Demonstrating biocompatibility with supercritical C02: Biphasic cultivation
of Bacillus spp. and probing acclimation mechanisms through proteome and
lipid analysis
MACHSTSINTTT
by
Kyle Creighton Peet
B.S. Worcester Polytechnic Institute (2008)
Submitted to the Department of Civil and Environmental Engineering in partial fulfillment of the requirements for the Degree of
Doctor of Philosophy in Environmental Biology at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY February 2015
C 2015 Massachusetts Institute of Technology. All rights reserved.
Signature redacted
A u th o r ... ... Department of Cii1 and Environmental Engineering
2015
Signature redacted
C ertified b y ... . ... .. ... ... ... . .. ..
/7
anelle R. Thompson Doherty Assistant Professor in Oce Utilization in Civil and Environmental EngineeringSignature redacted
Thesis SupervisorA ccepted by... ... Heidi Nepf Donald and Martha Harleman Professor of Civil and Environmental Engineering
Chair, Graduate Program Committee
MASSACHUSETTS INSTITUTE OF TECHNOLOLGY
MAY 0 5
2015
Demonstrating biocompatibility with supercritical C02: Biphasic cultivation
of Bacillus spp. and probing acclimation mechanisms through proteome and
lipid analysis
by
Kyle Creighton Peet
Submitted to the Department of Civil and Environmental Engineering On January 29', 2015, in partial fulfillment of the requirements for the Degree of
Doctor of Philosophy in Environmental Biology
Abstract
Supercritical (sc) CO2 usage is increasing globally with applications as a sterilizing agent, as a non-toxic solvent, and as the form of the greenhouse gas CO2 injected underground for
geologic carbon sequestration (GCS). In this thesis I have described the isolation of
microorganisms from three different carbon sequestration pilot sites through a novel method of successive scCO2 enrichments. I show that microorganisms of the genus Bacillus, including GCS site isolates, are resistant to the bactericidal properties of scCO2, and can germinate and grow in an aqueous phase incubated under scCO2 (Chapter 2). Bacterial resistance to scCO2 challenges the efficacy of scCO2 based sterilization and indicates that microbial activity may be harnessed in engineered environments containing scCO2 (e.g. biochemical catalysis involving scCO2 as a solvent or biofilm/biomineralized barriers to scCO2 leakage from GCS sites). In an effort to understand the physiology of acclimation to scCO2, I have sequenced and analyzed the genomes
of two GCS-site isolates, B. cereus MIT0214 and B. subterraneus MITOTI (Chapter 3). I have used genome-enabled analysis of the proteome combined with analysis of membrane lipids to ask whether cellular macromolecules are differentially represented in cells grown under different headspace and pressure conditions including CO2 and scCO2 (Chapters 4). In this chapter I have
examined the following three hypotheses regarding the mechanisms employed by Bacilli to resist scCO2: (1): Resistance to CO2 stress is governed by a similar response as acclimation to low pH stress. (2): Cell wall and membrane alterations promote bacterial growth under scCO2 by
modulating the cell's microenvironment. (3): Global expression of proteins mediating cellular homeostasis in viable but non-growing (stationary-phase) populations acclimated to scCO2 resembles a generalized profile of anaerobic growth, with notable exceptions of individual protein(s) that mediate acclimation. The results from this thesis enhance understanding of bacterial resistance to scCO2, enabling improved strategies for scCO2-based sterilization and
accelerating biotechnological applications of scCO2-biocompatible organisms.
Thesis Supervisor: Janelle R. Thompson
Acknowledgements
I have many people and agencies to thank for this thesis and I am sure that there are some who I forgetten to mention. Major funding sources for the research in this thesis include MIT and the MIT's Energy Initiative (MITEI), which funded both the research and my first year of
graduate school through the BP-MITEI fellowship. The US Department of Energy National Energy Technology Lab (DOE NETL) has also provided essential grants for this work. The Ralph M. Parsons Lab in the Department of Civil and Environmental Engineering has enabled this research to be conducted, and the MIT Ippen Fund has funded travel for presentation of portions of this work at American Geophysical Union (AGU) conferences.
My advisor, Janelle R. Thompson, has been the most important factor in the completion of this work, and without her mentorship, it would not have been possible. I'm forever indebted to her for taking me on and I have learned so much over the past 6.5 years. She has helped guide this research throughout many successes and failures, and has helped me see through perceived failures to see that positive results can be obtained where I did not initially see any. She has helped greatly with enhancing the presentation of these results, both in written form, and in presentation at conferences and my thesis defense.
Members of the Thompson lab past and present have been friends, mentors and great people to conduct science with over the years. Samodha Fernando and Hector Hernandez helped get me started in the lab and teach me many things in the beginning of graduate school. Adam Freedman has been a great lab and project mate over the years and I think we've both learned many things from each other as we struggled to grow microbes under supercritical CO2. Kevin Penn has also been very helpful in giving advice over the past few years. Other members of the lab who I must thank include Jia Yi Har, Jia Wang, Jean Pierre Nshimyimana, Tim Helbig, Hanny Rivera, Ju Young Lim, Luciane Chimetto, Eric Hill, and Carolina Bastidas. I enjoyed working with several UROPs who helped with parts of this work (Vanya Britto, Joseph Aboki, Holly Josephs, Tzipora Wagner, and Matthew Archer) and I hope I was able to teach them useful skills through their work.
My committee members, Martin Polz, Penny Chisholm, and Roger Summons have also provided essential advice for guiding the direction of my research and crafting the story of this thesis. All of my committee members have always welcomed me to use their lab facilities throughout the years, and members of their labs have taught me many techniques and methods. Particular members of their labs who were of great help include Kelden Pehr, Florence Shubotz, Carolyn Colonero, Michael Cutler, Jan Hendrick, Alison Takemura, Allison Coe, and Jessie Thompson. In addition to committee members and their lab members, many collaborators and other scientists have provided essential advice and assistance for this work, including: Jonathan Ajo-Franklin, Chris Boreham, Tommy Phelps, Susan Pfiffner, Mike Timko, Pete Wishnok, and Ravi Kodihalli.
The Parsons community also deserves recognition as a great place to work, with great people. Sheila Frankel, Jim Long, Darlene Strother, Vicky Murphy have all helped me and all help keep Parsons running. I have met numerous people who have helped me through friendship and discussing science. Anthony, James, Ben, Jeff, Gaj, Sean, Mitul, Ryan, Amy, Matt, Jessie, Teresa, Alison T, Jim, Robin, Mason, Chris M, Mike S, Ruby, Alex, Kesley, Jen, Sarah Jane, Dave G, Dave W, Sarah, Fatima, Katya, Illana, Alison H, Chris L, Jon, Patricia, Ali P, Mark S,
Parsons community, I must thank Kelly who has helped me throughout graduate school, both in science and in life. I am excited for many more adventures with Kelly.
Finally I must thank friends and family for their support outside of MIT. Joey, Duncan, Sergey, James, Ande, Andreas, George, Corey, Rachel, and Adam have all been great friends over the years. All of my aunts, uncles, and cousins have been positive parts of my life, encouraging me in whatever I have pursued. Especially Helene, Duncan, Nico, Janny, Jonnie, Jacob, Rebecca, and Hannah. Ling-Se has been the best sister and I'll always look up to her. Joey and my niece Helene are the best additions to my loving family. Lastly my Mom and Dad raised me, guided me, and inspired me and I'll always be in their debt.
