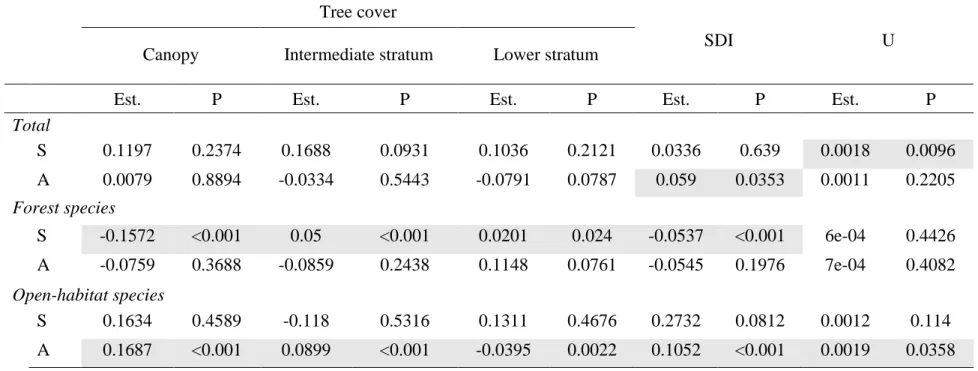
Different variation coefficients of forest covers and variation coefficient of stand density index had no significant impact on the total species richness of understory vegetation. However, total species richness was positively impacted by ungulate density, even if the magnitude of the effect was small (Tab. 4). Moreover, total abundance was positively impacted by the coefficient of variation of stand density index (Tab. 4).
3.2. Forest species
Forest species richness was impacted by the coefficient of variation of stand structure variables while abundance had no significant response. Forest species richness was negatively impacted by the coefficient of variation of SDI and canopy cover. On the other hand, intermediate stratum and low stratum covers had positive effects on species richness (Tab. 4). Canopy cover was the effect with the largest magnitude.
3.3. Open habitat species
No variable studied in this analysis had a significant impact on the open habitat species richness. However, the abundance of open habitat species showed a significant response for each variable studied. Open species abundance was positively impacted by hunting data, i.e. the increase in ungulate density had a significant positive effect on the abundance of open habitat species, even if the magnitude was rather small (Tab. 4). All stand structure variables had positive effect on open habitat species abundance except the coefficient of variation of lower stratum cover which had a negative effect on abundance (Tab. 4). The coefficient of variation of canopy cover was the effect with the highest magnitude while the coefficient of variation of SDI and ungulate density had the lowest magnitudes of the same order; 0,1 (Tab. 4).
To sum up, at gamma scale, i.e. at the forest scale, all the variables studied had an impact on the understory vegetation diversity, but the variables with significant effects were different depending on the diversity studied. Indeed, forest species richness and open species abundance were particularly affected by all stand structure variables while total species richness was impacted only by hunting data.
16
Tab. 4 Results of all SEM on gamma scale, i.e. the management modality. P-values and estimates are given for the same response variables in each ecological group and for the total, according to each stand structure variable (three forest cover and SDI) and browsing. Significant effects (p<0.05) have the grey box. SDI = Stand Density Index; U = Hunting data on ungulate populations; S = Species richness; A = Abundance.
Tree cover
SDI U
Canopy Intermediate stratum Lower stratum
Est. P Est. P Est. P Est. P Est. P
Total S 0.1197 0.2374 0.1688 0.0931 0.1036 0.2121 0.0336 0.639 0.0018 0.0096 A 0.0079 0.8894 -0.0334 0.5443 -0.0791 0.0787 0.059 0.0353 0.0011 0.2205 Forest species S -0.1572 <0.001 0.05 <0.001 0.0201 0.024 -0.0537 <0.001 6e-04 0.4426 A -0.0759 0.3688 -0.0859 0.2438 0.1148 0.0761 -0.0545 0.1976 7e-04 0.4082 Open-habitat species S 0.1634 0.4589 -0.118 0.5316 0.1311 0.4676 0.2732 0.0812 0.0012 0.114 A 0.1687 <0.001 0.0899 <0.001 -0.0395 0.0022 0.1052 <0.001 0.0019 0.0358
17
Discussion
We showed that management abandonment had an effect on stand structure at alpha scale but not at gamma scale. Indeed, management abandonment increased forest cover and forest closure (SDI). As shown in other studies, forest management changes stand structure (Wesely et al., 2018), particularly at the local scale (Kirby et al., 1991). Although the forest reserves studied are not considered old-growth stands, some stand characteristics appear to be similar (Paillet et al., 2015). Indeed, these results are consistent with forest characteristics with an absence of human disturbance because these forests have greater closure, cover and structural heterogeneity (Bauhus et al., 2009; Burrascano, Keeton, Sabatini, & Blasi, 2013). Changes, induced by forest management, modify biotic interactions and its influences on herbaceous plants of understory vegetation.
