Virulence Factors and TEM-Type
-Lactamases Produced by Two
Isolates of an Epidemic Klebsiella pneumoniae Strain
Frédéric Robin,a,b,cClaire Hennequin,a,dMarek Gniadkowski,eRacha Beyrouthy,b,c,fJoanna Empel,eLucie Gibold,a,b,c and Richard Bonneta,b,c
CHU Clermont-Ferrand, Centre de Biologie, Laboratoire de Bactériologie Clinique, Clermont-Ferrand, Francea; Clermont Université, Université d’Auvergne, Evolution des Bactéries Pathogènes et Susceptibilité Génétique de l’Hôte, Clermont-Ferrand, Franceb; INRA, USC2018, Clermont-Ferrand, Francec; Clermont Université, Université d’Auvergne, Laboratoire de Bactériologie, Faculté de Pharmacie, Clermont-Ferrand, Franced; National Medicines Institute, Warsaw, Polande; and Faculty of Public Health, Lebanese University, Tripoli, Lebanonf
Two Klebsiella pneumoniae isolates of the same strain, identified in Poland, produced either TEM-47 or TEM-68, which differed
by the Arg275Leu substitution. They harbored a few virulence factors, including an iron-chelating factor and capsule
overpro-duction, suggesting that these factors were sufficient to enhance their nosocomial potency. TEM-68 and TEM-47 had similar
en-zymatic activities, but TEM-68 was less susceptible to inhibitors than TEM-47. These results confirm the role of the Arg275Leu
substitution in the evolution of TEM enzymes.
K
lebsiella pneumoniae strains are responsible for
community-acquired and nosocomial infections. Different virulence
fac-tors have been involved in the infective potency of the
community-acquired strains, such as K1 and K2 capsular
sero-types, types 3 and 1 fimbriae, capsule synthesis, production of
iron-chelating agents, hypermucoviscosity, biofilm formation
ability, and the presence of the genomic island encoding the
bio-synthesis of the colibactin (3, 10, 11, 14, 17, 18, 20, 25, 27, 33, 40).
However, data concerning the virulence factors expressed by
nos-ocomial strains of K. pneumoniae are scarce. The strains
respon-sible for nosocomial epidemics are usually multiresistant to
anti-biotics, and most of them produce extended-spectrum
-lactamases (ESBLs) (8, 26). The largest ESBL family is the TEM
family (5). However, another subgroup of enzymes has appeared
that combine the substitutions observed in the ESBLs and in the
inhibitor-resistant TEM (IRT): its members were designated
com-plex mutant TEMs (CMTs). They have been observed mainly in
Escherichia coli and mostly in France (16, 29, 31, 34–37, 39). The
enzyme TEM-68 (CMT-2) was identified by Fiett et al. in an
epi-demic strain of K. pneumoniae and appeared to have emerged
from the ESBL TEM-47 (16). The clinical isolate that produced
the ESBL TEM-47 was isolated from eight patients hospitalized in
the neonatal ward of the University Hospital of Wrocław, Poland.
During this spread, another isolate, which produced TEM-68, was
isolated from the respiratory tract of a ninth newborn. All of the
isolates were indistinguishable by pulsed-field gel electrophoresis
(15). TEM-68 and TEM-47 differed from TEM-1 by the
substitu-tions Gly238Ser, Glu240Lys, and Thr265Met. TEM-68 differed
from TEM-47 by the substitution Arg275Leu observed in the IRT
TEM-103 (IRT-28) (1, 16).
In this work, we studied the virulence factors that could have
allowed their nosocomial dissemination and characterized the
en-zymes TEM-47, TEM-68, and TEM-103 to understand the role of
the Arg275Leu substitution in TEM evolution.
Multilocus sequence type analysis confirmed that all the
iso-lates belonged to the same new sequence type (ST), ST514 (9). The
clinical isolates 3144 and 3151, which produced TEM-47 and
TEM-68, respectively, were selected for further experiments. They
did not belong to the K1 or K2 genotypes (15). They were
inves-tigated for the presence of different virulence genes as
recom-mended by Brisse et al. (3), for the presence of the colibactin
genomic island (32), and for the production of the iron-chelating
factor aerobactin (28) and of type 1 fimbriae (19). They harbored
the genes wabG and uge, which are involved in lipopolysaccharide
synthesis and have been associated with virulence, the kfu gene,
which encodes an iron-chelating factor, and the gene mrkD, which
encodes a type 3 fimbria. They also expressed type 1 fimbriae.
