Decreased Expression of Cyclic Adenosine
Monophosphate-Regulated Aldose Reductase (AKR1B1)
Is Associated with Malignancy in Human Sporadic
Adrenocortical Tumors
ANNE-MARIE LEFRANC¸ OIS-MARTINEZ, JE´ ROˆME BERTHERAT, PIERRE VAL,
COLETTE TOURNAIRE, NICOLE GALLO-PAYET, DAVID HYNDMAN, GEORGES VEYSSIE` RE,
XAVIER BERTAGNA, CLAUDE JEAN, AND ANTOINE MARTINEZ
Unite´ Mixte de Recherche 6547 CNRS-Universite´ Blaise Pascal Clermont II (A.-M.L.-M., P.V., C.T., G.V., C.J., A.M.), Ge´ne´tique des Eucaryotes et Endocrinologie Mole´culaire, Complexe Universitaire des Ce´zeaux, 63177 Aubie`re, France; Institut National de la Sante´ et de la Recherche Me´dicale U-576, De´partement d’Endocrinologie (J.B., X.B.), Institut Cochin, Universite´ Rene´ Descartes-Paris V, 75014 Paris, France; Department of Endocrinology (N.G.-P.), Faculty of Medicine, University of Sherbrooke, Que´bec J1H 5N4, Canada; and Protein Function Discovery Facility (D.H.), Queen’s University, Ontario K7L 3N6, Kingston, Canada
The human aldose reductase, AKR1B1, participates in glucose metabolism and osmoregulation and is supposed to play a protective role against toxic aldehydes derived from lipid per-oxidation and steroidogenesis that could affect cell growth/ differentiation when accumulated. Adrenal gland is a major site of expression of AKR1B1, and we asked whether changes in its expression could be associated with adrenal disorders. Therefore, we examined AKR1B1 gene expression in human fetal adrenals, adrenocortical cell line, and tumors and com-pared the results with the expression of steroidogenic genes (StAR and CYP11A) and regulators of adrenal cortex devel-opment [steroidogenic factor-1 (SF-1) and dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1 (DAX1)]. Using specific antibodies, Northern blotting, and enzymatic assays, we present evi-dences that AKR1B1 detectable in 15-wk-old fetal glands is regulated by cAMP in NCI-H295 cells and thus that AKR1B1 is functionally related to the ACTH-responsive murine akr1b7/ mvdp gene rather than to its direct ortholog, the mouse aldose
reductase akr1b3 gene. Although low DAX1 expression in al-dosterone-producing adenomas (nⴝ 5) was confirmed (P < 0.05), no correlation was found between the expression of all other genes and the tumors endocrine activity. In contrast, relative abundance of AKR1B1 mRNA was decreased in adre-nocortical carcinomas (nⴝ 5; mean ⴞSEM, 0.95ⴞ 0.2) when compared with adenomas (nⴝ 12; 9.29 ⴞ 3.05; P < 0.001). Most (seven of eight) adrenocortical carcinomas (19.0ⴞ 5.4) had very low relative AKR1B1 protein levels when compared with benign tumors (cortisol-producing adenomas, nⴝ 5, 63.0 ⴞ 9.8; nonfunctional adenomas, n ⴝ 5, 58.0 ⴞ 10.4; aldosterone-producing adenomas, nⴝ 4, 65.3 ⴞ 7.7; P < 0.001), Cushing’s hyperplasia (nⴝ 5, 54.6 ⴞ 5.3; P < 0.01), or normal adrenals (n ⴝ 4; 37.1ⴞ 5.3; P < 0.001). These properties provide the first evidence that expression of cAMP-regulated AKR1B1 is de-creased in adrenocortical cancer. This might take part in ad-renal tumorigenesis and could be investigated as a marker of malignancy for the diagnosis of adrenal tumors. (J Clin En-docrinol Metab 89: 3010 –3019, 2004)
A
DRENAL TUMORS ARE fairly common because the prevalence of these lesions in the general population is around 1%, increases with age, and reaches 6% in the seventh decade (1). They are divided into benign adenomas, which are extremely frequent, and rare malignant carcino-mas, accounting for 0.05– 0.2% of all cancers and with an extremely poor prognosis, the survival rate being 20% at 5 yr (2). The distinction between benign and malignant adreno-cortical tumors is crucial for diagnosis and treatment of ad-renal disease. However, in the absence of absolutebiochem-ical, clinbiochem-ical, or histological criteria, discerning malignancy in adrenocortical tumors can be difficult. Although the molec-ular mechanisms of adrenocortical tumorigenesis are not well understood, it is clear that abnormalities in the im-printed region 11p15 are strongly associated with malignant phenotype of sporadic adrenal tumors (3–7). These abnor-malities result in an overexpression of the IGFII gene and a loss of the p57 (Kip2) cyclin-dependent kinase inhibitor gene and the H19 RNA gene expression. However, other loci are linked to malignancy of adrenocortical neoplasms (8 –12), and some of them have a prognostic value for the tumor recurrence (13).