1- Competition for resources at the expense of understory vegetation
1.1. Alpha-scale competition between strata modified by forest management.
Contrary to our hypothesis (i), at plot scale, we showed that increasing forest cover reduces species richness, abundance and diversity indices. However, stand density, measured by SDI, had no effect on understory vegetation. Moreover, at the ecological guild level, we showed that the effect concerns particularly on open habitat species. In addition, open habitat species are also sensitive to increased cover of intermediate and low strata, which is not the case for all species or forest species.
Upper stratum species, particularly canopy species, and understory vegetation compete directly for resources (Gilliam, 2007; Roberts & Gilliam, 1995). Canopy cover, and the entire stand structure, are subjected to disturbance and are therefore significantly modified by forest management. Changes in the canopy then have effects on the lower competitive strata (Montgomery & Chazdon, 2016). Our results are consistent with studies showing a decrease in understory vegetation diversity with increasing canopy cover (Vockenhuber et al., 2011). Indeed, Vockenhuber et al. (2011) showed a decrease in herbaceous richness and cover with increasing canopy cover. At the same time, forest management by modifying the canopy cover and intermediate stratum had a positive effect on forest species richness. Forest species need less light and are therefore favored, in competition with other species of open habitat species, by the increase of forest cover. However, in other studies, types of management (e.g. selective
18
cutting) can lead to strong and frequent openings that reduce the diversity of forest species (Decocq et al., 2004).
Open habitat species require more light than forest species, and are therefore more sensitive to increased cover. In this study, effects of increased forest covers are particularly important on the ecological group of open habitat species. Our results are consistent with the assumption that light constitutes the limiting resource in competition between forest strata (Borer et al., 2014). However, light can be a limiting resource for understory vegetation, but competition can also be in relation to soil resources, including nitrogen (Murray, Webster, & Bump, 2013). Indeed, different forest management types influence forest ecosystems by modifying the properties and nutrients available in the soil (Kovács et al., 2018) or local microclimates (Frey et al., 2016). However, these effects of forest management on soil were not studied here. Moreover, the characteristics of the forest reserves compared in this study are not the same according to the sites. Indeed, the area of management abandonment is considered as reserve when the abandonment period was at least 20 years, but some reserves are older than others. As the time since last harvesting is not homogeneous, the age and stage of the vegetation are not identical for all sites. The multisite approach may have led to undetected effects due to this heterogeneity. In other studies, comparing managed and unmanaged forests, the variations of species richness of understory vegetation are contradictory. Indeed, some studies observe a decrease in species richness in abandoned plots (Horvat et al., 2017; Schmidt, 2005; Von Oheimb & Härdtle, 2009), while others observe an increase in species richness (Burrascano, Rosati, & Blasi, 2009) or no difference (Sitzia et al., 2012). However, comparisons between forest management are not exactly the same, as the definition of abandonment varies between studies. Indeed, the forests unmanaged, according to Schmidt (2005), have been forest reserves for 10 years, whereas in Burrascano et al. (2009), unmanaged forest correspond to old-growth forest, abandoned for almost a hundred years. In addition, studies do not always compare the same types of management. Horvat et al. (2017) compared four management types according to management time while Von Oheimb & Härdtle (2009) compared three management types according to selection harvest. The definition of understory vegetation also differs from one study to another. Indeed, Von Oheimb & Härdtle (2009) have integrated woody species in the vegetation measurements of understory vegetation, while we chose to exclude them. However, several meta-analysis have shown that herbaceous diversity is higher in managed forests (Chaudhary, Burivalova, Koh, & Hellweg, 2016; Paillet et al., 2010).
19
1.2.Gamma scale competition
At the gamma scale, our results confirmed our second hypothesis (ii) which predicted that at landscape scale, disturbances induced by forest management increase understory vegetation diversity. We showed that coefficients of variation of stand structure had no effect on total diversity. However, coefficient of variation of canopy cover and the coefficient of variation of SDI had negative effects on the richness of forest species, but they had a positive effect on abundance of open habitat species. In addition, the coefficient of variation of low stratum cover had a positive effect on forest species richness but a negative effect on the open habitat species abundance. However, the effects of stand structure variables at this scale must be qualified, as the magnitudes of these effects are quite low.