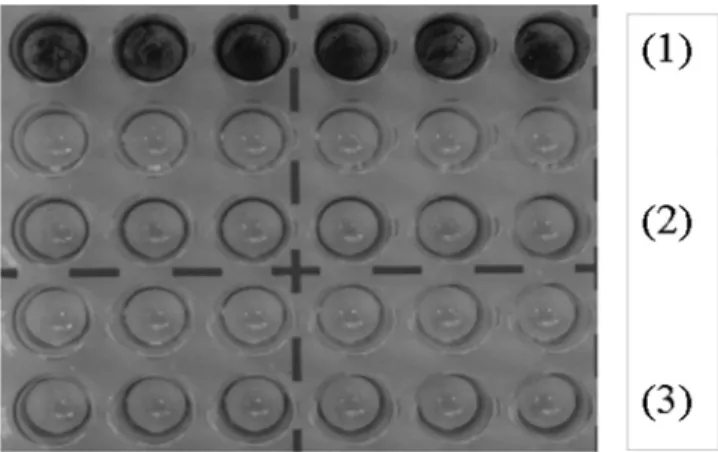
Using a microtiter plate experimental model (30), we showed that
the two isolates produced no biofilm (Fig. 1). Since the capsule is
one of the main virulence factors of nosocomial K. pneumoniae
strains, we investigated the amount of capsular polysaccharides
produced by the two isolates. They could both be categorized as
high-level producers (⬎15
g of glucuronic acid/0.5 ml of
cul-ture) (18). This high level of capsule could be important in the
virulence because the capsule plays an important role in
protect-ing K. pneumoniae from intracellular killprotect-ing, complement,
surfac-tant, oxidative stress, and phagocytosis (12, 13, 38). Lawlor et al.
observed in an intranasal infection model that their
capsule-defective mutant was not able to grow in lung or tracheal tissues
and was unable to progress to a disseminated infection (24). Our
results suggest that a nosocomial strain does not need to be highly
virulent but only requires factors that allow its subsistence in the
human body, such as iron-chelating factors, and that protect it
from host defenses, such as high production of capsule, especially
when the strain is associated with a high resistance to antibiotics.
E. coli DH5
␣ isogenic clones producing TEM-68, TEM-47,
TEM-103, and TEM-1 were obtained using the vector pBK-CMV,
as previously described (34). The MICs of the clinical strain and
the different E. coli DH5
␣ clones were determined in duplicate by
Received 13 June 2011 Returned for modification 31 July 2011 Accepted 12 November 2011
Published ahead of print 21 November 2011
Address correspondence to Frédéric Robin, frobin@chu-clermontferrand.fr. Copyright © 2012, American Society for Microbiology. All Rights Reserved.
doi:10.1128/AAC.05079-11
0066-4804/12/$12.00 Antimicrobial Agents and Chemotherapy p. 1101–1104 aac.asm.org 1101
a microdilution method and were interpreted according to the
guidelines of the CASFM (7) (Table 1). The clinical isolates 3144
and 3151 were highly resistant to penicillins and also
demon-strated resistance to cefuroxime, cefotaxime, ceftazidime, and
az-treonam. They were intermediate or resistant to cefepime but
re-mained susceptible to imipenem. K. pneumoniae 3144 was less
resistant to
-lactam–-lactamase inhibitor combinations than
K. pneumoniae 3151. Cefoxitin remained active only against
iso-late 3144. TEM-68-producing E. coli DH5␣ harbored high levels
of resistance to penicillins, close to those of the TEM-103- and
TEM-47-producing clones. However, although its MICs for
penicillin-clavulanate combinations were the same as those of the
TEM-47-producing clone, they were lower than those of the
TEM-103-producing clone. The piperacillin-tazobactam
combi-nation was active against the three clones. The cephalosporin and
aztreonam MICs of the TEM-68-producing clone were close to
those of the TEM-47-producing E. coli. Finally, as observed with
the TEM-47-producing clone, E. coli DH5␣(pBK-TEM-68) was
susceptible to all oxyimino
-lactam–clavulanate combinations.