Table of contents
Chapter 1: Review of supercritical CO2 industries that may involve microbial
activity and possible survival strategies to scCO2 9
1.1 Microbial impacts of Geologic Carbon Sequestration ... 9
1.2 Microbial activity in high pressure, C02-rich environments ... 12
1.3 Microbial sterilization and survival under scCO2 . . . . .. 13
1.4 Biocatalysis under supercritical CO2 ... . . . 15
1.5 Adaption mechanisms allowing survival in extreme environments, implications for organisms growing under sCO2 ... . . . 17
1.6 Acclimation mechanisms allowing survival in extreme environments, implications for growing under scCO2 . . . . ... 18
1.7 Research questions ... 22
Chapter 2: Microbial growth under supercritical CO2 27 2.1 Introduction ... 30 2.2 M ethods ... 32 2.3 R esults ... 42 2.4 D iscussion ... 53 2.5 Acknowledgements ... 59 2.6 Supplemental Figures ... 60
Chapter 3: Draft genome sequences of the supercritical CO2 tolerant bacteria Bacillus subterraneus MITOTI and Bacillus cereus MIT0214 70 3.1 M ain text ... 72
3.2 Acknowledgements ... 74
Chapter 4: Changes in lipid and proteome composition accompany growth of Bacillus spp. under supercritical CO2 and may promote acclimation to associated stresses 75 4.1 Introduction ... 78
4.2 M ethods ... 82
4.3 R esults ... 9 1
4.4 D iscussion ... 112
4.5 Supplemental Figures and Tables ... 119
Chapter 5: Conclusions 133
Chapter 1: Review of supercritical CO2 industries that may involve microbial activity and
possible survival strategies to scCO2
Growth and survival of microorganisms under high pressure CO2 has become an
increasingly important research topic with the use of supercritical phase CO2 (scCO2) as a sterilizing agent, bioengineering in geologic carbon sequestration (GCS), and interest in carrying out biocatalysis and product extraction with a scCO2 solvent. Supercritical phase fluids are space
filling, similar to a gas, but they are denser and able to solvate similar to a liquid. These properties help make scCO2 an effective sterilizing agent, and an industrially important,
non-toxic solvent, with growing applications involving scCO2 phase biocatalysis. Large
anthropogenic point sources of CO2 have led to increasing studies in reducing these greenhouse
gas emissions by capturing emitted CO2 followed by injection underground for GCS, creating subsurface environments where it is not yet known what the effects of microbial activity will be on the fate of injected CO2. The potential for microbial activity in these environments opens up possibilities for utilizing microorganisms in bioengineering solutions to enhance the permanence of CO2 storage. This thesis is the first demonstration and examination of microbial growth in the aqueous phase under a scCO2 headspace and includes analysis of potential acclimation
mechanisms that mediate growth under scCO2.
1.1 Microbial impacts on Geologic Carbon Sequestration
Geologic carbon sequestration has been increasingly cited as one solution to reduce atmospheric emissions from large point sources of CO2. In GCS, carbon dioxide from large C02
geologic formations where it remains trapped beneath an impermeable rock layer or cap-rock. After injection, CO2 remains a separate supercritical phase in most formations targeted for GCS (Benson et al., 2005), with supercritical CO2 present at temperatures above 31 'C and pressures above 72.8 atm. The major fates of injected CO2 are: (i) remain as a supercritical phase between the aqueous phase and cap-rock or in residually trapped pockets, (ii) dissolve into the aqueous phase and (iii) mineralize into carbonates and precipitate (Benson et al., 2005). The idea of geologic CO2 storage is not without precedent, as there are stable, natural geologic
accumulations of CO2 (Watson et al., 2004), but leakage rates from the GCS sites will need to remain below 1% per thousand years to effectively reduce CO2 emissions (Shaffer, 2010). However, there are legitimate concerns of leakage through cap-rock fractures, especially from injection-triggered earthquakes (Zoback and Gorelick, 2012) and along improperly sealed wellbores (Kutchko et al., 2007; Watson et al., 2009). There is a need for more research into microbial influences on CO2 permanence and the potential for bioengineering solutions to address leakage scenarios (Oldenburg et al., 2008).
The subsurface is one of the largest reservoirs of microbial biomass (Whitman et al., 1998), and microbial communities are both present and active in deep subsurface environments (Onstott, 2005; Kieft et al., 2005; Chapelle et al., 2002; Lavalleur and Colwell, 2013). These active populations raise questions of how microbial activity may affect human activities like enhanced oil recovery (EOR), fracking, or GCS in the subsurface, and whether microbes can be harnessed for bioengineered applications within these contexts.
While geochemical modeling suggests biological CO2 fixation is likely to be a negligible factor relative to the massive quantities of injected CO2 (Onstott, 2005), microbial activity can
bioremediation applications (Cunningham et al., 2003; Williams et al., 2005), and with the possibility of CO2 leakage from fractures and wellbores, there is a need for engineering solutions to address these leakage pathways. One bioengineering proposal to enhance structural trapping is
the biofilm-barrier, which has been demonstrated to reduce to the flow of scCO2 in a sandstone
core with pre-grown microbial biofilms (Mitchell et al., 2009). CO2 permanence would also be increased by the precipitation of CO2 into carbonate minerals, and microbial activity can effect the rate of precipitation by microbial mineral weathering which liberates metal cations necessary for incorporation of CO2 into carbonates (Mcmahon and Chapelle, 1991; Ferris et al., 1996; Barker et al., 1998). Microbially induced precipitation of carbonates is well documented (Wright and Oren, 2005; Mitchell and Ferris, 2006), which has led to the application of microbial mineral plugging (Ferris and Stehmeier, 1992), to the context of GCS (Cunningham et al., 2009).
Engineered microbial mineral plugging (or biomineralization) could reduce rock porosity and permeability through the addition of urea to injected CO2 as an energy source for
microorganisms, which will hydrolyze urea, resulting in increased pH and subsequent increased CaCO3 precipitation (Mitchell et al., 2010; Cunningham et al., 2011; Phillips et al., 2012;
Cunningham et al., 2013). While the use of biofilms and biomineralization to impede scCO2 flow
shows promise, current literature is limited to lower, sub-critical CO2 pressures (Mitchell et al., 2010; Phillips et al., 2012), which do not have the same inhibitory effects as scCO2.
Additionally, the greatly reduced growth rates in these deep subsurface environments (Phelps et al., 1994), may present a major obstacle in developing bioengineering solutions in situ.
1.2 Microbial activity in high-pressure, C02-rich environments
For bioengineering solutions to be realized, microorganisms will need to remain active in these deep formations after CO2 injection. Theoretical studies indicate microbial processes are thermodynamically favorable in deep subsurface formations both before and after CO2 injection (Onstott, 2005; Kirk, 2011). A study of the Ketzin CO2 sequestration site in Germany observed
that after an initial decrease in cell numbers (> 2 log orders) and a shift toward Archaea for several months after CO2 injection, the total cell numbers rebounded and microbial community
composition shifted to be dominated by sulfate reducing bacteria, suggesting the presence of an active community acclimating to near-critical levels of CO2 (Morozova et al., 2011). A recent
study of microbial diversity from the Otway Basin CO2 sequestration site also found changes in
community composition in formation water after CO2 injection, with the population shifting
from Firmicutes to Proteobacteria, again suggesting that at least a subset of these microbial populations are acclimating to (or differentially surviving) CO2 injection, although changes in
biomass were not reported in this study (Mu et al., 2014).