Forest management can be considered as a more or less intense disturbance (Bengtsson, 2000). As discussed in the intermediate disturbance hypothesis, forest management can therefore, at an intermediate intensity, increase diversity. On the contrary, the intermediate disturbance hypothesis suggests that low-intensity disturbances lead to low diversity through competitive exclusion of species, while high-intensity disturbances eliminate species unable to recolonize rapidly (Roxburgh, Shea, & Wilson, 2004; Wilkinson, 1999). Our results at the gamma scale coincide with the intermediate disturbance hypothesis, i.e., the intensity of forest management at the study sites (excluding clearcuts and exotic species plantations) corresponds to intermediate disturbances, and promotes diversity at the landscape scale. At intermediate levels, disturbances would promote habitat heterogeneity in forest landscapes (Swanson et al., 2011), thus increasing the diversity of several taxa (Schall et al., 2018). Indeed, the presence of many different conditions for herbaceous of understory vegetation, such as different soil pH, different soil moisture levels or litter properties, allow the presence of a higher diversity of herbaceous (Brunet, Fritz, & Richnau, 2010).
2- Facilitation of understory vegetation diversity by ungulates
2.1. Facilitation difference between the two ecological groups
All results, for the alpha scale, showed that the first part of hypothesis (i) was not validated. Indeed, contrary to our predictions, herbivory pressure increased the total abundance of species, and did not modify forest species diversity any more. There was no difference between the two ecological guilds, except the inverse of Simpson index for open habitat species. However, as
20
indicated in the first hypothesis (i), herbivory pressure increased total richness and higher canopy cover increased forest species richness.
We showed that the effects of browsing pressure on the Shannon and Simpson diversity indices were in the same order of magnitude. These two diversity indices were chosen because the Shannon diversity index gives more weight to rare species while the Simpson index gives more weight to abundant species. Our results seem to show that there is no difference in the effects of herbivory pressure depending on whether the herbaceous understory species is rare or dominant.
However, in this study, forest management abandonment through reserve establishment had no effect on herbivory or ungulate populations. In other words, there was no difference in herbivory pressure and ungulate density between managed forests and forest reserves. In the studied sites, forest reserves are not strict reserves for ungulates because they are regularly hunted, which may explain the lack of management effect on herbivory. Indeed, forest reserves may not represent a refuge area for ungulates (Meisingset et al., 2018; Parviainen, Bücking, Vandekerkhove, Schuck, & Päivinen, 2000), in particular because of human disturbance (Stankowich, 2008). Indeed, ungulates can resolve the compromise between the use of the resources, even of high quality, with the risk of predation, here the risk of hunting, by modifying their behavior of habitat use (Bonnot et al., 2013). The effects of ungulates differ according to the studies, which may suggest that other factors not tested may modify the effects of ungulates, such as the nutritional quality of focal and neighboring plants, the time of browsing or even behavior (Champagne, Tremblay, & Côté, 2016).
2.2.Gamma scale facilitation by ungulate populations
As hypothesized (ii), at the gamma scale, we showed that ungulate populations increased the diversity of understory vegetation. Indeed, ungulates density increase total species richness and abundance of open habitat species.
In this study, ungulate densities are not considered overabundant. Indeed, in accordance with the Intermediate Disturbance Hypothesis, the presence of ungulates, in intermediate density, increases the herbaceous diversity of understory vegetation (Faison, DeStefano, Foster, Motzkin, & Rapp, 2016). Ungulate populations increase the heterogeneity of the landscape by browsing, and consequently, by reducing the growth of fast-growing pioneer species (Murray et al., 2013; Royo, Collins, Adams, Kirschbaum, & Carson, 2010). Ungulates then have two facilitating effects: (1) direct, by not browsing certain plant and (2) indirect, by creating
21
heterogeneity. First, ungulates consume more palatable species that are more likely to suffer damage because of their characteristics (Gill, 1992b). Plant species responses to browsing are inevitably species-dependent (Kirby, 2001). The selection of a plant depends mainly on its species but also on the season, i.e. the availability of resources and the phenology (Gill, 1992b). Secondly, non-palatable species are then favored (Beguin, Pothier, & Côté, 2010; Díaz et al., 2007; Gill & Beardall, 2001). Because of their selectivity, ungulates create a heterogeneity that allows other species to establish. Ungulates then indirectly facilitate the establishment and presence of these no appetent open species. In this study, the abundance of open species is increasing. These plant species may be consumed less by ungulates because they have a low palatability.
