Mémoire de fin d’étude pour l’obtention du :
Thème :
Présenté Par: Dr. Safaa Fellous
Mémoire encadré par le Professeur Hanan Rkain
Service de Rhumatologie B
Pr. Fadoua Allali
Hôpital El Ayachi
One-year direct costs of biological therapy in
rheumatoid arthritis and its predictive factors: data
Summary
Résumé...3
Abstract ...4
Introduction...5
Material and methods...7
Data source: RBSMR registry Patient population and study design Direct costs Cost calculation Statistical analysis
Results ...12
Discussion...15
Conclusion ...17
References...18
One-year direct costs of biological therapy in rheumatoid arthritis
and its predictive factors: data from the Moroccan RBSMR registry
Safaa Fellous*1, Hanan Rkain1,2, Samir Ahid3, Latifa Tahiri1, Redouane Abouqal4,
Ihsane Hmamouchi4,5, Lahsen Achemlal6, Imane El Bouchti7, Abdellah El Maghraoui8,
Imad Ghozlani9, Hasna Hassikou10, Taoufik Harzy11, Linda Ichchou12, Ouafae
Mkinsi13, Radouane Niamane14, Rachid Bahiri15, Fadoua Allali1
1. Department of Rheumatology B, El Ayachi Hospital, Ibn Sina University Hospital, Salé, Morocco
2. Physiology laboratory; Faculty of Medicine and Pharmacy of Rabat, Mohammed V University, Rabat, Morocco.
3. Research Team of Pharmacoeconomics & Pharmacoepidemiology, Mohammed V University, Rabat, Morocco
4. Laboratory of Biostatistical, Clinical and Epidemiological Research, Faculty of Medicine and Pharmacy, Mohammed V University, Rabat, Morocco
5. Department of Rheumatology, Provincial Hospital of Temara, Morocco
6. Department of Rheumatology, Military Hospital Mohammed V, Ibn Sina University Hospital, Rabat, Morocco
7. Department of Rheumatology, Arrazi University Hospital, Marrakech, Morocco 8. Private Medical Office, Rabat, Morocco
9. Department of Rheumatology, University Hospital of Agadir, Morocco
10. Department of Rheumatology, Military Hospital Moulay Ismail, Hassan II University Hospital, Meknès, Morocco
11. Department of Rheumatology, Hassan II University Hospital, Fès, Morocco 12. Department of Rheumatology, Mohammed VI University Hospital, Oujda, Morocco
13. Department of Rheumatology, Ibn Rochd University Hospital, Casablanca, Morocco
14. Department of Rheumatology, Military Hospital Avicenne, Mohammed VI University Hospital, Marrakech, Morocco
Résumé
Objectif: L’objectif de l'étude était d'estimer les coûts directs annuels de la biothérapie dans la polyarthrite rhumatoïde (PR) et d'établir les éventuels facteurs associés à ces coûts.
Méthodes: La principale source de données était la registre des Biothérapies de la Société Marocaine de Rhumatologie (RBSMR). Nous avons inclus des patients avec des données disponibles à 1 an. Des données liées au statut socio-économique, à la maladie et à la biothérapie ont été recueillies. Les coûts directs comprenaient les prix des produits biologiques, les coûts des perfusions et des injections sous-cutanées. Les différences de coûts entre les groupes ont été testées par des tests de Mann-Whitney et Kruskal-Wallis. Une analyse des corrélations a été effectuée à la recherche de facteurs associés à des coûts élevés.
Résultats: Nous avons inclus 197 patients atteints de polyarthrite rhumatoïde. L'âge moyen était de 52,3 ± 11 ans, avec une prédominance féminine de 86,8%. Recevant l'un des traitements suivants: rituximab (n = 132), tocilizumab (n = 37) ou anti-TNF (n = 28). Les coûts directs médians sur un an étaient de 1 665 € [1 472 € - 9 879 €]. Les coûts directs annuels totaux s'élevaient à 978 494 €. Le rituximab, représentait 25,7% du budget annuel total. Les anti-TNF et le tocilizumab représentaient respectivement 27,3% et 47% de ce budget global. Bien que les coûts n'étaient pas significativement différents en termes de sexe ou de niveau d'études, le type d'assurance a eu une incidence significative sur l'estimation des coûts. Une corrélation positive a été retrouvée entre le coût direct annuel et l'indice de masse corporelle (r = 0,15, p = 0,04).
Conclusion: Au Maroc, pays en développement, les coûts directs annuels de la biothérapie sont élevés. Nos résultats peuvent contribuer à l'élaboration de stratégies pour une meilleure gouvernance de ces coûts.
