HAL Id: tel-01426126
https://tel.archives-ouvertes.fr/tel-01426126
Submitted on 4 Jan 2017HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
activity by spinal GABAergic sensory neurons in
zebrafish : connectivity mapping of an intraspinal
sensory feedback circuit
Kevin Fidelin
To cite this version:
Kevin Fidelin. Modulation of premotor circuits controlling locomotor activity by spinal GABAergic sensory neurons in zebrafish : connectivity mapping of an intraspinal sensory feedback circuit. Neu-rons and Cognition [q-bio.NC]. Université Pierre et Marie Curie - Paris VI, 2016. English. �NNT : 2016PA066200�. �tel-01426126�
Université Pierre et Marie Curie
École doctorale Cerveau-Cognition-Comportement (ED 158)
Institut du Cerveau et de la Moelle épinière (ICM)
Laboratoire Dissection Optogénétique des Circuits Spinaux Sous-Tendant la Locomotion
Modulation of premotor circuits controlling locomotor
activity by spinal GABAergic sensory neurons in zebrafish
Connectivity mapping of an intraspinal sensory feedback circuit
Kevin Fidelin
Thèse de doctorat - Sciences de la vie, spécialité Neurosciences
Présentée et soutenue publiquement le 30 Septembre 2016 devant un jury composé de
Dr. Claire Wyart
Directrice de thèse
Prof. Abdel El Manira
Rapporteur
Dr. Alessandra Pierani
Rapporteuse
Dr. Daniel Zytnicki
Examinateur
Dr. Philippe Faure
Examinateur
© 2016 – Kevin Fidelin All rights reserved
-I dedicate this thesis to my teachers, all of them, from middle school to college, and to my previous mentors. Thank you for everything.
Acknowledgements ... i
Abstract ... ii
Résumé ... iii
List of abbreviations ... iv
Introduction ... 1
I. Contribution of sensory feedback during locomotion in vertebrates ... 4
I.1. Sensory feedback as a driver of locomotor activity: spinal reflexes vs. half-center models ... 4
I.2. Integration of sensory inputs in spinal circuits ... 5
I.2.a. Descending pathways reconfigure the output of sensory afferents ... 7
I.2.b. State-dependent modulation of motor output by sensory feedback ... 9
I.2.c. Modulation of sensory signaling by presynaptic inhibition ... 10
I.3. Connectivity between primary afferent neurons and spinal premotor interneurons ... 11
I.4. Cellular and molecular investigation of proprioception in mammals ... 13
I.5. Sensory feedback in fish and amphibians ... 13
Key concepts of part I ... 16
Transition ... 16
II. Anatomical, genetic, and functional organization of locomotor central pattern generators ... 17
II.1. Basic organization of spinal circuits controlling the pattern and rhythm of locomotion ... 17
II.2. Inductive signals control the fate of spinal neuron specification during development ... 19
II.3. Contribution of genetically identified spinal neurons to locomotion ... 19
II.3.a. V0 neurons ... 19
II.3.b. V1 neurons ... 22
II.3.c. V2a neurons ... 23
II.3.c. V3 neurons ... 24
III.1. Identification and characterization of sensory neurons lining the central canal in the spinal cord .... 28
III.2. Molecular analysis of CSF-cNs across species reveals specific markers and a double developmental origin ... 30
III.3. Sensory modalities underlying the recruitment of CSF-cNs ... 32
III.4. Experimental strategies and optogenetic approaches to unravel the function of CSF-cNs ... 34
Key concepts of part III ... 35
IV. Aims of the thesis ... 36
Chapter 1 - Functional connectivity mapping between CSF-cNs and spinal premotor neurons controlling slow swimming ... 37
Predictions regarding the connectivity of CSF-cNs ... 37
Building the experimental paradigm ... 37
Highlights of the findings described in this chapter ... 38
Graphical abstract of the results ... 38
Published article: State-Dependent Modulation of Locomotion by GABAergic Spinal Sensory Neurons ... 39
Figures and Supplemental Information ... 54
Discussion and perspectives ... 65
On the specificity of CSF-cNs connectivity ... 65
CSF-cNs target V3 interneurons, a second class of ventral glutamatergic interneurons ... 69
On the modulation of bout generation ... 71
The problem of chloride homeostasis in developing neuronal networks ... 71
On the role of CSF-contacting neurons during slow swimming ... 73
Can CSF-cNs modulate distinct locomotor behaviors using circuit-specific mechanisms? ... 75
BoTxBLC-mediated silencing of V2a interneurons confirms their critical role in fast locomotion ... 78
Silencing of V2a interneurons decreases the locomotor frequency during spontaneous slow swimming ... 81
Topographic organization of CSF-cNs inputs onto V2a interneurons ... 83
Discussion and perspectives ... 85
Experimental strategy to probe the modulation of V2a neurons by CSF-cNs ... 85
Some assembly required: building a global picture of CSF-cNs-mediated modulation of spinal central pattern generators ... 86
Are V2a interneurons at the core of a global mechanosensory feedback loop? ... 86
Conclusions and future directions ... 89
Experimental procedures ... 90
References ... 96
Annex 1 ... 111
Annex 2 ... 139
This is it. The PhD is almost over! I would like to thank here all the people who made this journey a unique human and scientific experience. I hope to highlight each of your precious contributions as best as I can.
First and foremost, I wish to thank my thesis committee for evaluating my work and for taking the time to read this manuscript. Thank you Abdel for your support all along the PhD, your feedback at every step of the way has been precious to me. Thank you Philippe for asking the difficult questions when I was defending my project four years ago. It really helped me grasp the key concepts in my field of research (and thank you for the beer in Banyuls!). Thank you Daniel for your support and for gathering our labs together on multiple occasions, there is so much to share and it’s always a pleasure to brainstorm together. Thank you Alessandra for your advices regarding the postdoc decision, our discussion was very helpful. Thank you Elena for crossing the Channel and for your support and implication during TENSS 2014.
Claire, I will be brief even if I have a lot to say. Thank you for accepting me in your lab. Giving a neuroscience project to someone who barely knew what the brain was about (as of November 2011) was a risky choice, but we made it work. I am super proud of what we have accomplished as a team. Thank your for your constant enthusiasm, trust, support, and passion. You are an example to follow on many different levels and I hope we will continue to share our love for science in the future. Perhaps you will teach me how to ride a mechanical bull someday….?
My dear fantastic three, I remember being worried when I learned we would be so many students in the lab, the same year. It turned out to be the best thing ever. Djenoune, thanks for EVERYTHING, you’ve been the best lab buddy/friend I could dream of. Thank you for supporting me when all I could do was failing my recordings. Thanks for the food and for dealing with my shitty mood. AH OUI C’EST MOI! Thank you Ginna for being a super inspiring colleague and friend. You taught me never to give up and that’s a pretty good skill in science. Thank you Urs for being the smartest and the coolest. I wish I have learned more from you, especially when it comes to building stuff. See you in Boston!
Thank you Kris for being the kindest, can I say that? (and sometimes the craziest). You are the type of person who makes a lab a happy place and who is always available to help. Thank you for all the good cakes and for your energy. I do not thank you for converting me to GoT though… Thank you Laura for being the coolest handball player ever and for being supportive all the time. Thank you Andy for being the super-powered postdoc who is always there to help. May the chauffage concept live forever. Thank you Steven for cheating and ruining
dedication. Thank you Pierre for the good work and for the good spirit, it’s been a pleasure to work with you. Thank you PLuc for always being the wise one and for our many discussions. Thank you Jeff for bringing this unique Californian vibe to the lab. Thank you Sophie-la-sagesse for being our best lab and facility manager ever. Thank you Johanna for being the most dedicated lab engineer I have ever met. The lab still misses you a lot. Thank you Natalia and Bogdan for taking good care of our beloved fish, you are the best, I hope you know that. Thank you Audrey for being my nerdy friend and for mentioning the lab to me back in 2011, I owe you much. Thanks to the rest of the lab, past and present members, for being part of this adventure. Thank you Beyonce, for everything. You made it to the defense and you’re definitely a survivor, how cool is that?