TEM-68, TEM-47, TEM-103, and TEM-1 were overproduced in
E. coli BL21(DE3), as previously described (36). They were
puri-fied to homogeneity, and the level of purity was estimated to be
⬎95% by sodium dodecyl sulfate-polyacrylamide gel
electropho-resis (2, 23). Their kinetic parameters (Table 2) were determined
by computerized microacidimetry as previously described (22).
TEM-68 was slightly more active than TEM-47 against penicillins
but remained 6- to 52-fold less active than TEM-103, and TEM-1.
Its Km
values for these substrates were also slightly lower than
those of TEM-47. TEM-68’s catalytic efficiency against penicillins
was higher than that of TEM-47 but remained 3- to 10-fold lower
than those of TEM-103 and TEM-1. TEM-68 harbored hydrolytic
activity against cephalothin that was close to those of TEM-47 and
TEM-1 and 1.8-fold lower than that of TEM-103. The cephalothin
K
mvalue was 1.6- to 7.6-fold lower for TEM-68 than for TEM-47,
TEM-103, and TEM-1. TEM-68’s catalytic efficiency against this
substrate was 1.7- to 6.3-fold higher than the catalytic efficiencies
of the three parental enzymes. TEM-68’s hydrolytic activities
against ceftazidime, cefotaxime, cefepime, and aztreonam were
close to that of TEM-47. Its Km
values for these substrates were
close to or even lower than those of TEM-47. Finally, its catalytic
efficiency against cefotaxime was close to that of TEM-47, and
against ceftazidime and cefepime, its catalytic efficiencies were
1.5- to 2.2-fold higher than those of TEM-47. TEM-68 was 2- to
2.6-fold less susceptible to clavulanate and tazobactam than
TEM-47 (50% inhibitory concentrations [IC
50s], 0.02 and 0.08
versus 0.01 and 0.03
M), but it remained more susceptible than
TEM-1 and TEM-103 (IC
50s, 0.02 and 0.08 versus 0.08 and 0.13
FIG 1 K. pneumoniae 3144 (TEM-47) and 3151 (TEM-68) biofilm formation.
Biofilms were developed in Dulbecco’s modified Eagle’s medium at 37°C after 6 h of incubation and stained with 0.5% crystal violet. (1) Positive control K.
pneumoniae LM21; (2) K. pneumoniae 3144; (3) K. pneumoniae 3151.
TABLE 1 MICs of-lactam antibiotics for K. pneumoniae 3144 (TEM-47), K. pneumoniae 3151 (TEM-68) and E. coli DH5␣ recombinants carrying
the indicated plasmids
-Lactama MIC (g/ml) for: K. pneumoniae 3144 (TEM-47) K. pneumoniae 3151 (TEM-68) E. coli DH5␣ carrying:
pBK-TEM-47 pBK-TEM-68 pBK-TEM-103 pBK-TEM-1 pBK-CMV
Amoxicillin ⬎2,048 ⬎2,048 ⬎2,048 ⬎2,048 ⬎2,048 ⬎2,048 4 Amoxicillin⫹ CLA 8 ⬎1,024 8 8 ⬎1,024 16 4 Ticarcillin ⬎2,048 ⬎2,048 ⬎2,048 ⬎2,048 ⬎2,048 ⬎2,048 2 Ticarcillin⫹ CLA 32 ⬎1,024 128 128 ⬎1,024 32 2 Piperacillin 1,024 1,024 ⬎2,048 ⬎2,048 ⬎2,048 512 2 Piperacillin⫹ TZB 4 1,024 2 1 2 2 2 Cephalothin ⬎256 ⬎256 ⬎256 ⬎256 64 4 4 Cefoxitin 4 16 4 4 4 4 4 Cefuroxime 32 256 256 256 0.06 4 4 Cefotaxime 8 32 32 16 0.06 0.06 0.06 Cefotaxime⫹ CLA 0.06 32 0.06 0.06 0.06 0.06 0.06 Ceftazidime 256 512 1,024 512 0.12 0.12 0.12 Ceftazidime⫹ CLA 0.25 256 1 0.5 0.06 0.12 0.12 Aztreonam 128 128 32 32 0.12 0.12 0.12 Aztreonam⫹ CLA 0.12 128 0.12 0.12 0.12 0.12 0.12 Cefepime 2 32 4 4 ⬍0.06 ⬍0.06 ⬍0.06 Cefepime⫹ CLA 0.25 32 ⬍0.06 ⬍0.06 ⬍0.06 ⬍0.06 ⬍0.06 Imipenem 0.12 0.25 0.25 0.25 0.25 0.25 0.25
aCLA, clavulanic acid at 2g/ml; TZB, tazobactam at 4 g/ml. Robin et al.
