Exploration of Aldol Reactions Catalyzed by Stereoselective
Pyruvate Aldolases with 2-Oxobutyric Acid as Nucleophile
V. Laurent,
aA. Uzel,
aV. Hélaine,
aL. Nauton,
aM. Traïkia,
aT. Gefflaut,
aM. Salanoubat,
bV. de Berardinis,
bM. Lemaire,
a,* and C. Guérard-Hélaine
a,*
aUniversité Clermont Auvergne, CNRS, SIGMA Clermont, Institut de Chimie de Clermont-Ferrand, 63000 Clermont-Ferrand, France
E-mail: marielle.lemaire@uca.fr; christine.helaine@uca.fr b
Génomique Métabolique, Génoscope, Institut François Jacob, CEA, CNRS, Univ. Evry, Université Paris-Saclay, 91057 Evry, France
Manuscript received: January 28, 2019; Revised manuscript received: March 25, 2019; Version of record online: May 9, 2019
Supporting information for this article is available on the WWW under https://doi.org/10.1002/adsc.201900128
Abstract: Pyruvate aldolases from Pfam family PF03328, recently described to be able to use hydroxypyruvic acid as nucleophile substrate, were shown to also catalyse aldol adducts formation from 2-oxobutyric acid. A1H NMR-based assay was used to screen 21 aldolases for their activity towards 2-oxobutyric acid and to predict their stereoselectivity at position 3 of the aldol adducts. The best biocatalysts were then proved to be strictly (3S)-selective. Contrary to known enzymes from this family that are seldom stereoselective at position 4, three aldolases were highlighted as allowing a selective access to (3S,4R) or (3S,4S) aldols. Seven 3-deoxy-3-methylulosonic acids never described before were finally prepared and isolated with good yields and stereoselectivities.
Keywords: aldolase; aldolisation; ketoacids; oxobu-tyric acid; biocatalysis
Aldolisation reaction is a staple of C C bond creation in organic synthesis, enabling the preparation of complex molecules and generating chiral centres with controlled stereochemistry. Among the plethora of existing aldolisation methods,[1] processes involving aldolases offer the benefit of working in eco-friendly conditions (water as solvent, neutral conditions, room temperature), and avoiding protection and deprotection steps as well. In addition, the usefulness of these biocatalysts mainly resides in their capabilities to control the configuration of the newly created chiral centre(s). Nevertheless, the use of aldolases was mostly hampered by their high specificity towards their natural nucleophile substrate, thus limiting their
application scope. In an effort to push forward these boundaries, new natural or modified aldolases have appeared in recent years, displaying a broader nucleo-phile tolerance. An exceptional example is wild-type fructose-6-phosphate aldolase (FSA) and in particular, one of its variant, that was able to accept various non-hydroxylated nucleophiles such as acetone, ethanal, butanone and even cyclopentanone in addition to the initially identified substrates dihydroxyacetone, hy-droxyacetone, 1-hydroxybutan-2-one, and glycolaldehyde.[2–5] Furthermore, other aldolases from the pyruvate aldolase family were reported in the literature as being able to use fluoropyruvate, hydrox-ypyruvate or 2-oxobutyrate (ketobutanoate, KB) as nucleophiles, in addition to pyruvate.[6,7] It is note-worthy that pyruvate aldolases allow access to the α-keto acid moiety, which is found in various bio-logically active compounds notably involved in phys-iological functions (cellular recognition and communi-cation), displaying antimicrobial properties, or useful for the development of vaccine candidates.[8,9] While only one chiral centre is generated when pyruvate is the nucleophile substrate, two new ones (C3 and C4) result from the use of fluoropyruvate, hydroxypyruvate and KB (Scheme 1).
Scheme 1. Aldolase catalysed aldol addition of pyruvate and derivatives.