Thank you Caleb for teaching me how to perform VNR recordings, you’re an incredible teacher and I wish we had more time to work together. Jean, my dear Jean, you have no idea how important your support was to me. Thank you very much for sitting with me and taking the time to review my patch clamp protocols. Thank you Charlotte and Caroline for our many discussions. Big thank you to you Alberto for your support during the PhD. Thank you Richard for evaluating my project proposal early on and for believing in me. Thank you Patricia and Philippe for your help.
Thank you to my friends from the AJITES. William, Morwena, Maria-Belen, Morgane, Alizee, Elise, Aysegul, Sean, Typhaine, and everyone. It’s been super fun (and sometimes stressful) to run the association with you guys. It will probably sound pretentious but I think we’ve done a very good job together.
Merci à mes amis du lycée, du BTS, du Master et du CEA pour votre soutien sans faille tout au long de la thèse, et pour certain(e)s, bien avant qu’il ne soit question d’une thèse. Je veux vous dire combien je suis fier de vous avoir dans ma vie.
Merci à Marelly, Fredo, Emy, Alex, Marcus et la famille Dubus pour votre soutien indéfectible durant toutes ces années. Et pour ces nombreux weekends qui m’ont permis de décompresser, autour d’un bon Uby.
Merci à mes frères, Julien et Valentin, Nathalih, mon petit Kael et mes parents chéris. Je pourrais écrire des lignes et des lignes mais je préfère simplement vous dire merci, pour tout. Je vous aime.
Enfin merci à toi mon amour. Merci pour ton infinie patience, pour ton écoute, pour ton soutien. La vie est tellement belle à tes côtés. Je t’aime.
Locomotion is one of the most vivid expressions of the central nervous system in action.
Looking at people walking in the street or the ballet dancer on the stage, motion seems
effortless to the point that many movements are almost executed unconsciously. Indeed, the
generation of sophisticated motor behaviors relies on the complex interplay between
supraspinal brain structures and circuits in the spinal cord. Understanding how the central
nervous system generates a large repertoire of motor sequences, coordinate limbs and body
orientation in an ever-changing environment while adapting to a myriad of sensory cues
remains a central question in the field of systems neuroscience. The work presented here aims
to understand how local sensory neurons in the spinal cord contribute to the production and/
or the modulation of locomotor activity. We focused our attention on dissecting the circuit
architecture and function of a conserved class of spinal sensory neurons termed cerebrospinal
fluid contacting neurons (CSF-cNs). These neurons lie at the interface between the CSF and
spinal interneurons controlling motor output and represent an interesting yet poorly
understood sensorimotor loop in the vertebrate spinal cord. Recent studies have revealed that
cNs are responsive to pH variations and bending of the spinal cord, suggesting that
CSF-cNs are polymodal sensory neurons able to carry information from distinct sensory cues in a
context dependent manner. Furthermore, their remote activation using optogenetics in
zebrafish was shown to induce slow swimming, demonstrating the ability of CSF-cNs to
modulate spinal circuits controlling locomotion. However, the connectivity of CSF-cNs
remains completely uncharacterized. To understand how CSF-cNs modulate locomotion in
vertebrates, we combined genetics, imaging, optogenetics, electrophysiology, and behavior
analysis to map the functional connectivity of these sensory neurons and test their function in
the zebrafish larva. Our results demonstrate that CSF-cNs project onto several elements
thought to be part of the locomotor central pattern generator in zebrafish, including
glutamatergic spinal interneurons involved in slow and fast swimming. We show that
CSF-cNs can modulate the duration and occurrence of spontaneous locomotor events in a state
dependent manner and tune the frequency of evoked fast escape responses. Altogether our
work dissecting sensorimotor integration in the spinal cord bridged single cell function to
behavior in zebrafish in vivo and should contribute to a better understanding of the role of
sensory feedback during locomotion in vertebrates.
La capacité à se mouvoir est sans doute l’expression la plus évidente du système nerveux en
action. À observer les passants marcher dans la rue ou un spectacle de danse classique, le
mouvement semble si fluide et rapide qu’il parait être exécuté de manière inconsciente. Il n’en
est rien. La locomotion résulte d’interactions complexes entre de nombreuses structures
supra-spinales et les circuits de la moelle épinière. Comprendre les mécanismes mis en place
au sein du système nerveux pour générer des répertoires locomoteurs complexes, coordonner
les membres et l’orientation du corps dans l’espace, dans un environnement qui évolue à
chaque instant tout en intégrant des entrées sensorielles variées, reste l’un des grands défis des
neurosciences systémiques. Le travail présenté dans ce manuscrit vise à comprendre comment
les neurones de la moelle épinière contribuent à la production et à la modulation de l’activité
locomotrice. Pour répondre à ce problème, nous utilisons le poisson-zèbre comme organisme
modèle et avons développé de nouvelles approches génétiques et optiques afin de disséquer
l’architecture du circuit formé par une classe de neurones sensoriels de la moelle et qui est
conservée chez tous les vertébrés. Ces neurones sont appelés les neurones au contact du
liquide céphalo-rachidien (Nc-LCR) et nous proposons de sonder leur(s) fonction(s) in vivo.
Ces neurones sensoriels forment une interface unique entre le liquide céphalo-rachidien et le
réseau de neurones impliqué dans le contrôle du mouvement dans la moelle épinière. Nous
formulons donc l’hypothèse selon laquelle ces neurones participent à l’intégration
sensori-motrice dans la moelle épinière des vertébrés. Des études récentes ont démontré la capacité de
ces cellules à répondre aux variations locales de pH ainsi qu’à la torsion de la moelle épinière
durant le mouvement ce qui suggère que ces cellules sont polymodales, avec de potentielles
fonctions qui diffèrent selon le contexte dans lequel elles sont recrutées. De plus, leur
activation par des méthodes optogénétiques a révélé leur capacité à induire la nage lente chez
la larve du poisson, ce qui démontre que ces cellules peuvent moduler l’activité des neurones
de la moelle épinière. Cependant, leur diagramme de connectivité demeure complètement
inconnu. Afin de comprendre comment ces « Nc-LCR ou CSF-cNs » modulent la locomotion
chez les vertébrés, nous avons développé un projet combinant des approches génétiques,
électrophysiologiques, d’imagerie, et d’analyse du comportement, afin de cartographier le
circuit qu’elles forment avec les neurones de la moelle épinière. Nos résultats montrent que
les CSF-cNs projettent leurs axones sur de nombreux éléments du centre générateur de rythme
de la moelle, et en particulier sur les neurones excitateurs glutamatergiques impliqués dans la
locomotion lente et rapide. Notre approche révèle également la capacité des CSF-cNs à
moduler la locomotion selon l’état dans lequel se trouve l’animal, une propriété
caractéristique des circuits proprioceptifs dans la moelle épinière. Dans l’ensemble, ce travail
de dissection des circuits impliqués dans l’intégration sensorimotrice de la moelle épinière fait
le lien entre la fonction de neurones à l’échelle cellulaire, du réseau et du comportement, au
sein d’un animal intact. Ces résultats devraient apporter de nouveaux éléments permettant
d’appréhender et de mieux comprendre de rôle du retour sensoriel durant la locomotion chez
les vertébrés.