1102 aac.asm.org Antimicrobial Agents and Chemotherapy
M for TEM-1 and 0.72 and 0.22 M for TEM-103). The
stan-dard deviations for the IC
50results were lower than 15%. As
sug-gested by the MIC results, TEM-68 and TEM-47 have closely
re-lated kinetic parameters. However, TEM-68 was more active
against some substrates, including ceftazidime and aztreonam,
and harbored lower Km
values for cefotaxime and cefepime, which
allowed higher catalytic efficiencies against most of the oxyimino
-lactams tested. Increases in catalytic efficiencies against most
substrates, especially cephalothin, were also observed for the
Arg275Leu-harboring IRT TEM-103 in comparison to the
cata-lytic efficiencies of TEM-1. Brown et al. also reported that the
substitution Arg275Glu affected the catalytic efficiency of TEM
enzymes (4); the presence of the Glu275 residue decreased the
catalytic efficiency through an increase in K
m. In contrast, the
catalytic efficiencies of the Arg275Leu-harboring enzymes
TEM-68 and TEM-103 against most substrates were higher than
those of the parental enzymes, TEM-47 and TEM-1, respectively,
suggesting a role for Leu275 in the increase in catalytic efficiency.
There is little structural information about the Arg275Leu
substi-tution. Most authors consider that the Arg275Leu/Glu
substitu-tions probably modify the position of Arg244, as observed for the
substitutions at position 276 (6). Recently, Kather et al. and
Brown et al. observed that the Arg275Leu/Glu substitutions have a
stabilizing action in TEM enzymes (4, 21).
In conclusion, our study suggests that the production of a high
quantity of capsule and a few other virulence factors could be
sufficient to enhance the dissemination potency of a K.
pneu-moniae strain in a nosocomial setting, especially when associated
with the production of an ESBL. We also confirmed the role of the
Arg275Leu substitution in TEM-68 as a catalytic efficiency
en-hancer.
ACKNOWLEDGMENTS
We thank Marlene Jan and Rolande Perroux for their technical assistance, Sophie Quevillon-Cheruel for providing the modified pET9a plasmid, and Patrice Courvalin for providing us the clinical E. coli strain BM4511. This work was supported in part by grants from the Ministère de l’Enseignement Supérieur et de la Recherche (JE2526), INRA (USC2018), the Centre Hospitalier Régional Universitaire de Clermont-Ferrand, France, and the Ministère de la Santé, de la Jeunesse et des Sports.
REFERENCES
1. Alonso R, Gerbaud G, Galimand M, Courvalin P. 2002. TEM-103/ IRT-28-lactamase, a new TEM variant produced by Escherichia coli BM4511. Antimicrob. Agents Chemother. 46:3627–3629.
2. Bonnet R, et al. 2000. A novel class A extended-spectrum-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob. Agents Che-mother. 44:3061–3068.
3. Brisse S, et al. 2009. Virulent clones of Klebsiella pneumoniae: identifica-tion and evoluidentifica-tionary scenario based on genomic and phenotypic charac-terization. PLoS One 4:e4982.
4. Brown NG, Pennington JM, Huang W, Ayvaz T, Palzkill T. 2010. Multiple global suppressors of protein stability defects facilitate the evo-lution of extended-spectrum TEM-lactamases. J. Mol. Biol. 404: 832– 846.
5. Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 54:969 –976.
6. Chaibi EB, Sirot D, Paul G, Labia R. 1999. Inhibitor-resistant TEM -lactamases: phenotypic, genetic and biochemical characteristics. J. An-timicrob. Chemother. 43:447– 458.
7. Comité de l’Antibiogramme de la Société Française de Microbiologie. January 2010, posting date. Recommandations 2010. http://www.sfm -microbiologie.org/UserFiles/file/CASFM/casfm_2010.pdf.
8. De Champs C, et al. 1989. Prospective survey of colonization and infec-tion caused by expanded-spectrum--lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. J. Clin. Microbiol.
27:2887–2890.
9. Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multi-locus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178 – 4182.
10. Di Martino P, et al. 1995. Molecular characterization and adhesive prop-erties of CF29K, an adhesin of Klebsiella pneumoniae strains involved in nosocomial infections. Infect. Immun. 63:4336 – 4344.
11. Di Martino P, Cafferini N, Joly B, Darfeuille-Michaud A. 2003.
Kleb-siella pneumoniae type 3 pili facilitate adherence and biofilm formation on
abiotic surfaces. Res. Microbiol. 154:9 –16.
12. Domenico P, Salo RJ, Cross AS, Cunha BA. 1994. Polysaccharide capsule-mediated resistance to opsonophagocytosis in Klebsiella
pneu-moniae. Infect. Immun. 62:4495– 4499.
13. Domenico P, Tomas JM, Merino S, Rubires X, Cunha BA. 1999. Surface antigen exposure by bismuth dimercaprol suppression of Klebsiella
pneu-moniae capsular polysaccharide. Infect. Immun. 67:664 – 669.
14. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. 2004. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver ab-scess and septic metastatic complications. J. Exp. Med. 199:697–705. 15. Fang CT, et al. 2007. Klebsiella pneumoniae genotype K1: an emerging
pathogen that causes septic ocular or central nervous system complica-tions from pyogenic liver abscess. Clin. Infect. Dis. 45:284 –293. 16. Fiett J, et al. 2000. A novel complex mutant-lactamase, TEM-68,
iden-tified in a Klebsiella pneumoniae isolate from an outbreak of
extended-TABLE 2 Kinetic parameters of-lactamases TEM-47, TEM-68, TEM-103, and TEM-1a
-Lactam
TEM-68 (Ser238, Lys240, Met265, Leu275)
TEM-47 (Ser238, Lys240, Met265, Arg275)
TEM-103 (Gly238, Glu240, Thr265, Leu275)
TEM-1 (Gly238, Glu240, Thr265, Arg275) kcat (s⫺1) Km (M) kcat/Km (M⫺1· s⫺1) kcat (s⫺1) Km (M) kcat/Km (M⫺1· s⫺1) kcat (s⫺1) Km (M) kcat/Km (M⫺1· s⫺1) kcat (s⫺1) Km (M) kcat/Km (M⫺1· s⫺1) Benzylpenicillin 61.2 17.9 3.4 30.8 24.8 1.2 3,190 89.5 36 1,500 34 44 Amoxicillin 156.5 22.4 7 66.5 27.5 2.4 2,563 69.7 37 1,125 15 75 Ticarcillin 20.9 17.3 1.2 17.1 34.3 0.5 411 47.9 8.6 135 36 4 Piperacillin 172.7 45.3 3.8 150.1 52.6 2.8 1,228 30.15 41 1,250 55 23 Cephalothin 139.3 31.9 4.4 127.1 48 2.6 246 148 1.7 165 242 0.7 Ceftazidime 22.7 29.3 0.77 19.8 56.3 0.35 ⬍0.1 ND ⬍0.1 ND Cefotaxime 178.1 100.4 1.8 212.7 110.9 1.9 ⬍0.1 ND ⬍0.1 ND Aztreonam 0.9 17.2 0.05 ⬍0.2 40.5b ⬍0.1 ND ⬍0.1 ND Cefepime 18 30.5 0.59 34.8 89.5 0.39 ⬍0.1 ND ⬍0.1 ND
aThe standard deviation for analysis wasⱕ15%. ND, not determined. bK
mvalues were determined as the Kivalues by substrate competition with benzylpenicillin.
-Lactamases and Virulence of a K. pneumoniae Strain
February 2012 Volume 56 Number 2 aac.asm.org 1103
spectrum-lactamase-producing Klebsiellae. Antimicrob. Agents Che-mother. 44:1499 –1505.
17. Fung CP, et al. 2002. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endoph-thalmitis? Gut 50:420 – 424.
18. Hennequin C, Forestier C. 2007. Influence of capsule and extended-spectrum-lactamases encoding plasmids upon Klebsiella pneumoniae adhesion. Res. Microbiol. 158:339 –347.
19. Hennequin C, Forestier C. 2009. oxyR, a LysR-type regulator involved in
Klebsiella pneumoniae mucosal and abiotic colonization. Infect. Immun.
77:5449 –5457.
