A Dose-Response Study of a Live Attenuated Varicella-Zoster Virus (Oka Strain)
Vaccine Administered to Adults 55 Years of Age and Older
Rosemarie Berger, Emanuelle Trannoy, University Children’s Hospital, Basel, Switzerland; Pasteur Me´rieux Connaught, Lyon, and Pasteur Me´rieux Connaught, Georges Holla¨nder, Fabrice Bailleux, Christophe Rudin,
Marnes-la-Coquette, France and Herve´ Creusvaux
Decreased cell-mediated immune (CMI) response to varicella-zoster virus (VZV) is correlated with an increased risk of reactivation of latent virus from dorsal root sites, leading to herpes zoster. The cell-mediated and humoral immunogenicity of three concentrations (3200, 8500, and 41,650 pfu/dose) of a live attenuated VZV vaccine (Oka strain; VZV/Oka) was compared with a control pneumococcal polysaccharide vaccine in 200 healthy adults who were§55 years old. Six weeks after vaccination, the VZV-specific CMI response (as measured by stimulation index values and precursor cell frequencies) was enhanced in all VZV/Oka vaccine groups compared with the control group (for all VZV/Oka groups combined vs. controls, tested with VZV crude antigen: stimulation index, Põ .001; precursor cell frequency, P õ .001). Geometric mean titers of anti-VZV antibodies increased in all VZV/Oka vaccine groups but remained unchanged in the control vaccine group. No dose effect of VZV/Oka vaccine was observed for CMI or humoral responses.
Herpes zoster is a painful cutaneous eruption caused by decrease the severity of zoster episodes [6]. Of note, none of these previous studies included a control group.
reactivation of latent varicella-zoster virus (VZV), the agent
that causes chickenpox in children. It is estimated that over a Vaccination with live attenuated VZV vaccine thus resents a potential strategy for preventing zoster and/or pre-lifetime,Ç15% of persons who experienced clinical or
subclin-ical varicella infection will eventually develop zoster [1]. Her- venting or lessening zoster-associated pain in persons who may already harbor latent VZV [7]. This randomized, controlled pes zoster may be associated with severe neurologic
manifesta-tions and complicamanifesta-tions, mainly acute pain and postherpetic trial in 200 healthy older adults evaluated the cell-mediated and humoral immunogenicity and the safety of one of three neuralgia.
The risk of developing herpes zoster is inversely correlated to doses of a live attenuated VZV/Oka vaccine compared with a control vaccine.
the status of VZV-specific cell-mediated immunity. Progressive loss of cell-mediated immunity is a natural part of the aging
process and is correlated with an increase of zoster incidence Materials and Methods and more severe zoster complications (e.g., postherpetic
neural-Study population. Healthy adults were enrolled in this study gia and ophthalmic zoster) [1 – 3].
during routine out-patient visits. The main inclusion criteria were Enhancement of the VZV-specific immune response of
age§55 years, previous history of varicella confirmed by positive healthy adults by immunization with a live attenuated VZV
serology to VZV (screened using Enzygnost; Behringwerke, Mar-vaccine (Oka strain; VZV/Oka) was first demonstrated by
burg, Germany), and a competent immune system (no signs of Berger et al. [4]. Subsequently, these results were confirmed immunodeficiency). Main exclusion criteria were fever at the time by Levin et al. [5], who immunized a cohort of 202 healthy of selection, any previous zoster episode, seropositivity to human older subjects (§55 years) with one or two doses of a live immunodeficiency virus (screened using Genelavia mixt; Sanofi attenuated VZV/Oka vaccine at different concentrations (1140 Diagnostics Pasteur, France), any underlying immunodepressive condition, previous vaccination against varicella or zoster, any to 12,040 pfu/dose) and demonstrated that both humoral and
other recent vaccination, recent administration of any blood prod-cell-mediated VZV immunity could be boosted by active
im-uct, and sensitivity to neomycin. munization with VZV/Oka. Moreover, the 4-year follow-up
Study design. Study participants were randomly assigned to 1 of this cohort suggested that vaccination with VZV/Oka may
of 4 vaccine groups. Three groups received a subcutaneous injec-tion of a live attenuated VZV/Oka vaccine [8] manufactured by Pasteur Me´rieux Connaught (Lyon). Patients in these groups were randomly allocated one of three concentrations (3200, 8500, or
Informed consent was obtained from all subjects who participated in this
41,650 pfu/dose) of live VZV/Oka under double-blind conditions.
