The MIT Faculty has made this article openly available.
Please share
how this access benefits you. Your story matters.
Citation
Avilés-Pagán, Emir E., and Terry L. Orr-Weaver. “Activating
Embryonic Development in Drosophila.” Seminars in Cell &
Developmental Biology (February 2018).
As Published
http://dx.doi.org/10.1016/J.SEMCDB.2018.02.019
Publisher
Elsevier BV
Version
Final published version
Citable link
http://hdl.handle.net/1721.1/116724
Terms of Use
Creative Commons Attribution-NonCommercial-NoDerivs License
Contents lists available atScienceDirect
Seminars
in
Cell
&
Developmental
Biology
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / s e m c d b
Review
Activating
embryonic
development
in
Drosophila
Emir
E.
Avilés-Pagán,
Terry
L.
Orr-Weaver
∗WhiteheadInstituteandDept.ofBiology,MassachusettsInstituteofTechnology,Cambridge,MA,UnitedStates
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received19June2017 Receivedinrevisedform 21December2017 Accepted11February2018 Availableonlinexxx Keywords: Oocyte-to-embryotransition Oocytematuration Meiosis
MaternalmRNAtranslation Fertilization
a
b
s
t
r
a
c
t
Thetransitionfromoocytetoembryomarkstheonsetofdevelopment.Thisprocessrequirescomplex regulationtolinkdevelopmentalsignalswithprofoundchangesinmRNAtranslation,cellcyclecontrol, andmetabolism.Thiscontrolisbeginningtobeunderstoodformostorganisms,andresearchinthefruit flyDrosophilamelanogasterhasgeneratednewinsights.Recentfindingshaveincreasedour understand-ingoftherolesplayedbyhormoneandCa2+signalingeventsaswellasmetabolicremodelingcrucial forthistransition.Specializedfeaturesofthestructureandassemblyofthemeioticspindlehavebeen identified.Thechangesinproteinlevels,mRNAtranslation,andpolyadenylationthatoccurastheoocyte becomesanembryohavebeenidentifiedtogetherwithkeyaspectsoftheirregulation.Herewehighlight theseimportantdevelopmentsandtheinsightstheyprovideontheintricateregulationofthisdramatic transition.
©2018TheAuthors.PublishedbyElsevierLtd.ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Contents
1. Introduction/overview...00
2. Meiosisduringoocytematuration ... 00
2.1. Meioticprogression...00
2.2. Assemblyoftheoocytemeioticspindle...00
2.3. Regulationofgeneexpressionduringoocytematuration...00
3. Oocytemetabolismpriortoandduringmaturation...00
3.1. Lipidstorage...00
3.2. Glycogenaccumulation...00
3.3. Mitochondriaselectionandquiescence...00
4. Signalingpathwaysaffectinglateoogenesis...00
4.1. Ecdysonesignalingoflateoogenesisevents...00
4.2. Insulinsignaling...00
5. Eggactivation...00
5.1. Calciumsignaling ... 00
5.2. Completionofmeiosis...00
5.3. Changesingeneexpressionateggactivation...00
6. Fertilizationandearlydevelopment ... 00
6.1. Redoxstateandactivationofspermchromatin...00
6.2. Startofembryonicdivisions...00
7. Parallelstootherorganisms...00
8. Conclusions...00
Acknowledgments...00
References...00
∗ Correspondingauthor.
E-mailaddress:weaver@wi.mit.edu(T.L.Orr-Weaver).
https://doi.org/10.1016/j.semcdb.2018.02.019
1084-9521/©2018TheAuthors.PublishedbyElsevierLtd.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4. 0/).
1. Introduction/overview
Innearlyeveryanimaltheearlyembryonicdivisionsoccurinthe absenceoftranscriptionofthezygoticgenome.Inhumanembryos, transcription is not thoughtto initiateuntil after two or three embryonicdivisions.Inorganismsinwhichtheembryodevelops outsideofthemother,rapidembryogenesisisneededtoproduce motileprogeny, necessitatingan accelerateddivision cycle that precludesgrowthandtranscription.MaternalstockpilesofmRNAs, translationalmachinerycomponents,andnutrientspermitearly embryogenesistooccurintheabsenceofzygotictranscription.
Thisdevelopmentalstrategy requiresthatmaternalstoresbe depositedintotheoocyteduringoogenesis.Thisisaccomplished duringanarrestinmeiosis,whentheimmatureoocyteisarrestedin prophaseIofmeiosis.Releasefromthisarrestandmeiotic progres-sionthenoccurduringoocytematuration,leadingtoasecondary meioticarrest.Inmostvertebrates,theoocytecompletesthefirst meioticdivisionandhasasecondaryarrestatmetaphaseofmeiosis II.Ininsects,thissecondaryarrestisinmetaphaseI.Thesecondary meioticarrestisthoughttofacilitatecouplingofthecompletionof meiosistofertilization,anditisreleasedbyeggactivationand fertil-ization.Thus,developmentalcuesmustimpactmeioticprogression andregulatedepositionofmaternalproductsduringoogenesis.
Thecontroloftheoocyte-to-embryotransitioninDrosophila parallelsthatofotheranimals,butDrosophilaoffersexperimental advantagesasamodeltodeciphertheregulationofthiskey devel-opmentalevent.Inadditiontotheeaseofgeneticanalysisandtools forreversegenetics,thestagesofoocytematurationare morpho-logicallydistinct,permittingsufficientquantitiestobeisolatedfor biochemicalanalysesandimaging.Releaseofthesecondary mei-oticarrestandpromotionofthechangesintheproteomeandmRNA translationcanbetriggeredbyinvitroactivationofmatureoocytes. HerewedetailrecentadvancesfromDrosophilainour under-standingoftheonsetofmeioticdivisioninmeiosis,theformation of thespecialized meiotic spindle, themetabolic and signaling eventsaccompanyingoocytematuration,thecontrolofegg acti-vation by Ca2+ fluxes, and the consequences of fertilization to activateembryogenesis.Globalapproachesarediscussedthathave
defined the changes in protein levels, mRNA translation, and
polyadenylationthataccompanyoocytematurationandegg acti-vation.Ultimatelythe oocyte-to-embryotransition leadstothe activationofexpressionofthezygoticgenome,astepcalledthe
maternal-to-zygotictransition(MZT).Manyadvanceshavebeen
madeindefiningthecontrolofthisdevelopmentalhandoff,but thesehavebeenextensivelyreviewedrecently,sothe maternal-to-zygotictransitionisnotcoveredinthisreview[1–5].
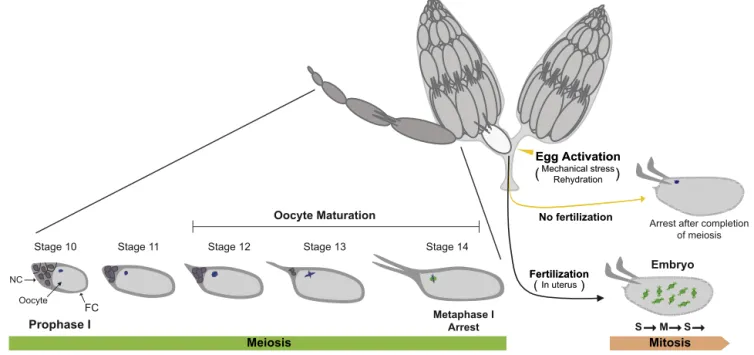
Priortodiscussingoocytematuration,anoverviewofthelate stagesofDrosophilaoogenesisiswarranted.Theoocytedevelops withinaneggchamberinaseriesoffourteenmorphologically dis-tinctstages,andtheeventsofoocytematurationoccurinstages 12–14 (Fig. 1). A single cell out of a group of 16 sister cells, whichremainattachedbyringcanalstosharethesamecytoplasm,
becomes anoocyte, while theother fifteen becomesupporting
nursecells.TheoocytearrestsinprophaseI,afterhomologous chro-mosomeshavepairedandareattachedphysicallybychiasmata(the productofacrossoverrecombinationevent)orheterochromatin links[6,7].Theselockedhomologpairsarecalledbivalents,and theycontainfourDNAduplexes,duetothetwosisterchromatids ofeachhomolog.
2. Meiosisduringoocytematuration
Oocytematurationistheprocessbywhichtheoocyteenters the first meiotic division, releasing the primary meiotic arrest inprophaseI.Duringoocytematuration, meiosisresumes,with
nuclearenvelopebreakdown(alsoreferredtoasgerminalvesicle breakdown,GVBD).Aspecializedmeioticspindleisassembled,and oneachhomologofthebivalentthesister-chromatidkinetochores aremono-orientedtowardsthesamepoleofthemeioticspindle. Becausethetwohomologsareheldtogetherbutareattachedto microtubules(MTs)fromoppositepoles,thisisastable configu-rationpresentatthemetaphaseIarrest inthematurestage14 oocyte.
2.1. Meioticprogression
ProgressionfromtheprophaseIarresttometaphaseIrequires cellcycleregulationtoactivatetheCyclinB/CDK1mitotickinase. TheroleofconservedregulatorsinactivatingCyclinB(CycB)/CDK1
during Drosophila oocyte maturation has been reviewed [8].
Briefly,theonsetofmaturationandGVBDaredictatedbythe tim-ingofCDK1activation,asinvertebrates,inwhichactiveCycB/CDK1 iscritical.InDrosophilatwoothermitoticCyclins,AandB3,also contributetooocytematuration[9].ThisrequirestheCdc25
phos-phataseTWINE,whoseactivationis dependentonPOLOkinase,
whoseactivityisinturninhibitedintheprophaseI-arrestedoocyte byMATRIMONY(MTRM)[10,11].AslevelsofPOLOproteinincrease [12], it is thought that it exceeds thepool of MTRM by stage 13ofoogenesis,thustriggeringmaturation.Consistentwiththis,
mutationsinMtrmdominantlycauseprematureGVBD.Maturation
additionallyiscontrolledbyEndosulfine(ENDOS)andthe Great-wallkinase[11,13].WhenphosphorylatedbyGreatwall,ENDOS caninhibitthePP2Aphosphatase[14,15],andthiscouldpromote theonsetofmeiosis.Infact,mutationsinendosresultindelayed GVBDandfailuretoprogresstometaphaseI.Inotherorganisms Greatwallisactivatedinafeed-forwardmechanismbyCycB/CDK1, butitisnotknownwhetherthisisalsotrueinDrosophilaoocytes. The developmental signals responsible for triggering oocyte
maturationand how these lead to the activation of POLO and
ultimatelyCycB/CDK1remainsunknown.Technicalhurdleshave
hinderedevaluationofknowncellcyclecomponents,asitis dif-ficulttoinactivatefunctionsspecificallyinstage13or14oocytes. Additionally,oncetheeggshellisdepositedinstage13,theoocyte cannotsuccessfullybetreatedwithmanyinhibitors.