ASIC Acid-sensing ion channel
5-HT 5-hydroxytryptamine
5-HTP 5-hydroxy-tryptophan
aIN Ascending interneuron
BMP Bone Morphogenetic Protein
Btx-LC or BTXB-LC Botulinum toxin light chain
CaP Caudal Primay motor neuron
Chx10 Ceh-10 homeodomain-containing homolog
ChR2 Channelrhodopsin
cIN Commissural interneuron
CiA Circumferential Ascending interneuron
CiD Circumferential Descending interneuron
CoBL Commissural Bifurcating Longitudinal interneuron
CoLA Commissural Longitudinal Ascending interneuron
CoPA Commissural Primary Ascending interneuron
CoSA Commissural Secondary Ascending interneuron
CPG Central pattern generator
CNS Central nervous system
CSF-cNs Cerebrospinal-fluid contacting neurons
dpf Day post fertilization
Dbx1 Developing brain homeobox 1
DRG Dorsal root ganglia
DsRed Discosoma sp. red fluorescent protein
DTA Diphteria toxin A
DV or D-V Dorso-ventral
eIN Excitatory interneuron
EM Electron microscopy
EMG Electromyogram
FRA Flexor reflex afferents
GABA gamma-Aminobutyric acid
GAD Glutamic acid decarboxylase
GFP Green fluorescent protein
Hz Hertz
IN Interneuron
IPSP Inhibitory post-synaptic potential
KCC2 Potassium-Chloride co-transporter
LCR Liquide céphalo-rachidien
LiGluR Light-gated glutamate receptor
L-DOPA L-3,4-dihydroxyphenyl-alanine
MAG-1 Maleimide-azobenzene-glutamate 1
MCoD Multipolar Commissural Descending interneuron
MN Motor neuron
ms millisecond
nAchR Nicotinic acetylcholine receptor
Nc-LCR Neurones au contact du liquide céphalo-rachidien
NKCC1 Sodium-Potassium-Chloride co-transporter
Nkx2.2 NK2 Homeobox 2
NpHR Halorhodopsin
NTR Nitroreductase
pH hydrogen potential
PAD Primary afferent depolarization
Pax Paired box
PIR Post-inhibitory rebound
Pkd2l1 Polycystic kidney disease-2 like-1
Pitx2 Paired like homeodomain trans 2
RB Rohon-Beard
RC Renshaw cell
TRP Transient Receptor Potential
UCoD Unipolar Commissural Descending interneuron
UAS Upstream Activating Sequence
VeMe Ventro-medial interneuron
Vgat Vesicular GABA transporter
Vglut Vesicular glutamate transporter
VIP vasoactive intestinal peptide
VNR Ventral nerve root
Introduction
Animals rely on locomotion to explore their environment, to feed, to find partners for reproduction, or to escape from predators. To perform these actions, the central nervous system generates elaborate goal-directed locomotor sequences that must be adapted to ever-changing environmental conditions. In particular, animals must be able to rapidly change their gait and to adapt their locomotion to various terrains while the movement is performed (Orlovsky, Deliagina, Grillner, 1999; McNeill, 2002; Bel-lardita and Kiehn, 2015; Kiehn, 2016). In this context, sensory information relative to the timing of movement (through the state of muscle contraction, referred to as proprioception, Dietz, 2002; Wind-horst, 2007) and sensory inputs from the external world (transmitted by cutaneous afferents mediating touch or by nociceptors and referred to as exteroception, Edin, 2001; Panek et al., 2014) are integrated by central circuits during movement execution (Figure I.1). This process, referred to as sensorimotor integration, contributes to optimizing or correcting the pattern of motor output to produce smooth movements (Pearson, 1995; Büschges and El Manira, 1998, McCrea, 2001; Rossignol et al., 2006).
Neuronal circuits controlling the generation of movement and receiving sensory feedback from the periphery are located in the spinal cord where they also receive descending inputs from supraspinal structures (Figures I.1, I.2, Shik et al., 1966; Armstrong, 1986; Jordan, 1998). The spinal cord can then be viewed as a neuronal hub, a site of intense neuronal processing as well as a structure driving the activation of muscles in complex and sequential manners (Figure I.2). Spinal cord injuries or dis-eases associated with motor or somatosensory processing dysfunctions affect or completely prevent forelimb, trunk, and/or hindlimb associated muscles from being activated, thus perturbing or abolish-ing movement generation and locomotion in particular (Sanes et al., 1985; Dietz, 2002; Holtz and Levi, 2010; Conte et al., 2013).
Dissecting the architecture of spinal circuits by identifying the constituent neurons responsible for muscle activation and the nature of descending and sensory inputs to spinal neurons is a critical step to
2
Figure I.1. Organization of glutamatergic sensorimotor circuits in the spinal cord. Schematic of neuronal circuits in a mouse spinal cord at embryonic day 18. The axons of sensory neurons project from the dorsal root ganglia to specific laminae in the dorsal horn and to the periphery. Pain and temperature are sensed by nociceptive neurons (red), and the messages are conveyed to laminae I and II. Touch is mediated by mecha-noreceptors (green) in the periphery, which connect to laminae III, IV and V. Proprioception is mediated by the sensory neurons that project through the dorsal horn to the ventrally located motor neurons (shown in blue) The motor neurons also connect directly back to the muscle in the periphery to drive movement (pink). Roman nu-merals indicate the laminae of the dorsal horn. From Caspary and Anderson, 2003.
Figure I.2. Anatomy of the human spinal cord.(A) Drawing of the spinal cord, the spinal roots and the corresponding vertebrates. Each spinal nerve is composed of nerve fibers that are related to the region of the muscles and skin that develops from one body somite (segment). A spinal segment is defined by dorsal roots entering and ventral roots exiting the cord, (i.e., a spinal cord section that gives rise to one spinal nerve is con-sidered as a segment.) (B) Map of dermatomes (area of muscle and skin supplied by peripheral nerve fibers originating from a single dorsal root ganglion). The numbers refer to the spinal segments by which each nerve is named C = cervical; T = thoracic; L = lumbar; S = sacral spinal cord segments. From Dafny, Neuroscience online, an electronic textbook for neurosciences, The University of Texas Medical School at Houston.
pathways by means of sensory afferent stimulations and intracellular recordings have brought to light mechanisms regulating the recruitment and activity of motor neurons by sensory feedback. These stud-ies have also uncovered the diagram of connectivity between sensory afferents, relay interneurons, and motor neurons, and ultimately identified principles governing muscle coordination in limbed verte-brates (reviewed in Burke, 1999; Hultborn 2001, Stuart and Hultborn, 2008). In parallel, the identifi-cation of interneurons responsible for the phasic activation of motor pools during locomotion and their genetic origin during development has improved our understanding of motor pattern generation by distinct subtypes of spinal neurons (reviewed in Jessell, 2000; Marder and Bucher, 2001; Grillner 2003; Goulding, 2009; Arber, 2012; Grillner and El Manira, 2015; Kiehn, 2016).
Despite a century of research on the topic, the connectivity and specific contribution of sensory path-ways during locomotion remains difficult to address in vivo. This is due to a lack of genetic access to desired neuronal population and because testing the dynamics of sensory integration, notably by re-cording single neurons in moving animals, is technically challenging (Dombeck et al., 2007; Naumann et al., 2010). As a consequence, the connectivity between sensory neurons modulating locomotor ac-tivity and spinal interneurons driving the rhythmic activation of motor pools remains poorly under-stood. While new tools and approaches are emerging in the field of systems neuroscience, new routes can be taken to unravel the nature and function of neuronal circuits controlling locomotion, including peripheral sensory circuits. In particular, genetic targeting of spinal neurons combined with electro-physiology and optogenetics open new paths to revisit and test some of the most challenging questions in the field (reviewed in Del Bene and Wyart, 2012; McLean 2013; Portugues et al., 2013; Fidelin and Wyart, 2014, Deisseroth, 2015; Montgomery et al., 2016).
The work presented in this manuscript aims to 1/ understand how sensory and central circuits in the spinal cord interact during locomotion, and 2/ determine how inputs from sensory feedback pathways shape locomotor activity. In this introduction I will start by reviewing important concepts about the control and modulation of locomotor activity by sensory feedback in vertebrates. I will then explore
4
ing the consequences of their modulation by sensory feedback. Finally, I will describe the sensory pathway investigated in this project and explain how it constitutes a novel and interesting circuit for the study of sensorimotor integration in vertebrates.