3- Conclusion
We showed that setting aside forest reserves increased forest cover and stand closure but, in our context, had no effect on ungulate populations, thus herbivory pressure. At the alpha scale, stand structure and ungulate populations have antagonistic effects. Increased forest cover decreased herbaceous diversity of understory vegetation while increased herbivory pressure increased herbaceous diversity. At the gamma scale, increased heterogeneity in stand structure had a positive effect on open habitat species. In addition, ungulate populations increased the total diversity of herbaceous diversity of understory vegetation. As hypothesized (iv), effects on herbaceous understory are dependent on ungulate density. The effects of forest management are therefore greater than the effects of herbivory pressure. Indeed, the effects of the coefficients of variation of stand structure variables are more often significant and the magnitudes of the effects are stronger. For example, the magnitude of the effect of ungulate density is almost a hundred times smaller than the magnitude of the effect of the canopy variation coefficient.
In various studies, herbivory pressure by ungulate populations and forest management are both considered disturbances (Côté et al., 2004; Franklin et al., 2002; Kirby et al., 1991). Many studies have measured the effects of ungulate populations, especially in situations of overabundance, to come out with recommendations for the management of ungulate populations. On the other hand, the interactions between herbivory pressure and forest management are poorly studied. Our results show that it is important to evaluate the interactions and their intensity between these two disturbances because the effects can be antagonistic. At
22
intermediate ungulate population levels, ungulates can have positive effects on understory vegetation herbaceous species diversity that make up much of the forest diversity, and is recognised as an important driver of the forest ecosystem (Gilliam, 2007). Indeed, certain herbaceous species seem to have a facilitating role in the growth and establishment of woody species of interest (Gómez, Zamora, Gómez, Hódar, & Castro, 2004). In addition, biodiversity conservation is one of the objectives of sustainable forest management (Lindenmayer, Margules, & Botkin, 2000). In order to achieve this objective, it is necessary to understand how disturbances, and their interactions, affect forest biodiversity (Bengtsson, 2000).
A
Bibliography
Apollonio, M., Belkin, V. V., Borkowski, J., Borodin, O. I., Borowik, T., Cagnacci, F., … Yanuta, G. (2017). Challenges and science-based implications for modern management and conservation of European ungulate populations. Mammal Research, 62(3), 209–217. https://doi.org/10.1007/s13364-017-0321-5
Arivin Rivaie, A. (2014). The effects of understory vegetation on P availability in Pinus radiata forest stands: A review. Journal of Forestry Research, 25(3), 489–500. https://doi.org/10.1007/s11676-014-0488-4
Augustine, D. J., & Mcnaughton, S. J. (1998). Ungulate effects on the functional composition of plant
communities: herbivore selectivity and plant tolerance. The Journal of Wildlife Management, 62(4), 1165– 1183. https://doi.org/10.2307/3801981
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R. H. B., Singmann, H., … Grothendieck, G. (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67, 1–48. https://doi.org/10.18637/jss.v067.i01
Bauhus, J., Puettmann, K., & Messier, C. (2009). Silviculture for old-growth attributes. Forest Ecology and
Management, 258(4), 525–537. https://doi.org/10.1016/j.foreco.2009.01.053
Beguin, J., Pothier, D., & Côté, S. D. (2010). Deer browsing and soil disturbance induce cascading effects on plant communities: a multilevel path analysis. Ecological Applications, 21(2), 439–451.
https://doi.org/10.1890/09-2100.1
Bengtsson. (2000). Biodiversity, disturbances, ecosystem function and management of European forests. Forest Ecology and Management 132, 39–50.
Bonnot, N., Morellet, N., Verheyden, H., Cargnelutti, B., Lourtet, B., Klein, F., & Hewison, A. J. M. (2013). Habitat use under predation risk: Hunting, roads and human dwellings influence the spatial behaviour of roe deer. European Journal of Wildlife Research, 59(2), 185–193. https://doi.org/10.1007/s10344-012-0665-8
Borer, E. T., Seabloom, E. W., Gruner, D. S., Harpole, W. S., Hillebrand, H., Lind, E. M., … Yang, L. H. (2014). Herbivores and nutrients control grassland plant diversity via light limitation. Nature, 508(7497), 517–520. https://doi.org/10.1038/nature13144
Boulanger, V., Dupouey, J.-L., Archaux, F., Badeau, V., Baltzinger, C., Chevalier, R., … Ulrich, E. (2018). Ungulates increase forest plant species richness to the benefit of non-forest specialists. Global Change
Biology, 24(2), e485–e495. https://doi.org/10.1111/gcb.13899
Brunet, J., Fritz, Ö., & Richnau, G. (2010). Biodiversity in European beech forests - a review with recommendations for sustainable forest management, (53), 77–84. Retrieved from
https://www.jstor.org/stable/41442021
B
attributes of moist temperate old-growth forests: A global review. Forest Ecology and Management, 291, 458–479. https://doi.org/10.1016/j.foreco.2012.11.020
Burrascano, S., Lombardi, F., & Marchetti, M. (2008). Old-growth forest structure and deadwood: Are they indicators of plant species composition? A case study from central Italy. Plant Biosystems - An
International Journal Dealing with All Aspects of Plant Biology, 142(2), 313–323.