Mots clés: polyarthrite rhumatoïde; Médicaments biologiques; Coût direct annuel; Registre RBSMR
Abstract
Objectives: The aim of the study was to estimate the annual direct costs of biological therapies in rheumatoid arthritis (RA), and to establish possible factors associated with those costs.
Methods: The main data source was the Moroccan registry of biological therapies in rheumatic diseases (RBSMR Registry). We included patients with available 1-year data. Variables related to socio-economic status, disease and biological therapy were collected. Direct costs included prices of biologics, costs of infusions, and subcutaneous injections. Differences in costs across groups were tested by Mann-Whitney and Kruskal-Wallis tests. Correlations analysis was performed in search of factors associated with high costs.
Results: We included 197 rheumatoid arthritis patients. The mean age was 52.3 ± 11 years, with female predominance 86.8%. Receiving one of the following therapies: rituximab (n=132), tocilizumab (n=37), or TNF-blockers (n=28). Median one-year direct costs per patient were €1,665 [€1,472 - €9,879]. The total annual direct costs were € 978,494. Rituximab, constituted 25.7% of the total annual budget. TNF-blockers and tocilizumab represented 27.3% and 47% of this overall budget, respectively. Although the costs were not significantly different in terms of gender or level of study, the insurance type significantly affected the cost estimation. A positive correlation was found between the annual direct cost and body mass index (r = 0.15, p=0.04).
Conclusion: In Morocco, a developing country, the annual direct costs of biological therapy are high. Our results may contribute to the development of strategies for better governance of these costs.
Keywords: Rheumatoid arthritis; Biological therapy; Annual direct cost; RBSMR registry
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by pain, chronic inflammation and joint destruction, resulting in a functional impairment and a reduced quality of life [1-3]. Not only does RA cause physical impairments, but also it places a huge economic burden on the patient, family, and society [4-6].
Advances in the treatment of RA diseases have improved the health-related quality of the life of patients [7]. The introduction of effective biological treatments transformed the expectations of inflammatory rheumatic disease management. However, the very high cost of these drugs limits their availability and their use in daily practice [8].
The Moroccan health system contains several health insurance organisations. The medical assistance scheme for the economically underprivileged (RAMED) constitutes one of the components of basic medical coverage, it is intended for economically disadvantaged people and not covered by any health insurance scheme. The other health insurance schemes are represented by compulsory health insurance for employees and pensioners in the private sector (CNSS), that of employees and pensioners in the public sector (CNOPS) and the private mutuals. For the military, the royal armed forces mutual (FAR) constitutes the basic health insurance [9,10].
Rheumatoid arthritis management recommendations from the Moroccan Society of Rheumatology (SMR) represent a reference for both rheumatologists and health insurance organizations in the country. By comparing these recommendations with international ones, the SMR proposes rituximab as the first line compared to other biological drugs. This could be explained by two points: firstly, tuberculosis is endemic in our country and rituximab poses fewer problems of reactivation of tuberculosis than other biotherapies. Secondly, rituximab price is cheaper compared to other biologic DMARDs [11-13].
in Morocco for treating RA: infliximab, etanercept, adalimumab, golimumab which are blockers of tumor necrosis factor-alpha (TNF-blockers); rituximab, an anti-CD20 monoclonal antibody; tocilizumab, an interleukin-6 receptor inhibitor; Anakinra, an IL1 inhibitor. In addition, a number of biosimilars are also currently available in Morocco.
In Morocco, which is a developing country, access to biotherapies is difficult because of their exorbitant prices. An international study published in 2010 QUEST-RA (Quantitative Standard Monitoring of Patients with RA) which encompassed 32 countries revealed that in Morocco the use of biotherapy was observed in less than 1% of Moroccan RA patients [14].
Assessing the costs of treatment is an integral part of a cost-effectiveness analysis [15-19]. Predicting the annual cost of biologic is complex due to the differences in dosing schedules. Furthermore, patients may not respond and may require dose escalation overtime or a switch between biologics. Some biologics can be administered by subcutaneous route while others are only administered at the hospital.
Several studies around the world have evaluated the direct costs of biological therapy [20-24]. The aim of this study is to estimate the annual direct costs of biotherapy (initiation or ongoing biotherapy) in rheumatoid arthritis and to establish possible factors associated with those costs. using the Moroccan biotherapy registry database RBSMR.
Methods
Data source: RBSMR registry
The data for our study were taken from the registry of biological therapies in rheumatic diseases of the Moroccan Society of Rheumatology (RBSMR Registry). The RBSMR registry is a historical, prospective and multicenter registry, which includes 10 departments of rheumatology in university medical centres in Morocco. The age of patients recruited in the registry was over 18 years old. They were diagnosed with Rheumatoid Arthritis or spondyloarthritis (SpA) and treated by biotherapy (initiation and ongoing biotherapy). Patients who were prescribed biotherapy for any indication other than rheumatoid arthritis or spondyloarthritis as well as patients with juvenile idiopathic arthritis were excluded.