I.
Contribution of sensory feedback during locomotion in vertebrates
I.1. Sensory feedback as a driver of locomotor activity: spinal reflexes vs. half-center models
Studies from the mid-19th century reported that spinal birds and mammals could use the body parts
innervated by spinal circuits below the site of transection to produce patterns of locomotion (Flourens, 1824; Freusberg, 1874; Unzer, 1771). These observations led to further studies demonstrating that inputs from sensory afferents elicited by mechanical or electrical skin stimulations were sufficient to produce rhythmic contraction of hindlimb flexor and extensor muscles, thus recapitulating scratching in spinal cats and dogs (Sherrington, 1906a, 1910). At the time, it was suggested that the sequential activation of muscles during these evoked movements might be directly driven by the feedback from muscle contraction itself. Sherrington and his peers formulated the hypothesis that rhythmic move-ments such as stepping in cats were generated by a succession of these so-called “reflexes” in the ab-sence of descending “activating” inputs from the brain (Sherrington, 1906a, 1906b; 1910; reviewed in McCrea, 2001; Hultborn, 2006). Although it was clear that inputs from cutaneous or proprioceptive afferent fibers (Table 1, Figure I.3, reviewed in Rossignol et al., 2006) could provide powerful excita-tion to spinal centers and enable the contracexcita-tion of limb muscles, it was later shown that spinal cats with deafferented hindlimbs could still present rhythmic patterns of motor output (Brown, 1911, 1914). This observation followed by the work of many other groups suggested that sensory feedback was not necessary for the generation of rhythmic contraction of hindlimb muscles (Brown, 1911, 1914; Brown and Sherrington, 1912; reviewed in Delcomyn, 1980; Stuart and Hultborn; 2008; McCrea and Rybak, 2008). As a matter of fact, Sherrington himself had gathered similar evidence when he found that spinal dogs with deafferented limbs could still perform scratching, a movement that resembles stepping (Sherrington, 1906b).
Altogether this series of pioneer studies gave rise to the idea that the spinal cord contains autonomous “half-center” circuits composed of limb and muscle-specific excitatory neurons that are responsible for the rhythmic activation of limb muscles. These circuits were later defined as single-level locomotor central pattern generators by Wilson and Wyman from their work in the locust (Wilson and Wyman, 1965) and by Grillner and colleagues following their work in the lamprey (Grillner, 1969; reviewed in Grillner, 2003; McCrea and Rybak, 2008; Grillner and Jessell, 2009; Grillner and El Manira, 2015). This large body of work also led to the general understanding that locomotion, while initiated by su-praspinal brain centers, is generated and maintained in the spinal cord.
Even though sensory inputs are not necessary for the production of motor output, they may be im-portant for muscle coordination and for the modulation of circuit activity, notably by interacting with descending circuits and by tuning the excitability of local spinal circuits (Grillner and Rossignol, 1978; Duysens and Pearsons, 1980; Hiebert et al., 1996; Lam and Pearson, 2001). Eliminating inputs from sensory feedback pathways has dramatic effects on locomotion and postural control (Akay, 2014; Takeoka; 2014), as depicted in the example of the disembodied lady from Oliver Sacks, a patient who may have suffered from a rare form of sensory neuritis targeting dorsal root ganglia.
I.2. Integration of sensory inputs in spinal circuits
The work from Sherrington, Brown, and colleagues revealed that spinal circuits underlying locomo-tion and sensory pathways emerging from muscles and tendons had complex interaclocomo-tions during movement. Because of the lack of genetic tools and techniques for recording individual neurons, it was not technically possible to probe the cellular organization of spinal circuits receiving these senso-ry signals. This limitation prevented them from understanding to what extent sensosenso-ry and descending inputs are dynamically integrated at the spinal neuron level in order to generate different types of mo-tor output. The development of intracellular recording techniques allowed researchers to further dis-sect the architecture of afferent feedback circuits and the function of proprioception during locomotion (Brette and Destexhe, 2012; reviewed in Jankoswka and Hammar, 2002; Hultborn, 2006).
6
Table 1. Proprioceptors in limbed vertebrates. Proprioceptors are located in muscles, tendons, joint lig-aments and in joint capsules. There are no specialized sensory receptor cells for body proprioception. In skeletal (striated) muscle, there are two types of encapsulated proprioceptors, muscle spindles (Ia and II) and Golgi tendon organs (Ib), as well as numerous free nerve endings (III). Within the joints, there are encapsulated end-ings similar to those in skin, as well as numerous free nerve endend-ings. Neuroscience online, an electronic textbook for neurosciences, The University of Texas Medical School at Houston.
Figure 1.3. Location of proprioceptors in the body. (A) The Golgi tendon organ is located at the junction
of muscle and tendon. Afferent terminal fibers are intertwined with collagenous fibers of the tendon and the entire organ is encapsulated in a fibrous sheath. (B) A muscle spindle with its sensory and motor innervation. The primary muscle spindle afferent terminates as annulospiral endings in the central area of the intrafusal mus-cles whereas the secondary muscle spindle afferent terminates as flower spray endings in more polar regions of intrafusal muscles. (C) The joint receptors are free nerve endings encapsulated in the joint capsule and joint ligaments. Adapted from Dougherty, Neuroscience online, an electronic textbook for neurosciences, The Univer-sity of Texas Medical School at Houston.
Of particular relevance, the work of many labs, including the labs of Sir John Eccles, Anders Lundberg, and Elzbieta Jankowska revisited the concept of spinal half-centers developed a few dec-ades earlier by T. G Brown, notably by identifying spinal interneurons forming rhythmic circuits that would also be part of reflex circuits (reviewed in Jankowska, 2001; McCrea, 2001; Hultborn, 2006; Jankowska 2008; Stuart and Hultborn, 2008).
I.2.a. Descending pathways reconfigure the output of sensory afferents
After the identification of monoaminergic terminals in the spinal cord (Carlsson et al., 1964), Lundberg and colleagues probed the influence of monoaminergic descending inputs on the regulation of spinal processing by performing intravenous injections of the noradrenergic precursor L-3,4-dihydroxyphenyl-alanine (L-DOPA) and 5-hydroxy-tryptophan (5-HTP) in spinal cats. They found that these compounds modified the pattern of motor activity elicited by stimulation of sensory afferent fibers (Anden et al., 1963, 1964; Jankoswka, et al., 1965). Instead of measuring a short-latency depo-larization of flexor motor neurons, at it is the case for typical reflex responses, Jankowska and col-leagues observed delayed, long-lasting and rhythmic depolarizing volleys in flexor motor neurons, which were reminiscent of a locomotor-like state (Jankoswka, et al., 1967a, 1967b). These results sug-gest that inputs from sensory pathways have different effects on motor pools when supraspinal de-scending neurons are active compared to when they are not (Figure I.4). This observation led to the idea that inputs from descending neurons may either directly modulate the activity of sensory affer-ent’s target motor neurons or activate sets of spinal neurons that would in turn modify the recruitment or excitability of motor neurons in response to sensory stimulations. Alternatively, Jankowska and colleagues found that flexor motor neurons did receive direct reciprocal inhibition when contralateral flexor motor neurons were active. This pioneering work identified the first diagram of connectivity of spinal interneurons with ipsilateral excitatory interneurons recruited by the combination of descending and sensory inputs. These interneurons were proposed to be responsible for the rhythmic activation of motor neurons, while inputs from inhibitory interneurons were similarly recruited to ensure alternation of activity from one side of the body to the other (Jankowska, 1967a, 1967b, reviewed in Hultborn,
8
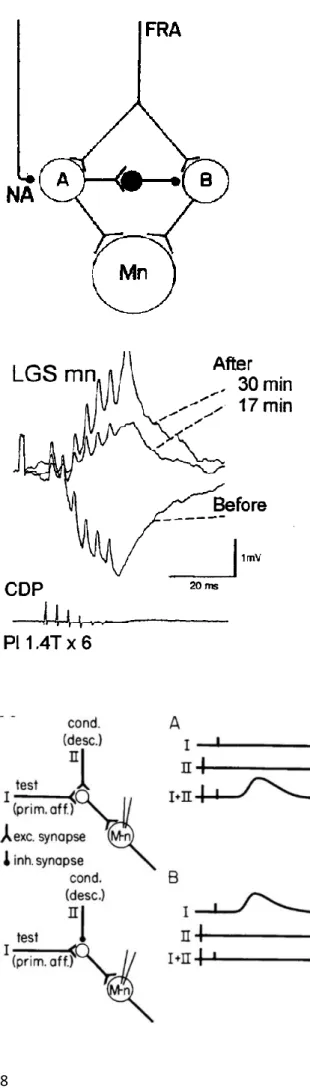
Figure I.4. Schematic representation of neu-ronal pathways transmitting excitatory action to motoneurones (Mn) from flexor reflex af-ferents (FRA).