https://doi.org/10.1080/11263500802150613
Burrascano, S., Rosati, L., & Blasi, C. (2009). Plant species diversity in Mediterranean old-growth forests: A case study from central Italy. Plant Biosystems - An International Journal Dealing with All Aspects of
Plant Biology, 143(1), 190–200. https://doi.org/10.1080/11263500802709699
Chaudhary, A., Burivalova, Z., Koh, L. P., & Hellweg, S. (2016). Impact of Forest Management on Species Richness: Global Meta-Analysis and Economic Trade-Offs. Scientific Reports, 6, 1–10.
https://doi.org/10.1038/srep23954
Côté, S. D., Rooney, T. P., Tremblay, J.-P., Dussault, C., & Waller, D. M. (2004). Ecological Impacts of Deer Overabundance. Annual Review of Ecology, Evolution, and Systematics, 35(1), 113–147.
https://doi.org/10.1146/annurev.ecolsys.35.021103.105725
Decocq, G., Aubert, M., Dupont, F., Alard, D., Saguez, R., Wattez-Franger, A., … Bardat, J. (2004). Plant diversity in a managed temperate deciduous forest: understorey response to two silvicultural systems.
Journal of Applied Ecology, 41(6), 1065–1079. https://doi.org/10.1111/j.0021-8901.2004.00960.x
Díaz, S., Lavorel, S., de Bello, F., Quétier, F., Grigulis, K., & Robson, T. M. (2007). Incorporating plant functional diversity effects in ecosystem service assessments. Proceedings of the National Academy of
Sciences of the United States of America, 104(52), 20684–20689. https://doi.org/10.1073/pnas.0704716104
Dieler, J., Uhl, E., Biber, P., Müller, J., Rötzer, T., & Pretzsch, H. (2017). Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zone of Europe. European
Journal of Forest Research, 136(4), 739–766. https://doi.org/10.1007/s10342-017-1056-1
Faeth, S. H., Marussich, W. A., Shochat, E., & Warren, P. S. (2005). Trophic Dynamics in Urban Communities.
BioScience, 55(5), 399. https://doi.org/10.1641/0006-3568(2005)055[0399:TDIUC]2.0.CO;2
Faison, E. K., DeStefano, S., Foster, D. R., Motzkin, G., & Rapp, J. M. (2016). Ungulate browsers promote herbaceous layer diversity in logged temperate forests. Ecology and Evolution, 6(13), 4591–4602. https://doi.org/10.1002/ece3.2223
Franklin, J. F., Spies, T. A., Pelt, R. V., Carey, A. B., Thornburgh, D. A., Berg, D. R., … Chen, J. ; (2002). Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. Forest Ecology and Management, 155, 399–423.
https://doi.org/10.1016/S0378-1127(01)00575-8
Frey, S. J. K., Hadley, A. S., Johnson, S. L., Schulze, M., Jones, J. A., & Betts, M. G. (2016). Spatial models reveal the microclimatic buffering capacity of old-growth forests. Science Advances, 2(4), e1501392–
C
e1501392. https://doi.org/10.1126/sciadv.1501392
Fuller, R. J., & Gill, R. M. A. (2001). Ecological impacts of increasing numbers of deer in British woodland.
Forestry, 74(3), 193–199. https://doi.org/10.1093/forestry/74.3.193
Gill, R. M. A. (1992a). A review of damage by mammals in north temperate forest: 3. Impact on Trees and Forests. Forestry, 65(4), 363–388.
Gill, R. M. A. (1992b). A Review of Damage by Mammals in North Temperate Forests: 1. Deer. Forestry, 65(2), 145–169. https://doi.org/10.1093/forestry/65.2.145
Gill, R. M. A., & Beardall, V. (2001). The impact of deer on woodlands: The effects of browsing and seed dispersal on vegetation structure and composition. Forestry, 74(3), 209–218.
https://doi.org/10.1093/forestry/74.3.209
Gilliam, F. S. (2007). The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems.