The inclusion period in the registry was from May 2017 to January 2019 and the follow up was 3 years. The registry enrolled 441 patients and 419 patients were eligible (225 RA, 194 SpA). The primary objective of the RBSMR registry was to assess the tolerability of the patients with RA or SpA treated by biotherapy in rheumatology [25-27].
The study was approved by the Ethics Committee of the Faculty of Medicine, Mohammed V Rabat University, as well as the National Committee for the Protection of Personal Data. All patients included in the study gave their prior written consent. Study design and population
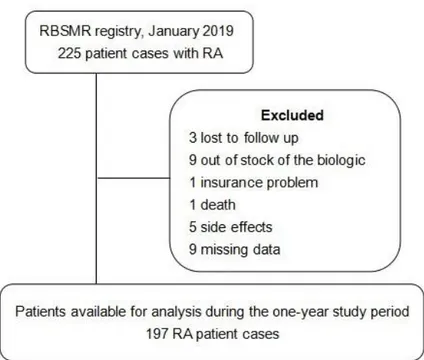
From the RBSMR registry, we included patients with RA (initiation or ongoing biotherapy), who completed 1 year of the same biologic or switched to another biologic during this year. We excluded patients who discontinued the biologic during the first 12 months of their inclusion in the registry (Figure 1). Assessments were performed at baseline, at 6 months, and at 1 year.
Figure 1: Patient selection flowchart
The socio-demographic and anthropometric data of the included patients were collected (age, gender, weight and Body Mass Index (BMI), level of education, profession, Insurance plan type) and the characteristics of RA (disease duration, disease activity, conventional synthetic DMARDs and biologic DMARDs used at inclusion in the registry). The disease activity score DAS 28-ESR score at inclusion and at 12 months was calculated. The difference between the DAS 28 at inclusion in the registry and at a 1-year follow-up was noted. The Health Assessment Questionnaire (HAQ) at baseline was collected.
Direct costs
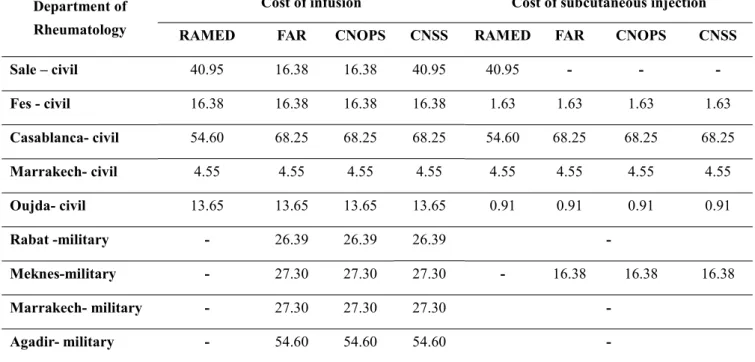
Direct costs are defined as prices of biological agents, costs of injection for intravenous (IV) and subcutaneous biologics. Those costs depend on the type of department of rheumatology (civil, military) and insurance plan type(tables 1 and 2). The drug prices were taken from the local pharmacy of the university medical center
insurance. Prices of conventional DMARDs or other treatments (corticosteroids, Non-steroidal anti-inflammatory drugs (NSAIDs)) were not taken into account in the analysis.
The costs are presented in Euro using an exchange rate of (1 Moroccan Dirham = 0.091Euro)
Cost calculation
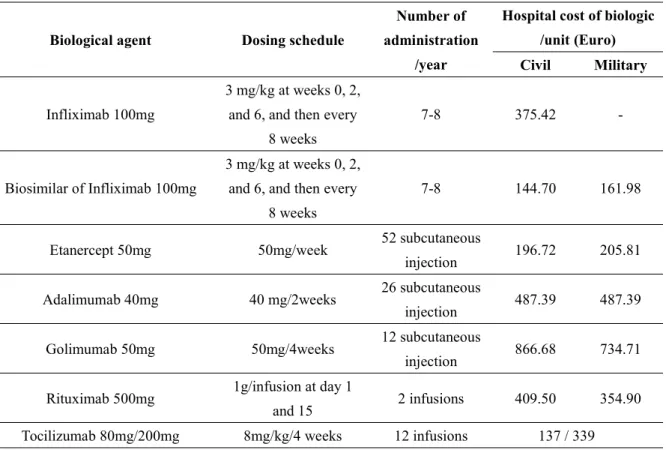
The dosing schedule for biological agents and the number of hospitalizations per year are presented in table 2. In the case of tocilizumab and infliximab, which are dosed by weight, the cost used corresponds to the number of vials administered to the patient. The total direct cost per treated patient was defined as the sum of biologic price and cost of infusion or subcutaneous injection during a total period of 12 months.