study. The trial was approved by the Ethics Committee of the University
Hospital, Basel, Switzerland. The fourth group was vaccinated subcutaneously with a licensed
Financial support: Pasteur Me´rieux Connaught, Lyon, France. pneumococcal polysaccharide vaccine (Pneumo 23, lot L0042; Reprints or correspondence: Dr Herve´ Creusvaux, Pasteur Me´rieux
Con-Pasteur Me´rieux Connaught, Lyon; distributed in France by Con-Pasteur
naught, 3 ave. Pasteur, B.P. 10, 92430 Marnes-la-Coquette, France.
Me´rieux Se´rums et Vaccins, Marnes-la-Coquette). This vaccine
The Journal of Infectious Diseases 1998; 178(Suppl 1):S99 – 103
was administered under single-blind conditions and was used as q 1998 by the Infectious Diseases Society of America. All rights reserved.
Vaccine recipients noted the occurrence of any adverse reactions Table 1. Percentage of subjects (nÅ 200) with local adverse reac-tions during 6 weeks after vaccination with Oka VZV/Oka vaccine, to vaccination in individual monitoring diaries and were followed
according to vaccine group. by means of medical visits at days 3 and 42 after vaccination. If
vesicular rash or zoster occurred, subjects returned to the study
VZV/Oka vaccine
center for an additional medical visit, and vesicles were sampled for VZV identification by polymerase chain reaction (PCR) and
3200 8500 41,650 Pneumo 23*
restriction analysis [9]. Injection-site reaction pfu pfu pfu vaccine
Vaccine. The Pasteur Me´rieux Connaught VZV/Oka vaccine
is freeze-dried and has been demonstrated to have good stability None 60.0 58.8 73.5 34.0
for at least 2 years at 57C [10]. The vaccine should be reconstituted §1 reaction 40.0 41.2 26.5 66.0 Induration (diameter§2 cm) 18.0 21.6 8.2 16.0
with diluent (NaCl, 4 g/L) immediately before subcutaneous
injec-Pain (all) 28.0 37.2 24.5 58.0
tion. Three lots corresponding to the three concentrations were
Pain (probably vaccine related) 14.0 19.0 12.0 29.0
used for the study: S2885, 3200 pfu/dose; S2887, 8500 pfu/dose;
Redness (diameter§2 cm) 26.0 17.6 12.2 26.0
and S3061, 41,650 pfu/dose. These concentrations of VZV/Oka
Pruritus 10.0 7.8 8.2 6.0
vaccine were chosen in order to obtain widely differing virus titers
Vesicle 0 2.0 0 0
between the 3 groups (i.e., a difference of at least 0.3 in log10
plaque-forming units [pfu]). The control, a purified polyvalent * Pneumococcal polysaccharide vaccine (licensed by Pasteur Me´rieux
Con-Streptococcus pneumoniae polysaccharide liquid vaccine, was ad- naught, Lyon, France, and distributed in France by Pasteur Me´rieux Se´rums et Vaccins, Marnes-la-Coquette).
ministered subcutaneously.