BioScience, 57(10), 845–858. https://doi.org/10.1641/B571007
Gómez, L., Zamora, R., Gómez, J. M., Hódar, J. a, & Castro, J. (2004). Applying Plan Facilitation To Forest Restoration In Mediterranean Ecosystems: A Meta-Analysis Of The Shrubs As Nurse Plants. Ecological
Applications, 14(4), 1118–1138. https://doi.org/10.1890/03-5084
Hartley, M. J. (2002). Rationale and methods for conserving biodiversity in plantation forests. Forest Ecology
and Management, 155(1–3), 81–95. https://doi.org/10.1016/S0378-1127(01)00549-7
Horvat, V., Biurrun, I., & García-Mijangos, I. (2017). Herb layer in silver fir – beech forests in the western Pyrenees: Does management affect species diversity? Forest Ecology and Management, 385, 87–96. https://doi.org/10.1016/j.foreco.2016.11.037
Jon, A., Byrnes, J., & Grace, J. (2018). Package ‘ piecewiseSEM .’
Kaufmann, S., Hauck, M., & Leuschner, C. (2017). Comparing the plant diversity of paired beech primeval and production forests: Management reduces cryptogam, but not vascular plant species richness. Forest
Ecology and Management, 400, 58–67. https://doi.org/10.1016/j.foreco.2017.05.043
Kirby, K. J. (2001). The impact of deer on the ground flora of British broadleaved woodland. Forestry, 74(3), 219–229. https://doi.org/10.1093/forestry/74.3.219
Kirby, K. J., Webster, S. D., & Antczak, A. (1991). Effects of forest management on stand structure and the quantity of fallen dead wood: some British and Polish examples. Forest Ecology and Management, 43(1– 2), 167–174. https://doi.org/10.1016/0378-1127(91)90083-8
Kovács, B., Tinya, F., Guba, E., Németh, C., Sass, V., Bidló, A., & Ódor, P. (2018). The Short-Term Effects of Experimental Forestry Treatments on Site Conditions in an Oak–Hornbeam Forest. Forests, 9(7), 406. https://doi.org/10.3390/f9070406
Lefcheck, J. S. (2016). piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods in Ecology and Evolution, 7(5), 573–579. https://doi.org/10.1111/2041-210X.12512
D
Lindenmayer, D. B., Margules, C. R., & Botkin, D. B. (2000). Society for Conservation Biology Indicators of Biodiversity for Ecologically Sustainable Forest Management. Source: Conservation Biology, 14(4), 941– 950.
Marcon, E. (2016). Mesures de la diversité. https://doi.org/https://hal.archives-ouvertes.fr/cel-01205813v2/document
Meier, M., Stöhr, D., Walde, J., & Tasser, E. (2017). Influence of ungulates on the vegetation composition and diversity of mixed deciduous and coniferous mountain forest in Austria. European Journal of Wildlife
Research, 63(1), 1–10. https://doi.org/10.1007/s10344-017-1087-4
Meisingset, E. L., Loe, L. E., Brekkum, Ø., Bischof, R., Rivrud, I. M., Lande, U. S., … Mysterud, A. (2018). Spatial mismatch between management units and movement ecology of a partially migratory ungulate.
Journal of Applied Ecology, 55(2), 745–753. https://doi.org/10.1111/1365-2664.13003
Montgomery, R. A., & Chazdon, R. L. (2016). Forest Structure , Canopy Architecture , and Light Transmittance in Tropical Wet Forests Authors ( s ): Rebecca A . Montgomery and Robin L . Chazdon Published by : Wiley Stable URL : http://www.jstor.org/stable/2679955 Accessed : 24-03-2016 22 : 32 UTC Yo, 82(10), 2707–2718.
Murray, B. D., Webster, C. R., & Bump, J. K. (2013). Broadening the ecological context of ungulate-ecosystem interactions: The importance of space, seasonality, and nitrogen. Ecology, 94(6), 1317–1326.