The aldo-keto reductases (AKR) belong to an oxidoreduc-tase superfamily that catalyze the reduction of a wide variety of substrates including aldoses, aliphatic and aromatic alde-hydes and ketones, prostaglandins, and xenobiotics (14). Among the AKR1B subfamily, aldose reductase (AKR1B1 in human, AKR1B3 in mouse), known as the first enzyme of the polyol pathway of sugar metabolism, is one of the most Abbreviations: ACC, Adrenocortical carcinoma; APA,
aldosterone-producing adenoma; CPA, cortisol-aldosterone-producing adenoma; DAX1, dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1; HNE, 4-hydroxynonenal; MVDP, mouse vas deferens protein; NADPH, nicotinamide adenine dinucleotide phos-phate reduced; NFA, nonfunctional adenoma; PKA, protein kinase A; SF, steroidogenic factor; StAR, steroidogenic acute regulatory protein.
JCEM is published monthly by The Endocrine Society (http://www. endo-society.org), the foremost professional society serving the en-docrine community.
doi: 10.1210/jc.2003-031830
3010
studied because of its role in diabetic complications (15, 16). Although being ubiquitously expressed in both species, the most abundant source of aldose reductase in human tissues is the adrenal gland (17). This suggests an important role for AKR1B1 in this specialized organ in which its isocaproalde-hyde reductase activity could be recruited (18). In human and rodent adrenals, the toxicity of isocaproaldehyde, the prod-uct of cholesterol side chain cleavage by the cytochrome P450scc (CYP11A gene), is mainly neutralized by the reduc-tase activity of AKR1B1 and AKR1B7/mouse vas deferens protein (MVDP), respectively (18, 19). Moreover, we have shown that in rodents, the akr1b7 gene expression is under control of ACTH through cAMP pathway (20 –23). Thus, full steroidogenic activity should require the coordinate regula-tion by ACTH/cAMP of genes involved in cholesterol trans-port and steroid conversion but also of scavenger genes detoxifying harmful byproducts of steroidogenesis e.g. iso-caproaldehyde (19) and free radicals (24). Until now the murine AKR1B7 is the only aldose reductase-like protein showing ACTH responsiveness. A possible regulation of the aldose reductase AKR1B1 by ACTH/cAMP has not been investigated yet in human adrenal. To shed more light on the role of AKR1B1 in adrenal steroidogenesis and pathophys-iology, we compared the expression of AKR1B1 gene with those of genes involved in steroidogenesis e.g. steroidogenic acute regulatory protein gene (StAR) and CYP11A (25–27), or in adrenal differentiation, e.g. steroidogenic factor (SF)-1 and dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1 (DAX1) (28, 29), in human adrenocortical tumor cell line and malignant or benign adrenocortical neoplasms with different endocrine profiles. These comparisons should reveal whether AKR1B1 along with steroidogenic genes is coordinately controlled and whether its expression could be correlated with the malignancy and/or the endocrine status of the tumors.