Excitatory terminals are indicated by two branches, inhibitory by filled circles. Termination of inhibitory interneurones on a cell body merely indicates inhibi-tory action and there is no commitment as to whether the inhibition is postsynaptic on cell bodies or pre-synaptic on terminals of interneurones. Pathway A is activated in the acute spinal cat (without DOPA) and inhibits pathway B so that no effect is transmitted via this route. After DOPA, transmission in pathway A is partially or completely inhibited (by liberation of transmitter from a descending noradrenergic path-way) thereby removing the inhibition of pathway B through which the late longlasting EPSP is evoked in the motoneurone. From Jankowska et al., 1967a.
Figure I5. Slow excitation of extensor moto-neurons by extensor group I afferents. Rever-sal of the influence of group I afferents from plantaris (PL) on a motor neuron supplying either the lateral gastrocnemius or soleus muscle (LCS) following the administration of L-DOPA in an acute spinal cat. Before L-DOPA, the response was predominantly hyperpolarizing due to summation of disynaptic group lb IPSPs. After L-DOPA, the response was depolarizing due to the opening of an additional ex-citatory pathway. Adapted from Gossard et al., 1994 and from Pearson, 1995.
Figure I.6. Diagrams illustrating convergence from primary afferents (I, prim aff) and de-scending pathways (II, desc) onto common INs projecting to MNs. Neuronal circuits shown on the left with (A) showing excitatory convergence from both sources whereas (B) shows excitation from primary afferents and inhibition from a descending pathway. INs (open circles) represent populations of these cells with similar convergence. Traces in the right-side diagrams show idealized IC records from a single MN after a test stimulus (I, prim aff), a condi-tioning (cond) stimulus (II, desc) and their combina-tion (I+II). From Stuart and Hultborn, 2008.
Along the same line, stimulation of Ib fibers led to different outputs on motor neurons with or without L-DOPA (Gossard et al., 1994). While stimulating group Ib fibers at rest was associated with large volleys of inhibition in recorded target motor neurons, the same stimulation after addition of L-DOPA in the preparation triggered large depolarization of the same motor neurons (Figure I.5, Gossard et al., 1994; Pearson and Collins, 1993; Whelan et al., 1995; reviewed in Pearson, 1995). This phenomenon, termed reflex reversal, demonstrates that sensory afferents are fully integrated within rhythmic spinal circuits and that the effect of their activation depends on the state of spinal circuit’s activation and as a consequence the nature of active spinal neurons at the time of the stimulation. Conversely, these re-sults also indicate that neuromodulators such as dopamine (and additionally for noradrenaline and serotonin) can dramatically modify the output of both spinal circuits and sensory feedback pathways. This body of work is important because it gave rise to some of the most fundamental concepts in the field of sensorimotor integration. First, these results showed that descending circuits interact with sen-sory afferents and can change the output of motor neurons following sensen-sory stimulation. Then, it brought to light the architecture of sensorimotor connectivity by revealing the existence of premotor circuits, which are not active when L-DOPA is absent but seems nonetheless responsible for the rhythmicity observed in these experiments. Finally, it showed that descending commands can modu-late the configuration of active circuits during sensory processing by adding or derecruiting spinal interneurons (Figure I.4). Altogether, these data and a series of follow-up papers (described in Lundberg, 1975 and Burke, 1985 and a long series of papers from Jankowska and colleagues) demon-strated that descending and sensory inputs converge onto spinal microcircuits with the ability to modi-fy their output in a context-dependent manner (Stuart and Hultborn, 2008; Figure I.6). The latter con-cept is particularly important for the work presented in this manuscript.
I.2.b. State-dependent modulation of motor output by sensory feedback
Using L-DOPA to induce locomotor-like states in decerebrate and acute spinal cats allowed research-ers to investigate the effects of activating sensory pathways during different contexts, either at rest or during fictive locomotion. It was demonstrated that the timing of Ia or group II afferents activation
10
determine whether these sensory inputs could entrain the rhythm if they are elicited at rest (without L-DOPA) or reset the rhythmicity of spinal neurons in L-DOPA (Figure I.7, Conway et al., 1987; Kriel-laars et al., 1994; Guertin et al.,1995). Thus, sensory feedback can participate in controlling the transi-tion from one phase to the next during the step cycle. This work led to the concept that sensory feed-back and local circuits in the spinal cord interact in a complex and state-dependent manner to control the timing of muscle coordination during complex motor sequences (McCrea, 2001). One interpreta-tion for these findings is that sensory feedback could compensate for perturbainterpreta-tions occurring in the locomotor environment or correct non-linear mechanics during movement (Stuart, 1999; McCrea, 2001).
I.2.c. Modulation of sensory signaling by presynaptic inhibition
Eccles and colleagues found that pre-stimulating group I afferents led to a decrease of amplitude of elicited EPSPs in recorded target motor neurons during subsequent afferent stimulations (Eccles et al., 1962a, 1962b, 1962c). In addition, they observed a reduction of the overall monosynaptic reflex am-plitude without noticeable modification of motor neurons’ membrane resistance (Eccles et al., 1962a, 1962b, 1962c). These data revealed the existence of a tight regulation of the excitability of motor
neu-Figure I.7. Extension enhancement and reset-ting evoked by extensor group I afferents. The records are rectified-integrated neurograms obtained during fictive locomotion evoked by stimulation of the midbrain in a decerebrate cat and show rhythmic alternating activity in ankle flexor (TA) and extensor (MG) nerves. The intervals (ms) between subsequent discharges in the MG nerve are indicated below the MG recording. A, stimulation of the plantaris nerve (twice threshold (2T), 22 shocks, 200 Hz) during flexion initiates the extension phase of locomotion (i.e. resets to extension). B, the same stimulation delivered during extension prolongs the duration and enhances the amplitude of extensor activity. C, aver-aged rectified integrated neurogram of SmAB activi-ty during fictive locomotion. The traces were aligned at the onset of stimulation and show the effects of LGS nerve stimulation (200 Hz) at different intensi-ties on the activity of these hip extensor motoneu-rones. Note the persistence of activity well beyond the end of the stimulus train. Adapted from Guertin
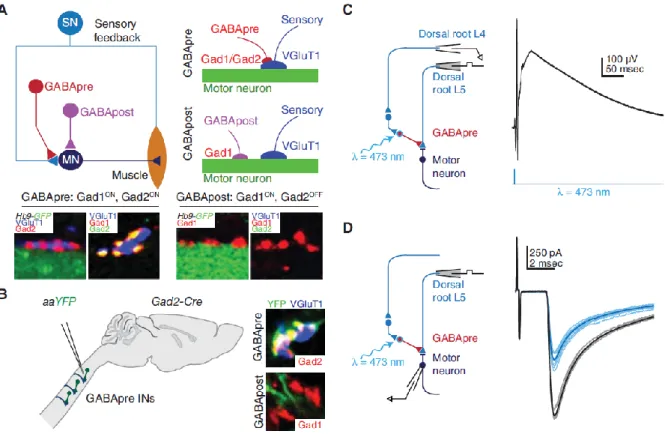
rons occurring at the premotor level, which was directly related to the timing of sensory inputs in an-tagonist motor pools. Recording of Ia afferents during these experiments revealed a strong depolariza-tion at their terminals termed “primary afferent depolarizadepolariza-tion” (PAD), which was sufficient to dis-charge primary afferents and consequently abolish the excitation of target motor neurons (Marlinskii, 1983; Rudomin and Schmidt, 1999; Rudomin, 2009). This mechanism is crucial for movement coor-dination and stability because it limits the gain of proprioception and prevents undesired excitation of motor neurons during movement when these neurons are not supposed to be recruited. However, the nature of spinal neurons mediating this effect in vivo remained elusive for a long time. Combining electrophysiology, optogenetics, and behavior analysis, a recent study tackled this particular point by identifying local Gad2+ spinal neurons as the cellular substrate mediating GABAergic presynaptic
inhibition during skilled reaching in mouse (Betley et al., 2009; Fink et al., 2014; Figure I.8). The authors tested the role of Gad2+ neurons by specifically eliminating this subpopulation and found that
reaching tasks were altered in mice lacking Gad2+ neurons (Fink et al., 2014).