https://doi.org/10.1890/12-1582.1
Ohse, B., Seele, C., Holzwarth, F., & Wirth, C. (2017). Different facets of tree sapling diversity influence browsing intensity by deer dependent on spatial scale. Ecology and Evolution, 7(17), 6779–6789. https://doi.org/10.1002/ece3.3217
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., Mcglinn, D., … Oksanen, M. J. (2018). Package “vegan.” Retrieved from
https://github.com/vegandevs/vegan/issues%0Ahttps://github.com/vegandevs/vegan/issues%0Ahttps://gith ub.com/vegandevs/vegan/issues
Paillet, Y., Bergès, L., Hjältén, J., Ódor, P., Avon, C., Bernhardt-Römermann, M., … Virtanen, R. (2010). Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conservation Biology, 24(1), 101–112. https://doi.org/10.1111/j.1523-1739.2009.01399.x
Paillet, Y., Pernot, C., Boulanger, V., Debaive, N., Fuhr, M., Gilg, O., & Gosselin, F. (2015). Quantifying the recovery of old-growth attributes in forest reserves: A first reference for France. Forest Ecology and
Management, 346, 51–64. https://doi.org/10.1016/j.foreco.2015.02.037
Parviainen, J., Bücking, W., Vandekerkhove, K., Schuck, A., & Päivinen, R. (2000). Strict forest reserves in Europe: efforts to enhance biodiversity and research on forests left for free development in Europe (EU-COST-Action E4). Forestry, 73(2), 107–118. https://doi.org/10.1093/forestry/73.2.107
E
Its Expression at Various Hierarchical Levels. Oikos, 54(2), 129. https://doi.org/10.2307/3565258
Poirier, V., Coyea, M. R., Angers, D. A., & Munson, A. D. (2016). Silvicultural treatments and subsequent vegetation impact long-term mineral soil biogeochemistry in mixedwood plantations. Forest Ecology and
Management, 368, 140–150. https://doi.org/10.1016/j.foreco.2016.03.016
Reimoser, F. (2003). Steering the impacts of ungulates on temperate forests. Journal for Nature Conservation,
10(4), 243–252. https://doi.org/10.1078/1617-1381-00024
Reimoser, F., & Gossow, H. (1996). Impact of ungulates on forest vegetation and its dependence on the silvicultural system. Forest Ecology and Management, 88(1–2), 107–119. https://doi.org/10.1016/S0378-1127(96)03816-9
Reineke, L. H. (1933). Perfecting a stand-density index for even-aged forests. J. Agric. Res., 46(7), 627–638. https://doi.org/http://cmapspublic3.ihmc.us/rid=1N4TSLMJK-JNTN9D-14QK/Perfecting%20a%20stand-density%20index%20for%20even-aged%20forests.PDF
Roberts, M. R. (2002). Effects of forest plantation management on herbaceous-layer composition and diversity.
Canadian Journal of Botany, 80(4), 378–389. https://doi.org/10.1139/b02-023
Roberts, M. R., & Gilliam, F. S. (1995). Patterns and Mechanisms of Plant Diversity in Forested Ecosystems: Implications for Forest Management. Ecological Applications, 5(4), 969–977.
https://doi.org/10.2307/2269348
Roxburgh, S. H., Shea, K., & Wilson, J. B. (2004). THE INTERMEDIATE DISTURBANCE HYPOTHESIS: PATCH DYNAMICS AND MECHANISMS OF SPECIES COEXISTENCE. Ecology, 85(2), 359–371. https://doi.org/10.1890/03-0266
Royo, A. A., Collins, R., Adams, M. B., Kirschbaum, C., & Carson, W. P. (2010). Pervasive interactions between ungulate browsers and disturbance regimes promote temperate forest herbaceous diversity.
Ecology, 91(1), 93–105. https://doi.org/10.1890/08-1680.1
Schall, P., Gossner, M. M., Heinrichs, S., Fischer, M., Boch, S., Prati, D., … Ammer, C. (2018). The impact of even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. Journal of Applied Ecology, 55(1), 267–278. https://doi.org/10.1111/1365-2664.12950
Schmidt, W. (2005). Herb layer species as indicators of biodiversity of managed and unmanaged beech forests.
Diversity, 125(1/2), 111–125.