Materials and Methods
Hormonal investigations, tissue collection, and histological diagnosis on human biopsies
Adrenal tissues were obtained during surgery and were immediately dissected by the pathologist, frozen, and stored in liquid nitrogen until use. Hormonal investigation and diagnosis were performed as previ-ously reported (30). For malignant tumors staging was performed as previously reported (31). Adrenal tumors were diagnosed by the use of classical histological criteria and molecular genetic markers as previ-ously reported by the Cortico et Medullosurre´nale: Etude des Tumeurs Endocrines (COMETE) network (13), which is dedicated to the study of adrenal tumors. All the carcinomas are primitive tumors and exhibited IGF-II overexpression and a histological Weiss score 4 or more. None of the patients with carcinomas have been submitted to mitotane therapy before surgery. Among these patients during a 6- to 24-month follow-up period, five (numbered b, 2, 37, 39, and 40 in Table 1) presented with tumor recurrence or distant metastasis. The Weiss score was 1 or less in all adrenal adenomas. Normal adrenal cortex tissue was obtained from normal glands removed during the surgery of adjacent nonendocrine tumors (kidney tumors or incidentalomas). Aldosterone adenomas were removed from patients exhibiting a primary hyperaldosteronism and a small benign adrenocortical adenoma (all these Conn’s adenomas have a diameterⱕ 15 mm). Adrenal tissues from ACTH-dependent Cushing’s syndrome were obtained during bilateral adrenalectomy performed for Cushing disease. Informed consent was given for adrenal tissue collec-tion as part of a protocol approved by the Institucollec-tional Review Board of the Cochin Hospital.
Fetal adrenal glands were obtained from fetuses between 15 and 19
wk old (post fertilization) at the time of therapeutic abortion. Fetal ages were estimated by foot length and time after last menstruation, accord-ing to Streeter (32). The project was approved by the Human Subject Review Committee of the University of Sherbrooke, Quebec, Canada. Recombinant protein production
AKR1B1 cDNA was isolated by RT-PCR, starting from 2g human adrenal total RNAs, using Moloney murine leukemia virus reverse tran-scriptase (Promega, Charbonnier, France) and Taq polymerase (Pro-mega) and outside primers containing engineered 5⬘ B1 NdeI primer (5⬘-CGGCAGCCATATGGCAAGCCGTC-3⬘) and 3⬘ B1 EcoRI primer (5⬘-CGGAATTCGGGCTTCAAAACTCTTCATGG-3⬘). AKR1B7 cDNA was obtained by PCR amplification with MVDP pUC13 (33) as template and with outside primers containing engineered 5⬘ B7 NdeI primer (5⬘-CG-GCAGCCATATGGCCACCTTCGT-3⬘) and 3⬘ B7 BamHI primer (5⬘-CG-GCATCCCGTCAGTATTCCTCGTGG-3⬘).
AKR1B8 cDNA was isolated by RT-PCR, starting from 2g mouse adrenal total RNAs, using Moloney murine leukemia virus reverse tran-scriptase and Taq polymerase (Promega) and outside primers containing engineered 5⬘ B8 NdeI primer (5⬘-CGGCAGCCATATGGCCACGTTC-GTGG-3⬘) and 3⬘ B8 EcoRI primer (5⬘-CGGGATCCCGGGGCTGACT-CAGCTTCA-3⬘).
Recombinant AKR1B1, AKR1B7, and AKR1B8 were expressed in
Escherichia coli after inserting their corresponding cDNA into the Nde1
and EcoRI sites of the PET 28a vector (Novagen, Tebu, Le Perray-en-Yvelines, France) to produce N-terminal fusions with six histidine res-idues. AKR1B10 cDNA was inserted into PET 16 vector (gift of Dr. D Hyndman, Queen’s University, Ontario, Canada). Recombinant AKR1B1, AKR1B7, AKR1B8, and AKR1B10 proteins were produced in BL21(DE3) pLys S cells upon isopropyl--d-thiogalactopyranoside in-duction and purified by nickel affinity chromatography according to the manufacturer’s instructions (Novagen). For each protein, column frac-tions were analyzed by SDS-PAGE, and those containing the purified protein were pooled and stored at 4 C.
Antibodies and Western blot experiments
For production of the L3 antibody, rabbits were injected with a glu-tathione S-transferase fusion of the 17 C-terminal amino acid residues of the murine AKR1B7 protein, and the antibody was obtained and tested as previously described (34). L3 antibody is both specific for murine AKR1B7 and human AKR1B1 (Fig. 1). Western blots were per-formed as previously described (22). Blots were treated with primary L3 antibody at a 1:3000 dilution for 1 h at room temperature. Peroxidase-conjugated antimouse or antirabbit secondary antibodies were added at 1:15000 dilution for 1 h at room temperature. Peroxidase activity was detected with the enhanced chemiluminescence system (Amersham Bio-siences, Buckinghamshire, UK). Densitometric analysis of the immuno-reactive protein bands obtained in Western blots were performed on nonsaturated signals using Molecular Analyst software (Bio-Rad Lab-oratories, Inc., Marnes la Coquette, France).