In addition to studies of carbon sequestration sites, marine seeps may form CO2 rich
environments with active microbial populations. The Okinawa Trough off the coast of Japan and Taiwan is a hydrothermal system with active CO2 seeps in sediments, which include liquid and
hydrate CO2. Community composition at the CO2 - sediment interface was primarily composed of methanotrophic Archaea and chemolithotrophic Epsilonproteobacteria (Inagaki et al., 2006). A more recent study of the Okinawa Trough verified that microbial communities in these CO2 rich sediments are active, through reverse transcribed 16S ribosomal RNA, with increasing fractions of Deltaproteobacteria and Euryarchaeota in deeper sediments with higher CO2
the interface with the liquid CO2 (from 109 to 107 cm-3 direct cell counts), with decreasing diversity in sediments with CO2 concentration. These studies on CO2 rich environments indicate
that high concentrations of CO2 will alter the community composition, and in some cases result in decreased biomass, suggesting that some microbial population may not easily acclimate to high concentrations of CO2.
1.3 Microbial sterilization and survival under scCO2
It is well documented that high pressure C02, particularly in supercritical phase is an
effective sterilizing agent, which has been utilized by food and biomedical industries for over a decade as a lower temperature alternative to traditional heat-based sterilization methods.
Supercritical CO2 is effective at sterilizing a diverse range of vegetative cells, including fungi,
gram-positive, and gram-negative bacteria (Garcia-Gonzalez et al., 2009; Dillow et al., 1999; Zhang et al., 2006). In yeast strains, cell death in response to CO2 follows a sigmoidal curve with increasing CO2 pressure, with major decreases in viability occurring at phase changes of CO2 (from gas to liquid and liquid to supercritical fluid) (Isenschmid et al., 1995). The mechanism by which scCO2 inactivates cells is theorized to result from a combination of factors where the
initial step is the influx of high concentrations of dissolved CO2 which will permeabilize cell membranes (Tamburini et al., 2014; Spilimbergo et al., 2008; Zhang et al., 2006; Hong and Pyun, 1999), in part due to the lipid disordering effects of high concentrations of dissolved gases (Chin et al., 1976). Following permeabilization, cytoplasmic acidification will occur (Tamburini et al., 2014; Spilimbergo et al., 2008; Zhang et al., 2006; Hong and Pyun, 1999), and some microorganisms (especially gram negative species) will experience cell wall collapse (Oule et al., 2006; Dillow et al., 1999). Finally, inactivation of enzymes and leakage of intracellular contents
via scCO2 extraction (Bertoloni et al., 2006; Kim et al., 2008), and in some cases physical cell rupture will occur (Oule et al., 2006). It is important to note that some effects of scCO2
sterilization may be partly effects of decompression, as extremely rapid depressurization (from 260 atm to 1 atm) will lyse cells; however depressurization over 5 min time scales results in much lower lysis rates (Park and Clark, 2002). Figure 1 is a schematic of the mechanisms of sCCO2 sterilization that combines membrane permeabilization, cytoplasm acidification, scCO2
extraction, and cell rupture.
SC
2
Co
2Aqueous
. .0 .- je - - .. 0 -. * * .~ 0 . 0.. 0 ..-0 0 001t
4W
*0 0 0~CO2 + H2 - HCO~ + H+ = H2CO~
Cytosol
ItceulrpoinFigure 1. Conceptual model of scCO2 sterilization documenting processes of membrane permeabilization, cytoplasm acidification, supereritical extraction, and cell rupture. CO2 is a
small, uncharged molecule that readily diffuses across the cell lipid membrane. High cytoplasmic CO2 may inhibit metabolic processes and lead to acidification due to carbonate chemistry.
Non-While vegetative cells show high degrees of sterilization upon scCO2 exposure,
microorganisms residing in biofilms (Mitchell et al., 2008) and spores show resistance to scCO2 sterilization (Kamihira et al., 1987; Dillow et al., 1999; Ballestra and Cuq, 1998; Enomoto et al., 1997; Watanabe et al., 2003; Zhang et al., 2006b). Sterilization of spores by scCO2 often requires additional methods including extended exposure time, higher temperatures, pressure cycling, and addition of co-solvents or oxidizing agents (Ishikawa et al., 1997; Watanabe et al., 2003; Shieh et al., 2009; Zhang et al., 2006a,b). Recent work suggest that additional factors such as mineral matrices may enhance microbial survival to scCO2 exposure by providing substrates for biofilm
formation and/or by creating buffered microenvironments (Wu, 2010; Santillan, 2013). However, these studies were conducted on shorter timescales (less than 20 hours) and most experiments used sub-supercritical CO2.
In an effort to simulate CO2 sequestration in a lab environment Frerichs et al. (2014)
incubated formation fluids from a natural gas well under a scCO2 headspace and found that during scCO2 exposure, microbial populations were static with no apparent activity, but upon
removal of scCO2, there was an outgrowth of spore-forming Clostridiales with active sulfate reduction. These studies, and the extensive sterilization literature indicate that certain
physiologies, including organisms capable of spore-formation will be more resistant to initial scCO2 exposure.
1.4 Biocatalysis under supercritical CO2
Part of the mechanism that makes scCO2 an effective sterilizing agent is its solvating property, and this solvent capability also makes scCO2important industrially. ScCO2has been
used for some time as an environmentally friendly solvent for extracting chemicals (especially from plants), but enzymes can often be inactivated due to effects of both scCO2 and
depressurization (Kasche et al., 1988). Despite this, research efforts have developed scCO2 (and other solvents) as non-aqueous solvents for enzyme catalysis, using both purified enzymes as well as whole cells (Baiker, 1999). ScCO2 enzyme catalysis allows for interesting applications
like the modification of compounds that are not soluble in the aqueous phase, as the exact
solvent properties of scCO2 can be altered by changing temperature and pressure, and continuous removal of catalysis products with immobilized enzymes (Wimmer and Zarevucka, 2010).
Examples of scCO2-phase catalysis include esterification reactions with lipase (Nakamura et al., 1986; Marty et al., 1991), carboxylation of pyrrole with Bacillus megaterium cells (Matsuda et al., 2001), reduction of ketones with Geotrichum candidum cells (Matsuda et al., 2008) and hydrolysis of carboxymethyl cellulose with cellulase (Paljevac et al., 2007). These reactions are a few of the multitude of enzymatic reactions demonstrated under supercritical CO2, with many
more possibilities including alkylation, amination, isomerization, oxidation, and dehydrogenation reactions (Baiker, 1999; Wimmer and Zarevucka, 2010). One major limitation to biocatalysis under scCO2 is the variable stability of enzymes under scCO2, and even more stable enzymes
eventually need to be replaced after they lose activity. Use of microorganisms capable of growth in reactors containing scCO2, may help reduced these limitations by protecting the stability of
enzymes and allowing enzyme renewal through growth. The development of enzymatic catalysis
under scCO2, combined with apparent biologic activity under high pressure, high CO2
environments as demonstrated in this thesis suggest that new possibilities are on the horizon for supercritical catalysis.