2.2. Assemblyoftheoocytemeioticspindle
ThemeioticspindleformsasGVBDoccurs,anditislocalized nearthedorsalsurfaceoftheoocyte.Thisspindleisdistinguished fromthemeioticspindleinspermatocytesorfrommitotic spin-dles.Themicrotubuleorganizingcenter(MTOC),thecentrosome, iscomposedofapairofcentrioles surroundedbypericentriolar material(PCM)[16].Inmostanimaloocytes,however,centrioles arelostandtheoocyteassemblesanacentrosomalspindle,which formsintheabsenceofcentrioles.DuringDrosophilaoogenesis, thecentriolesfromthenursecellsmigrateintotheoocytetoform alargeMTOCwith60centriolesthatorganizestheMTsnecessary forlocalizationofpatterningproteinsandRNAs[17],butallofthese centriolesareultimatelylostasoogenesisproceeds.
Recentadvancesinimagingtechnologies,incombinationwith fluorescentlytaggedproteinfusionsforkeycentrioleandPCM com-ponents,havefacilitatedanalysisoftheeventsleadingtocentriole lossduringDrosophilaoogenesis[17].ThePCMstartsshrinking duringmid-oogenesis(stages7/8),priortooocytematuration,and thiscorrelateswithareductionofPOLOlevelsattheMTOC. Centri-olenumberdecreasesonceoocytematurationbegins,presumably asaconsequenceofPCMloss[17].POLOplaysakeyrolein cen-triolelossinoocytes,asreductionofPOLOproteinlevelsbyRNAi ledtoprematurelossofcentriolemarkersduringstage10,whereas tetheringofPOLOtothecentriolebyfusingittothePACTcentriole proteinledtocentrioleretentioneveninmatureoocytes.Notably,
Fig.1.OverviewofDrosophilaoogenesisandearlydevelopment.Drosophilahastwoovaries,eachofwhichcontains20–30ovarioles.Eachovarioleislikeaproductionline, wheretheoocytedevelopswithinaneggchamberinaseriesoffourteenmorphologicallydistinctstages.Asinglecelloutofagroupof16sistercells,whichremainattached byringcanalstosharethesamecytoplasm,becomesanoocyte,whiletheotherfifteenbecomesupportingnursecells(NCs).Thedevelopingoocyteissurroundedbyalayer ofpolyploidfolliclecells(FCs),whichplayaroleinpatterninginearlystagesandareresponsiblefordepositingtheeggshellduringlateoogenesis.Theoocytearrestsin prophaseI,andthisarrestisreleaseduponoocytematuration(stages12–14),withmeiosisprogressingtoasecondaryarrestpointinmetaphaseIinmatureoocytes.As oocytematurationproceeds,NCsdumptheircontentintotheoocyteandthenproceedtoundergocelldeath.Uponovulation,thematureoocytedescendsintotheoviduct, wheremechanicalforcesandhydrationinduceeggactivation.Afterfertilizationintheuterus,thematernalandpaternalhaploidnucleifuseandstarttoundergothirteen divisioncycles,characterizedbyrapidS-andMphases,inacommoncytoplasm.Ifeggactivationoccursbuttheeggisnotfertilized,thendevelopmentisarrestedafter completionofmeiosis,andthefourfemalemeioticproductscongregateasapolarbody.Inadditiontocompletionofmeiosis,thechangesintranslationofmaternalmRNAs occurduringeggactivation,independentoffertilization.DNAisrepresentedinblue,spindlesingreen.
Fig.2.Fertilizationandthestartofembryonicdivisions.Theactivatedeggisfertilizedonceitreachestheuterus.Insetsrepresentprogressionofcytoplasmiceventsfollowing fertilization.First,asinglespermenterstheeggthroughthemicropyle,andremainsattheanterioroftheoocyte(darkblue),asfemalemeiosisiscompletedintheegg andfourmeioticproducts(pink)areproduced.Thespermcontributesthecentrosomes(green)andpaternalhaploidgenomiccontent(darkblue)totheembryo.Initially themalepronucleusisinahighly-condensedchromatinstateduetothepresenceofprotamines.Thespermnuclearmembranebreaksdown,andprotaminesareevicted fromthespermDNAandreplacedbyhistonesduringspermnucleusactivation,inaprocessthatrequiresthehistonechaperoneHIRA(notshown)andthematernally providedthioredoxinDHD(shown).Afterwards,thepaternalcentriolesandMTOCs(greendots)initiatetheformationofamitoticaster(brightgreen),whichincreasesin sizeuntilitreachesthefemalemeioticproductintheclosestproximity.Thefemalepronucleusisthenpulledtowardsthemalepronucleusduringpronuclearapposition. Thefirstdivisionfollows,inwhichthereisnointermixingofthematernalchromosomes(lightpink)andpaternalchromosomes(lightblue)untiltelophase.Theremaining femalemeioticproductsundergochromosomecondensationandremainarrestedaspolarbodies(darkpink)atthecortexoftheegg.Subsequentmitoticdivisionsoccurin acommoncytoplasm,orsyncytia,startingatthemiddleoftheembryoandthenexpandingtowardsthesurfacewithincreasednuclearnumber.Thesyncytialdivisionsare drivenbymaternallyprovidedproteinsandmRNAs.
apartfromdemonstratingPOLOprotectscentrioles,thispermitted testingtherequirementofcentriolelossintheoocyte.Retentionof centriolesinmatureoocytesresultedindefectsduringearly embry-onicdivisions[17],supportingtheideathatcentrioleeliminationin
theoocyteiscrucialforensuringthatthereareonlytwocentrioles inthefertilizedembryo,bothderivedfromthesperm(Fig.2).
AsGVBDoccursintheoocyte,thechromosomescanbeobserved tobindMTs,servingasthecenterforspindleorganization.This
Fig.3.Signalingeventsandmetabolicchangesduringoocytematuration.Lipidsandcarbohydratestoresthatwillserveasenergysourcesaccumulateinastepwisemanner duringlateoogenesis.Attheonsetofmaturation,lipidsarepresentintheformofdroplets(yellowcircles)inthecytoplasm.Thislipidaccumulationispromotedbyecdysone signaling.Theinsulinsignalingpathwayisactiveatthisstage,leadingtotheinactivationofGSK3.Asoocytematurationproceeds,insulinsignalingisnolongerpresent,and GSK3nowisabletoregulatechangesincarbohydratemetabolism,particularlytheaccumulationofglycogen(greenstars)duringmaturation.Inaddition,GSK3mediates theentryofmitochondriaintoaquiescentstate,characterizedbyinactivationoftheelectrontransportchain,mitochondrialmembranedepolarization,andachangein mitochondrialmorphology(depicted).GSK3alsoisresponsibleforactivatingphosphorylationofthecalcipressinSRA(notshown),addingalevelofcoordinationbetween insulinandthesubsequentCa2+signalingeventsateggactivation.Ecdysonesignalingactsonceagainonmatureoocytes,thistimetostimulateovulation,whichwillresult
intheoocyteproceedingthrougheggactivation.Thesupportingnursecells(darkgray)alsoprovidestockpilesofmaternalmRNAs,whiletheoocyteprogressesinmeiosis toametaphaseIarrest(spindleingreen,chromosomesinblue).
requiresamechanismtoproduceabipolarspindlewithpolesand toensurebipolarattachmentofthehomologstothespindle.The
ChromosomePassenger Complex(CPC)plays animportant role
inmeioticspindleformationandcorrectchromosomeattachment [18,19],withcomponentsoftheCPClocalizingtothemiddleofthe formingspindle.TheMT-bundlingactivityofthekinesinSUBITO alsoisrequiredforspindleformation[20],anditmayacttorecruit andstabilizeotherMT-bundlingproteinstothespindle.SomePCM proteinspresentatthepolesmayacttotaperthepoles.
Depletion and mutation of kinetochore proteins have
pro-videdcriticalinsightsintokinetochorefunctioninmeioticspindle formation[21]. Although theinitialformation of thespindle is kinetochore-independent,boththecentralspindleandthe kine-tochoresbecomenecessarytostabilizethespindle.Itisproposed thatinitiallateralattachmentsoftheMTstothekinetochoreare followedbyend-onattachmentsashomologsattainstablebipolar kinetochoreattachmentsonthespindle.TheCPCandothercentral spindleproteinsarethoughttofacilitatekinetochore-MTbinding intheprometaphasebeltmodel,whichspeculatesthatthecentral spindleactsasabelttoconcentratetheplusendsofMTsnearthe kinetochores[19].
2.3. Regulationofgeneexpressionduringoocytematuration Oocytematurationoccursintheabsenceoftranscriptionwith stablelevelsofmRNAs[22].Hence,theeventsofmaturationmust beregulatedposttranscriptionally.Theenigmaofhowmaternal mRNAscanbeloadedintotheoocyteandstablymaintainedfor pro-longedperiods,yetnottranslated,wasexplainedbytheproposal thatmaternalmRNAsaremaskedbyshortpoly(A)tails,whichboth stabilizemRNAsandpreventtheirtranslation.Thus,aninitiating eventofoocytematurationwouldbecytoplasmicpolyadenylation
andtranslationofselectedmRNAs.Thisindeedhasbeenshown
tobethecaseinXenopusandmouse,inwhichpolyadenylation
leadstotranslationofmitoticCyclinsthatactivateCyclin/CDKand promotetheonsetofmeiosis[23,24].
Recentglobalanalyseshaveidentifiedthechangesinpoly(A) taillengthandmRNAtranslationduringmaturation,fromstage 11throughstage14oocytes,scoringabout4500mRNAs[22,25].
These studies revealed that regulation of translation in this
developmentalwindowismorecomplexthansimple
polyadeny-lationand unmaskingof maternalmRNAs.Hundredsof mRNAs
aretranslationallyupregulated, buthundredsaretranslationally
downregulated.These changesin translationare dynamic,with
somemRNAs, suchas cyclinB and fizzy(Cdc20) mRNAs,being
progressivelytranslationallyupregulatedinstages11–14,others not upregulated until stage 14, and others being translated in
stages11and12andthendownregulated.Poly(A)taillengthalso dynamicallychangesduringoocytematuration[22,25],withsome mRNAsexhibitingincreasedpoly(A)taillengthbutothers under-goingpoly(A)tailshortening.Ingeneral,forboth upand down regulatedmRNAs,translationalefficiencycorrelateswithpoly(A)
lengthchanges.TheGLD-2likecytoplasmicpoly(A)polymerase
encodedbywispy(wisp)isresponsibleforpoly(A)taillengthening duringoocytematurationandeggactivation[22,25–28].