Table 1 : Unit cost of administering biological agents (Euro)
RAMED: Medical Assistance Scheme for the Economically Underprivileged
CNOPS: National Fund for Social Providence Organizations CNSS: National Social Security Fund
FAR Mutual : Royal Armed Forces Mutual
(1 Moroccan dirham= 0.091euro) Department of
Rheumatology
Cost of infusion Cost of subcutaneous injection RAMED FAR CNOPS CNSS RAMED FAR CNOPS CNSS Sale – civil 40.95 16.38 16.38 40.95 40.95 - - -Fes - civil 16.38 16.38 16.38 16.38 1.63 1.63 1.63 1.63 Casablanca- civil 54.60 68.25 68.25 68.25 54.60 68.25 68.25 68.25 Marrakech- civil 4.55 4.55 4.55 4.55 4.55 4.55 4.55 4.55 Oujda- civil 13.65 13.65 13.65 13.65 0.91 0.91 0.91 0.91 Rabat -military - 26.39 26.39 26.39 -Meknes-military - 27.30 27.30 27.30 - 16.38 16.38 16.38 Marrakech- military - 27.30 27.30 27.30 -Agadir- military - 54.60 54.60 54.60
-Table 2 : Characteristics of biological agents
Biological agent Dosing schedule
Number of administration
/year
Hospital cost of biologic /unit (Euro) Civil Military
Infliximab 100mg
3 mg/kg at weeks 0, 2, and 6, and then every
8 weeks
7-8 375.42
-Biosimilar of Infliximab 100mg
3 mg/kg at weeks 0, 2, and 6, and then every
8 weeks
7-8 144.70 161.98
Etanercept 50mg 50mg/week 52 subcutaneous
injection 196.72 205.81 Adalimumab 40mg 40 mg/2weeks 26 subcutaneous
injection 487.39 487.39 Golimumab 50mg 50mg/4weeks 12 subcutaneous
injection 866.68 734.71 Rituximab 500mg 1g/infusion at day 1
and 15 2 infusions 409.50 354.90 Tocilizumab 80mg/200mg 8mg/kg/4 weeks 12 infusions 137 / 339
Statistical analysis
The statistical study was conducted according to the database frozen in January 2020. The statistical analysis was performed using SPSS software, version 24. The data were represented as mean and standard deviation or median and quartile, and as numbers and percentages for categorical variables.
The differences between the groups in terms of direct medical costs were estimated using non-parametric tests (Mann-Whitney, Kruskal-Wallis). The correlations analysis was examined using the Spearman test. p values less than 0.05 were considered statistically significant.
Results
Baseline patient characteristics
A total of 197 patients met all inclusion criteria. Of these patients, 26,9 % were new initiators and 73,1 % were continuing therapy. 44.2% of included patients have the RAMED medical assistance scheme. 45.7% of the patients were illiterate.
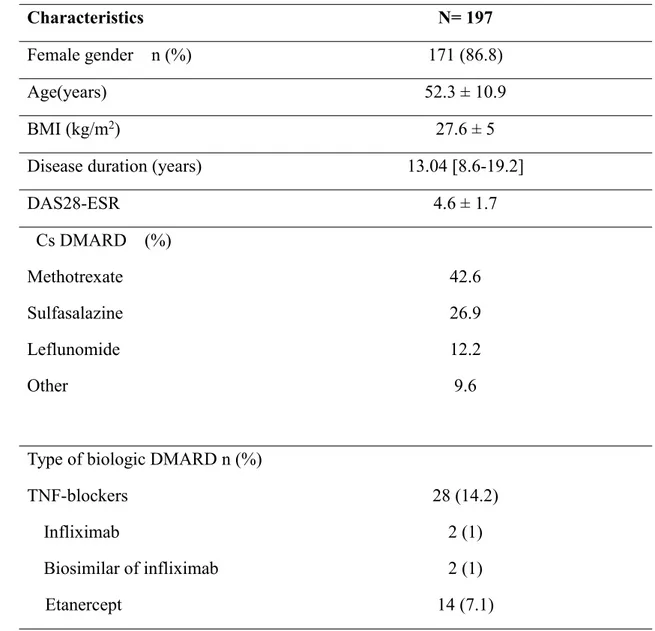
The biologic DMARDs most widely administered for patients with RA during the study period were rituximab, tocilizumab and etanercept in 67%, 18,8% and 7,1%, respectively. The Baseline characteristics of the study population are summarized in table 3.