Some synthetic examples involving fluoropyruvate were reported in the literature. They mainly concerned sialic acid aldolases,[10,11] the most studied pyruvate aldolases to date, and trans-o-hydroxybenzylidene pyruvate aldolases.[12] In the case of KB, pyruvate aldolase activities were mainly reported on analytic scale. Surprisingly, no product characterizations, puri-fications nor yields were mentioned, although these data are crucial to unambiguously establish product identity stereochemical outcome, and finally assess the real efficiency of an enzyme regarding yield, reaction time and biocatalyst quantity.[13–17] To our knowledge, only one preparative scale study was reported on an engineered 2-keto-3-deoxy-l-rhamnonate aldolase (YfaU W23V/L216A) fused with a maltose binding protein. It was used among others to catalyse the reaction between KB and various aldehydes.[18]A good stereoselectivity (S configuration) was observed at position 3 (C3) but with some disappointing results at C4 depending on the electrophile used and mostly in favour of (S) configuration. Therefore, there is a real need to explore the potential of pyruvate aldolases in organic synthesis, particularly the stereocontrol possi-bilities at position 4.
Recently, we have reported the discovery of 22 pyruvate aldolases from biodiversity belonging to the same Pfam family (PF03328) that are able to use hydroxypyruvate as nucleophile, with high efficiency (Scheme 1, R1= OH).[7] Most of these enzymes were annotated as HpcH (also known as HpaI). These 22 pyruvate aldolases are of class II. Indeed, the nucleophile substrate is activated in the active site as a cation-stabilised enolate. Class-II pyruvate aldolases received little attention because they were generally considered as poorly stereoselective enzymes.[15] How-ever, since some enzymes of our set were found stereoselective and versatile towards the nucleophile, we decided to further investigate the substrate promis-cuity and stereoselectivity of this 22 pyruvate aldolases in order to highlight the most useful catalysts. KB was chosen as an interesting nucleophile substrate for several reasons: i) as indicated above, little was described concerning the use of this pyruvate analogue as nucleophile, ii) two chiral centres result from the aldolisation reaction thus affording an exciting chal-lenge of stereoselectivity control, iii) a substantial activity with KB would demonstrate that these enzymes would be able to accommodate both a hydrophobic (KB) and hydrophilic (hydroxypyruvate) group substituting pyruvic acid, thus increasing their application scope, iv) the obtained chemical moiety (3-methyl-2-keto acid) is a valuable building block for drugs syntheses.[18]
Firstly, a1H NMR-based screening experiment was carried out, to evidence the pyruvate aldolases effi-ciency and selectivity for KB enolate formation.[15]The most promising detected enzymes were then used in
synthetic experiments, using d-glyceraldehyde as elec-trophile in order to determine yields and stereoselectiv-ities. Finally, the most efficient biocatalysts were further engaged in reactions with other aldehydes to give a first overview of their substrate specificities and application scope.
A series of 21 pyruvate aldolases from the above cited 22 enzymes set was tested[19] for the ability to catalyse the formation of an enolate from KB. One enzyme of the set (Uniprot Id. B7NJZ1) was discarded due to its redundancy with Garl (Uniprot Id. P23522) (99% of identity). Considering that the enolate can be formed within the active site by either proR or proS proton abstraction at position 3 of KB, isotope exchange in that position was monitored by 1H NMR. Hence, simple spectrum recording afforded useful informations: i) KB was considered as a substrate if the signal integration of hydrogens at position 3 was decreased over time, ii) the enzyme was expected to be stereoselective if only one proton at position 3 was exchanged, and iii) high activity and stereoselectivity were expected if only one proton was exchanged after both a short and a long reaction time. Isotope exchange experiments were carried out without buffer, using 1 mg/mL pyruvate aldolase, 20 mM KB in D2O, pH 7.5 and 1H NMR spectra were recorded after 1 h and 24 h of incubation. Results are summarized in Figure 1. KB was found to be a substrate for all the 21 studied enzymes. For some of them, (e. g. Uniprot Id.