Acomplementarystudyemployedquantitativemass
spectrom-etrytomeasurechangesinthelevelsofeachproteinbetweenstage 11and14[12].Approximately30%of3400scoredproteins signif-icantlychangeinlevels,withhundredsofproteinsincreasingand
hundredsdecreasing.Theweakconcordancebetweenchangesin
proteinlevelsandchangesintranslationefficiencyfortheirmRNAs indicatedthatproteinlevelsareaffectedbothbymRNA transla-tionandposttranslationally,likelybydegradation.Proteinsthat increaseinabundancearethoseexpectedtoactduringthe mei-oticdivisions, asisthecase for severalMT-associatedproteins. Proteinswhoseactivityisnotrequireduntiltheonsetof embryoge-nesisalsoincreaseduringoocytematuration,whichsuggeststhat itmaybenecessarytostockpiletheseproteinspriortoegg activa-tiontoensuresufficientquantitiesfortherapidearlyembryonic divisions.ComponentsoftheDNAreplicationmachinery, centro-somalproteins,andembryonicpatterningregulatorsareexamples ofproteinsinthisclass.
3. Oocytemetabolismpriortoandduringmaturation
Oogenesisresultsintheoocytebeingstockpilednotonlywith
mRNAsbutalsowithnutrientsandorganelles.Recentadvances
haveshedlightontheregulationofnutrientdepositionand uncov-eredactiveregulationofmitochondriaduringDrosophilaoogenesis (Fig.3).
3.1. Lipidstorage
Accumulationoftriglyceridesandsterolsintheoocytehasbeen observedinotherorganismsaswellasinDrosophila.Theseare storedintheformofcytoplasmiclipiddropletsintheoocyte[29],
andapartfromtheiruseinATPproductionandmembranelipid
biosynthesisduringembryogenesis[30],theselipiddropletscan haveotherroles,suchasanchoringandstoringhistones[31]. Accu-mulationoflipidsstartsasearlyasstage10ofoogenesis,andis regulatedinpartbythetranscriptionfactorSREBP[32].SREBP reg-ulatestheexpressionoftheLDLreceptorhomologLpR2,whichis requiredforlipiduptakeinthegermline[33],inadditiontoother targetgenesinvolvedintheuptakeandstoringoflipids.Thestores
oflipidsaccumulatedduringoogenesisserveasanenergysource
duringembryonicdevelopment.
3.2. Glycogenaccumulation
Carbohydratereservesalsoareamassedduringoogenesisand
maternally provided to the egg. Together with lipids
accumu-latedearlierinoogenesis,thesecarbohydratestoresaretheenergy sources for embryogenesis [34]. The accumulation of carbohy-dratesoccursintheformofanincreaseinglycogenlevelsduring late oogenesis, coinciding with theonset of oocyte maturation [35].Theincreaseinglycogenisaccompaniedbyincreasesin gly-colyticandcitricacidcycleintermediates[35],suggestingthatflow throughthesecatabolicpathwaysisredirectedtowardsglycogen anabolism.Consistentwiththis,mutanteggchambersforthe glu-coneogenesiscomponentpepckexhibitdecreasedglycogenlevels [35].However,asthemembraneoftheoocytedoesnotbecome impermeabletosmallsolutesuntileggactivation,itremains pos-siblethatextracellularmetabolitesmightbetransportedintothe
oocyte.Thus, remodeling of carbohydrate metabolismcouldbe
accompaniedbythetransportofmetabolitesforproperglycogen accumulation.Theconsequencesofinterferingwiththeglycogen pathwayspecificallyanditseffectsonearlyembryogenesisremain tobedetermined.
3.3. Mitochondriaselectionandquiescence
MitochondriaproducemostoftheATPineukaryoticcellsand are the exclusive site of oxidative phosphorylation in the cell.
Mitochondriacontaintheirown genomeor mitochondrial DNA
(mtDNA), and mutations in mtDNA can lead to mitochondrial
dysfunctionandtodeleteriouseffectsonorganismalhealth[36]. Mitochondria,andthereforemtDNA,areinheritedmaternally[36],
as paternally derived mitochondriaare degradedeither during
spermatogenesisor,suchasinDrosophila,shortlyafter fertiliza-tion[37,38].Therefore,thestate,respiratoryactivityandquality ofmitochondriadepositedduringoogenesisdeterminethe mito-chondriapopulationinheritedbythefutureembryo.
Mitochondrial selection occurs early in oogenesis, so the
mitochondrial diversity inherited by the egg has already been
established in mature oocytes. Competition between different
mtDNAsoccursaftergermlinestemcell(GSC)divisions[39], coin-cidingwithmtDNA replication[40].Althoughthereispurifying selectionagainstdefectivemtDNAviacompetition,some defec-tivegenomesarenotcompletelyeliminated[39],beingpresumably
maintainedbycomplementationwithothermtDNAsinthesame
organelleorinthepopulation.
Anotherstepofselectiondetermineswhichmitochondriaform partofthepolecells,andthusthefuturegermline.Mitochondria accumulateattheposterioroftheoocyte,thefuturesiteof primor-dialgermcell(PGC)formation,followingstage10andcontinuing throughmaturation[41],aprocessthatrequiresthegermplasm componentoskar[41].Mitochondriafromthisposteriorly accumu-latedpoolwillbelater,duringembryogenesis,incorporatedinto theemergingpolecells.Whetherspecificmitochondrialgenomes areselected forthis localization basedonfitnessor iftheyare selectednon-specificallyfromthepopulationsubjectedto purify-ingselectionearlierinoogenesisremainsunclear.
Therespiratoryactivityofmitochondriaalsoissubjectto regula-tion.Itwasobservedthatmitochondriaenterastateofquiescence during late oogenesis, and do not regain activityuntil later in embryonicdevelopment[35].Inearlyoogenesis,whenselection occurs,mitochondriaexhibitaperinuclearlocalizationandhave
astrongmembranepotential,asmeasuredusingthe
mitochon-drialmembranepotentialdyeTMRE.Incontrast,followingstage10, mitochondriabecomedisperse,arestructurallydifferent,andtheir
membranebecomesdepolarized,seeminglythrough
downregula-tionofmostelectrontransportchain(ETC)complexactivityduring lateoogenesis.Theentryofmitochondriaintothisquiescencent stateappearstoberegulatedbyinsulinsignaling[35],and
coin-cideswiththeglycogenaccumulationandmetabolicremodeling
occurringduringoocytematuration.Thus,quiescenceof mitochon-driamaybeameanstomodifythemetabolicoroxidativestateof oocytestosupporttheenergyrequirementsoftheearlyembryo.
4. Signalingpathwaysaffectinglateoogenesis
Althoughthecuesthattriggertheonsetofoocytematuration inDrosophilaremaintobeidentified,steroidandinsulinsignaling havebeendemonstratedtoaffectnutrientdepositioninto matur-ingoocytes.SteroidsignalingplaysaparallelroleinDrosophilaas inmammalsinpromotingruptureofthefolliclecelllayerduring ovulation(Fig.3).
4.1. Ecdysonesignalingoflateoogenesisevents
Signalingthroughtheecdysonereceptor(EcR)anditsligand,the activeformofthesteroidhormoneecdysone,20-hydroxyecdysone (20E),isinvolvedinmultipleprocessesduringlateoogenesis.The ovaryisthemainsourceofecdysoneproduction,with20Ebeing producedbythefolliclecell(FC)layerintheadultovary[42].In additiontoactingearlierin oogenesis,ecdysonepromoteslipid
accumulation beginning at stage 10 of oogenesis by activating
SREBP.Inadditiontothislocalsignaling,geneticanalysisofa dom-inantnegativeEcRgenedriventobeexpressedsolelyinneurons revealedthat20Eproducedbythegermlinemightalsomodulate feedingbehaviorinfemaleflies[43].Thishasbeenproposedtobe
amechanismthroughwhichlocalandsystemicmetabolic
modu-lationbyecdysonecreatesahomeostaticmetabolicstate,whereby
behavioral changes and directed lipid uptake by the germline
ensureoptimallipidstorageintheoocyte[43].
Apartfromthisearlierroleinlipidstorage,ecdysoneactsalso duringovulation.Duringovulation,amatureoocytefromoneofthe ovariolesentersthelateraloviduct,causingtheruptureoftheFC layer[44],andcoincidingwitheggactivation[45].Theruptureof theFClayerrequirestheactionofSHADE,whichconvertsecdysone intothe20E formin theposteriorFCsoftheeggchamber[46]. Theresultingincreasein20Eproductionleadstoautocrine activa-tionofaformofEcR,whichinturnactivatesexpressionofgenes intheposteriorfolliclecellsthatpromoteruptureoftheFClayer [46].Additionalcontrolofthisprocesscomesfromactivationof OAMBinposteriorFCs[47],a receptorfortheneurotransmitter octopamineknowntoregulateovulation,egglayingandresponse tomaleaccessoryproteins[48,49].Together,thesefindingssuggest thatecdysoneandoctopaminesignalingarecoordinatedtocontrol oocytereleaseduringovulation.
4.2. Insulinsignaling
Insulin signaling regulates germline stem cell divisions and manyotheraspectsofeggchamberdevelopment[50,51].Although activethroughoutearlyoogenesis,theinsulinsignalingpathwayis shutoffinmaturingeggchamberstoallowforglycogen accumu-lation.InDrosophila, eightinsulin-likepeptides(dILPs)serveas ligandsfortheinsulinreceptors,InRandLgr3,intargettissues,and theeffectsofthesedILPsvarybetweentissues[52].Intheovary, dilp8and dilp5arereportedly expressed[52], withdilp5mRNA detectedbyinsituhybridizationinFCsduringmid-oogenesis[53]. TheeffectorkinaseAKT,downstreamofInR,initiallyantagonizes Glycogensynthasekinase3(GSK3),anotherdownstream com-ponentofinsulinsignalingthatpromotesglycogenaccumulation [35]. Inmaturingoocytes,AKT activityis decreased andGSK3
Fig.4.OverviewofeggactivationinDrosophila.Passageoftheoocytethroughtheoviducttriggerseggactivation.Duringactivation,asinglewaveofcalciummovesthrough thecytoplasm,startingfromthepolesandmovingtowardsthemiddleoftheegg.TheincreaseinintracellularCa2+requiresaninfluxofextracellularCa2+,presumably
viaactivationofmechanosensitivechannelsandareleaseofCa2+fromERstores.TheincreaseinintracellularCa2+leadstoactivationinCa2+signalingcomponentsategg
activation,suchasSRA,calcineurin,andpotentiallyCAMKII,whichthenregulatedownstreamtargets.ThepreparationoftheoocytefortheconsequentCa2+signalingategg
activationappearstobesetupduringoocytematuration,asphosphorylationofSRAbyGSK3duringlateoogenesisisrequiredforpropereggactivation.Completionof meiosisislinkedtotheactivationofCa2+signalingintheoocyte,andapotentialtargetofthispathwayistheAPC/C,whoseactivationleadstoadecreaseinCycB/Cdk1activity
andmeioticprogression.AshighCycB/Cdk1inthematureoocyteleadstoinhibitoryphosphorylationofGNU,anactivatingsubunitofthePNGcomplex,thedecreaseof CycB/Cdk1levelsatactivationallowsfordephosphorylationofGNU,whichthenbindsandactivatesthePNGcomplex.ThePNGcomplexthenmediatestranslationalcontrol ofmanymaternalmRNAsduringeggactivation.Atactivation,changestothecytoskeletonandcrosslinkingofthevitellinemembranealsoensue(notdepicted).