1.5 Adaption mechanisms allowing survival in extreme environments, implications for organisms growing under scCO2
For microorganisms to be utilized in carbon sequestration bioengineering solutions or industrial biocatalysis, we need a better understanding of the adaptations and acclimation mechanisms employed by cells to survive and grow under stresses associated with supercritical
CO
2.Adaptations, or evolved changes in an organism, are frequently part of the explanation for how a microbe survives and grows in a specific environment. Some physiological adaptations are more specific to a subset of microorganisms such as the ability to form endospores, which is largely observed in Firmicutes. The adaptation of many Firmicutes to form spores allows those organisms to survive in a dormant state, withstanding high temperatures, desiccation, and many other stresses. While Frerichs et al. (2014) suggested that the ability to form endospores was crucial to the enrichment of Clostridiales after scCO2 incubations, Mu et al. (2014) observed anincrease in Proteobacteria after sCCO2 injection into the deep subsurface, suggesting that non
spore forming organisms may also survive scCO2 exposure.
Other adaptations are more broadly distributed across phyla, including the adaptive regulation by Sigma factors, which regulate general transcription and transcriptional responses to a variety of specific stresses including heat, cold, acid, starvation, nitrogen limitation and
pressure (Merrick et al., 1993; Gaidenko et al., 1998; Wemekamp-Kamphuis et al., 2004). Specialized adaptations can also often be conferred through mobile genetic elements, such as the ability to degrade xenobiotics that may be transferred by plasmids and transposons (Top and
Springael, 2003), or resistance to phage through CRISPRs (Barrangou et al., 2007).
Adaptations may also include changes that do not manifest in additional gene content. Differences have been observed in the amino acid composition between halophiles and
non-halophiles (Paul et al., 2008), high and low pressure adapted microorganisms (Di Giulio, 2005; Simonato et al., 2006), and mesophiles and thermophiles (McDonald et al., 1999). While specific amino acids may be selected for during adaptation to different environments, there are also specific gene changes that have been identified as adaptations to certain environments. For example, coding changes in specific regions of malate dehydrogenase (Saito et al., 2006) and
16S rRNA changes (Lauro et al., 2007) have been observed in high-pressure adapted
microorganisms. With coding changes occurring throughout genomes, differences in the ratio of nonsynonymous (coding) changes to synonymous (non-coding) changes (dN/dS or Ka/Ks), can be used to determine specific genes that are under selection (McDonald and Kreitman, 1991). With this method, Campanaro et al. (2008) identified numerous genes involved in solute transport and nucleotide transport and metabolism are under selection in deep-sea bacteria. Further investigations in using dN/dS have developed Selective Signature Analysis, which
improves on the traditional dN/dS to estimate genes under selection by controlling for mutation rate variation in different genomes and gene families (Shapiro and Alm, 2008). However, while novel gene content, changing amino acid content and coding changes may confer adaptations to different environments, many survival strategies rely on non-heritable acclimation mechanisms.
1.6 Acclimation mechanisms allowing survival in extreme environments, implications for organisms growing under scCO2
The high pressure, solvent effects, and pH decrease accompanying scCO2 exposure suggest that microbes will need to adjust aspects of their physiology to acclimate. It is well documented that changes in temperature, salinity, pH, and headspace all force cells to alter their membrane lipid composition to maintain a liquid crystalline membrane that can regulate
osmolarity, intracellular pH, and membrane protein folding (Beales, 2004; Beranova et al., 2010; Guerzoni et al., 2001; Kieft et al., 1994; Mukhopadhyay et al., 2006). Short duration exposures
to scCO2, before inactivation occurs, of S. enterica and E coli results in few changes to lipid acyl chains (Kim et al., 2009; Tamburini et al., 2014), but . coli does shows changes in lipid head groups, with a reduction in phosphatidylglycerol lipids (Tamburini et al., 2014). However, there are limited studies on lipid changes under scCO2, as growth under scCO2 has not been documented until this thesis. Thus, examining previous studies that have focused on conditions with some similarities to scCO2 (e.g. pressure and acid stress) may help elucidate acclimation mechanisms necessary for growth under scCO2. High-pressure gases (not just C02) have been
documented to alter membrane phospholipid ordering (Chin et al., 1976), providing evidence that cells may alter their membranes in the presence of scCO2. High pressure conditions tend to have the effect of compressing and decreasing fluidity of membranes, which cause bacteria to
compensate by producing more unsaturated lipids in order to maintain the fluidity of their membranes (Kato and Hayashi, 1999). Similar to high pressures, cold temperatures decrease membrane fluidity, and microbes acclimate to this by increasing the proportion of unsaturated and/or branched lipids (particularly the anteiso form) that have lower melting points due less dense packing (Miladi et al., 2013; Russell and Fukunaga, 1990; Beales, 2004; Klein et al., 1999). Cells under cold conditions may also respond by decreasing the average chain length (McGibbon and Russell, 1983).
The alteration of pH further complicates cell membrane adjustments. Acid stressed B. subtilis will increase the rigidity of their membranes by producing fewer branched and
unsaturated lipids (Petrackova et al., 2010). The previous study also observed that cells
determine if this was due to membrane diffusion or proton pumps. Clostridium acetobutylicum responds to pH reduction with a similar reduction in unsaturated lipids, but also increases the lipids containing cyclopropane rings (LePage et al., 1987). Acid stressed Streptococcus mutans have a different strategy, as they shift from shorter chain saturated fatty acids to longer chain monounsaturated fatty acids, which would seem to increase membrane fluidity (Fozo and Quivey, 2004). B. subtilis also alters membrane lipids under anaerobic conditions, which result in increased chain length and increases in the anteiso to iso ratio when compared to aerobically grown cells (Beranova et al., 2010). Another important class of membrane compounds is hopanoids, which are 5 membered rings that may be involved in stress response. A
Rhodopseodomonas palustris mutant that could not produce hopanoids was highly sensitive to acid and alkali stress (Welander et al., 2009).