A0A081HJP9, Q0K203, B2TGJO, Q16DB5,
A9BQY3, A3K1H1, Q2K7V6, Q9RYM1, P76469 and B1IS70), proR and ProS protons were exchanged, predicting a poor stereoselectivity. It is worth noting that, in our previous studies, when hydroxypyruvate was used as nucleophile with these enzymes, the corresponding aldol adducts, when formed, were also isolated as a mixture of epimers at C3.[7] More interestingly, half of the tested pyruvate aldolases (Uniprot Id. B4XH86, A5VH82, A9CJL2, A5VAX1, A9EOU5, A3SLS0, B2T1L6, Q1QUJ4, A7HU24, I7DKY0 and P23522) were able to catalyse the exchange of only one of the two enantiotopic protons. Although annotated as HpcH, these 11 enzymes displayed a higher selectivity compared to HpcH from E. coli (B1IS70) (Figure 1).
To confirm the promising results of the NMR screening, and to determine the most selective enzymes, preparative scale reactions were carried out in the presence of an electrophile, in order to form, purify and characterize the aldol adduct. Six enzymes were chosen for that purpose, each leading to 50% isotope exchange after one hour (Figure 1), without further proton abstraction after 24 h (Uniprot Id. B4XH86, A5VH82, A9CJl2, A5VAX1, A9E0U5 and I7DKY0). To allow configuration assignment of the newly created stereocenters, optically pure d-glycer-aldehyde was selected as the electrophile thus
provid-ing a stereochemical reference point in the aldol adducts, which were cyclised in water. To ensure a kinetic control, aldolisation reactions were monitored by KB titration (spectrophotometric assays, see SI) and stopped at around 80% conversion, after a 2 h reaction time. Products were purified by ion exchange chroma-tography and were analysed by 1H, 13C and NOESY NMR experiments. To note, during the purification process, all the products adsorbed on the exchange anionic resin were eluted in one unique fraction and isolated as mixture of stereoisomers to enable a complete overview of the stereoselectivity of each enzyme. The results are summarized in Table 1.
A thorough analysis of the NMR spectra of the products obtained with the six different aldolases indicated that the same two aldol diastereoisomers (5 and 6), existing in solution as furanose and pyranose
forms, were formed in all cases (Figure 2 and Fig-ure 2S). Considering that the 6 aldolases were chosen based on their ability to catalyse a stereoselective isotopic exchange at position 3 of KB, it was concluded that all the enzymes gave the same configuration at position 3 (aldol adduct has three asymmetric centres, carbon three being fixed according to the deuterium exchange assay) along with carbon 5 (determined by d-glyceraldehyde). Consequently, each enzyme gave two diastereoisomers, different on the configuration of carbon 4. The absolute configurations of 5 and 6 could be assigned by analysing NOE and coupling constants for the different forms of both isomers. Notably, in the case of the major isomer (> Figure 1. Isotopic exchange at position 3 of KB in D2O catalyzed by the various pyruvate aldolases: 50% corresponds to one proton removal, 100% to two protons removal. All experiments were run with 11 μmol KB (22 mM final concentration), 13 μL MgCl2 (stock solution 50 mM) and 0.5 mg of aldolase in D2O (500 μL) at pH 7.5.1H NMR spectra were recorded at 400 MHz.
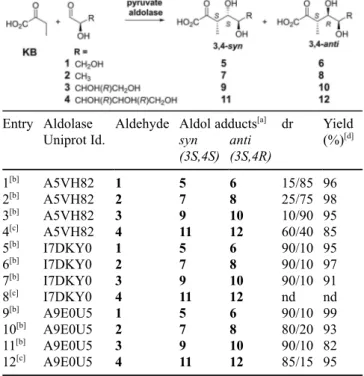
Table 1. Enzymatic aldolisations with 2-oxobutyric acid (KB) and d-glyceraldehyde.[a]
aldolase Uniprot Id. Conversion[a] (%) B4XH86 80 65 35 A5VH82 84 < 10 > 90 A9CJL2 88 55 45 A5VAX1 75 70 30 A9E0U5 78 90 10 I7DKY0 74 > 90 < 10
[a]Reaction conditions KB (20 mM, 0.25 mmol),
d-glyceralde-hyde (80 mM), MgCl2 (2 mM), pyruvate aldolase (5 mg),
H2O, pH 7.5, room temperature, 2 h. Conversions were
determined by KB titration (see SI).