Fig.5.DevelopmentallyregulatedproteinlevelchangesduringtheDrosophilaoocyte-to-embryotransition.Theoocyteistranscriptionallysilentduringoocytematuration andeggactivation,socontrolofgeneregulationoccursataposttranscriptionallevel.ChangestotheproteomecanresultfromchangesinmRNAtranslationorprotein degradation.Acomprehensivequantitativeanalysisofproteinlevelchangesduringoocytematurationandeggactivationuncoveredsubsetsofproteinswhosechanges suggestdevelopmentalregulation.421proteins,GroupI(purple),accumulateduringmaturationandremainconstantinlevelsafteractivation.Thisgroupincludesthe translationalregulatorsPNGandPLU,inadditiontomostMCMs(MCM2-3andMCM5-7,withtheexceptionofMCM4)andYA,aproteinnecessaryforthefirstmitosis.A similargroupof43proteins(notshown),whichincludesDNApolymeraseandORCsubunits,accumulatesduringmaturationandshowsafurtherincreaseinlevelsafter eggactivation.Bothofthesegroupssuggestthatthedriversoftheearlymitoticdivisionsaresetupduringoocytematuration.GroupII(red)includes66proteinsthatare upregulatedduringmaturationandthendecreaseinlevelsateggactivation,apatternconsistentwithrolesinlateoogenesis,maturation,oreggactivation.Theseproteins likelyrequiredownregulationateggactivationbecausetheymaybedetrimentaltomitosisandearlyembryogenesis.ThisgroupincludesthePNGcomplexsubunitGNU,the thioredoxinDHDandGEMININ.Anothergroupof117proteins,GroupIII(blue),remainsconstantinlevelsduringmaturationbutisupregulatedateggactivation,implying arolefortheseproteinsineventspost-activation.ThisgroupincludesSTIM,theSCFsubunitLIN19andthetranscriptionfactorSTAT92E.TheY-axisrepresentstherelative proteinlevels,andtheX-axisrepresentsdevelopmentalstageineachrepresentativediagram.Thedottedlineshowswheneggactivationoccurs.
becomesactive.Premature inactivationofinsulin, byInRorakt
RNAi,leadstoprematureglycogenaccumulation,whereasGSK3ˇ
RNAileadstodecreasedglycogenlevelsinmatureoocytes[35], suggestingthatinsulinsignalingisantagonistictoglycogen
accu-mulation.Notsurprisingly, GSK3ˇknockdown duringoogenesis
leadstodefectsinearlyembryogenesis,althoughitremainsunclear whetherthesedefectsareduetoimproperglycogenaccumulation ortoglycogen-independentGSK3functions,suchasitsabilityto
phosphorylateandactivateSRA(seebelow)[54].Insulinsignaling caninteractwithotherendocrinesignalingpathways[55],soit remainspossiblethatthecoordinatedactivitiesofmultiple path-waysareexertingtightcontrolontheeventsunderlyingoocyte maturation.
5. Eggactivation
The mature Drosophila oocyte is arrested at metaphase I.
CycB/CDK1activityishighandnecessaryforthemetaphaseIarrest [9].Matureoocytescanbeheldintheovaryforprolongedperiods ifthefemalehasnotmatedorifenvironmentalconditionsarenot good.Suchheldoocytesbecomedehydrated.Astheoocytepasses intotheoviduct,dramaticchangesoccurthataregroupedunder theheadingofeggactivation[56].Theseincludethereleaseofthe metaphaseIarrestandthecompletionofmeiosis,promotedbythe inactivationofCycB/CDK1throughCycBdestructionbyactivation oftheAPC/C[57,58].Swellingandhydrationoftheegginadditionto changesinthecytoskeletonatthecortexoccur[56].Asegg activa-tionresetstheeggforembryogenesis,itisaccompaniedbymassive changes in gene expression, all controlledposttranscriptionally [59].ThisisassociatedwithdisassemblyoflargecytoplasmicRNP granules[60].Inthenextsections,wefocusonrecentadvancesin ourunderstandingofthemolecularregulationoftheeventsofegg activation(Fig.4).
5.1. Calciumsignaling
Asinotherorganisms,anincreaseinintracellularCa2+occurs duringeggactivationinDrosophilamelanogaster.Manyaspectsof Ca2+signalingandrolesforCa2+effectors,suchasDrosophila cal-cipressinencodedbythesarah (sra)gene,havebeenpreviously reviewed[61],sowefocusourdiscussiononrecentinsightsinto thiseventofeggactivation.Briefly,asinmanyothermetazoans,an intracellularCa2+increaseoccursatactivation,andthisactivates asignalingcascadethatcontrolsmanyaspectsofeggactivation. Aninflux ofexogenousCa2+occursinvivoastheoocytepasses intotheoviductduringovulation[62],coincidingwiththetime when activationis occurring[45]. Invitroactivation of oocytes expressingtheintracellularCa2+sensorGCAMPrevealedthatthis riseinintracellularCa2+occursintheformofasinglewave dur-ingactivation[62,63].Moreover,consistentwithpreviousstudies showingthatmechanicalstimulicanelicitactivationofDrosophila oocytes[64], theinfluxof extracellular Ca2+can beblockedby pharmacologically inhibiting transient receptor potential (TRP) mechanosensitivechannels[62].However,blockingtheCa2+wave byvariousmanipulationsdoesnotimpedeoocyteswelling, sug-gestingthathydrationoftheoocyteiseitherparalleltoorprecedes theCa2+wave.
InternalstoresofCa2+releasedfromtheendoplasmic reticu-lum(ER)andtheactincytoskeletonalsocontributetotheCa2+ wave.ThereleaseofCa2+fromERstoresoccursthroughactivation ofinositoltriphosphatereceptors(IP3R),andseemstobe impor-tantforwavepropagationinsteadofinitiation.IP3RRNAioocytes areabletoinitiatethewavebutareunabletosustainit[62]. Inter-estingly,astudyonthedynamicsoftheERusingaGFP-tagged reporteroftheERresidentproteinPDIfoundthatERorganizationin embryosdiffersfromthatinmatureoocytes[65].Incontrasttothe sheet-likeorganizationinearlyembryos,theERinlateoogenesis exhibitsareticulatedorganizationthatincreasesinvolumeasthe oocytematures.TheprevalenceofdifferentERstructuresinother celltypeshasbeensuggestedtorelatetospecializedfunctionsof thisorganelle[66].WhereasERorganizationhasbeenimplicated increatingCa2+microdomainsduringembryonicmitosis[67],its structuralorganizationduringoogenesismayreflectrolesofthe
ERinproteinsynthesis,metabolism,andapreparationfortheCa2 waveatactivation.
Additionally,arolefortheactincytoskeletonintheCa2+flux alsohasbeenproposed. Treatingoocyteswithcytochalasin-D,a potentactinpolymerizationinhibitor,resultsinastutteredCa2+ increaseduringinvitroactivation[63].Thissuggeststhattheactin cytoskeletonalsocouldacttosustainthepropagatingCa2+wave, althoughthemechanismbywhichthisoccursisstillunclear.It wassuggested,however,thattheactincytoskeletonactsinCa2+ releasethroughinteractionswithTRPorIP3Rs[63],whichcould representawayofcouplingthemechanicalstimulationthatoccurs duringovulationtotheinductionof theCa2+wave.Thisideais consistentwithobservationsthatTRPchannelscanbemodulated bymechanicalsignalsfromthecytoskeletonthroughinteractions withMT-andactin-bindingproteins[68],orthatactininteractions withIP3RsmediateCa2+transientsinmammaliancells[69,70]. 5.2. Completionofmeiosis
Invertebrates,theCa2+fluxatfertilizationleadsto inactiva-tionoftheAPC/CinhibitorEmi2,thuspromotingdegradationof SecurinandCycBtoreleasethemeioticarrest.Althoughtherole ofAPC/CinhibitorsinmeioticarrestintheDrosophilaoocytehas notyetbeenevaluated,onepossibletargetforregulationbyCa2+ isactivationoftheAPC/CviaCORTEX(CORT).CORTisamemberof theCdc20familyofAPC/Cactivatorsthatisoocytespecific[57,58].
Whereas both Cdc20s, FIZZY and CORT, are redundant for the
metaphaseI/anaphaseItransition,onlyCORTisuniquelyrequired forthemetaphaseII/anaphaseIItransition[57].CORTproteinis presentinmatureoocytes,yetapparentlyinactiveuntilegg acti-vation[58].Eggslaidbyfemaleswithamorphicmutationsincort arrestinmetaphaseIIofmeiosisandshowafailuretoreduceCycB levels[58].Thisissimilartothephenotypeobservedinmutantsfor theCa2+effectorsra,indicatingthatCORTcouldindeedbeaCa2+ target.Inadditiontotriggeringthemeioticdivisions,APC/CCORT bindsandtargetsmanyproteinsfordegradation,whichcould con-tribute toregulationof many indirecttargetsbyCa2+ signaling [12,71].
Aftermeioticcompletion,themostinternalof thefour mei-oticproductsbecomesthefemalepronucleus.Theunusedmeiotic products,thepolarbodies,arenotbuddedoffthroughcytokinesis asinvertebrateoocytes(Fig.2).Thus,inDrosophilaan asymmet-ricalpositioningofthemeioticspindlewithrespecttotheoocyte cytoplasmisnotrequired.Thefirstmeioticdivisionoccurs par-alleltotheoocyteplasmamembrane,whereasthesecondoccurs perpendiculartothemembrane.
5.3. Changesingeneexpressionateggactivation
Thechangesinproteinlevels,mRNAtranslation,and
polyadeny-lation during egg activation have been globally mapped by
comparing mature oocytes to laid, activated eggs. Changes in
maternal mRNAtranslation ategg activationwere analyzedby
bothribosomefootprintingandpolysomeanalysis[59], uncover-ingextensivechangesinmRNAtranslation.Thematureoocytewas foundtobetranslationallyactive,notquiescent,andategg activa-tion,hundredsofmRNAsbecometranslationallyrepressed,while hundredsofothermRNAsbecometranslationallyactivated.