Table 3: Baseline characteristics of included patients
Characteristics N= 197
Female gender n (%) 171 (86.8)
Age(years) 52.3 ± 10.9
BMI (kg/m2) 27.6 ± 5
Disease duration (years) 13.04 [8.6-19.2]
DAS28-ESR 4.6 ± 1.7 Cs DMARD (%) Methotrexate Sulfasalazine Leflunomide Other 42.6 26.9 12.2 9.6 Type of biologic DMARD n (%)
TNF-blockers Infliximab
28 (14.2) 2 (1)
Adalimumab Golimumab Rituximab Tocilizumab 9 (4.6) 1 (0,5) 132 (67) 37 (18.8)
BMI: body mass index; DAS: disease activity score; ESR: Erythrocyte sedimentation rate; Cs DMARD: conventional synthetic Disease-modifying anti-rheumatic drug conventional synthetic
Direct costs
The total direct cost of biological agents in the RBSMR registry during the 1-year of follow-up was 10,752,868 dirhams (≈ € 978,494). Rituximab was prescribed in 67% of patients and constituted 25.7% of the total annual budget. TNF-blockers and tocilizumab were prescribed in 14.2% and 18.8% of cases and represented respectively 27.3% and 47% of this overall budget(figure 2).
The distribution of costs between biologics prices and administration costs is shown in table 4.
Table 4 : Annual total direct cost of rheumatoid arthritis patients
N=197
Total cost Overall %
Total drug prices € 954,684 97.5%
Total cost of infusions € 23,000.79 2.4%
Total cost of subcutaneous injection € 809.21 0.1%
Annual total direct cost € 978,494 100%
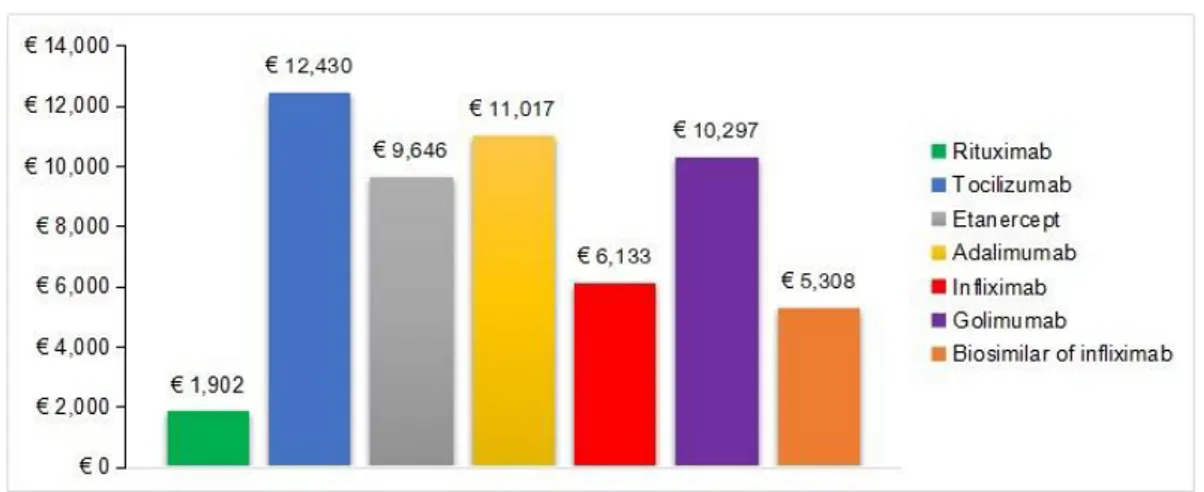
The median annual direct cost per patient was 18,300 [16,180-108,561] dirhams (≈ € 1,665). For all patients, the mean annual direct cost per treated patient for each biological agent is summarized infigure 3
Figure 3: Annual direct cost per treated patient Predictors of direct costs of biological agents
There was no statistically significant difference in costs between men and women, between illiterate and no-illiterate. There was a statistically significant difference in costs between patients with RAMED and other health insurance (p<0.01). A significant difference of the direct cost was found between biological agents (p<0.01)
Discussion
This study provides a global overview of the annual direct costs of biological therapies linked to rheumatoid arthritis from the Moroccan registry of biological therapies in rheumatic diseases (RBSMR Registry), and to establish possible factors associated with those costs.