Figure 2. Assignment of the absolute configuration of the asymmetric centres with NOESY experiments, based on the known C5 configuration provided by d-glyceraldehyde nucleo-phile substrate.
90%) obtained with A5VH82, the significant NOE observed between H3, H4 and H5 in the furanose forms along with the coupling constants measured for the pyranose form allowed to assign unambiguously the (3S,4R) configuration to aldol 6. So finally, the six aldolases displayed variable selectivity at position 4 and led to the same 3S configuration with high selectivity. Thus, only one enzyme from Sphingomonas wittichii (A5VH82) led to a large proportion (> 90%) of (4R)-aldol adduct. On the other hand, (4S) config-uration was predominantly obtained with two enzymes A9E0U5 from Sulfitobacter indolifex, and I7DKY0 from Phaeobacter inhibens. These three most selective enzymes were used again to catalyse the aldol reaction between KB and d-glyceraldehyde but this time, the reaction was stopped after complete conversion of KB. Aldols were obtained in excellent yields (> 95%, Table 2, entries 1, 5 and 9) without any significant decrease in the diastereomeric ratio. To note, similar results were obtained in our previous studies when
these enzymes were used with HPA and
glyceraldehyde.[7]
To further study the substrate tolerance of these three enzymes, three others aldehydes were evaluated as electrophile: lactaldehyde, erythrose and d-ribose. Aldolisation reactions were run with 20 mM KB and 80 mM aldehyde and monitored by KB titration. d-ribose was the sole substrate leading to a low conversion even after a long reaction time. Indeed, less than 50% conversion was obtained after 72 h, whereas a complete conversion of KB was observed after 24 h with the two other electrophiles. In order to optimize the reaction conditions with ribose as electro-phile, KB and ribose concentrations were increased using the two pyruvate aldolases showing the best activity with ribose (A5VH82 and A9E0U5). However, substrate inhibition was observed for KB concentra-tions over 20 mM. Finally, the best-tested condiconcentra-tions turned out to be 100 mM ribose along with 10 mM KB. In these conditions, conversions over 80% were reached after 24 h. The reactions were performed on 50 mg scale and stopped just after total conversion of KB, to avoid equilibration that could increase the proportion of the minor epimer. As summarized in Table 2, all the electrophiles led to the expected products in good yields. NMR coupling constants determination on pyranose forms, along with NOESY experiments, enabled to systematically determine the configuration of C4.
By this way, (3S) configuration was also confirmed for each product, in accordance with the previous results from isotope exchange and reactions with d-glyceraldehyde. When lactaldehyde and erythrose were used as electrophiles, one aldolase (A5VH82) afforded major products of (3S, 4R) configuration as it was already observed with d-glyceraldehyde (entries 1–3). The same observation was done for the two other enzymes which gave mostly the (4S) epimers. Even though ribose appeared as a poor substrate, good yields could be obtained with two enzymes (A5VH82 and A9E0U5, 85 and 95% respectively, entries 4 and 12) whereas I7DKY0 did not give significant conversion (entry 8) under the optimized conditions. Surprisingly, the stereoselectivity of A5VH82 (entry 4) was largely decreased and reversed, when compared to the other electrophiles (entries 1–3).
In this study, KB was shown to be a good nucleophile substrate for a set of 21 pyruvate aldolases (representative of PF03328 diversity) previously shown to use hydroxypyruvate as nucleophile. An NMR-based method already used for predicting the activity of pyruvate aldolases with pyruvate analogues,[15]was shown to constitute a smart approach to predict the stereoselectivity of aldolases. Three pyruvate aldolases were highlighted with complemen-tary stereoselectivities. The efficiency of these bio-catalysts was demonstrated in aldolisation reactions Table 2. Enzymatic aldolisations with KB and various
alde-hydes.