Assays for VZV immunity. Blood samples were obtained for the measurement of cell-mediated immune (CMI) and humoral
immune responses before and 42 days after vaccination. was taken to be 500/106
cells. After log10transformation, geometric
T cell recognition of VZV antigen was determined by incubating means of the SI and PCF were calculated for each vaccine group. peripheral blood mononuclear cells (105
/well) with dilutions of Since statistical tests did not reject the homogeneity of the regres-inactivated crude VZV antigen (Pasteur Me´rieux Connaught, sion lines for pre- and postvaccination responses between the 4 Lyon), purified VZV glycoproteins (Viro Research, Rockford, IL), vaccine groups (slope test: P§ .184 for SI and PCF), data were purified VZV immediate early protein 62 (IE62; Viro Research), analyzed by use of a covariance analysis model. This statistical and MRC-5 substrate antigens (negative control) and tetanus tox- procedure enabled adjustment for prevaccination values. The fol-oid (positive control) (Pasteur Me´rieux Connaught, Lyon) in RPMI lowing comparisons were made: all VZV/Oka vaccine groups medium with 10% human serum for 6 days [5, 11]. The inactivated against control, highest dose of VZV/Oka vaccine against control, VZV crude antigen and MRC-5 antigen were each prepared at and trend effect for the different VZV/Oka doses (dose response). protein concentrations of 3.5 mg/mL. All antigen dilutions were Contrasts were done at the 2.5% level of significance, adjusting made with lymphocyte culture medium. Optimal antigen concen- for three related contrasts.
trations in cultures were 1:3000 of the initial solution for inacti-vated VZV crude antigens and MRC-5 antigen (precise quantita-tion of antigen was not possible), 417 ng/mL for VZV
Results glycoproteins, 167 ng/mL for IE62, and 50 mg/mL for tetanus
toxoid. Proliferation was detected by [3
H-methyl]thymidine uptake Study population. Two hundred older adult subjects (age
during 6 h of incubation. range, 55 – 88 years), comprising 82 women and 118 men, were
The stimulation index (SI) was calculated as the ratio of the enrolled in the trial. Slightly fewer women (34%) and a higher mean counts per minute (cpm) in antigen-specific stimulated wells
mean age (67.7 years) were found in the 3200-pfu VZV/Oka to the mean cpm in the respective negative control wells. Four
vaccine group compared with the other groups (women, 41% – replicates per measurement were performed.
45%; mean age, 64.7 – 65.2 years); however, the higher age in The precursor cell frequency (PCF) analysis was done by use
this group can be explained by the advanced age (77 and 88 of the limiting dilution culture as described by Levin et al. [5] and
years) of 2 members. Although a possible potential selection Hayward et al. [11], except that cultures were incubated for 6
bias cannot be ruled out, between-group differences were rather than 10 days. PCF was expressed as the number of VZV
antigen – specific cells per 106
lymphocytes. mostly likely random, and the age and gender distributions
Humoral immune response. Determination of IgG antibodies were not considered different among the 4 groups.
to VZV were measured before vaccination and 6 weeks after vacci- Reactogenicity and safety. Adverse reactions observed dur-nation (day 42) using an in-house glycoprotein ELISA [12] at ing the 6 weeks following vaccination are summarized in table Pasteur Me´rieux Connaught (Laboratoire Se´ro-Immuno Clinique, 1. Overall, the VZV/Oka vaccine was well tolerated compared Val de Reuil, France).
with the pneumococcal polysaccharide control vaccine. At least Data analysis. Data were dual entered in database software
one adverse reaction was experienced by 26.5% – 41.2% of the (Climed; Simed, Cre´teil, France). Statistical analysis was done by
subjects in the groups that received one of the doses of the the Biometry Department of Pasteur Me´rieux Connaught
(Marnes-VZV/Oka vaccine, compared with 66% of subjects who re-la-Coquette) using SAS software (SAS Institute, Cary, NC); SI
ceived the control vaccine. Pain at the injection site that was values and PCFs were estimated before vaccination and at day 42.
was reported less frequently after VZV/Oka vaccination (12% – in all VZV/Oka vaccine groups and remained unchanged in the pneumococcal polysaccharide control vaccine group. The 19% of subjects) than after the pneumococcus control
vaccina-tion (29%). Incidences of redness, pruritus, and induravaccina-tion were geometric mean titer ratios (day 42/before vaccination) and 95% CIs were 1.4 (95% CI, 1.2 – 1.7), 1.4 (95% CI, 1.2 – 1.7), no more frequent in the VZV/Oka groups than in the control
group. and 1.3 (95% CI, 1.2 – 1.7) in the 3200-pfu, 8500-pfu, and
41,650-pfu groups, respectively, compared with 1.0 (95% CI, No subject had a fever during the 72 h following vaccination.