Cell culture
Human NCI-H295 (35) adrenocortical cells were routinely cultured in DMEM/nutrient mixture F12 (DMEM-F12) supplemented with 3% fetal calf serum, 2% Ultroser G (InVitrogen, Cergy-Pontoise, France), 1% ITS (insulin, transferrin, sodium selenite) (InVitrogen), penicillin (100 U/ml), and streptomycin (100g/ml). Human placental JEG-3 cells were grown in DMEM-F12 supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100g/ml). When required, 10⫺5m forskolin were added in the culture medium for the indicated time.
Northern blot analysis
Total RNAs from human biopsies or cellular samples were individ-ually extracted with trizol (InVitrogen). Northern blots were individu-ally performed with 25g of the RNAs as described previously (22). Probes used were the 3⬘ untranslated region of AKR1B1 cDNA segment from position 1032 to 1350 pb, the human StAR cDNA fragment from position 467 to 908, and the bovine CYP11A pvuII cDNA fragment of 1.8
kb (kindly provided by Dr. Waterman M, Vanderbilt University, Nash-ville, TN). To normalize the loading of RNAs, Northern blots were stripped and rehybridized with a mouse-actin probe extracted from pGEM-7ZF--actin by EcoRI/BamHI digestion. Hybridization signals were quantified by phosphor imager using Quantity One software (Bio-Rad Laboratories).
Enzymatic assays
The standard reaction mixture for the reductase activities contained 0.1 m sodium phosphate buffer (pH 6.6), 0.4 m ammonium sulfate, 100 mm nicotinamide adenine dinucleotide phosphate reduced (NADPH2),
appropriate isocaproaldehyde, and 5g of recombinant AKR1B1 or 200 g of NCI-H295 cytosolic proteins. The reaction was carried out at 25 C, and the decrease in NADPH2was monitored by spectrophotometer at
340 nm. Reactions were routinely started by the addition of enzyme or protein extracts. Controls without substrate or without enzyme were run simultaneously. One enzyme unit is defined as the change at 340 nm corresponding to the oxidation of 1mol NADPH2.
Results
Human adrenal tissue contains large amounts of AKR1B1 protein
The ACTH-responsive-AKR1B7 protein is responsible for the reduction of isocaproaldehyde in mouse adrenocortical cells (19). Such an isocaproaldehyde reductase function has not been assigned in the human adrenal cortex yet, although AKR1B1, the human aldose reductase, is able to reduce iso-caproaldehyde in vitro (18). We thus undertook the isolation of a human ortholog for the akr1b7 gene. Attempts to isolate AKR1B7 cDNA in human adrenal cortex samples by re-peated RT-PCR experiments using primers complementary to conserved sequences within AKR1B subfamily or by bioin-formatic searches within genomic or EST databases using BLAST failed to isolate a strict ortholog (not shown). Indeed, extensive sequence analyses of RT-PCR products showed that all amplified cDNAs corresponded to AKR1B1. TABLE 1. Clinical, hormonal, and histological classification
Patient No. Age/sex Hormonal
pattern
Histological data
Tumor type Tumor weight (g) Tumor stage at diagnosis
Group 1 (n⫽ 10) 1 46/F NS ACC 314 L 2 53/M GC⫹E ACC 827 M 4 30/F GC⫹A ACC 72 L c 52/F GC ACC 141 L b 80/F GC ACC 190 R 36 25/F GC⫹E ACC 50 L 37 40/M GC ACC 103 M 38 44/M GC⫹A ACC 738 L 39 53/F GC⫹A ACC 495 R 40 30/M GC ACC 400 M Group 2 (n⫽ 5) 5 41/F GC CPA 8 6 36/F GC CPA 8 7 50/F GC CPA 46 9 42/F GC CPA 14 11 58/F GC CPA 10 Group 3 (n⫽ 5) 8 68/F NS NFA 22 10 69/F NS NFA 30 12 36/F NS NFA 19 13 46/F NS NFA 14 14 68/M NS NFA 30
Group 4 (n⫽ 5) 15 56/F Aldo APA 8
16 55/F Aldo APA 8 17 41/F Aldo APA 5 18 33/F Aldo APA 10 19 43/F Aldo APA 10 Group 5 (n⫽ 8) 21 59/M GC CD 10 22 32/F GC CD 11 23 37/F GC CD 7 31 45/F GC CD 6 32 19/M GC CD 10 33 19/F GC CD 14 34 63/F GC CD 6 35 60/F GC CD 16 Group 6 (n⫽ 4) 27 25/F Control 28 21/F Control 29 40/F Control 30 47/F Control
F, Female; M, male; NS, nonsecreting; GC, glucocorticoid secretion; E, estrogen secretion; A, androgen secretion; Aldo, aldosterone secretion; CD, Cushing’s disease. For malignant tumors staging was performed as described in Materials and Methods. L, Localized; R, regional; M, metastatic.