20. Izquierdo L, et al. 2003. The Klebsiella pneumoniae wabG gene: role in biosynthesis of the core lipopolysaccharide and virulence. J. Bacteriol.
185:7213–7221.
21. Kather I, Jakob RP, Dobbek H, Schmid FX. 2008. Increased folding stability of TEM-1-lactamase by in vitro selection. J. Mol. Biol. 383: 238 –251.
22. Labia R, Andrillon J, Le Goffic F. 1973. Computerized microacidimetric determination of-lactamase Michaelis-Menten constants. FEBS Lett.
33:42– 44.
23. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680 – 685.
24. Lawlor MS, Hsu J, Rick PD, Miller VL. 2005. Identification of Klebsiella
pneumoniae virulence determinants using an intranasal infection model.
Mol. Microbiol. 58:1054 –1073.
25. Ma LC, Fang CT, Lee CZ, Shun CT, Wang JT. 2005. Genomic hetero-geneity in Klebsiella pneumoniae strains is associated with primary pyo-genic liver abscess and metastatic infection. J. Infect. Dis. 192:117–128. 26. Markowitz SM, Veazey JM, Jr, Macrina FL, Mayhall CG, Lamb VA.
1980. Sequential outbreaks of infection due to Klebsiella pneumoniae in a neonatal intensive care unit: implication of a conjugative R plasmid. J. Infect. Dis. 142:106 –112.
27. Nassif X, Fournier JM, Arondel J, Sansonetti PJ. 1989. Mucoid pheno-type of Klebsiella pneumoniae is a plasmid-encoded virulence factor. In-fect. Immun. 57:546 –552.
28. Nassif X, Sansonetti PJ. 1986. Correlation of the virulence of Klebsiella
pneumoniae K1 and K2 with the presence of a plasmid encoding
aerobac-tin. Infect. Immun. 54:603– 608.
29. Neuwirth C, et al. 2001. TEM-89-lactamase produced by a Proteus
mirabilis clinical isolate: new complex mutant (CMT 3) with mutations in
both TEM-59 (IRT-17) and TEM-3. Antimicrob. Agents Chemother. 45: 3591–3594.
30. O’Toole GA, Kolter R. 1998. Flagellar and twitching motility are neces-sary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol.
30:295–304.
31. Poirel L, Mammeri H, Nordmann P. 2004. TEM-121, a novel complex mutant of TEM-type-lactamase from Enterobacter aerogenes. Antimi-crob. Agents Chemother. 48:4528 – 4531.
32. Putze J, et al. 2009. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect. Immun. 77:4696 – 4703.
33. Regue M, et al. 2004. A gene, uge, is essential for Klebsiella pneumoniae virulence. Infect. Immun. 72:54 – 61.
34. Robin F, et al. 2006. CMT-type -lactamase TEM-125, an emerging problem for extended spectrum-lactamase detection. Antimicrob. Agents Chemother. 50:2043–2048.
35. Robin F, et al. 2007. TEM-158 (CMT-9), a new member of the CMT-type extended-spectrum-lactamases. Antimicrob. Agents Chemother. 51: 4181– 4183.
36. Robin F, et al. 2005. TEM-109 (CMT-5), a natural complex mutant of TEM-1-lactamase combining the amino acid substitutions of TEM-6 and TEM-33 (IRT-5). Antimicrob. Agents Chemother. 49:4443– 4447. 37. Robin F, et al. 2007. Evolution of TEM-type enzymes: biochemical and
genetic characterization of two new complex mutant TEM enzymes, TEM-151 and TEM-152, from a single patient. Antimicrob. Agents Che-mother. 51:1304 –1309.
38. Sahly H, et al. 2000. Capsule impedes adhesion to and invasion of epi-thelial cells by Klebsiella pneumoniae. Infect. Immun. 68:6744 – 6749. 39. Sirot D, et al. 1997. A complex mutant of TEM-1-lactamase with
mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob. Agents Che-mother. 41:1322–1325.
40. Tarkkanen AM, Virkola R, Clegg S, Korhonen TK. 1997. Binding of the type 3 fimbriae of Klebsiella pneumoniae to human endothelial and urinary bladder cells. Infect. Immun. 65:1546 –1549.
Robin et al.
1104 aac.asm.org Antimicrobial Agents and Chemotherapy