One subject in the 8500-pfu VZV/Oka group presented with a 0.9 – 1.1) in the control group. Increases were significant in all VZV/Oka groups (lower boundary of 95% CIs,ú1.0), but not mild vesicular rash at the injection site (õ10 vesicles) that
occurred 1 day after vaccination and lasted 7 days. PCR analy- in the control group (lower boundary of 95% CIs,õ1.0). A correlation analysis revealed that the antibody response sis of the vesicular fluid was negative for VZV.
CMI response to VZV. Analysis of the geometric mean of was not correlated with the SI response, irrespective of the
VZV antigen used (rÅ .05, Pearson’s correlation coefficient). SI values showed that the VZV antigen – specific proliferative
response was significantly enhanced in all VZV/Oka vaccine groups after vaccination but remained unchanged in the control
Discussion group. On day 42, the mean proliferative responses in the
groups receiving VZV/Oka vaccine, adjusting for prevaccina- The results from this randomized, controlled study confirmed previous observations in uncontrolled trials that vaccination tion titers, were 5.62 – 7.24 for crude VZV antigen (vs. 2.88 in
the controls), 2.29 – 3.09 for purified VZV glycoproteins (vs. with live attenuated VZV/Oka vaccine is safe and enhances CMI to VZV in older adult subjects [4, 5]. The VZV/Oka 1.41 in controls), and 1.38 – 1.58 for IE62 (vs. 1.05).
SI ratios (day 42/before vaccination) and 95% confidence vaccine used in this study was well tolerated, and vaccinated subjects presented few local and general reactions, even with intervals (95% CIs) were 1.6 (95% CI, 1.1 – 2.2), 1.5 (95% CI,
1.1 – 2.1), and 2.5 (95% CI, 1.9 – 3.3) in the 3200-pfu, 8500- the highest dose of VZV/Oka vaccine used (41,650 pfu). In fact, the incidence of local reactions was lower in the group pfu, and 41,650-pfu groups, respectively, compared with 0.85
(95% CI, 0.6 – 1.2) in the control group. A covariance analysis of subjects who received the highest dose of VZV/Oka vaccine (26.5%) compared with the other 2 VZV/Oka groups (3200 model with prevaccination SI values as covariables did not
detect any statistically significant dose effect for the different pfu, 40.0%; 8500 pfu, 41.2%), although these differences were not tested statistically. In particular, induration was reported VZV/Oka concentrations in the CMI response to VZV, as
mea-sured by the adjusted geometric mean SI at day 42 (trend test less frequently in the 41,650-pfu group (8.2% compared with 18.0% – 21.6%), and redness tended to decrease as the vaccine not significant for any VZV antigen: trend effect P§ .177).
Nonetheless, considering all VZV/Oka groups together, ad- dose increased (26.0% – 12.2%). The reason for these descrip-tive differences between the different doses of VZV/Oka vac-justed geometric mean SI values were statistically significantly
higher after VZV/Oka vaccination than control vaccination for cine is not clear but might be due to chance or other unknown factors, since the safety evaluation was done under double-all VZV antigens (P õ .001, crude VZV; P õ .001, VZV
glycoproteins; Põ .003, IE62). blind conditions. Of importance, the local reactogenicity of each dose of VZV/Oka was better than that of the widely used The PCFs before and after (day 42) vaccination are presented
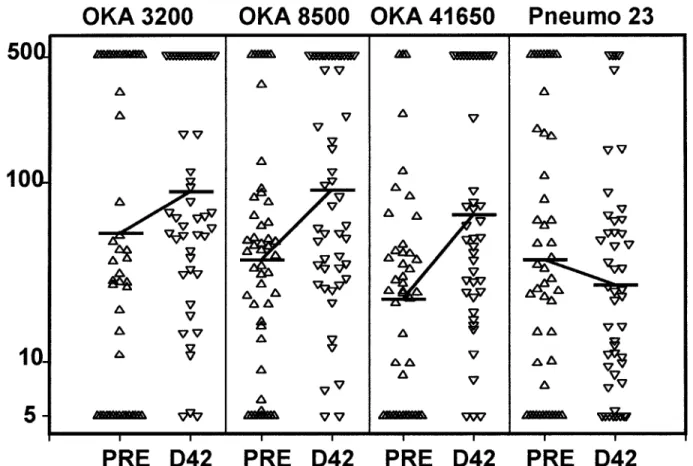
in figure 1. Geometric mean PCF (per 106cells) increased after pneumococcal polysaccharide control vaccine.