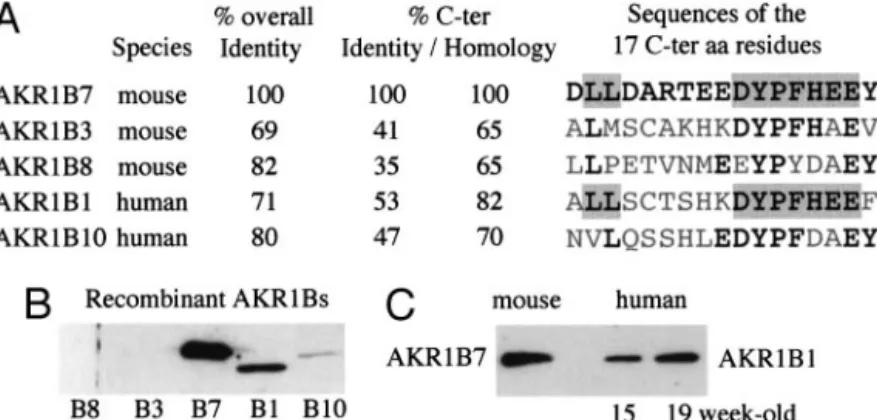
It has been previously shown that the C-terminal region of aldose reductase is critical to substrate specificity (36). In agreement with these data, the C-terminal domains were divergent among the AKR1B subfamily members (Fig. 1A). Interestingly, in the C-terminal region, AKR1B7 exhibited more homologies with human aldose reductase AKR1B1 (82%) than with mouse aldose reductase AKR1B3 (65%), suggesting that AKR1B7 and AKR1B1 might have similar substrate specificities.
Polyclonal antibodies were raised against AKR1B7 C-terminal peptide. As illustrated by the Western blot analysis shown in Fig. 1B, both AKR1B7 and AKR1B1 recombinant proteins were strongly recognized by the anti-AKR1B7 an-tibody (Fig. 1B). The anan-tibody does not cross-react with AKR1B3 or AKR1B8 and cross-reacts only slightly with AKR1B10. Because akr1b7 gene expression, first detected at embryonic d 13.5, was shown to follow the onset of glu-cocorticoid synthesis in mouse fetal adrenal (37), we looked at AKR1B1 expression in developing adrenals of human fe-tuses. The Western blot in Fig. 1C showed that AKR1B1 is detected in adrenals from fetuses aged 15–19 wk, a time at which fetal adrenal is potentially capable to produce cor-tisol (38).
Altogether these data prompted us to conclude that there was no akr1b7/mvdp ortholog gene in human, and we pos-tulated that AKR1B1 might fulfill the same role in human adrenals.
AKR1B1 expression in human adrenocortical cells is regulated by cAMP
To examine a possible regulation of AKR1B1 expression by the protein kinase A (PKA) transduction pathway, steady-state levels of AKR1B1 mRNA were measured in human NC1-H295 cells cultured for increasing periods of time in the presence of forskolin, an activator of cAMP synthesis, that was preferred to the natural inducer because these cells are poorly responsive to ACTH (39). The pattern of forskolin induction was compared with that of steroidogenic genes such as CYP11A and StAR. The action of forskolin was time
dependent and first detectable after 6 h of treatment (Fig. 2, A and C). Maximal induction of AKR1B1 mRNA was 2.1-fold (P ⬍ 0.05, compared with untreated cells) after 12 h. The kinetic and magnitude of forskolin induction of CYP11A mRNA level followed a very similar pattern. Note that StAR mRNA level showed only a 1.3-fold increase on forskolin induction. The low responsiveness of StAR in NCI-H295 cells was already mentioned (40). AKR1B1 cDNA had first been cloned in the human placenta (41). As expected, AKR1B1 was expressed in trophoblastic JEG-3 cells (Fig. 2B). Interestingly, forskolin did not affect AKR1B1 mRNA expression in these cells, indicating that the forskolin responsiveness seems to be at least specific of the adrenocortical cells.