I.3. Connectivity between primary afferent neurons and spinal premotor interneurons
The dissection of reflex circuits have enabled researchers to unravel the organization of premotor in-terneurons integrating sensory inputs, and revealed how these neurons could shape the pattern of mo-tor activity. In particular, Ia interneurons that receive monosynaptic excitation from Ia muscle spindle afferents became the center of intense investigation (reviewed in Jankowska, 1992). Work in cats and humans revealed that Ia neurons form a pathway providing monosynaptic excitation to homonymous motor neurons while also activating contralateral inhibitory interneurons responsible for the reciprocal inhibition of antagonists motor neurons, thus forming a disynaptic inhibitory circuit (Jankoswka et al., 1981; Baldissera et al., 1987). As a consequence, Ia interneurons form a relay pathway, which in addi-tion to presynaptic inhibiaddi-tion, controls the alternaaddi-tion of antagonistic pairs of motor pools. Interesting-ly, Ia interneurons also integrate descending inputs including direct inputs from corticospinal neurons and from other local interneurons (Hultborn and Udo, 1972a, 1972b; Illert and Tanaka, 1978). In par-ticular, inputs from Renshaw cells to Ia interneurons are thought to down regulate the Ia-mediated
12
Figure I.8. Gad2-expressing neurons mediate presynaptic inhibition. (A) Proprioceptive sensory neurons (SN) convey sensory feedback signals from muscle to motor neurons (MN). Presynaptic inhibitory (GABApre) neurons contact sensory afferent terminals, whereas postsynaptic inhibitory (GABApost) neurons contact motor neurons directly. GABApre neurons express Gad2 (top schematic and left two images; far left: red Gad2ON contacts on blue VGluT1+ sensory afferent terminal, adjacent green GFP+ motor neuron labeled in Hb9-GFP mice). GABApost neurons express Gad1 (bottom schematic and right two images; second from right: red
Gad1ON contacts adjacent green GFP+ motor neuron). Although GABApre neurons express both Gad1 and Gad2
(second image from left: yellow Gad1ON/Gad2ON puncta adjacent VGluT1+ sensory terminal), GABApost
neu-rons express Gad1 alone (far right image: red Gad1ON/ Gad2OFF puncta). (B) Injection of Cre-dependent virus
(AAV-FLEX-ChR2-YFP) into cervical cord of adult Gad2-Cre mice labels GABApre neurons (top, YFP+, Gad2+
contact adjacent VGluT1+ sensory terminal) and not GABApost neurons (bottom, YFP negative red puncta).
Viral injection marks ≈80% of GABApre and ≈1% of GABApost boutons. (C) After AAV-FLEX-ChR2-YFP injection in lumbar spinal cord of neonatal Gad2-Cre mice, recordings in isolated spinal cord from sensory affer-ents (dorsal root L4, extracellular) reveal primary afferent depolarization, and (D) recordings from motor neu-rons (whole cell patch clamp) reveal suppression of monosynaptic sensory-evoked excitatory postsynaptic cur-rents after photostimulation (black, control; blue, 473-nm wavelength (λ) photostimulation). At early ages Gad2 is also expressed in GABApost boutons; therefore, all behavioral experiments were performed after viral injec-tion in adult mice, when Gad2 expression is specific for GABApre neurons. Adapted from Betley et al. 2009; Azim et al., 2014b, Fink et al., 2014.
inhibition in order to favor co-contractions of antagonistic muscles, which is necessary to maintain body position in space (McCrea et al., 1980; Pratt and Jordan, 1987). The connectivity of spinal cir-cuits with either descending circir-cuits and/or sensory pathways remains particularly challenging to map but new approaches combining genetic targeting with the use of viral approaches should drastically improving our ability to map the connectivity of sensory neurons in the spinal cord (Wickersham et al., 2015a, 2015b).
I.4. Cellular and molecular investigation of proprioception in mammals
A recent study took advantage of the early growth response 3 (Egr3) mutant line, in which group Ia and II afferents are impaired, to investigate the contribution of sensory feedback during locomotion (Tourtellotte and Milbrandt, 1998; Akay et al., 2014). The authors found that eliminating these inputs altered stepping, precise locomotor pattern on ladders, and swimming activity, indicating that inputs from Ia, Ib and II afferents pathways act in concert to allow coordinated patterns of muscle activation during locomotion (Akay et al., 2014). A study published the same year confirmed these results and found that inputs from these sensory neurons are indeed critical for the functional recovery of spinal circuits after spinal cord hemi-sections (Takeoka et al., 2014). At the molecular level, Piezo2, a non-selective cation channel has been recently identified as the main mechanosensory channel responsible for the activation of mammalian proprioceptors. Accordingly, mice lacking this channel in DRG neu-rons display severe limb coordination problems (Woo et al., 2015).
I.5. Sensory feedback in fish and amphibians
In anamniotes such as the lamprey, the organization of sensory feedback circuits differs from mam-mals. Instead of relying on proprioceptive afferent pathways located at the periphery to detect muscle activation, swimming vertebrates have developed other sensory strategies that enable them to sense their environment. These systems can take the form of sensory cells on the skin that can detect hydro-dynamics and pressure, or mechanoreceptor cells that can sense axial muscle contraction, body ten-sion, or stretch. Lamprey possess intraspinal proprioceptors that are also referred to as stretch recep-tors or “edge cells” (Grillner et al., 1984, Hsu et al., 2013). One common strategy to investigate the contribution of sensory feedback in these animals consists in paralyzing muscles using blockers of the neuromuscular junction and analyzing the profile of fictive locomotion. In this configuration it re-mains possible to record the rhythmic activity of spinal neurons at the level of ventral nerve roots (VNR) but this activity does not translate into movement anymore due to the inactivation of muscle contraction (Masino and Fetcho, 2005). Comparison between the locomotor activity obtained with electromyogram (EMG) recordings during ‘active’ locomotion and the profile of ‘fictive’ locomotion
14
liams, 1984). These results suggest that sensory feedback in swimming animals is critical for setting the optimal rhythm, by modulating the timing or muscle contraction and propagation of excitation from head to tail (Mullins et al., 2011). Regarding the contribution of stretch receptor cells, a pioneer-ing experiment from the Grillner lab demonstrated the influence of stretchpioneer-ing the spinal cord in lam-prey. Such manipulation was shown to induce rhythmic discharges of motor neurons in the fictive configuration, regardless of the side where the stimulation was applied (McClellan and Grillner, 1983). This study is important because it shows that sensory feedback, like in mammals, has the prop-erty to entrain locomotion under certain contexts in lower vertebrates.