Thetranslationofapproximately90%ofthesemRNAsis
con-trolled by the PAN GU (PNG) kinase complex, whose activity
recentlyhasbeenfoundtobelinkedtocompletionofmeiosisinthe oocyte[59,72].ThePNGcomplexiscomposedofaser/thrkinase catalyticsubunitPNGandtwoactivatingsubunits,GIANTNUCLEI
(GNU) and PLUTONIUM (PLU). Although all three subunits are
andtherebyinhibitedfrombindingandactivatingPNG.Uponegg
activationanddegradationofCycB,GNUbecomes
dephosphory-lated,andactivePNGkinasecomplexassembles.Theactivationis onlytransient,however,asGNUbecomesdegradedafteregg acti-vationinaPNG-dependentmanner[72].PNGphosphorylatesGNU andthismaytargetitfordegradation.Thislevelof developmen-talcontroltemporarilyrestrictsPNGcomplexactivityandpermits
massivetranslationalchangesoveranarrowtimewindow.This
regulationiscoordinatedwithmeioticcompletionviatheinfluence ofCycB/CDK1onPNGcomplexactivation.
Pronouncedchangesinpoly(A)taillengthsalsooccur,andegg activationis thedevelopmentalperiodwhenpoly(A) taillength ismosttightlycoupledtotranslationalefficiency[22]. Coupling persistsduringthefirstthreehoursofembryogenesis,butthetwo processesbecomeunlinkedaszygotictranscriptioninitiatesand geneexpressionisnolongercontrolledposttranscriptionally.
Polyadenylation at egg activation is WISP-dependent, with
nearly everymRNA showing a reduction of poly(A) taillength
inwispmutants[22,25,27]. ExceptionsarethemRNAsfor ribo-somalproteins.Strikingly,therelationshipbetweentranslational efficiencyandpoly(A)taillengthisnotdifferentbetween wild-typeandwispmutantlaideggs,implyingthatselectivepoly(A)-tail shorteningiswhatprimarilyspecifiestranslationalchangesduring eggactivation.Giventhatwispmutanteggshavedefectivemeiotic divisionsandarrestfollowingactivationitcannot,however,bethe casethatpoly(A)taillengtheningiscompletelydispensable[26,28]. Ateggactivation,manyproteinsincreaseinabundance,whereas
many others decrease [59]. Among the proteins whose levels
increasearethoseneededforearlyembryonicpatterning,division, andzygoticgeneexpression.Proteinswhoselevelsdecreasedonot fallintospecificGOclasses,butoneinterestinggroup(Fig.5)is proteinswhoselevelsincreaseatmaturationbutthendecreaseat activation[12].CORTbelongstothisgroupofproteins,pointingto ahand-offofregulatedproteolysistootherAPC/CformsortoSCF. Anotherexampleistheoocyte-specificthioredoxinencodedbythe deadhead(dhd)gene[73],hintingthatchangesinredoxregulation maybecriticalatthis time.LikeDHDandCORT,manyproteins inthisgrouparelikelyneededformeiosisorotheraspectsoflate oogenesis,andcontinuedpresenceoftheseproteinscouldbe detri-mentaltoembryogenesis.Thishasbeenshowntobethecasefor MTRM,thePOLOinhibitor,becauseifMTRMisnotdegradedategg activationdefectsintheembryonicmitoticdivisionsresult[71].
AcomparisonoftheproteomeandmRNAtranslationdatasets
revealedthatincreasedmRNAtranslationcanaccountforincreased proteinlevels,buttranslationalinhibitionfailstoaccountformost decreasesinproteinabundance[59].Thesediscrepanciesbetween
changes in protein levels and mRNA translation highlight the
importanceofposttranslationalcontrolduringeggactivationand pointtoa crucialroleforproteolysis.Astherealsoareproteins thatarerobustlyactivatedfortranslationyetwhoseproteinlevels donotincrease,itappearsthatincreasedtranslationisneededto replaceproteinsthatwerepresentinoocytesbutbecamedegraded ateggactivation,possiblyasamechanismtoresettheproteome.
6. Fertilizationandearlydevelopment
Fertilization marks thefinal phase of the oocyte-to-embryo
transition,as thematernal and paternalDNA contributions are combinedtogenerateadiploidzygoticgenome.Themergerofthe maternalandpaternalchromosomesoccursintelophaseofthefirst mitoticdivision,andthisisfollowedbyrapid,synchronousmitotic divisions.Asexpressionfromthezygoticgenomedoesnotbegin untilthematernal-to-zygotictransition,theearlyembryonic divi-sionsarecontrolledbymaternalstockpilesofmRNAsandproteins.
6.1. Redoxstateandactivationofspermchromatin
The completion of female meiosis and other egg activation
eventsoccurindependentlyofspermentry,buttheonsetof embry-onicdivisionsanddevelopmentisdependentonfertilization.Many aspectsoffertilizationandearlydevelopmenthavebeenpreviously reviewed[74],sowefocusonrecentinsightsintothisintricate pro-cess.Whileeggactivationoccursduringthedescentoftheoocyte throughtheoviduct,fertilization occursin theuterus,withthe releaseofasinglespermfromthespermathecaanditsentryinto theeggcytoplasmthroughthemicropyleattheanterioroftheegg.
Thespermprovidestwoexclusiveelementstothenewembryo:
thepaternalhaploidDNAcontentandasinglecentriole(Fig.2). SpermentryiscoordinatedwithcompletionofmeiosisII,suchthat
themostproximalfemalepronucleusmigratestowardsthemale
pronucleus,leadingtopronuclearappositionandthestartofthe firstmitoticdivision.
Followingspermentry,themalepronucleusisactivated,the
protaminesareevictedfromspermchromatinandexchangedfor
histonesin aprocess thatdepends onmaternally provided fac-tors,suchasthehistonechaperoneHIRA[74]andDHD.Eggslaid bydhdmutantmothersarrestwithspermchromatinthatfailsto decondense[75,76],theresultofadelayinprotamineevictionand exchangefor histones.Additionally, thefemalepronucleus fails
tomigratetowardsthemalepronucleus,andtheembryosbegin
todevelopashaploid[75].Thespermdecondensationphenotype
canberescuedwithwild-typeDHDbutnotwithamutantDHD
in which one of two catalytic cysteines is mutatedto create a substratetrap[75],consistentwithredoxfunctionbeingrequired
forspermdecondensation.Moreover,whereasoxidationof
pro-taminescanleadtotheiroligomerization,addingDHD leadsto protaminereductionandtheirevictionfromDNA[76].Thus,DHD
mayregulatespermchromatindecondensationviareductionof
sulfide-bridgesbetweenprotamines,thoughthisregulationmay
beindirect,eitherbyactivationofadisulfidereductasespecialized forprotaminereductionorregulationofaglobalredoxstateby DHDduringeggactivation.
6.2. Startofembryonicdivisions
Afterfertilizationandpronuclearapposition,theembryoisset tostartdevelopment.DuringDrosophilaearlyembryogenesis,the embryoundergoes13roundsofrapid,synchronizednuclear divi-sioncyclesinacommoncytoplasm,leadingtotheformationof asyncytialblastodermthatcontains∼6000nucleipriorto cellu-larblastodermformation.Theseearlydivisionslackgapphases, insteadbeingcyclesofS-andM-phasesdrivenbyfluctuationsof highCDK1activityandrelyingonmaternalmRNAsandproteins[5]. Thesedivisionsoccurwithoutsignificantzygotictranscription,as theembryoislargelytranscriptionallysilentuntilthefirstwaveof zygotictranscriptionattheonsetofthematernal-to-zygotic tran-sition(MZT)[4].S-phaseisgraduallylengthenedduringcycles11 through13,slowingtheoveralltimeforeachnucleardivision,with apronouncedG2phasebeingaddedduringcellularization,when thezygoticgenomebecomesfullyactivatedfortranscriptionand maternalmRNAsaredegraded.Thefollowingthreedivisionsafter cellularizationoccurinmitoticdomainsregulatedbypatterning genes[5].
Thestartoftheembryonicdivisionsdependsonspecialized reg-ulators,providedmaternallyandactivatedateggactivation.One
exampleis YOUNGARREST(YA),a nuclearproteinofunknown
molecularfunction,whichisactivatedaftereggactivationandis necessaryforthefirstmitoticdivision [77].Anotherexampleis thePNGcomplex.Loss-of-functionmutationsforanyofthe
sub-unitsof thecomplex resultin arrested embryosin which DNA
requirementforPNGactivitytopromotecycBmRNAtranslation, therebyrestoringCycBproteinlevelsaftermeioticcompletionto restartmitosisintheembryo[79].PNGalsoaffectsthelaterMZT,as itisrequiredfortranslationofsmaugmRNA[80].SMAUGprotein
thenpromotesdeadenylationanddegradationofmaternalmRNAs
aszygotictranscriptioninitiates[59,81]. PNGfunction addition-allyisneededfordegradationattheMZTofseveralmaternally depositedRNA-bindingproteinsthatrepresstranslation,although itisnot knownwhethertheeffectofPNGisdirect[82]. Atthe oocyte-to-embryotransition,thecoordinatedactivityofYA,PNG andotherknownandyettobeuncoveredregulators,togetherwith theirdownstreamtargets,ensureapropertransitioninto embryo-genesis.
Recent work points to the importance of metabolic
regula-tion during early Drosophila development.The SHMT enzyme,
requiredforsynthesis ofpurines,thymidinenucleotides,and S-adenosylmethionine,isnecessaryfordivisionsbeyondcycle13 [83].Itis postulatedthat thisisthetimewhenmaternal
stock-piles of these metabolites become depleted. Analysis of dNTP
levelsinearlyembryos,controlledbyRibonucleotideReductase (RNR),demonstratedthatmaternalstockpilesarenotsufficientto supportaccuratedivisionsbeyondcycle11.ItappearsthatRNR becomesallostericallyactivatedaslevelsofmaternally-provided dNTPsbegintodropasDNAreplicationproceeds,thereby provid-ingthedNTPsneededforadditionaldivisions[84].Futurestudies ofmetabolismduringearlyembryogenesisarelikelytouncover additionalregulatorymechanisms.
7. Parallelstootherorganisms
Hormonesignalingisaconservedfeaturedofoocyte
develop-ment. Gonadotrophinsand sexsteroids inmammals and frogs,
andthesteroidhormoneecdysoneinDrosophilacontrolvarious aspectsofoogenesis.InDrosophila,apartfromactinginearly ooge-nesis[85],ecdysonealsoactslaterinoogenesistocontrolchanges inlipidmetabolism[43,86]andovulation[46].Incontrastto
ver-tebrates,where a surge in luteinizinghormone induces oocyte
maturation,thesignalformaturationinflieshasnotbeen deter-minedandaroleforhormonesignalinginthisprocessisunclear[8]. Similarlytomammalianovulation,Drosophilaovulationrequires theruptureoftheFClayer[46]andgenerationofacorpusluteum [44].Inbothmammalsandflies,hormonesignalingmayserveto modulateoogenesisbyanorganismalregulationofmetabolismand behavior.