The overall cost of biological agents according to the Moroccan register is very high, it corresponds to almost 1 million Euros to treat 197 patients, which corresponds to a median of €1,665 for each patient, with an annual direct cost varies between €1,472 and €9,879. Those high costs contrast with the Moroccan interprofessional guaranteed minimum wage (SMIG) which is 263 Euros per month on the basis of 191 working hours per month, with an average salary, all co-founded sectors of 222 Euros. In addition, the Gross Domestic Product (GDP) in Morocco was worth 106 billion Euros in 2019. The GDP value of Morocco represents 0.10 percent of the world economy [28,29]. In the literature, there is a disparity between the countries concerning the costs of biological therapies, in relation to the differences in socio-economic development and the budget allocated to the health sector [30-35].
Regarding the profile of the prescription of biological therapy, our study showed that two-thirds of patients were treated with rituximab and one-third was fairly comparable between tocilizumab and TNF-blockers. Only 2 patients were on infliximab biosimilar. The weak prescription of biosimilars would be explained by the recent advent of biosimilars in our country [36].
Our study found that the patients had equal access to biotherapies regardless of their gender, level of study or even social coverage. In recent years, the Moroccan ministry of Health has allocated a substantial budget for biologic DMARDs for patients with RAMED medical assistance scheme only accessible in the tertiary structures. This would also explain that these patients have access to biotherapies in a manner
The prescription of rituximab in the majority of patients could be explained by the fact that rituximab is cheaper, and by the intra-hospital policy to rationalize access to biological therapy and to expand the offer of care. Other biologic DMARDs including TNF-blockers or tocilizumab were also prescribed to one-third of the patients. Rituximab, which was prescribed to 67% of patients, constitutes a quarter of the total annual budget in the Moroccan registry. Likewise, TNF-blockers and tocilizumab, which were only prescribed to 14.2% and 18.8%, respectively, represent 27.3% and 47% of the overall biological therapy budget.
The analysis showed a positive correlation between the total direct cost of biological therapy and body mass index. This situation is not applicable for rituximab and concerns the prescription of TNF-blockers and tocilizumab since obesity automatically generates an increase in the doses administered. Active patients or patients with severe functional repercussions consume a lower biological therapy budget. This could probably be explained by the choice that this category of patients would be taken charge of in a hospital environment and would be administered rituximab rather than other biologics in particular subcutaneous drugs.
The evolution of RA activity in the registry patients after one year of follow-up was comparable between patients treated with rituximab and those receiving tocilizumab or TNF-blockers. Through these results, the therapeutic benefit of the different biologics could appear comparable. However, the type of our study as a retrospective and prospective register does not allow us to establish such conclusions methodologically.
Our study is the first Moroccan study to assess the medico-economic aspect of biological therapy in Morocco, with data from real-life follow-up of 1 year of patients
users of biological therapy and does not give anterior information about biotherapies in all cases. Secondly, this medico-economic assessment was only concerned with the direct costs of biotherapy, other expenses were not assessed. Finally, the database did not include assessment of the quality of life and sparing of corticosteroids which seem important elements to be considered in the daily management of rheumatoid arthritis.
Conclusion
This study presents a global overview of the direct costs of biological therapy associated with RA patients, taking as a source the Moroccan registry of biological therapies in rheumatic diseases. These data may contribute to the understanding and estimation of the economic impact of the disease and to the development of strategies for a better governance of medico-economic management in a developing country.
References
1. Benbouazza K, Benchekroun B, Rkain H, Amine B, Bzami F, Benbrahim L, Atouf O, Essakalli M, Abouqal R, Dougados M, Hajjaj-Hassouni N (2011) Profile and course of early rheumatoid arthritis in Morocco: a two-year follow-up study. BMC Musculoskeletal Disorder 12:266.https://doi.org/10.1186/1471-2474-12-266.
2. Badsha H, Fathi N.A, Hamoud H, Hajjaj-Hassouni N et al (2009) Profile of patients with rheumatoid arthritis in rarely-reported locations: North Africa. Ann Rheum Dis 68(Suppl3):352
3. Ibn Yacoub Y, Amine B, Laatiris A, Wafki F, Znat F, Hajjaj-Hassouni N (2012) Fatigue and severity of rheumatoid arthritis in Moroccan patients. Rheumatol Int 32:1901–1907.
https://doi.org/10.1007/s00296-011-1876-0.