Entry Aldolase Uniprot Id.
Aldehyde Aldol adducts[a] dr Yield (%)[d] syn (3S,4S) anti (3S,4R) 1[b] A5VH82 1 5 6 15/85 96 2[b] A5VH82 2 7 8 25/75 98 3[b] A5VH82 3 9 10 10/90 95 4[c] A5VH82 4 11 12 60/40 85 5[b] I7DKY0 1 5 6 90/10 95 6[b] I7DKY0 2 7 8 90/10 97 7[b] I7DKY0 3 9 10 90/10 91 8[c] I7DKY0 4 11 12 nd nd 9[b] A9E0U5 1 5 6 90/10 99 10[b] A9E0U5 2 7 8 80/20 93 11[b] A9E0U5 3 9 10 90/10 82 12[c] A9E0U5 4 11 12 85/15 95
[a]Products are drawn in linear form for better understanding
but they were observed as cyclic forms in water.
[b]Reaction conditions KB (20 mM, 0.3 mmol, 35 mg),
alde-hyde (80 mM), MgCl2 (2 mM), pyruvate aldolase (5 mg),
H2O, pH 7.5.
[c]Reaction conditions KB (10 mM, 0.15 mmol, 17.5 mg),
d-ribose (100 mM), MgCl2(2 mM), pyruvate aldolase (10 mg),
H2O, pH 7.5.
[d]Yields were calculated from the mass of the stereomers
with KB as nucleophile and various hydroxylated aldehydes as electrophile substrates. Various 3-deoxy-3-methylulosonic acids were prepared for the first time and isolated with good to high stereoselectivity and very good yields. Although HpcH enzymes were often described to be poorly stereoselective at position 4 when using pyruvate as nucleophile, good stereo-selectivities were obtained with the three selected pyruvate aldolases and KB as nucleophile. Finally, in light of our previous work, we can state that these enzymes are showing interesting substrate promiscuity, tolerating various nucleophiles such as hydroxypyru-vate and KB and various hydroxylated electrophiles. Further work is ongoing in our lab to evaluate other couples of substrates and to understand the stereo-selectivity by molecular modelling.
Experimental Section
General Procedure for Synthesis of Compounds 5, 6, 7, 8, 9 and 10
To a solution of 20 mM KB (35 mg, 0.30 mmol), 2 mM MgCl2, 80 mM aldehyde (d-glyceraldehyde, d-erythrose or d-lactalde-hyde) in water (12 mL) at pH 7.5, was added the aldolase (5 mg, Uniprot Id A5VH82, A9E0U5 or I7DKY0). The pH was controlled every 20 min over 2 h and adjusted to 7.5, if necessary, with 0.1 M NaOH. KB consumption was monitored as described in the SI section until completion of the reaction. The aldol adduct was then purified by anion exchange chromatography: the reaction mixture was loaded on a short column of Dowex® 1 × 8 (HCO3 form, 5 mL), the column was washed with H2O (20 mL) and the aldol adduct was eluted with a 0.1 M ammonium bicarbonate solution. The aldol-containing fractions (as shown by TLC using 32% aqueous NH3/EtOH (3:7) as eluent and a mixture of 0.2 M p-anisaldehyde, 0.2 M AcOH and 0.6 M H2SO4in EtOH as the revealing agent) were concentrated under reduced pressure. The residue was repeat-edly dissolved in water (2 mL) and concentrated to completely remove ammonium bicarbonate. Yields and NMR character-isations are described in the SI section.
General Procedure for Synthesis of Compounds 11 and 12
To a solution of 10 mM KB (17.5 mg, 0.15 mmol), 2 mM MgCl2, 100 mM d-ribose in water (12 mL), pH 7.5, was added the aldolase (10 mg, Uniprot Id A5VH82 or A9E0U5). The pH was adjusted to 7.5 and maintained over 72 h before lyophi-lized. Water was added to the residue, the resulting suspension was centrifuged, the pellet discarded and the aldol was purified by anion exchange chromatography as described above.