Signalingbyinsulinhasbeenproposedasanevolutionary
reg-ulatorof femalereproduction.Components ofthis pathwayare
highlyconserved,andaspectscontrolledbyinsulin,suchasearly germlinestemcelldivisionsandlocalandsystemicmetabolic reg-ulation,aresimilarinbothflyandvertebrateoocytedevelopment [50,86,87].Controlofglycogenaccumulationduringlateoogenesis byinsulinalsowasdescribedinXenopusoocytes[35].
Interest-ingly, in frogs, fish and worms, the ERK branch of the insulin
signalingpathwaycaninduceoocytematurationindependently
ofhormonesignaling[50].ERKisa downstreamtargetofMOS, andalthoughtheflyhomologdmosisnotessentialinDrosophila [88]andtheRAS/ERKbranchdownstreamofinsulinhasnotbeen evaluated in flies[50], it remains possible that components of thisbranchmayacttocontroloocytematuration.Theinsulinand ecdysonepathwayscaninteract,andcooperationbetweeninsulin
andgonadotrophinsignalingtomodulateoocytematurationhas
beendocumentedin mammals[50]. Hence,it remainspossible
thatcooperationbetweeninsulinandecdysone,perhaps synergis-ticallywithanothersignalingpathway,couldbeunderlyingoocyte maturationinDrosophila.
ParallelsbetweenDrosophilaandC.eleganshighlightacrucial roleforproteinkinasesincontrollingmRNAtranslationandthe
proteomeattheoocyte-to-embryotransition.TheMBK-2kinase
inC.eleganscontrolsproteolysisthroughtheSCFE3ubiquitin lig-asetodegradeoocyteproteinsthatcouldimpedeembryogenesis [89].ItalsoaffectsRNPgranuledynamicsandthuscouldinfluence maternalmRNAtranslation[90].Interestingly,activationof MBK-2depends ontheactivationofAPC/Cand isthereforelinkedto thecompletionofmeiosis[91].Theconservedstrategyoflinking alterationsin theproteomenecessaryfor theoocyte-to-embryo transitiontothemeiotic cellcyclevia theactivationofprotein kinasesraisesthequestionofwhethersuchamechanismmayalso beemployedinvertebrates.
8. Conclusions
Theadvancesinresearchontheoocyte-to-embryotransition inDrosophilainthepastfewyears havesignificantlyenhanced
ourunderstandingwhile openingnewareasand questionsthat
meritfurtherinvestigation.Themechanismsofnutrient deposi-tionduringDrosophilaoogenesisandtheinfluenceofinsulinand ecdysonesignalingnowaremorefullyunderstood.Recent
stud-ieshaveuncoveredbothselectionmechanismsformitochondria
and regulationof mitochondrial activity.The discoveriesof the dependenceofactivationandmeioticcompletiononaCa2+wave andsignalingpathwayareamajoradvanceinourunderstanding oftheoocyte-to-embryotransitioninDrosophila.Theglobal delin-eationofmRNAtranslationchangesaccompanyingeggactivation revealedthecentralrolethatthePNGcomplexplaysincontrolling mRNAtranslationandhowdevelopmentalcontrolofthiscomplex restrictsthisregulationofmRNAtranslation toa narrow
devel-opmentalwindow.Comparisonoftheproteomeandtranslatome
changesatmaturationandactivationimplicateamajorrolefor proteolysisininfluencingthesedevelopmentalevents.
Importantareasstilltobeexploredinclude:1)findingthe sig-nalsthatleadtooocytematurationandtheonsetofthemeiotic divisions;2)definingtherole,regulationandmRNAcomponents ofRNPgranulesduringmaturationandeggactivation;3) identify-ingthekeytargetsofCa2+signalingresponsibleforthemyriadof eventsoccurringateggactivation;4)elucidatingthemechanism throughwhichPNGcangloballyalterthetranslationofhundreds
of mRNAs;and 5)delineatingthecontrolof metabolicchanges
inearlyembryogenesis.Futureworkinthefieldtoaddressthese issuespromisesadeeperunderstandingintothecomplexityofthe Drosophilaoocyte-to-embryotransitionandinsightsinto regula-tionofthistransitioninotheranimals.
Acknowledgments
Weapologizetoourcolleagueswhoseworkcouldnotbecited
due to space limitations. We thank Jessica Von Stetina, Peter
NichollsandGabrielNeurohrfortheirhelpfulcommentsonthe
manuscript.ThisworkwassupportedbyNIHgrantGM118098to
TO-W.TO-WisanAmericanCancerSocietyResearchProfessor.
References
[1]J.D.Laver,A.J.Marsolais,C.A.Smibert,H.D.Lipshitz,Regulationandfunction ofmaternalgeneproductsduringthematernal-to-zygotictransitionin Drosophila,Curr.Top.Dev.Biol.113(2015)43–84,http://dx.doi.org/10.1016/ bs.ctdb.2015.06.007.
[2]M.M.Harrison,M.B.Eisen,Transcriptionalactivationofthezygoticgenomein Drosophila,Curr.Top.Dev.Biol.113(2015)85–112,http://dx.doi.org/10. 1016/bs.ctdb.2015.07.028.
[3]S.A.Blythe,E.F.Wieschaus,Coordinatingcellcycleremodelingwith transcriptionalactivationattheDrosophilaMBT,Curr.Top.Dev.Biol.113 (2015)113–148,http://dx.doi.org/10.1016/bs.ctdb.2015.06.002.
[4]W.Tadros,H.D.Lipshitz,Thematernal-to-zygotictransition:aplayintwo acts,Development136(2009)3033–3042,http://dx.doi.org/10.1242/dev. 033183.
[5]J.A.Farrell,P.H.O’Farrell,Fromeggtogastrula:howthecellcycleisremodeled duringtheDrosophilamid-blastulatransition,Annu.Rev.Genet.48(2014) 269–294,http://dx.doi.org/10.1146/annurev-genet-111212-133531. [6]K.S.McKim,J.K.Jang,W.E.Theurkauf,R.S.Hawley,Mechanicalbasisofmeiotic
metaphasearrest,Nature362(1993)364–366,http://dx.doi.org/10.1038/ 362364a0.
[7]S.E.Hughes,W.D.Gilliland,J.L.Cotitta,S.Takeo,K.A.Collins,R.S.Hawley, Heterochromaticthreadsconnectoscillatingchromosomesduring
prometaphaseIinDrosophilaoocytes,PLoSGenet.5(2009)e1000348,http:// dx.doi.org/10.1371/journal.pgen.1000348.
[8]J.R.VonStetina,T.L.Orr-Weaver,T.L.OrrWeaver,Developmentalcontrolof oocytematurationandeggactivationinmetazoanmodels,ColdSpringHarb. Perspect.Biol.3(2011)a005553,http://dx.doi.org/10.1101/cshperspect. a005553.
[9]M.Bourouh,R.Dhaliwal,K.Rana,S.Sinha,Z.Guo,A.Swan,Distinctand overlappingrequirementsforcyclinsA,BandB3inDrosophilafemale meiosis,G3(Bethesda)58(2016)3711–3724,http://dx.doi.org/10.1534/g3. 116.033050.
[10]Y.Xiang,S.Takeo,L.Florens,S.E.Hughes,L.J.Huo,W.D.Gilliland,S.K. Swanson,K.Teeter,J.W.Schwartz,M.P.Washburn,S.L.Jaspersen,R.S.Hawley, TheinhibitionofpolokinasebymatrimonymaintainsG2arrestinthemeiotic cellcycle,PLoSBiol.5(2007)2831–2846,http://dx.doi.org/10.1371/journal. pbio.0050323.
[11]J.R.VonStetina,S.Tranguch,S.K.Dey,L.A.Lee,B.Cha,D.Drummond-Barbosa, ␣-Endosulfineisaconservedproteinrequiredforoocytemeioticmaturation inDrosophila,Development135(2008)3697–3706,http://dx.doi.org/10. 1242/dev.025114.
[12]I.Kronja,Z.J.Whitfield,B.Yuan,K.Dzeyk,J.Kirkpatrick,J.Krijgsveld,T.L. Orr-Weaver,Quantitativeproteomicsrevealsthedynamicsofprotein changesduringDrosophilaoocytematurationandtheoocyte-to-embryo transition,Proc.Natl.Acad.Sci.U.S.A.111(2014)16023–16028,http://dx. doi.org/10.1073/pnas.1418657111.
[13]V.Archambault,X.Zhao,H.White-Cooper,A.T.C.Carpenter,D.M.Glover, MutationsinDrosophilaGreatwall/scantrevealitsrolesinmitosisand meiosisandinterdependencewithpolokinase,PLoSGenet.3(2007) 2163–2179,http://dx.doi.org/10.1371/journal.pgen.0030200. [14]B.C.Williams,J.J.Filter,K.A.Blake-Hodek,B.E.Wadzinski,N.J.Fuda,D.
Shalloway,M.L.Goldberg,Greatwall-phosphorylatedendosulfineisbothan inhibitorandasubstrateofPP2A-B55heterotrimers,eLife2014(2014) e01695,http://dx.doi.org/10.7554/eLife.01695.
[15]S.Vigneron,P.Robert,K.Hached,L.Sundermann,S.Charrasse,J.C.Labbé,A. Castro,T.Lorca,Themastergreatwallkinase,acriticalregulatorofmitosis andmeiosis,Int.J.Dev.Biol.60(2016)245–254,http://dx.doi.org/10.1387/ ijdb.160155tl.
[16]J.Fu,I.M.Hagan,D.M.Glover,Thecentrosomeanditsduplicationcycle,Cold SpringHarb.Perspect.Biol.7(2015)a015800,http://dx.doi.org/10.1101/ cshperspect.a015800.
[17]A.Pimenta-Marques,I.Bento,C.A.M.Lopes,P.Duarte,S.C.Jana,M. Bettencourt-Dias,Amechanismfortheeliminationofthefemalegamete centrosomeinDrosophilamelanogaster,Science353(2016),http://dx.doi.org/ 10.1126/science.aaf4866(80)aaf4866-aaf4866.
[18]S.J.Radford,J.K.Jang,K.S.McKim,Thechromosomalpassengercomplexis requiredformeioticacentrosomalspindleassemblyandchromosome biorientation,Genetics192(2012)417–429,http://dx.doi.org/10.1534/ genetics.112.143495.
[19]S.J.Radford,A.L.Nguyen,K.Schindler,K.S.McKim,Thechromosomalbasisof meioticacentrosomalspindleassemblyandfunctioninoocytes,Chromosoma 126(2017)351–364,http://dx.doi.org/10.1007/s00412-016-0618-1. [20]S.J.Radford,A.M.M.Go,K.S.McKim,Cooperationbetweenkinesinmotors
promotesspindlesymmetryandchromosomeorganizationinoocytes, Genetics205(2017)517–527,http://dx.doi.org/10.1534/genetics.116.194647. [21]S.J.Radford,T.L.Hoang,A.A.Głuszek,H.Ohkura,K.S.McKim,Lateraland
end-onkinetochoreattachmentsarecoordinatedtoachieveBi-orientationin Drosophilaoocytes,PLoSGenet.11(2015)e1005605,http://dx.doi.org/10. 1371/journal.pgen.1005605.