While membrane changes in response to scCO2 exposure seem likely, microbial stress responses include numerous changes in expression. Among the most common expression changes across stresses is the upregulation of general and specific sigma factors (Browne and Dowds, 2002; Foster, 1999; Ferreira et al., 2003; Gaidenko and Price, 1998). In B. subtilis, both the general stress response transcription factor (sigmaB) and the sporulation transcription factor (sigmaH) enhance survival under acid and alkaline stress (Gaidenko and Price, 1998). Listeria monocytogenes also expresses sigmaB (Ferriera et al., 2003), while Salmonella typhimurium expresses sigmaS in response to acid (Foster, 1995). Another acid tolerance mechanism
demonstrated in Streptococci is the increased expression of FoF1 ATPase, which pumps protons out of the cell (Martin-Galiano et al., 2001). Additional transporters are also involved such as glutamate and arginine transporters and decarboxylases, which transport amino acids into cells
and subsequently decarboxylate them, consuming protons in the process (Richard and Foster, 2004; Cotter et al., 2001). Amino acids may also be subject to deiminase enzymes that remove carboxyl and amino groups resulting in ammonia and CO2 which act to buffer the pH (Foster,
1999). Similarly, enzymes like arginase and urease produce the alkaline products ornithine and urea, and ammonia, respectively which can aid in buffering intracellular pH (Chen et al., 1998; Casiano-Colon et al., 1988; Curran et al., 1995; McGee et al., 1999). A recent study of D. vulgaris exposed to high pressures of CO2 observed upregulation of genes involved in production of leucine and isoleucine before cells were inactivated (Wilkins et al., 2014),
indicating that amino acid metabolism may be important in a scCO2 stress response, either for
neutralization of pH, or for use as compatible solutes for osmotic regulation (Csnonka, 1989). Similar to acid stress, high-pressure stress also results in induction of sigma factors (Abe et al., 1999), and various proteins with chaperone activity (Welch et al., 1993; Ishii et al., 2005). Not surprisingly, organisms adapted to high pressures (barophiles), do not show elevated levels
of chaperone expression under high pressure, as those organisms are adapted to growing under high pressure (Boonyaratanakornkit et al., 2007). However, elevated pressure is a different physical stress than acidity, with different expression patterns, aside from general stress
responses like sigma factors and chaperone proteins. In a study of Lactobacillus sanfranciscensis proteomes from heat, cold, acid, salt, starvation, and pressure stressed cells, high-pressure stress shared more common expression patterns with cold and osmotic stresses than acid stress
(Hormann et al., 2006). In experiments with E coli under pressure stress, transcription,
translation, and nucleotide metabolism all show upregulation under pressure stress (Ishii et al., 2005). Interestingly, barophilic organisms appear to show evidence of pressure-regulated
metabolism, as multiple barophiles have shown increases in certain respiratory proteins when grown under pressure (Vezzi et al., 2005; Abe et al., 1999).
1.7 Research Questions
While it is clear that supercritical CO2 presents a unique combination of stresses to microorganisms, existing studies of high pressure, C02-rich environments suggest that
microorganisms are active in close proximity to pure-phase
CO
2.
I sought to determine ifmicroorganisms can in fact grow under a scCO2 headspace, and to understand how
microorganisms survive and acclimate to grow under scCO2. The questions I address in this
thesis are:
1) Are microorganisms isolated from GCS sites capable of growth in bioreactors consisting of an aqueous phase under a supercritical CO2 headspace? What are the taxonomic identities and physiological characteristics of recovered scCO2-tolerant organisms (Chapter 2)
2) What are the genomic characteristics of scCO2 tolerant isolates B. cereus MIT0214 and B. subterraneus MITOT1? (Chapter 3)
3) What are the changes in membrane lipids and expressed proteins that encompass the acclimation response to growth under scCO2? (Chapter 4)
Regarding my first question (are isolates from GCS sites capable of growth in an aqueous phase under a scCO2 headspace?), I hypothesized that certain microorganisms will be able to
grow in the aqueous phase under a scCO2 headspace. This hypothesis has been supported by recent studies of CO2 sequestration pilot sites that show changes in microbial community after
scCO2 exposure (Mu et al., 2014; Morozova et al., 2011), and deep ocean CO2 seeps that contain
2012). Second, while CO2 does impose a range of stresses on vegetative cells, vegetative cells tested in current literature (Tamburini et al., 2014; Wilkins et al., 2014) are not acclimated to high concentrations of C02, and the resistance of spores opens up the possibility that some spores may eventually germinate. Third, microorganisms are incredibly diverse and able to grow under a variety of stresses including: considerably higher temperatures (Takai et al., 2008) and pressures (Boonyaratanakornkit et al., 2007) and far lower pH's (Johnson, 1998) than associated with the critical point of CO2. Finally, modeling predicts that growth in scCO2 containing environments is thermodynamically favorable (Onstott, 2005; Kirk, 2011). To test this
hypothesis, I incubated microorganisms that I isolated through scCO2 enrichments (notably only
Bacilli were isolated) and surface isolated strains in growth media under a scCO2 headspace and demonstrated that a variety of Bacilli (both subsurface and surface isolates) are able to grow under a scCO2 headspace. Additionally, I determined that microbial growth from spores under
sCCO2 is stochastic and varies as a function of inocula density and incubation time.
To address my second research question, (what are the genomic characteristics of scCO2
tolerant isolates B. cereus MIT0214 and B. subterraneus MITOTI) I hypothesized that supercritical CO2 tolerant organisms MIT0214 and MITOTI will show characteristics and
evidence of adaptations that would enhance their fitness in the deep subsurface. As both of these organisms are capable of sporulation and were isolated from samples collected from the deep subsurface through successive passages of scCO2 enrichment passages, it stands to reason they tolerate growth at different pressures, and can survive drastic environmental changes as a spore. Additionally, the anoxic nature of the subsurface and the enrichment method in this thesis suggests these strains may be able to grow anaerobically. These microorganisms may also contain other adaptations of novel genes or differentially evolving genes unique to slow growth
in the nutrient poor subsurface (Phelps et al., 1994). To examine this hypothesis I sequenced and annotated the genomes of scCO2 tolerant isolates B. cereus MIT0214 and B. subterraneus MITOT 1. I did not observe substantial differences between MIT0214 and closely related B. cereus isolates from surface environments, as their genomes are highly similar. MITOTI is more distantly related to genome-sequenced strains and contains more unique gene content than MIT0214, with some content involved in respiratory processes that may enable the strain to access alternative electron acceptors for anaerobic respiration.
To address my final research question (what are the changes in membrane lipids and
expressed proteins that encompass the acclimation response to growth under scCO2?), I
hypothesized that MIT0214 and MITOT 1 will show changes in membrane lipids in response to headspace conditions and in response to different pressures, but that these responses will not necessarily manifest similarly for pressure and headspace. Among the acclimation responses described above, one method that acid stressed cells use to acclimate is through reducing
membrane fluidity, via reduced branching (Petrackova et al., 2010). As the referenced study was performed with B. subtilis, and both MIT0214 and MITOT 1 are Bacilli, I would hypothesize that a similar response may be observed to CO2. As the response to pressure and cold is generally to
increase membrane fluidity, through increased unsaturated lipids (Kato and Hayashi, 1999) or increased branching (Miladi et al., 2013), I would hypothesize that MIT0214 and MITOTI may increase the fluidity of their membranes under elevated pressure. Based on the present literature, the effects of CO2 and pressure may be somewhat opposing each other, leading to a scCO2 phenotype that is intermediate between only CO2 and only high pressure.
To acclimate to scCO2, I hypothesized that protein expression will also change in
is the cell membrane region, which is affected by both acid and pressure, albeit in different manners. I would hypothesize that some cell membrane localized proteins will be involved in acclimation to scCO2. Additionally, the effects of pressure and high CO2 concentration are both stresses that may upregulate general stress response mechanisms like sigma factors and
chaperone proteins (Browne and Dowds, 2002; Welch et al., 1993). Aside from those responses, I would expect that other acclimation mechanisms will not necessarily be related to both CO2 and pressure, as CO2 stress (similar to acid stress) often involves various mechanisms to
neutralize the acid or pump protons out of the cell (Cotter and Hill, 2003). Depending on the pressure tolerance of MIT0214 and MITOTI, the effects of elevated pressure may not be a stress to these strains, as they were isolated from higher pressure, deep subsurface environments. To
examine this hypothesis, I analyzed lipids from MITOTI and MIT0214 and proteomes from MITOTI under 1 atm and 100 atm pressures of N2 and CO2 headspaces. I observed that both
MIT0214 and MITOT 1 respond to CO2 by reducing branched lipids and increasing average acyl chain lengths, similar to patterns observed in Bacilli under acid stress. In proteomes of MITOTI under these conditions, I observed similar profiles to other Bacillus proteomes. Proteomes separated by headspace and pressure along the first two components of principal component analysis and amino acid metabolic proteins including proteins in the glycine cleavage system were enriched in CO2 headspace samples.