Schulze, E. D., Bouriaud, O. B., Wäldchen, J., Eisenhauer, N., Walentowski, H., Seele, C., … Teodosiu, M. (2014). Ungulate browsing causes species loss in deciduous forests independent of community dynamics and silvicultural management in Central and Southeastern Europe. Annals of Forest Research, 57(2), 1. https://doi.org/10.15287/afr.2014.273
Siitonen, J. (2001). Forest Management , Coarse Woody Debris and Saproxylic Organisms : Fennoscandian Boreal Forests as an Example Author ( s ): Juha Siitonen Source : Ecological Bulletins , No . 49 , Ecology of Woody Debris in Boreal Forests ( 2001 ),. Ecological Bulletins, (49), 11–41. Retrieved from
F
http://www.jstor.org/stable/20113262
Sitzia, T., Trentanovi, G., Dainese, M., Gobbo, G., Lingua, E., & Sommacal, M. (2012). Stand structure and plant species diversity in managed and abandoned silver fir mature woodlands. Forest Ecology and
Management, 270, 232–238. https://doi.org/10.1016/j.foreco.2012.01.032
Stankowich, T. (2008). Ungulate flight responses to human disturbance: A review and meta-analysis. Biological
Conservation, 141(9), 2159–2173. https://doi.org/10.1016/j.biocon.2008.06.026
Swanson, M. E., Franklin, J. F., Beschta, R. L., Crisafulli, C. M., DellaSala, D. a, Hutto, R. L., … Swanson, F. J. (2011). The forgotten stage of forest succession: Early- successional ecosystems on forest sites. Frontiers
in Ecology and the Environment, 9(2), 117–125. https://doi.org/10.1890/090157
Takada, M., Asada, M., & Miyashita, T. (2001). Regional differences in the morphology of a shrub
Damnacanthus indicus: An induced resistance to deer herbivory? Ecological Research, 16(4), 809–813. https://doi.org/10.1046/j.1440-1703.2001.00436.x
Tanentzap, A. J., Burrows, L. E., Lee, W. G., Nugent, G., Maxwell, J. M., & Coomes, D. A. (2009). Landscape-level vegetation recovery from herbivory: Progress after four decades of invasive red deer control. Journal
of Applied Ecology, 46(5), 1064–1072. https://doi.org/10.1111/j.1365-2664.2009.01683.x
Vockenhuber, E. A., Scherber, C., Langenbruch, C., Meißner, M., Seidel, D., & Tscharntke, T. (2011). Tree diversity and environmental context predict herb species richness and cover in Germany’s largest connected deciduous forest. Perspectives in Plant Ecology, Evolution and Systematics, 13(2), 111–119. https://doi.org/10.1016/j.ppees.2011.02.004
Von Oheimb, G., & Härdtle, W. (2009). Selection harvest in temperate deciduous forests: Impact on herb layer richness and composition. Biodiversity and Conservation, 18(2), 271–287. https://doi.org/10.1007/s10531-008-9475-4
Wesely, N., Fraver, S., Kenefic, L. S., Weiskittel, A. R., Ruel, J., Thompson, M. E., & White, A. S. (2018). Structural Attributes of Old-Growth and Partially Harvested Northern White-Cedar Stands in Northeastern North America. https://doi.org/10.3390/f9070376
Wilkinson, D. M. (1999). The Disturbing History of Intermediate Disturbance. Oikos, 84(1), 145. https://doi.org/10.2307/3546874
Abstract
Ungulate herbivory pressure and forest management are both considered forest ecosystems disturbances. Many studies analysed the effects of ungulate populations, particularly in situations of overabundance, however the joint effects of herbivory and forest management abandonment on herbaceous understory vegetation have rarely been studied in a causal framework. In order to identify the joint effects of forest management and herbivory pressure in managed and unmanaged forests, we worked on 212 plots in 15 massifs throughout France. We used 21 Structural Equation Models to assess effects at local scale (plot scale) and at a larger scale (forest management scale). We showed that, at the alpha scale, stand structure and ungulate populations have antagonistic effects. Increased forest cover decreases herbaceous diversity of understory vegetation while increased herbivore pressure increases herbaceous diversity. At the gamma scale, the increased heterogeneity of stand structure had a positive effect on open habitat species. In addition, ungulate populations increased the total diversity of herbaceous diversity of understory vegetation. At intermediate levels of ungulate populations, herbivory pressure can have positive effects on the diversity of herbaceous species of understory vegetation which constitutes a large part of forest diversity and is recognized as an important driver of forest ecosystem biodiversity.
Résumé
La pression d’herbivorie par les ongulés et la gestion forestière sont considérées comme des perturbations des écosystèmes forestiers. De nombreuses études ont analysé les effets des populations d'ongulés, en particulier dans les situations de surabondance, mais leurs effets conjoints sur la végétation herbacée de sous-bois ont rarement été étudiés dans un cadre causal. Afin d'identifier leurs effets dans les forêts gérées et non gérées, nous avons travaillé sur 212 placettes réparties dans 15 massifs français. Nous avons utilisé 21 modèles d'équations structurelles pour évaluer les effets à l'échelle de la placette et à l'échelle de la gestion forestière. Nous avons montré qu'à l'échelle alpha, la structure du peuplement et les populations d'ongulés ont des effets antagonistes. L'augmentation du couvert forestier diminue la diversité herbacée tandis que l'augmentation de la pression d’herbivorie augmente la diversité. A l'échelle gamma, l'augmentation de l’hétérogénéité de la structure de peuplement a eu un effet positif sur les espèces d’habitat ouvert. En outre, les ongulés ont augmenté la diversité totale des herbacées. A des niveaux intermédiaires de populations d'ongulés, la pression d’herbivorie peut avoir des effets positifs sur la diversité des herbacées qui constitue une grande partie de la diversité forestière et est reconnue comme un important moteur de la biodiversité des écosystèmes forestiers.