[22]S.W.Eichhorn,A.O.Subtelny,I.Kronja,J.C.Kwasnieski,T.L.Orr-Weaver,D.P. Bartel,mRNApoly(A)-tailchangesspecifiedbydeadenylationbroadly reshapetranslationinDrosophilaoocytesandearlyembryos,eLife5(2016) 714–724,http://dx.doi.org/10.7554/eLife.16955.
[23]J.D.Richter,CPEB:alifeintranslation,TrendsBiochem.Sci.32(2007) 279–285,http://dx.doi.org/10.1016/j.tibs.2007.04.004.
[24]L.Weill,E.Belloc,F.-A.Bava,R.Méndez,Translationalcontrolbychangesin poly(A)taillength:recyclingmRNAs,Nat.Struct.Mol.Biol.19(2012) 577–585,http://dx.doi.org/10.1038/nsmb.2311.
[25]J.Lim,M.Lee,A.Son,H.Chang,V.N.Kim,mTAIL-seqrevealsdynamicpoly(A) tailregulationinoocyte-to-embryodevelopment,GenesDev.30(2016) 1671–1682,http://dx.doi.org/10.1101/gad.284802.116.
[26]P.Benoit,C.Papin,J.E.Kwak,M.Wickens,M.Simonelig,PAP-andGLD-2-type poly(A)polymerasesarerequiredsequentiallyincytoplasmic
polyadenylationandoogenesisinDrosophila,Development135(2008) 1969–1979,http://dx.doi.org/10.1242/dev.021444.
[27]J.Cui,C.V.Sartain,J.A.Pleiss,M.F.Wolfner,Cytoplasmicpolyadenylationisa majormRNAregulatorduringoogenesisandeggactivationinDrosophila, Dev.Biol.383(2013)121–131,http://dx.doi.org/10.1016/j.ydbio.2013.08.013. [28]J.Cui,K.L.Sackton,V.L.Horner,K.E.Kumar,M.F.Wolfner,Wispy,the
DrosophilahomologofGLD-2,isrequiredduringoogenesisandegg activation,Genetics178(2008)2017–2029,http://dx.doi.org/10.1534/ genetics.107.084558.
[29]M.A.Welte,Proteinsundernewmanagement:lipiddropletsdeliver,Trends CellBiol.17(2007)363–369,http://dx.doi.org/10.1016/j.tcb.2007.06.004. [30]M.H.Sieber,C.S.Thummel,TheDHR96nuclearreceptorcontrols
triacylglycerolhomeostasisinDrosophila,CellMetab.10(2009)481–490,
http://dx.doi.org/10.1016/j.cmet.2009.10.010.
[31]Z.Li,K.Thiel,P.J.Thul,M.Beller,R.P.Kühnlein,M.A.Welte,Lipiddroplets controlthematernalhistonesupplyofDrosophilaembryos,Curr.Biol.22 (2012)2104–2113,http://dx.doi.org/10.1016/j.cub.2012.09.018. [32]M.H.Sieber,C.S.Thummel,Coordinationoftriacylglycerolandcholesterol
homeostasisbyDHR96,CellMetab.10(2012)481–490,http://dx.doi.org/10. 1016/j.cmet.2009.10.010.
[33]E.Parra-Peralbo,J.Culi,Drosophilalipophorinreceptorsmediatetheuptake ofneutrallipidsinoocytesandimaginaldisccellsbyan
endocytosis-independentmechanism,PLoSGenet.7(2011)e1001297,http:// dx.doi.org/10.1371/journal.pgen.1001297.
[34]J.M.Tennessen,N.M.Bertagnolli,J.Evans,M.H.Sieber,J.Cox,C.S.Thummel, CoordinatedmetabolictransitionsduringDrosophilaembryogenesisandthe onsetofaerobicglycolysis,G3(Bethesda)4(2014)839–850,http://dx.doi. org/10.1534/g3.114.010652.
[35]M.H.Sieber,M.B.Thomsen,A.C.Spradling,Electrontransportchain remodelingbyGSK3duringoogenesisconnectsnutrientstateto
reproduction,Cell164(2016)420–432,http://dx.doi.org/10.1016/j.cell.2015. 12.020.
[36]J.B.Stewart,P.F.Chinnery,ThedynamicsofmitochondrialDNAheteroplasmy: implicationsforhumanhealthanddisease,Nat.Rev.Genet.16(2015) 530–542,http://dx.doi.org/10.1038/nrg3966.
[37]M.Sato,K.Sato,MaternalinheritanceofmitochondrialDNAbydiverse mechanismstoeliminatepaternalmitochondrialDNA,Biochim.Biophys. Acta—Mol.CellRes.1833(2013)1979–1984,http://dx.doi.org/10.1016/j. bbamcr.2013.03.010.
[38]Y.Politi,L.Gal,Y.Kalifa,L.Ravid,Z.Elazar,E.Arama,Paternalmitochondrial destructionafterfertilizationismediatedbyacommonendocyticand autophagicpathwayinDrosophila,Dev.Cell29(2014)305–320,http://dx. doi.org/10.1016/j.devcel.2014.04.005.
[39]H.Ma,H.Xu,P.H.O’Farrell,Transmissionofmitochondrialmutationsand actionofpurifyingselectioninDrosophilamelanogaster,Nat.Genet.46(2014) 393–397,http://dx.doi.org/10.1038/ng.2919.
[40]J.H.Hill,Z.Chen,H.Xu,SelectivepropagationoffunctionalmitochondrialDNA duringoogenesisrestrictsthetransmissionofadeleteriousmitochondrial variant,Nat.Genet.46(2014)389–392,http://dx.doi.org/10.1038/ng.2920. [41]T.R.Hurd,B.Herrmann,J.Sauerwald,J.Sanny,M.Grosch,R.Lehmann,Long
oskarcontrolsmitochondrialinheritanceinDrosophilamelanogaster,Dev.Cell 39(2016)560–571,http://dx.doi.org/10.1016/j.devcel.2016.11.004. [42]O.Uryu,T.Ameku,R.Niwa,Recentprogressinunderstandingtheroleof
ecdysteroidsinadultinsects:germlinedevelopmentandcircadianclockin thefruitflyDrosophilamelanogaster,Zool.Lett.1(2015)32,http://dx.doi.org/ 10.1186/s40851-015-0031-2.
[43]M.H.Sieber,A.C.Spradling,Steroidsignalingestablishesafemalemetabolic stateandregulatesSREBPtocontroloocytelipidaccumulation,Curr.Biol.25 (2015)993–1004,http://dx.doi.org/10.1016/j.cub.2015.02.019.
[44]L.D.Deady,W.Shen,S.A.Mosure,A.C.Spradling,J.Sun,Matrix
metalloproteinase2isrequiredforovulationandcorpusluteumformationin Drosophila,PLoSGenet.11(2015)e1004989,http://dx.doi.org/10.1371/ journal.pgen.1004989.
[45]Y.Heifetz,J.Yu,M.F.Wolfner,OvulationtriggersactivationofDrosophila oocytes,Dev.Biol.234(2001)416–424,http://dx.doi.org/10.1006/dbio.2001. 0246.
[46]E.Knapp,J.Sun,Steroidsignalinginmaturefolliclesisimportantfor Drosophilaovulation,Proc.Natl.Acad.Sci.U.S.A.114(2017)699–704,http:// dx.doi.org/10.1073/pnas.1614383114.
[47]L.D.Deady,J.Sun,Afollicleruptureassayrevealsanessentialrolefor follicularadrenergicsignalinginDrosophilaovulation,PLoSGenet.11(2015) e1005604,http://dx.doi.org/10.1371/journal.pgen.1005604.
[48]C.D.Rubinstein,M.F.Wolfner,Drosophilaseminalproteinovulinmediates ovulationthroughfemaleoctopamineneuronalsignaling,Proc.Natl.Acad. Sci.U.S.A.110(2013)17420–17425,http://dx.doi.org/10.1073/pnas. 1220018110.
[49]H.G.Lee,C.S.Seong,Y.C.Kim,R.L.Davis,K.A.Han,OctopaminereceptorOAMB isrequiredforovulationinDrosophilamelanogaster,Dev.Biol.264(2003) 179–190,http://dx.doi.org/10.1016/j.ydbio.2003.07.018.
[50]D.Das,S.Arur,Conservedinsulinsignalingintheregulationofoocytegrowth, development,andmaturation,Mol.Reprod.Dev.84(2017)444–459,http:// dx.doi.org/10.1002/mrd.22806.
[51]D.Drummond-Barbosa,A.C.Spradling,Stemcellsandtheirprogenyrespond tonutritionalchangesduringDrosophilaoogenesis,Dev.Biol.231(2001) 265–278,http://dx.doi.org/10.1006/dbio.2000.0135.
[52]D.R.Nässel,O.I.Kubrak,Y.Liu,J.Luo,O.V.Lushchak,Factorsthatregulate insulinproducingcellsandtheiroutputinDrosophila,Front.Physiol.4 (September)(2013)252,http://dx.doi.org/10.3389/fphys.2013.00252.
[53]T.Ikeya,M.Galic,P.Belawat,K.Nairz,E.Hafen,Nutrient-dependent expressionofinsulin-likepeptidesfromneuroendocrinecellsintheCNS contributestogrowthregulationinDrosophila,Curr.Biol.12(2002) 1293–1300,http://dx.doi.org/10.1016/S0960-9822(02)01043-6. [54]S.Takeo,S.K.Swanson,K.Nandanan,Y.Nakai,T.Aigaki,M.P.Washburn,L.
Florens,R.S.Hawley,Shaggy/glycogensynthasekinase3andphosphorylation ofSarah/regulatorofcalcineurinareessentialforcompletionofDrosophila femalemeiosis,Proc.Natl.Acad.Sci.U.S.A.109(2012)6382–6389,http://dx. doi.org/10.1073/pnas.1120367109.
[55]D.R.Nässel,J.VandenBroeck,Insulin/IGFsignalinginDrosophilaandother insects:factorsthatregulateproduction,releaseandpost-releaseactionof theinsulin-likepeptides,Cell.Mol.LifeSci.73(2016)271–290,http://dx.doi. org/10.1007/s00018-015-2063-3.
[56]V.L.Horner,M.F.Wolfner,Transitioningfromeggtoembryo:triggersand mechanismsofeggactivation,Dev.Dyn.237(2008)527–544,http://dx.doi. org/10.1002/dvdy.21454.
[57]A.Swan,T.Schupbach,TheCdc20(Fzy)/Cdh1-relatedprotein,Cort, cooperateswithFzyincyclindestructionandanaphaseprogressionin meiosisIandIIinDrosophila,Development134(2007)891–899,http://dx. doi.org/10.1242/dev.02784.