To our knowledge, this is the first time such a high vaccine vaccination in all VZV/Oka groups but remained unchanged
in the control group. Increases in geometric mean PCFs (per dose (41,650 pfu) has been administered to VZV-seropositive subjects, and it confirms the innocuous nature of the VZV/ 106cells) from before vaccination to day 42 were as follows
for the different VZV/Oka doses: from 52.5 to 89.1 in the Oka strain. Only 1 subject (in the 8500-pfu group) presented postvaccination vesicles (õ10), which might have been caused 3200-pfu group, from 37.2 to 91.2 in the 8500-pfu group, and
from 22.4 to 66.1 in the 41,650-pfu group. In the control group, by the vaccine; however, the vesicles were VZV-negative as determined by PCR analysis of vesicular fluid. No subject pre-the geometric mean PCF decreased slightly from 37.2 before
vaccination to 26.9 at day 42. This very small decrease does sented any varicella-like rash or zoster during the 6-week fol-low-up.
not appear related to the pneumococcal polysaccharide control
vaccine since the same effect was also observed with the con- All in vitro measurements of cell-mediated immunity (SI and PCF) and VZV glycoprotein – specific antibody titers were trol tetanus toxoid antigen (data not shown). After adjustment
for prevaccination PCFs, no statistically significant dose effect significantly increased 6 weeks after vaccination (day 42) in the 3 VZV/Oka groups, compared with the control group. The was found for the different VZV/Oka doses in the geometric
mean PCF at day 42. Still, the adjusted geometric mean PCF adjusted geometric means for SI values at day 42 were about two times higher in the VZV/Oka groups compared with the was statistically significantly higher when all VZV/Oka groups
together were compared against the control group. control group, and, likewise, adjusted geometric mean PCFs were approximately three times higher in the active vaccine
Humoral immune response. After vaccination, geometric
Figure 1. Individual precursor cell frequencies (PCFs; no./106
cells) in 4 groups of subjects before (PRE) and 42 days after (D42) vaccination with various doses (in plaque-forming units) of Oka strain of VZV or with Pneumo 23 (pneumococcal polysaccharide vaccine; Pasteur Me´rieux Connaught, Lyon, France; distributed in France by Pasteur Me´rieux Se´rums et Vaccins, Marnes-la-Coquette). Solid horizontal bars Å geometric mean PCFs.
expected, crude VZV antigen elicited the best proliferative Although prevaccination VZV antibody titers tended to be high in the subjects, this did not prevent vaccination with VZV/ response. Proliferative response to purified IE62 VZV antigen
was observed only in a minority of vaccinees, and all of these Oka from boosting the CMI and humoral immune responses. Six weeks after vaccination with VZV/Oka vaccine, VZV-responders to IE62 demonstrated a proliferative response to
VZV glycoproteins. Furthermore, all responders to VZV glyco- antibody levels had increased byÇ30% – 40%, and a significant proliferative response was obtained. No boost of VZV glyco-proteins demonstrated a response to crude VZV antigen.