My findings suggest that microbial growth in close proximity with supercritical CO2 is
indeed possible, but that injection of scCO2 into the subsurface may initially select for
organisms, particularly spore forming organisms, that can survive the associated stresses. For bioengineering solutions (e.g. biofilm and biomineralized barriers to scCO2 flow) to be realized
non-native scCO2 tolerant microorganisms be injected. My results suggest that it may be
advantageous to select spore forming strains for bioengineering applications involving scCO2,
and Bacillus strains I have isolated may be potentially useful in these applications. The
importance of microbial growth under scCO2 also extends to other areas of biotechnology (e.g. biocatalysis) where scCO2 is an important industrial solvent. Finally this thesis provides insight into the physiological plasticity of the Bacillus genus, which might extend to closely related spore-forming organisms, and expands the known range of conditions under which these organisms can grow.
Chapter 2: Microbial growth under supercritical CO2
Microbial growth under supercritical CO2
Kyle C. Peet', Adam J.E. Freedman, Hector H. Hernandez'*, Vanya Britto', Chris Boreham2,3
Jonathan B. Ajo-Franklin4 and Janelle R. Thompson, **
Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA, 02139
2 Geoscience Australia, GPO Box 378, Canberra, ACT 2601, Australia.
3 CO2CRC, GPO Box 463, Canberra, ACT 2601, Australia
4 Earth Science Division, Lawrence Berkeley National Laboratory, #1 Cyclotron Rd. MS 74R0 120 Berkeley, CA 94720
* Microbial and Environmental Chemical Engineering Laboratory (MECEL), iEnergy Center,
Masdar Institute of Science and Technology, PO Box 54224, Abu Dhabi, United Arab Emirates.
** Corresponding author:
Janelle R. Thompson. Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA, 02139. Telephone: 617.324.5268. Fax: 617.258.8850 Email: jthompson@mit.edu
Abstract
Growth of microorganisms in environments containing CO2 above its critical point is unexpected
due to a combination of deleterious effects including cytoplasmic acidification and membrane destabilization. Thus, supercritical (sC) CO2 is generally regarded as a sterilizing agent. We report isolation of bacteria from three sites targeted for geologic carbon dioxide sequestration (GCS) that are capable of growth in pressurized bioreactors containing scCO2. Analysis of 16S
rRNA genes from scCO2 enrichment cultures revealed microbial assemblages of varied
complexity including representatives of the genus Bacillus. Propagation of enrichment cultures under scCO2 headspace led to isolation of six strains corresponding to B. cereus, B.
subterraneus, B. amyloliquefaciens, B. safensis, and B. megaterium. Isolates are spore-forming, facultative anaerobes, and capable of germination and growth under a scCO2 headspace. In addition to these isolates, several Bacillus type strains grew under scCO2, suggesting this may be
a shared feature of spore-forming Bacilli. Our results provide direct evidence of microbial activity at the interface between scCO2 and an aqueous phase. Since microbial activity can influence the key mechanisms for permanent storage of sequestered CO2 (i.e. structural, residual,
solubility, and mineral trapping), our work suggests that during GCS microorganisms may grow and catalyze biological reactions that influence the fate and transport of CO2 in the deep
2.1 Introduction
Geologic carbon dioxide sequestration (GCS) is an emerging strategy to abate CO2
emissions associated with the burning of fossil fuels by capture, compression, and subsurface
injection of generated CO2 (Lal, 2008; Metz et al., 2005). Although many subsurface geologic
formations targeted for storage of compressed CO2 are known to be biologically active
environments (Chapelle et al., 2002; Kieft et al., 2005; Lavalleur and Colwell, 2013; Onstott,
2005), the extent to which biological processes may play a role in the fate and transport of CO2
remains unknown (Gaus, 2010). CO2 exists as a supercritical fluid (scCO2) at the temperature
and pressures of the vast majority of reservoirs targeted for sequestration (i.e. >31 'C and 72.9
atm). Aqueous fluids in contact with scCO2 may have dissolved CO2 concentrations on the order
of 3 M (Table 1). ScCO2 has generally been regarded as a microbial sterilizing agent due to a
combination of factors including cytoplasm acidification, increased CO2 anion concentration,
osmotic stress, membrane permeabilization and leakage via CO2 extraction, and physical cell
rupture (Bertoloni et al., 2006; Dillow et al., 1999; Hong and Pyun, 1999; Kamihira et al., 1987;
Spilimbergo et al., 2008; Wu et al., 2010; Zhang et al., 2006).
While there has been no direct evidence that microorganisms can sustain metabolic
activity and grow in environments containing scCO2, previous work indicates this possibility.
Survival of spores and biofilms after short-term sCCO2 exposure (i.e., minutes to hours)
(Ballestra and Cuq, 1998; Dillow et al., 1999; Mitchell et al., 2008; Zhang et al., 2006) is
well-documented, and recent studies show that mineral matrices may enhance microbial survival to
scCO2 exposure by providing substrates for biofilm formation and/or by creating buffered
that diverse forms of microbial metabolism are thermodynamically favorable under reservoir conditions post-CO2 injection (Kirk, 2011; Onstott, 2005). Furthermore, high-pressure incubations to simulate reservoir conditions with elevated (but not supercritical) CO2 have
demonstrated activity of acetoclastic methanogens (under 49.3 atm pressure with 86.4 mM C02) (Mayumi et al., 2013) and homo-acetogens (under 395 atm total pressure with 126 mM C02)
(Ohtomo et al., 2013). Recently, field studies at the Ketzin CO2 sequestration site in Germany
and the Otway Basin site in Australia provide evidence that changes in microbial community composition occur following CO2 injection, suggesting that a combination of processes including
differential survival, and possibly growth, occur in the subsurface after exposure to near- and super-critical levels of CO2 (Morozova et al., 2011; Mu et al., 2014).
Whether microorganisms survive and remain active post-CO2 injection is relevant for predicting the fate and stability of the injected CO2. Microbial activity can influence the various
trapping mechanisms that are crucial to permanent storage of sequestered CO2. Prior work has
documented that microbial biofilms can be employed to plug pore spaces and impede the flow of sCCO2 through sandstone cores, providing a means of "structural trapping" for a mobile CO2 phase (Mitchell et al., 2008; 2009). Trapping of CO2 residuals in pore spaces by capillary forces (residual trapping) may be affected by biosurfactant effects on wetting (Jenneman et al., 1983). Bacterial surfaces may provide sites for carbonate mineral nucleation, while bacterial activity can increase the rate of mineral weathering and therefore liberate the metal cations necessary for incorporation of CO2 into carbonate minerals (mineral trapping) (Barker et al., 1998; Ferris et al., 1996; McMahon and Chapelle, 1991). Finally, increased dissolution of CO2into an aqueous
phase (solubility trapping) has been demonstrated by pH increases induced by bacterial ureolysis under high pCO2 (Mitchell et al., 2010).