4. Fazal SA, Khan M, Nishi SE, Alam F, Zarin N, Bari MT, Ashraf GM (2018) A Clinical Update and Global Economic Burden of Rheumatoid Arthritis. Endocr Metab Immune
Disord Drug Targets 13;18(2):98-109.
https://doi.org/10.2174/1871530317666171114122417
5. Rkain, H, Allali, F, Jroundi, I, & Hajjaj-Hassouni, N. (2006). Socioeconomic impact of rheumatoid arthritis in Morocco. Joint bone spine, 73(3), 278–283.
https://doi.org/10.1016/j.jbspin.2005.03.021
6. Ghabri, S, Lam, L, Bocquet, F, & Spath, H. M. (2020). Systematic Literature Review of Economic Evaluations of Biological Treatment Sequences for Patients with Moderate to Severe Rheumatoid Arthritis Previously Treated with Disease-Modifying Anti-rheumatic
Drugs. PharmacoEconomics, 38(5), 459–471.
https://doi.org/10.1007/s40273-020-00887-6
7. Law, S. T., & Taylor, P. C. (2019). Role of biological agents in treatment of rheumatoid
arthritis. Pharmacological research, 150, 104497.
https://doi.org/10.1016/j.phrs.2019.104497
8. Pisetsky D. S. (2017). Advances in the Treatment of Rheumatoid Arthritis: Costs and Challenges. North Carolina medical journal, 78(5), 337–340.
https://doi.org/10.18043/ncm.78.5.337
obligatoire au Maroc [The characteristics of the population covered by Compulsory Health Insurance (CMI) in Morocco]. Pan Afr Med J 8;30:266.
https://doi.org/10.11604/pamj.2018.30.266.13209
11. Smolen, J. S, Landewé, R, Bijlsma, J et al (2020). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Annals of the rheumatic diseases, 79(6), 685–699. https://doi.org/10.1136/annrheumdis-2019-216655
12. Daien C, Hua C, Gaujoux-Viala C, Cantagrel A, Dubremetz M, Dougados M, Fautrel B, Mariette X, Nayral N, Richez C, Saraux A, Thibaud G, Wendling D, Gossec L, Combe B (2019) Update of French society for rheumatology recommendations for managing
rheumatoid arthritis. Joint Bone Spine 86(2):135-150.
https://doi.org/10.1016/j.jbspin.2018.10.002
13. Niamane, R, Bahiri, R, Bouchti, I et al (2014). Recommandations de la Société Marocaine de Rhumatologie pour la prise en charge de la polyarthrite rhumatoïde : mise à jour du référentiel de 2011. Rev Mar Rhum 2014;30: 3-13
14. Sokka T, Kautiainen H, Pincus T et al (2010) Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther 12(2):R42. https://doi.org/10.1186/ar2951
15. Verhoef, L. M., Bos, D., van den Ende, C., van den Hoogen, F., Fautrel, B., Hulscher, M. E., Kievit, W., & den Broeder, A. A. (2019). Cost-effectiveness of five different anti-tumour necrosis factor tapering strategies in rheumatoid arthritis: a modelling study.
Scandinavian journal of rheumatology, 48(6), 439–447.
https://doi.org/10.1080/03009742.2019.1613674
16. Pazmino, S., Boonen, A., Stouten, V., De Cock, D., Joly, J., Van der Elst, K., Westhovens, R., & Verschueren, P. (2020). Two-year cost-effectiveness of different COBRA-like intensive remission induction schemes in early rheumatoid arthritis: a piggyback study on the pragmatic randomised controlled CareRA trial. Annals of the rheumatic diseases, 79(5), 556–565. https://doi.org/10.1136/annrheumdis-2019-216874
17. Patel, A., Heslin, M., Scott, D. L., Stringer, D., Birrell, F., & Ibrahim, F. (2020). Cost-Effectiveness of Combination Disease-Modifying Antirheumatic Drugs Versus Tumor Necrosis Factor Inhibitors in Active Rheumatoid Arthritis: A Pragmatic, Randomized, Multicenter Trial. Arthritis care & research, 72(3), 334–342.
https://doi.org/10.1002/acr.23830
18. Navarro, F., Martinez-Sesmero, J. M., Balsa, A., Peral, C., Montoro, M., Valderrama, M., Gómez, S., de Andrés-Nogales, F., Casado, M. A., & Oyagüez, I. (2020).
treatment of rheumatoid arthritis in Spain. Clinical rheumatology, 39(10), 2919–2930.
https://doi.org/10.1007/s10067-020-05087-3
19. Drosos, A. A., Pelechas, E., Kaltsonoudis, E., & Voulgari, P. V. (2020). Therapeutic Options and Cost-Effectiveness for Rheumatoid Arthritis Treatment. Current rheumatology reports, 22(8), 44.https://doi.org/10.1007/s11926-020-00921-8
20. Martínez-López-de-Castro, N., Álvarez-Payero, M., Samartín-Ucha, M et al (2020). Direct costs in patients with chronic inflammatory arthropathies on biological therapy: a real-world data study. Clinical and experimental rheumatology, Advance online publication.