protein – specific antibodies or CMI response was seen in the Six weeks after vaccination, there were no significant
differ-control group. ences in VZV-specific immune responses between the different
virus concentrations tested. This indicated that either the lowest The vaccine concentrations tested were selected on the basis of infectivity titer (pfu/dose), not on the inactive viral antigen. dose (3200 pfu) is sufficient to boost the CMI or that other
inactive viral components of the VZV/Oka preparation used The three concentrations were chosen to obtain significantly different pfu contents per dose, according to the accuracy of may compensate for the lower pfu content by additionally
en-hancing the immune response [13]. As previously reported by the plaque assay. On the basis of our data, the lowest dose of VZV/Oka vaccine (3200 pfu) is sufficient to significantly boost Levin et al. [5], the VZV CMI response early after vaccination
was not related to the dose of the vaccine given. However, a both CMI and antibody response to VZV.
difference may appear between doses after several years of A long-term follow-up of the vaccinated subjects is being follow-up, with a longer-lasting enhancement of immune re- conducted both to determine whether the enhanced VZV-spe-sponse being obtained with high vaccine doses [6]. Further cific CMI response obtained after a single vaccination with long-term assessment of CMI and humoral response after vacci- VZV/Oka is long lasting and also to monitor the zoster inci-dence. It is not known whether a boost of VZV cell-mediated nation with VZV/Oka vaccine will be needed to confirm this
4. Berger R, Luescher D, Just M. Enhancement of varicella-zoster – specific seems more likely, will lessen the severity of a zoster episode
immune responses in the elderly by boosting with varicella vaccine. J [6, 7]. A large, double-blind, placebo-controlled trial is needed
Infect Dis1984; 149:647.
to assess the efficacy of this vaccine in the prevention or reduc- 5. Levin M, Murray M, Rotbart H, Zerbe G, White CJ, Hayward AR. Immune tion of the severity of zoster in a healthy adult population. After response of elderly individuals to a live, attenuated varicella vaccine. J
Infect Dis1992; 166:253 – 9. such a study, additional research will be needed to improve
6. Levin MJ, Murray M, Zerbe GO, White CJ, Hayward AR. Immune re-our knowledge of the immune mechanisms underlying VZV
sponses of elderly persons 4 years after receiving a live, attenuated latency and reactivation.
varicella vaccine. J Infect Dis1994; 170:522 – 6.
7. Oxman MN, Immunization to reduce the frequency and severity of herpes zoster and its complications. Neurology1995; 45:S41 – 6.
8. Takahashi M, Otsuka T, Okuno Y, et al. Live vaccine used to prevent the Acknowledgment
spread of varicella in children in hospitals. Lancet1974; 2:1288 – 90. 9. LaRussa P, Lungu O, Gershon A, Steinberg SP, Silverstein S. Restriction
We thank Susan Wood for her help in the preparation of this
fragment length polymorphism chain reaction products from vaccine
manuscript.
and wild-type varicella-zoster virus isolates. J Virol1992; 66:1016 – 20. 10. Fanget B, Franc¸on A. A varicella vaccine stable at 57C. Dev Biol Stand
1996; 87:167 – 71.
11. Hayward AR, Zerbe GO, Levin MJ. Clinical application of responder cell References
frequency estimates with four years of follow-up. J Immunol Methods 1. Gershon A, Silverstein S. Live, attenuated varicella vaccine for prevention 1994; 170:27 – 36.
of herpes zoster. Biologicals1997; 25:227 – 30. 12. Provost PJ, Krah DL, Kuter BJ, et al. Antibody assays suitable for assessing 2. Ragozzino MW, Melton LJ III, Kurland LT, et al. Population-based study immune responses to live varicella vaccine. Vaccine1991; 9:111 – 6.
of herpes zoster and its sequelae. Medicine1982; 61:310 – 6. 13. Bergen RE, Diaz PS, Arvin AM. The immunogenicity of the Oka/Merck 3. Berger R, Florent G, Just M. Decrease of the lymphoproliferative response varicella vaccine in relation to infectious varicella-zoster virus and
rela-tive viral antigen content. J Infect Dis1990; 162:1049 – 54. to varicella zoster virus antigen in the aged. Infect Immun1981;32:24–7.