In this study we tested whether environmental microbes could be isolated with the ability to survive and exhibit microbial activity (growth) during exposure to scCO2. We performed a
series of experimental enrichment cultures inoculated with subsurface fluid filtrate or well core samples from three subsurface environments targeted for CO2 sequestration: the Frio-2 site near
Liberty, TX (Hovorka et al., 2006), the Otway Basin site in Southeastern Australia (Dance et al., 2009; Mu et al., 2014; Sharma et al., 2011), and the King Island site near Stockton, CA (Downey and Clinkenbeard, 2010). These three sites are geologically attractive as prospective CO2
injection zones because they consist of high porosity/permeability sandstone formations overlaid by low-permeability sealing layers capable of structurally trapping buoyant scCO2 in the
underlying zone. Enrichment cultivation was followed by isolation and characterization of strains able to grow under scCO2. Microbial growth under sCCO2 is surprising given its inhibitory
properties, and indicates the possibility that microbial activities will influence CO2 trapping
during geologic carbon dioxide sequestration.
2.2 Methods
Subsurface sample collection and storage. Samples from GCS sites were utilized as inocula for microbial enrichment cultures using scCO2 as the selective agent. Samples from the Frio-2 site were collected as part of the Frio-2 project and shared courtesy of Dr. Tommy Phelps (Oak Ridge National Laboratory). For sample collection, 10 to 20 L of formation fluids were collected
by U-tube from the Frio-2 CO2 sequestration site near Liberty, TX before, during and after CO2
injection (Freifeld et al., 2005; Hovorka et al., 2006). Frio-2 formation fluids from 1,528 to 1,534 m depth were filtered through borosilicate glass filters (nominal pore size 0.8 pm) and frozen on site. Three samples screened for this study correspond to samples collected prior to CO2
injection, 7.5 hours after injection and 372 days post-injection. Otway Basin samples consisted of rock cores from 929 to 1,530 m depth from the Pemble, Paaratte and Skull Creek formations (Sharma et al., 2011). Otway cores were collected as part of the CO2CRC project in Southeast Australia (www.co2crc.com.au). The King Island core sample was obtained from the
Mokelumne River Formation at ~1447 m depth during drilling of the Citizen Green #1 deep characterization well by the West Coast Regional Sequestration Partnership (WESTCARB), San Joaquin County, CA. Rock cores were kept refrigerated at 4'C prior to analysis.
Enrichment cultivation. Inocula for enrichment cultures were prepared in an anaerobic glove bag with 02 and H2 monitor (95% CO2 / 5% H2) and added to 4 or 10 ml pressure vessels
containing a 50% volume of growth media (below). For Frio-2 samples, inocula consisted of 10 pL of hydrocarbon and particulate residue from the surface of glass fiber filters, collected with a sterile scalpel. For Otway Basin cores, drilling fluid tracer penetration data was used to guide collection from the core interior where contamination was least likely. The selected regions of Otway cores were pulverized with a stainless steel mortar and pestle and 1 gram of crushed rock was used as inoculum. For the King Island formation core, no tracer-free interior could be identified, as the sediment was highly permeable and unconsolidated. Thus, a representative
sample from the center of the King Island core was used as inoculum and processed in the same manner as Otway cores.
Media for enrichment cultivation of Frio-2 samples was modified GYP Sodium Acetate Mineral Salts Broth (GYP) consisting of (in g/l) 2.0 glucose, 1.0 yeast extract, 1.0 tryptic peptone, 1.0 sodium acetate, 0.2 MgSO4.7H20, 0.01 NaCl, 0.01 MnSO4.4H20, 0.01
FeSO4.7H20. Both GYP and MS media were used for enrichment cultivation from the Otway Basin and King Island cores with supplements targeting different microbial functional groups added to MS medium (Colwell et al., 1997). MS medium consisted of (in g/l) 0.5 yeast extract, 0.5 tryptic peptone, 10.0 NaCl, 1.0 NH4Cl, 1.0 MgCl2.6H20, 0.4 K2HPO4, 0.4 CaCl2, 0.0025
EDTA, 0.00025 CoCl2.6H20, 0.0005 MnCl2.4H20, 0.0005 FeSO4.7H20, 0.0005 ZnCl2, 0.0002
AlCl3.6H20, 0.00015 Na2WoO4.2H20, 0.0001 NiSO4.6H20, 0.00005 H2SeO3, 0.00005 H3B03, and 0.00005 NaMoO4.2H20. MS medium supplements consisted of: 0.5 g/l glucose for
fermenters; 1.3 g/l MnO2, 2.14 g/l Fe(OH)3, and 1.64 g/l sodium acetate for metal reducers; 0.87
g/l K2SO4, 0.83 g/l FeSO4, 0.82 g/l sodium acetate for sulfate reducers; or 1.3 ml trimethylamine
and 0.82 g/l sodium acetate to target methanogens (Colwell et al., 1997). Culture media were added to serum bottles and degassed with a stream of 100% CO2 or 100% N2 gas for 30 minutes prior to pressurization. Na2S (at 0.25 g/l), a reducing agent to maintain anaerobicity, and
resazurin (at 0.001 g/l), a visual redox indicator, were added to culture media. Following inoculation, vessels were pressurized and incubated for 2-4 weeks at 37 'C for Frio-2 samples and 37 and 60 'C for Otway Basin and King Island samples. In addition to the above media, Luria-Bertani Broth (LB) (Difco) and LB agar were used for strain cultivation at 37C under ambient aerobic conditions.
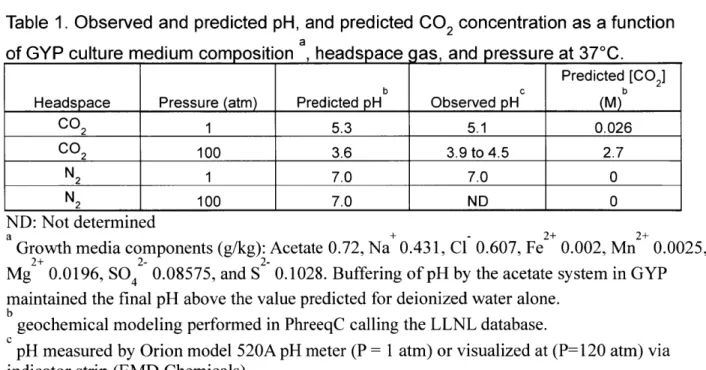
The pH of ambient and CO2 saturated media was measured at 1 atm and 21 'C using an
Orion model 520A pH meter. The pH of media under a scCO2 headspace was measured by visualization of a pH indicator strip (EMD Chemicals) through the sapphire window of a 25 ml
view cell (Thar Technologies, 05422-2). In addition, PHREEQC Version 2 was used to predict the equilibrium pH and potential precipitation of chemical species in the growth media (Table 1) under a CO2 or N2 atmosphere and as a function of temperature and pressure. Thermodynamic
data was obtained from the Lawrence Livermore National Library (LLNL) database.
Table 1. Observed and predicted pH, and predicted CO2 concentration as a function
a
of GYP culture medium composition , headspace as, and pressure at 370C.