[58]J.A.Pesin,T.L.Orr-Weaver,Developmentalroleandregulationofcortex,a meiosis-specificanaphase-promotingcomplex/cyclosomeactivator,PLoS Genet.3(2007)e202,http://dx.doi.org/10.1371/journal.pgen.0030202. [59]I.Kronja,B.Yuan,S.W.Eichhorn,K.Dzeyk,J.Krijgsveld,D.P.Bartel,T.L.
Orr-Weaver,Widespreadchangesintheposttranscriptionallandscapeatthe Drosophilaoocyte-to-embryotransition,CellRep.7(2014)1495–1508,
http://dx.doi.org/10.1016/j.celrep.2014.05.002.
[60]T.T.Weil,R.M.Parton,B.Herpers,J.Soetaert,T.Veenendaal,D.Xanthakis,I.M. Dobbie,J.M.Halstead,R.Hayashi,C.Rabouille,I.Davis,Drosophilapatterning isestablishedbydifferentialassociationofmRNAswithPbodies,Nat.Cell Biol.14(2012)1305–1315,http://dx.doi.org/10.1038/ncb2627. [61]C.V.Sartain,M.F.Wolfner,CalciumandeggactivationinDrosophila,Cell
Calcium53(2013)10–15,http://dx.doi.org/10.1016/j.ceca.2012.11.008. [62]T.Kaneuchi,C.V.Sartain,S.Takeo,V.L.Horner,N.A.Buehner,T.Aigaki,M.F.
Wolfner,CalciumwavesoccurasDrosophilaoocytesactivate,Proc.Natl.Acad. Sci.U.S.A.112(2015)791–796,http://dx.doi.org/10.1073/pnas.1420589112. [63]A.H.York-Andersen,R.M.Parton,C.J.Bi,C.L.Bromley,I.Davis,T.T.Weil,A
singleandrapidcalciumwaveateggactivationinDrosophila,Biol.Open4 (2015)553–560,http://dx.doi.org/10.1242/bio.201411296.
[64]V.L.Horner,M.F.Wolfner,Mechanicalstimulationbyosmoticandhydrostatic pressureactivatesDrosophilaoocytesinvitroinacalcium-dependent manner,Dev.Biol.316(2008)100–109,http://dx.doi.org/10.1016/j.ydbio. 2008.01.014.
[65]Y.Bobinnec,C.Marcaillou,X.Morin,A.Debec,Dynamicsoftheendoplasmic reticulumduringearlydevelopmentofDrosophilamelanogaster,CellMotil. Cytoskelet.54(2003)217–225,http://dx.doi.org/10.1002/cm.10094. [66]D.S.Schwarz,M.D.Blower,Theendoplasmicreticulum:structure,function
andresponsetocellularsignaling,Cell.Mol.LifeSci.73(2016)79–94,http:// dx.doi.org/10.1007/s00018-015-2052-6.
[67]H.Parry,A.McDougall,M.Whitaker,Endoplasmicreticulumgenerates calciumsignallingmicrodomainsaroundthenucleusandspindleinsyncytial Drosophilaembryos,Biochem.Soc.Trans.34(2006)385–388,http://dx.doi. org/10.1042/BST0340385.
[68]T.Smani,N.Dionisio,J.J.López,A.Berna-Erro,J.A.Rosado,Cytoskeletaland scaffoldingproteinsasstructuralandfunctionaldeterminantsofTRP channels,Biochim.Biophys.Acta—Biomembr.1838(2014)658–664,http:// dx.doi.org/10.1016/j.bbamem.2013.01.009.
[69]M.R.Turvey,K.E.Fogarty,P.Thorn,Inositol(1,4,5)-trisphosphatereceptor linkstofilamentousactinareimportantforgeneratinglocalCa2+signalsin pancreaticacinarcells,J.CellSci.118(2005)971–980,http://dx.doi.org/10. 1242/jcs.01693.
[70]J.K.Foskett,C.White,K.-H.Cheung,D.-O.D.Mak,Inositoltrisphosphate receptorCa2++releasechannels,Phys.Rev.87(2007)593–658,http://dx.doi. org/10.1152/physrev.00035.2006.
[71]Z.J.Whitfield,J.Chisholm,R.S.Hawley,T.L.Orr-Weaver,Ameiosis-specific formoftheAPC/Cpromotestheoocyte-to-embryotransitionbydecreasing levelsofthePolokinaseinhibitorMatrimony,PLoSBiol.11(2013)e1001648,
http://dx.doi.org/10.1371/journal.pbio.1001648.
[72]M.Hara,B.Petrova,T.L.Orr-Weaver,ControlofPNGkinase,akeyregulatorof mRNAtranslation,iscoupledtomeiosiscompletionateggactivation,eLife6 (2017)e22219,http://dx.doi.org/10.7554/eLife.22219.
[73]H.K.Salz,T.W.Flickinger,E.Mittendorf,A.Pellicena-Palle,J.P.Petschek,E.B. Albrecht,TheDrosophilamaternaleffectlocusdeadheadencodesa thioredoxinhomologrequiredforfemalemeiosisandearlyembryonic development,Genetics136(1994)1075–1086,doi:PMC1205864.
[74]B.Loppin,R.Dubruille,B.Horard,TheintimategeneticsofDrosophila fertilization,OpenBiol.5(2015)150076,http://dx.doi.org/10.1098/rsob. 150076.
[75]S.Tirmarche,S.Kimura,R.Dubruille,B.Horard,B.Loppin,Unlockingsperm chromatinatfertilizationrequiresadedicatedeggthioredoxininDrosophila, Nat.Commun.7(2016)13539,http://dx.doi.org/10.1038/ncomms13539. [76]A.V.Emelyanov,D.V.Fyodorov,Thioredoxin-dependentdisulfidebond
reductionisrequiredforprotamineevictionfromspermchromatin,Genes Dev.30(2016)2651–2656,http://dx.doi.org/10.1101/gad.290916.116. [77]K.L.Sackton,J.M.Lopez,C.L.Berman,M.F.Wolfner,YAisneededforproper
nuclearorganizationtotransitionbetweenmeiosisandmitosisinDrosophila, BMCDev.Biol.9(2009)43,http://dx.doi.org/10.1186/1471-213X-9-43. [78]D.D.Fenger,J.L.Carminati,D.L.Burney-Sigman,H.Kashevsky,J.L.Dines,L.K.
Elfring,T.L.Orr-Weaver,PANGU:aproteinkinasethatinhibitsS-phaseand promotesmitosisinearlyDrosophiladevelopment,Development127(2000) 4763–4774.
[79]L.Vardy,T.L.Orr-Weaver,TheDrosophilaPNGkinasecomplexregulatesthe translationofcyclinB,Dev.Cell12(2007)157–166,http://dx.doi.org/10. 1016/j.devcel.2006.10.017.
[80]W.Tadros,A.L.Goldman,T.Babak,F.Menzies,L.Vardy,T.Orr-Weaver,T.R. Hughes,J.T.Westwood,C.A.Smibert,H.D.Lipshitz,SMAUGisamajor regulatorofmaternalmRNAdestabilizationinDrosophilaanditstranslation isactivatedbythePANGUkinase,Dev.Cell12(2007)143–155,http://dx.doi. org/10.1016/j.devcel.2006.10.005.
[81]L.Chen,J.G.Dumelie,X.Li,M.H.Cheng,Z.Yang,J.D.Laver,N.U.Siddiqui,J.T. Westwood,Q.Morris,H.D.Lipshitz,C.A.Smibert,GlobalregulationofmRNA translationandstabilityintheearlyDrosophilaembryobytheSmaug RNA-bindingprotein,GenomeBiol.15(2014)R4,http://dx.doi.org/10.1186/ gb-2014-15-1-r4.
[82]M.Wang,M.Ly,A.Lugowski,J.D.Laver,H.D.Lipshitz,C.A.Smibert,O.S. Rissland,ME31BgloballyrepressesmaternalmRNAsbytwodistinct mechanismsduringtheDrosophilamaternal-to-zygotictransition,eLife6 (2017)e27891,http://dx.doi.org/10.7554/eLife.27891.
[83]F.Winkler,M.Kriebel,M.Clever,S.Gröning,J.Großhans,Essentialfunctionof theserinehydroxymethyltransferase(SHMT)geneduringrapidsyncytialcell cyclesinDrosophila,G3(Bethesda)7(2017)2305–2314,http://dx.doi.org/10. 1534/g3.117.043133.
[84]Y.Song,R.A.Marmion,J.O.Park,D.Biswas,J.D.Rabinowitz,S.Y.Shvartsman, DynamiccontrolofdNTPsynthesisinearlyembryos,Dev.Cell42(2017) 301–308,http://dx.doi.org/10.1016/j.devcel.2017.06.013,e3.
[85]X.Belles,M.D.Piulachs,Ecdysonesignallingandovariandevelopmentin insects:fromstemcellstoovarianfollicleformation,Biochim.Biophys. Acta—GeneRegul.Mech.1849(2015)181–186,http://dx.doi.org/10.1016/j. bbagrm.2014.05.025.
[86]M.H.Sieber,A.C.Spradling,Theroleofmetabolicstatesindevelopmentand disease,Curr.Opin.Genet.Dev.45(2017)58–68,http://dx.doi.org/10.1016/j. gde.2017.03.002.
[87]J.Dupont,R.J.Scaramuzzi,Insulinsignallingandglucosetransportinthe ovaryandovarianfunctionduringtheovariancycle,Biochem.J.473(2016) 1483–1501,http://dx.doi.org/10.1042/BCJ20160124.
[88]I.Ivanovska,E.Lee,K.M.Kwan,D.D.Fenger,T.L.Orr-Weaver,TheDrosophila MOSorthologisnotessentialformeiosis,Curr.Biol.14(2004)75–80,http:// dx.doi.org/10.1016/j.cub.2003.12.031.
[89]S.Robertson,R.Lin,Theoocyte-to-embryotransition,Adv.Exp.Med.Biol.757 (2013)351–372,http://dx.doi.org/10.1007/978-1-4614-4015-4-12. [90]J.T.Wang,J.Smith,B.C.Chen,H.Schmidt,D.Rasoloson,A.Paix,B.G.Lambrus,
D.Calidas,E.Betzig,G.Seydoux,RegulationofRNAgranuledynamicsby phosphorylationofserine-rich,intrinsicallydisorderedproteinsinC.elegans, eLife3(2014)e04591,http://dx.doi.org/10.7554/eLife.04591.
[91]J.M.Parry,A.Singson,EGGmoleculescoupletheoocyte-to-embryotransition withcellcycleprogression,SpringerBerlinHeidelberg,ResultsProbl.Cell Differ.53(2011)135–151,http://dx.doi.org/10.1007/978-3-642-19065-07.