In Xenopus and zebrafish, early born Rohon-Beard (RB) cells are dorsally located mechanosensory neurons expressing piezo2b that respond to light touch on the skin and mediate touch-evoked escape responses (Clarke and Roberts, 1984b, Spitzer, 1984, Kohashi and Oda, 2008, Douglass et al., 2008, Faucherre et al., 2013). Rostral RB cells send axonal projection directly to the Mauthner cell, the main component of the escape response circuit, while more caudal RB neurons project on commissural pri-mary ascending interneurons (CoPA) in zebrafish and dorsolateral commissural interneurons (dlc) in Xenopus, which are monosynaptically connected to contralateral primary motor neurons (Clarke and Roberts, 1984a, 1984b, Sillar and Roberts, 1988, 1992, Roberts and Sillar, 1990, Bernhardt, 1990, Li et al., 2004, Palanca et al., 2013, Knogler and Drapeau, 2014). RB neurons were thought to degenerate early during development but it is now accepted that RB neurons can be visualized in two weeks old zebrafish suggesting that their role is not restricted to the embryo (Reyes et al., 2004, Palanca et al., 2013). CoPA neurons are silenced by ascending inhibitory interneurons (aINs in Xenopus and CiA in zebrafish) during fictive swimming, a mechanisms thought to prevent the sensory activation of the escape response when animals are engaged in swimming (Li et al, 2003, Higashijima et al., 2004a, 2004b). Interestingly, Pietri et al reported putative anatomical contacts between axons of CoPA inter-neurons onto the soma of V2a interinter-neurons, which are rhythmically active premotor interinter-neurons driv-ing slow and fast swimmdriv-ing (Pietri et al., 2009). Whether the RB-CoPA loop modulates spontaneous locomotion remains to be determined. Interesting experiments revealed that RB neurons could trigger different types of locomotor responses depending on the intensity of touch stimuli (Soffe et al., 1991,
1997), pointing to more complex role from RB neurons during locomotion. While a brief light touch can trigger fictive swimming, repetitive stimulations of the RB pathway triggers struggling, a strong motor response where activity propagates from tail to head in Xenopus and zebrafish (Soffe et al., 1991, 1997, Liao and Fetcho, 2008).
Trigeminal neurons are sensory neurons with “free” nerve endings in the head of Xenopus tadpoles that respond to mechanical inputs and relay these sensory signals to downstream hindbrain circuits and in the spinal cord (Roberts, 1980, Hayes and Roberts, 1983). Interestingly, trigeminal neurons form two independent pathways in Xenopus, one glutamatergic and one GABAergic, that can differentially modulate locomotor activity. Activation of the glutamatergic pathway can entrain locomotion while the recruitment of long projecting GABAergic neurons by the second pathway was shown to stop lo-comotion, an effect thought to recapitulate the “stopping” behavior of young tadpoles hitting solid structures (Boothby and Roberts, 1995; Perrins et al., 2002; Buhl and al., 2015). Altogether, these studies show that the state-dependent modulation of locomotion by sensory feedback pathways is pre-sent both in mammals and lower vertebrates. In each case, inputs from sensory neurons can entrain the activity of neurons involved in rhythm and pattern generation, again highlighting the strong interaction between circuits referred to as spinal central pattern generators and sensory feedback pathways. How-ever, the circuit architecture underlying such interactions is not fully understood and more work is required to extract the cellular and circuit mechanisms engaged in modulatory effects from sensory feedback.
16
Key concepts of part I
- The modulation of spinal circuits by sensory feedback is often complex and state-dependent. - Inputs from sensory pathways can entrain or reset the phase of locomotor activity.
- Sensory pathways provide direct excitation to motor neurons and project onto spinal interneu-rons mediating recurrent and reciprocal inhibition.
- The interaction between sensory pathways and central circuits are important for muscle coor-dination, patterning of locomotor activity, and movement stability.
- Mapping of connectivity between descending, spinal and sensory neurons is key to understand sensorimotor integration during locomotion.
Transition
Altogether, the work reviewed in this section led to a basic functional connectivity diagram between central, motor, and peripheral sensory neurons thought to be at the basis of motor pattern generation in the spinal cord. These studies have also revealed that individual spinal interneurons are highly wired and that the net effect of their recruitment following sensory afferent stimulation is both state and con-text-dependent. However, the spinal cord is a complex structure containing many types of spinal neu-rons and only a small fraction of spinal neuneu-rons has been investigated. In order to understand how sensory feedback shapes locomotor activity by acting on rhythmically active spinal interneurons, it remains critical to better understand the nature of central pattern generating circuits, their molecular, physiological, and anatomical properties as well as their function in rhythm and pattern generation. The part II describes current knowledge on CPG organization and function.
II.
Anatomical, genetic, and functional organization of locomotor central
pattern generators
Sensory feedback closely interacts with central circuits controlling the pattern of activity and rhythm of spinal motor neurons. Thus it is important to identify the elements forming the so-called central pattern generators. In cat preparations, the identification of interneurons was mainly done after record-ing their responses to sensory stimuli, after mapprecord-ing their anatomy and connectivity to other interneu-rons and/or motor neuinterneu-rons (Harrison et al., 1984). Since cat spinal cords are not amenable to genetics and microscopy, it was difficult to rely on specific markers of interneurons or reconstruct the mor-phology and projections of recorded neurons even if they were filled with dyes. The use of smaller preparations (mainly in lampreys, tadpoles, or in genetic model organisms such as rodents and zebrafish), with the development of immunohistochemistry and molecular genetics for labeling, imag-ing, and manipulating the fate of spinal neurons complemented our understanding of the functional organization of locomotor CPGs.
II.1. Basic organization of spinal circuits controlling the pattern and rhythm of locomotion
Recordings of spinal interneurons in cats, lampreys, and tadpoles allowed the identification of the basic architecture of motor circuits by revealing monosynaptic connections between excitatory and inhibitory interneurons, and motor neurons. Monitoring the activity of spinal interneurons during in-duced fictive locomotion has revealed the nature of spinal neurons active during rhythmic oscillations of motor neurons (Buchanan and Grillner, 1987; Grillner, 2003, Liao and Fetcho, 2008; Berkowitz et al., 2010). Data acquired in several species allowed the identification of the core central pattern gener-ator, which is composed of: ipsilateral descending excitatory interneurons that provide rhythmic drive to motor neurons through direct monosynaptic connections; ipsilateral ascending inhibitory interneu-rons that also project onto motor neuinterneu-rons and modulate their activity as well as modulating the re-sponse to sensory circuits, and contralateral descending and ascending glutamatergic and glycinergic interneurons that regulate the alternation of activity from one side of the body to the other (Figure I.9, Buchanan and Grillner, 1988; Buchanan et al., 1989; Ohta et al., 1991; Biro et al., 2008;
18
Figure I.9. Basic organization of locomotor CPG in swimming vertebrates. Four functional classes of neurons make up the swimming central pattern generator (CPG) in lamprey (see the figure): Segmentally organized motor neurons (MNs) that innervate each adjacent axial myotome. Glycinergic commissural interneu-rons (CINs) project to the opposite side of the spinal cord. During swimming, inhibitory connections provide the mid-cycle inhibition that ensures that the axial muscles on each side of the body contract out of phase with those on the opposite side. Ipsilaterally-projecting inhibitory L-interneurons (IINs) that provide inhibition to motor neurons and to CINs. Their exact role in swimming has not been defined. Excitatory glutamatergic neurons (EINs) that project to all three other CPG neuron cell types. These cells, or a proportion of these cells, are rhythmically active and provide rhythmic drive to motor neurons and other CPG neurons during swimming. Excitatory commissural neurons are also present in the lamprey cord, however their function is not known. From Goulding, 2009.