21. Gu, T, Mutebi, A, Stolshek, B. S, & Tan, H (2018). Cost of biologic treatment persistence or switching in rheumatoid arthritis. The American journal of managed care, 24(8 Spec No.), SP338–SP345.
22. Best, J. H, Vlad, S. C, & Pei, J (2020). Comparative Cost per Response for 4 Clinical Endpoints with Tocilizumab Monotherapy vs Adalimumab Monotherapy in a Head-to-Head Randomized Double-Blind Superiority Trial (ADACTA) in Patients with Rheumatoid Arthritis. Rheumatology and therapy, 7(1), 165–171.
https://doi.org/10.1007/s40744-019-00191-6
23. Ohinmaa AE, Thanh NX, Barnabe C, Martin L, Russell AS, Barr SG, Maksymowych WP (2014) Canadian estimates of health care utilization costs for rheumatoid arthritis patients with and without therapy with biologic agents. Arthritis Care Res (Hoboken) 66(9):1319-1327.https://doi.org/10.1002/acr.22293
24. González Fernández, M, Villamañán, E, Jiménez-Nácher, I, Moreno, F, Plasencia, C, Gayá, F, Herrero, A, & Balsa, A (2019). Cost evolution of biological drugs in rheumatoid arthritis patients in a tertiary hospital: Influential factors on price. Reumatologia clinica,
S1699-258X(19)30162-7. Advance online publication.
https://doi.org/10.1016/j.reuma.2019.10.004
25. Benchérifa S, Amine B, Elbinoune I et al (2019). Radiographic axial versus nonradiographic axial spondyloarthritis: Comparison of the disease activity parameters and the disease activity and functional scores: RBSMR study. International Journal of Clinical Rheumatology, 14, 282.
et al (2019) The Moroccan registry of biological therapies in rheumatic diseases (RBSMR): methods and preliminaries results. Rev Mar Rhum 49:32-7
28. High Commission for Planning of Morocco (2020) Keys Figures. https://www.hcp.ma/Accessed 05 November 2020.
29. High Commission for Planning of Morocco (2020) LA SITUATION ÉCONOMIQUE NATIONALE EN 2019. https://www.hcp.ma/Accessed 05 November 2020.
30. Sugiyama N, Kawahito Y, Fujii T, Atsumi T, Murata T, Morishima Y, Fukuma Y (2016) Treatment Patterns, Direct Cost of Biologics, and Direct Medical Costs for Rheumatoid Arthritis Patients: A Real-world Analysis of Nationwide Japanese Claims Data. Clin Ther 38(6):1359-1375.e1.https://doi.org/10.1016/j.clinthera.2016.03.022
31. Nolla JM, Martín E, Llamas P, Manero J, Rodríguez de la Serna A, Fernández-Miera MF, Rodríguez M, López JM, Ivanova A, Aragón B (2017) An estimate of the cost of administering intravenous biological agents in Spanish day hospitals. Ther Clin Risk Manag 14;13:325-334.https://doi.org/10.2147/TCRM.S112062
32. Naqvi, A. A, Hassali, M. A, Naqvi, S, Kachela, B, & Khan, I (2020). Estimation of direct cost of managing rheumatoid arthritis treatment to Pakistani patients using real-world follow-up data. International journal of rheumatic diseases, 23(3), 325–333.
https://doi.org/10.1111/1756-185X.13776
33. Souliotis, K, Golna, C, Kani, C, Nikolaidi, S, & Boumpas, D (2019). Real world, big data cost of pharmaceutical treatment for rheumatoid arthritis in Greece. PloS one, 14(12), e0226287.https://doi.org/10.1371/journal.pone.0226287
34. Ohinmaa AE, Thanh NX, Barnabe C, Martin L, Russell AS, Barr SG, Maksymowych WP (2014) Canadian estimates of health care utilization costs for rheumatoid arthritis patients with and without therapy with biologic agents. Arthritis Care Res (Hoboken) 66(9):1319-1327.https://doi.org/10.1002/acr.22293
35. Baser O, Burkan A, Baser E, Koselerli R, Ertugay E, Altinbas A (2013) Direct medical costs associated with rheumatoid arthritis in Turkey: analysis from National Claims Database. Rheumatol Int 33(10):2577-2584.https://doi.org/10.1007/s00296-013-2782-4
36. Eddaoudi M, Rostom S, Hmamouchi I, et al. (2020) The first biological choice in patients with rheumatoid arthritis: data from the Moroccan register of biotherapies. Research Square. DOI: 10.21203/rs.3.rs-51813/v1