Predicted [CO2]
b c b
Headspace Pressure (atm) Predicted pH Observed pH (M)
CO2 1 5.3 5.1 0.026 CO2 100 3.6 3.9 to 4.5 2.7 N2 1 7.0 7.0 0 N2 100 7.0 ND 0 ND: Not determined a + 2+ 2+
Growth media components (g/kg): Acetate 0.72, Na 0.43 1, Cl 0.607, Fe 0.002, Mn 0.0025,
2+ 2-
2-Mg 0.0196, SO4 0.08575, and S 0.1028. Buffering of pH by the acetate system in GYP maintained the final pH above the value predicted for deionized water alone.
b
geochemical modeling performed in PhreeqC calling the LLNL database.
C
pH measured by Orion model 520A pH meter (P = 1 atm) or visualized at (P=120 atm) via indicator strip (EMD Chemicals).
High-pressure incubation. Vessels for high-pressure growth (Supplemental Fig. 10) were 316 stainless steel HPLC column bodies or 316 stainless steel tubing (4 and 10 ml capacity). Vessels were fitted with ball valves (Supelco) or quarter turn plug valves (Swagelok or Hylok). Vessels were filled to 1/2 capacity (2 or 5 ml) with cultivation media, and following inoculation, the headspace of the stainless steel culture vessels, representing 50% of the total vessel volume, was pressurized at a rate of 2-3 atm min' to 100-136 atm with industrial grade N2 gas (Airgas) or
with extraction grade CO2 gas (Airgas) with a helium (He) head pressure such that the final gas mixture was 97-99%
CO
2. Pressurized vessels were incubated in a 37'C warm room, shaking atwere connected with 316 stainless steel tubing and fittings to Swagelok pressure gauges to measure the final vessel pressure before samples were depressurized at a rate of 3-5 atm min over approximately 30 min. Generally, culture vessels with initial headspace pressures of>100 atm lost between 5-25 atm of pressure, due to slow leakage through fittings, over the course of a multiple-week incubation with greater losses associated with longer incubations. Unless
specifically noted, all vessel incubation data reported herein maintained scCO2 headspace pressures of >72.9 atm, the critical pressure for CO2 mixed with <3% inert Helium at 37'C (Roth, 1998), or for incubations at lower pressures Pfinal was > 70% of Pinitial. All pressures were measured at room temperature (21 'C). Based on the ideal gas law we can estimate a maximum pressure increase of 6% associated with incubation of reactors at 37'C, although since scCO2 is a
non-ideal gas this may be an overestimate. Following depressurization, cultures were transferred to an anaerobic chamber (Coy lab products) containing a 95% CO2/5% H2 atmosphere for
sub-sampling and passaging. All pressure vessels and valves were cleaned and autoclaved between uses, and high-pressure tubing was flushed before use with 10% bleach for 30 minutes, rinsed with milliQ-H20, rinsed with 100% ethanol, and dried with CO2 gas.
Enrichment cultures from the Frio-2, Otway and King Island sites were serially passaged by diluting 10% v/v of the previous culture in fresh growth media under a 95% CO2 / 5% H2
atmosphere, pressurizing to 120 atm with C02, followed by incubation at 37'C. The contents of
enrichment vessels were analyzed for cell abundance at the end of passages and in the inocula prior to incubations. Frio-2 passages 1-3 incubated for 15 days each, while passage 4 incubated for 60 days; subsequent passages were incubated for 9 to 15 days (Table 2). Otway and King Island passages were incubated for longer time periods (i.e. 1-2 months) to increase the likelihood of cellular growth based on earlier observations. Samples from each passage were
subjected to microscopic enumeration and archived as a glycerol stocks at -800C.
Enumeration of cell density. To quantify biomass, we used a combination of methods including direct cell counts, viable cell counts and optical density. Cell density at the beginning and end of incubations was determined by microscopic epifluorescent cell staining using 4',6-diamidino-2-phenylindole (DAPI, Sigma) or SYTO9 (Invitrogen) with shaking for 10 minutes in the dark. 500 pl to 1 ml of sample was then filtered onto 25 mm, 0.2 pm pore size, black polycarbonate filters (Nucleopore), followed by 2 washes with 1 ml of Phosphate Buffered Saline (PBS). PBS was incubated on the filtered sample for 1 minute to help wash off excess dye. Filters were laid on slides under microscope immersion oil with a cover slip (Thermo Scientific), and were stored at 4'C in the dark until counting. The cell density (in cells/ml) was calculated by multiplying the mean cell counts (in one 10 x 10 microscope grid) by the dilution factor and then by 3.46x104 (as one 10 x 10 grid at 1000X magnification corresponds to 1/3.46x104 of a 25 mm filter). Samples were visualized on a Zeiss Axioplan fluorescent microscope. Images were captured on a Nikon D100 camera using the NKRemote live imaging software. Viability counts, i.e. Colony Forming Unit (CFU) plating was performed using Luria Broth Agar. Viable spore counts were carried out by heating aliquots to 80'C for 10 minutes to kill vegetative cells (Setlow, 2006) prior to plating on Luria Broth Agar. Culture optical density (600nm) was measured on a Bausch and Lomb spectrophotometer (1 cm path length) or via 96-well microplate reader (BioTek Synergy 2) (200 pI per well). Optical density was not measured for incubations using metal reducer medium due to confounded readings from solid particulate content.
For MIT0214, growth was defined by increased cell density and evidence of vegetative cell morphologies by microscopy. Growth-positive cultures had at least 45-fold increased direct
cell counts relative to initial inocula less than lx 106 spores/ml, or at least 5-fold increase in direct cell counts for cultures with inocula greater than lx106 spores/ml, as MIT0214 final cell
densities generally varied between 1x107 and 1x108 cells/ml. For MITOT1, growth was defined
by observation of culture turbidity accompanied by at least a 4-fold increase in viable cell counts (CFU/ml) above the initial spore density (on average, increases were >50-fold) and at least
25-fold higher viable counts compared to replicates without observed turbidity since samples without evident growth all showed a decline in viable counts relative to the initial viable counts
of the inoculated cultures, presumably due to a loss of spore viability during the incubation period.
Extraction of DNA and analysis of 16S rRNA gene diversity in enrichment cultures. DNA extraction from Frio-2 sample enrichment passages was performed using a protocol modified for gram-positive bacteria (Lessard et al., 2004). DNA extraction from Otway Basin project
passages was performed using two methods, the Qiagen Blood and Tissue DNA extraction kit protocol for gram-positive cells (Qiagen), or the MoBio Soil DNA extraction kit (MoBio). Amplification of 16S rRNA genes from extracted DNA was performed using universal Bacterial primers 515F 5'- GTG CCA GCM GCC GCG GTA A- 3' and 1406R 5'-ACG GGC GGT GWG TRC AA- 3' (Frio-2 passages 1, 2 and 7) and 27F 5'- AGA GTT TGA TCM TGG CTC AG- 3' and 1492R 5'-TAC GGY TAC CTT GTT ACG ACT T- 3' (Frio-2 passage 9, Otway Passage 3, and colony-purified isolates). PCR mixtures (20 gL per reaction) contained 25 to 75 ng of genomic DNA, IX Phusion Polymerase buffer, 0.4 gM of each primer (IDT), 0.4 pM
deoxynucleotide mixture and 1 U Phusion Polymerase (New England Biolabs). Thermal cycling conditions consisted of an initial 3 minutes at 95 C followed by 35 cycles of 95C for 30 sec,