Table 2. Putative phylogenetic relationship between spinal cord neurons. The table below illustrates what we know about the relationship between neurons identified in the spinal cords of different species. aINs and CiA neurons seem to be equivalent to the inhibitory L- interneurons of the lamprey and V1 neurons in the mouse. In Xenopus, the dIN glutamatergic neurons seem to be the major source of ipsilateral excitatory input in the hindbrain and ventral spinal cord. dIN neurons might be homologous to CiD neurons in zebrafish, which express the Chx10 transcription factor. CiD neurons, are rhythmically active during fictive swimming and pro-vide the main source of on-cycle excitation to the swimming central pattern generator. Glycinergic inhibitory commissural interneurons have an essential role in generating these alternating outputs between each half of the spinal cord. In Xenopus, the commissural interneurons (CINs) that mediate reciprocal inhibition have been char-acterized in some detail. They typically fire in phase with ipsilateral motor neurons once per swimming cycle. There are multiple anatomically, distinct populations of CINs, including CoPA, CoSA, CoLA, UCoD and CoBL cells in the zebrafish spinal cord. Although these cells are largely characterized anatomically and to a lesser extent molecularly, their functions in locomotion have not been described. CoBL and Evx2+ MCoD neurons are
both active during swimming. CoBL cells are bifurcating glycinergic neurons. The excitatory commissural MCoD neurons are preferentially recruited during slow swimming movements. They seem to be necessary for slow swimming, but are dispensable for coordinating the left–right alternation of segmental motor neurons dur-ing fast swimmdur-ing movements. The inhibitory CINs, excitatory interneurons (EINs) and L-interneurons in the lamprey have not been molecularly characterized. The zebrafish homologues of V3 neurons might be VeMe and UCoD cells, but they are yet to be identified. UCoD neurons are similar to commissural V3 interneurons (glu-tamatergic with descending axons). From Goulding, 2009.
Green and Soffe, 1998; Roberts et al., 1998, 2000; Li et al., 2001; Hale et al., 2001; Liao and Fetcho, 2008; Berkowitz et al., 2010, Table 2). Studies investigating the molecular and cellular basis of CNS development combined with novel genetic strategies led to the identification of specific sets of mark-ers labelling each class of spinal neurons and allowed probing the contribution of each of these neu-ronal classes to the regulation of locomotion.
II.2. Inductive signals control the fate of spinal neuron specification during development
Spinal neurons acquire their identity during early developmental stages, soon after the neural tube formation (Jessell, 2000; Lee and Pfaff, 2001; Arber, 2012; Gouti et al., 2015). During this intense period of cellular proliferation and differentiation, concentration gradients of morphogens dictate the identity of progenitor cells along the dorsoventral axis of the developing CNS. On the ventral side, the notochord and the floor plate cells release Sonic hedgehog (Shh) while roof plate cells on the dorsal side secrete Bone Morphogenetic Proteins (BMPs). The interplay between these signaling molecules condition the expression of homeodomain proteins and other transcription factors along the dorso-ventral (DV) axis (Jessell, 2000). The given concentration of each of these signaling molecules and the combinatorial expression of transcription factors gives rise to discrete progenitor domains along the DV axis from which derive each of the five populations of ventral spinal neurons, respectively termed V0, V1, V2, MN, and V3 neurons (Figure I.10, I.11, Jessell, 2000; Grillner, 2003; Goulding and Pfaff, 2005; Alaynick et al., 2011; Goulding, 2009; Arber, 2012). The generation of mutant lines tar-geting single or multiple transcription factors allowed specific elimination of populations of spinal neurons and probing of their contribution to the control of locomotor activity.
II.3. Contribution of genetically identified spinal interneurons to locomotion
II.3.a. V0 neurons
V0 neurons are commissural premotor interneurons expressing the dbx1 transcription factor and can be divided in dorsal inhibitory Evx1-/Pax7+ and ventral excitatory Evx1+/Pax7- subpopulations
20
Figure I.10. Early development of the spinal cord. Schematic cross-sections through the developing mouse spinal cord showing the patterning and specification of early spinal cord progenitors and their neuronal progeny. At embryonic day 9 (E9), a gradient of Sonic hedgehog (red) (ventrally) and Bone Morphogenetic Proteins (BMPs) and Growth Differentiation Factor 7 (GDF-7) (yellow) (dorsally) provide instructive positional signals to dividing progenitors in the ventricular zone. This leads to the restricted activation of patterning factors in discrete dorsoventral domains, including Nkx6.1 (ventral), Pax6 (intermediate), and Pax3 and Pax7 (dorsal). At E11, eleven early classes of post-mitotic neuron are present in the embryonic spinal cord. dI1-dI5 neurons that are derived from dorsal progenitors (grey) primarily contribute to sensory spinal pathways, whereas dI6, MN and V0–V3 neurons arising from intermediate/ventral progenitors (yellow) are involved in the locomotor circuit-ry. Some of the post-mitotic transcription factors that serve to identify each of the eleven early generic popula-tions are indicated. From Goulding, 2009.
Figure I.11 Identified spinal interneurons in the embryonic mouse and zebrafish spinal cord.
Similar neuronal cell types are present in the embryonic spinal cords of aquatic and terrestrial vertebrates. The putative zebrafish homologues of V0, V1, V2 and V3 locomotor interneurons are indicated by the same colour. These include V0 and CoSA neurons (light blue), V1 and CiA neurons (dark green), V2a and CiD neurons (or-ange), V2b and VeLD neurons (turquoise), and V3, UCoD and VeMe neurons (red). From Goulding, 2009.
cord preparations (Lanuza et al., 2004). This result suggests that V0 inputs to contralateral motor neu-rons are critical for the timing of activation of antagonist motor units in mammals (Lanuza et al., 2004). In this study, animals died early on because V0 neurons are also present in the brainstem and control respiration (Talpalar et al., 2013). Selective ablation of spinal V0 neurons enabled Talpalar and colleagues to look at the behavioral consequence of losing either the entire V0 population or only the ventral or the dorsal subpopulation (Talpalar et al., 2013). Mice lacking spinal V0 neurons lose the ability to alternate limbs and display rabbit-like hopping locomotor patterns regardless of the speed of locomotion. In contrast, the elimination of dorsal inhibitory dorsal V0D neurons triggers hopping at
slow locomotor frequencies but alternation is conserved during fast locomotion while the phenotype reverses when ventral glutamatergic V0V neurons are selectively ablated (Talpalar et al., 2013;
Bel-lardita and Kiehn; 2015; Kiehn, 2016). These results demonstrate that the V0 population, through commissural projections, plays a critical role in setting the basic pattern of terrestrial locomotion while also regulating the speed at which alternation between the left and the right side occur (Bellardita and Kiehn, 2015). However, it remains difficult to understand how individual V0 neurons regardless of their transmitter phenotype and projections pattern shape the activity of contralateral motor neurons. Future work should investigate the connectivity of V0 neurons with descending neurons and with spi-nal neurons to better understand their role in speed modulation.
In zebrafish V0 neurons also express Dbx1 and Evx1 (Satou et al., 2012). The V0 population is also composed of both excitatory and inhibitory commissural interneurons that can either send ascending or descending projections. Little is known of the role of zebrafish V0 neurons during locomotion ex-cept for glycinergic commissural bifurcating longitudinal (CoBL) that are rhythmically active during slow swimming but not during rapid escape responses (Liao and Fetcho, 2008) and multipolar com-missural descending (MCoD) interneurons that are premotor interneurons specifically active during slow swimming (Hale et al., 2001; Ritter et al., 2001; McLean et al., 2007, 2008; Satou et al., 2012; Fidelin et al., 2015). Interestingly, MCoD neurons are de-recruited during the transition from slow to fast fictive swimming in zebrafish suggesting that they might receive inhibitory inputs by spinal