Acknowledgements
We thank the French National Centre for Scientific Research (CNRS) and University Clermont-Auvergne for financial sup-port.
References
[1] Y. Yamashita, T. Yasukawa, W.-J. Yoo, T. Kitanosono, S. Kobayashi, Chem. Soc. Rev. 2018, 47, 4388–4480. [2] K. Fesko, M. Gruber-Khadjawi, ChemCatChem 2013, 5,
1248–1272.
[3] R. Roldán, I. Sanchez-Moreno, T. Scheidt, V. Hélaine, M. Lemaire, T. Parella, P. Clapés, W.-D. Fessner, C. Guérard-Hélaine, Chem. Eur. J. 2017, 23, 5005–5009. [4] R. Roldán, K. Hernandez, J. Joglar, J. Bujons, T. Parella,
I. Sánchez-Moreno, V. Hélaine, M. Lemaire, C. Guérard-Hélaine, W.-D. Fessner, ACS Catal. 2018, 8, 8804–8809. [5] D. Güclü, A. Szekrenyi, X. Garrabou, M. Kickstein, S. Junker, P. Clapés, W.-D. Fessner, ACS Catal. 2016, 6, 1848–1852.
[6] P. Clapés, X. Garrabou, Adv. Synth. Catal. 2011, 353, 2263–2283.
[7] V. de Berardinis, C. Guérard-Hélaine, E. Darii, K. Bastard, V. Hélaine, A. Mariage, J.-L. Petit, N. Poupard, I. Sánchez-Moreno, M. Stam, Green Chem. 2017, 19, 519–526.
[8] D. G. Gillingham, P. Stallforth, A. Adibekian, P. H. Seeberger, D. Hilvert, Nat. Chem. 2010, 2, 102–105. [9] P. Clapés, W.-D. Fessner, G. A. Sprenger, A. K. Samland,
Curr. Opin. Chem. Biol. 2010, 14, 154–167.
[10] J. Stockwell, A. D. Daniels, C. L. Windle, T. A. Harman, T. Woodhall, T. Lebl, C. H. Trinh, K. Mulholland, A. R. Pearson, A. Berry, Org. Biomol. Chem. 2016, 14, 105– 112.
[11] H. A. Chokhawala, H. Cao, H. Yu, X. Chen, J. Am. Chem. Soc. 2007, 129, 10630–10631.
[12] J. K. Howard, M. Müller, A. Berry, A. Nelson, Angew. Chem. Int. Ed. 2016, 55, 6767–6770; Angew. Chem.
2016, 128, 6879–6882.
[13] H. P. Meloche, B. A. Parekh, E. O’Connell, J. Protein Chem. 1983, 2, 411–423.
[14] M. C. Shelton, I. C. Cotterill, S. T. A. Novak, R. M. Poonawala, S. Sudarshan, E. J. Toone, J. Am. Chem. Soc.
1996, 118, 2117–2125.
[15] W. Wang, P. Baker, S. Y. K. Seah, Biochemistry 2010, 49, 3774–3782.
[16] H. Ling, G. Wang, Y. Tian, G. Liu, H. Tan, Biochem. Biophys. Res. Commun. 2007, 361, 196–201.
[17] J. Ogawa, H. Yamanaka, J. Mano, Y. Doi, N. Horinouchi, T. Kodera, N. Nio, S. V. Smirnov, N. N. Samsonova, Y. I. Kozlov, Biosci. Biotechnol. Biochem. 2007, 71, 1607– 1615.
[18] K. Hernández, J. Joglar, J. Bujons, T. Parella, P. Clapés, Angew. Chem. Int. Ed. 2018, 57, 3583–3587; Angew. Chem. 2018, 130, 3645–3649.
[19] C. Guérard-Hélaine, V. de Berardinis, M. Besnard-Gonnet, E. Darii, M. Debacker, A. Debard, C. Fernandes, V. Hélaine, A. Mariage, V. Pellouin, ChemCatChem