Activation of the PKA signaling pathway increases isocaproaldehyde reductase activity in NC1-H295 cell line
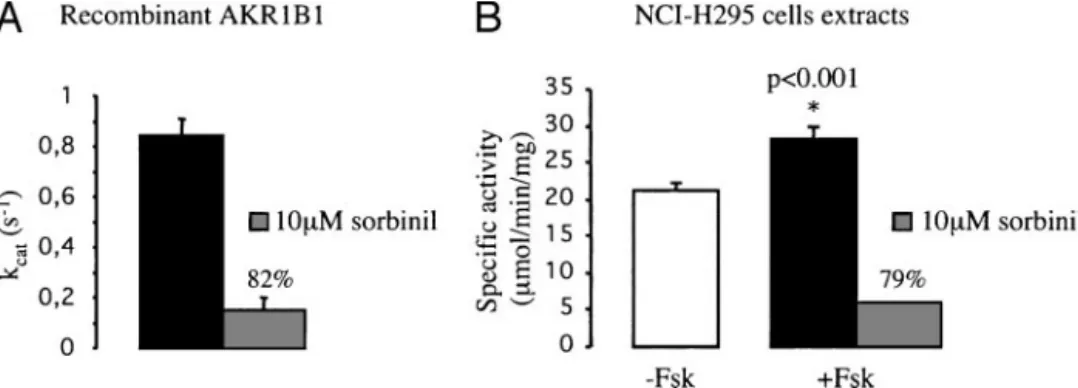
Aldose reductase (AKR1B1) has been described as a major reductase for isocaproaldehyde in adrenal glands (19). As shown in Fig. 2, forskolin (10⫺5 m) induced a significant increase in AKR1B1 mRNA levels. To assess the physiolog-ical relevance of this effect, isocaproaldehyde reductase ac-tivity was measured in NCI-H295 adrenocortical cells. Figure 3A shows that the recombinant AKR1B1 had the ability to reduce isocaproaldehyde with a constant of molecular ac-tivity (Kcat) value in accordance with those previously re-ported (18). Recombinant AKR1B1 isocaproaldehyde reduc-tase activity was strongly inhibited by sorbinil, a potent inhibitor of aldose reductase (Fig. 3A). In contrast, AKR1B7 showed no sensitivity to this compound (19). Isocaproalde-hyde reductase activities, measured in cytosolic protein ex-tracts from NCI-H295 cells, were significantly enhanced by forskolin treatment and inhibited by sorbinil (Fig. 3B). Malignant and benign tumors exhibit different
AKR1B1 profiles
Table 1 outlined clinical data from the patients examined. Expression of SF-1, DAX1, CYP11A, StAR, and AKR1B1 mRNAs was analyzed by Northern blot experiments in 20 of FIG. 1. A, Sequence comparison between the 17 C-terminal amino acid residues involved in substrate specificity of the human and murine AKR1B family members. Amino acid residues conserved among the five members are in black characters, those conserved between AKR1B1 and B7 are shaded. The C-terminal parts of AKR1B1 and B7 shared the most closely related sequence, whereas the two proteins exhibited a less degree of overall identity. B, Cross-reactivity of the L3 antibody with the different AKR1B proteins. Equal amounts (150 ng) of recombinant AKR1Bs from mouse (B3/murine aldose reductase; B7/MVDP; B8/FR-1) (70) or human origin (B1/human placenta aldose reductase; B10/ ARL1/HSI) (57, 71) were detected by Western blot using the L3 antiserum raised against the 17 C-terminal residues. C, Immunodetection of AKR1B1 protein in human fetal adrenal tissue and AKR1B7 in adult mouse adrenals by Western blot using L3 antiserum. Twenty micrograms protein extracts were loaded per lane.
the 37 tissues available for RNA extraction. Although the levels of their mRNAs varied among the tumors, expression of SF-1, DAX1, CYP11A, StAR, and AKR1B1 genes was de-tectable in all samples analyzed (Fig. 4). The relative amounts of mRNAs from all specimens studied were measured (Table 2) and did not correlate with endocrine activity (Tables 1 and 2). When considered together, data from cortisol-producing adenomas (CPAs) and nonfunctional adenomas (NFAs) re-vealed that the relative levels of all the tested genes, with the exception of DAX1, were significantly higher in this group than in malignant tumors [adrenocortical carcinomas
(ACCs)] (Fig. 5). In accordance with previous data (42), relative mRNA levels of DAX1 were significantly lower in aldosterone-producing adenomas (APAs) than in other ad-enomas (CPA⫹NFA).
Interestingly, abundant amounts of AKR1B1 transcripts were detected in CPAs, NFAs, APAs, and Cushing’s hy-perplasias (Fig. 4). In contrast, ACCs had low to barely undetectable levels of AKR1B1 mRNA, compared with all other groups (Fig. 4). If individual values from the pop-ulation of tumors were divided into benign or malignant, statistical analysis revealed that the relative AKR1B1 mRNA levels measured in carcinomas (ACCs) (0.95⫾ 0.2 sem) were significantly lower (P⬍ 0.001) than the overall mean value calculated from adrenocortical adenomas (CPA⫹NFA⫹APA) (9.29 ⫾ 3.05). Moreover, as shown in Fig. 5, the values measured in carcinomas were lower than those determined in CPAs plus nonfunctional adenomas (P⬍ 0.01) as well as in APAs (P ⬍ 0.05). The relative values measured in ACCs slightly varied, from 0.5 to 1.3 arbitrary units, and were always lower than those observed in other pathological adrenal tissues (from 2.2 to 21.0). No signif-icant differences in relative AKR1B1 mRNA levels were measured in CPAs (15.1⫾ 2.9) and nonfunctional adeno-mas (9.5⫾ 2.5), suggesting that there was no correlation between expression of the AKR1B1 gene and the hormonal status of the neoplasm.
AKR1B1 protein content is decreased in ACCs
AKR1B1 protein expression was evaluated by Western blotting using the antibodies described above. As shown in Fig. 6A, AKR1B1 could be detected in both normal and most of neoplastic adrenal tissues but with marked quantitative differences. AKR1B1 was markedly accumulated in CPAs, NFAs, APAs, and Cushing’s hyperplasias. By contrast, most ACCs (seven of eight) exhibited low or hardly detectable amounts of AKR1B1 protein. Quantification of AKR1B1 sig-nals showed significantly lower concentrations of the en-zyme in malignant tumors than in normal tissue, adenomas, or Cushing’s hyperplasias (Fig. 6B). The results of Western blot studies were consistent with those of mRNA assays.
Discussion
In murine adrenocortical cells, AKR1B7, an aldose reduc-tase-like enzyme, rather than AKR1B3, the murine aldose reductase, is the main reductase for isocaproaldehyde (a product of side-chain cleavage of cholesterol) and 4-hydroxynonenal (HNE, a lipid peroxidation product) (19). Whereas high levels of AKR1B7 have been detected in rodent adrenal cortex (20, 23, 43), we found here that there is no human ortholog to murine akr1b7 gene. However, several lines of evidence suggest that AKR1B1, the human aldose reductase, is structurally and functionally homologous to AKR1B7. First, the AKR1B7-specific polyclonal antibody directed against the C-terminal end of the protein (residues 300 –316) shows only significant cross-reaction with AKR1B1. Thus, although not being encoding by ortholog genes, these two proteins have structurally related ends, sharing antigenic determinants. Second, in agreement with these data, the human AKR1B1 and murine AKR1B7 proteins FIG. 2. Regulation by forskolin of AKR1B1 mRNA accumulation in
human cell cultures. Time-dependent effects of 10⫺5Mforskolin (Fsk)
on CYP11A, StAR, and AKR1B1 mRNA expression in cultures of adrenocortical NCI-H295 cells (A) and AKR1B1 mRNA expression in trophoblast JEG-3 cells (B). The Northern blots were prepared with 25g total RNA in each lane and transferred onto a nylon membrane, and the filter was sequentially hybridized with the indicated32
P-labeled probes. C, The quantification⫾SDof the corresponding mRNA
signals from NCI-H295 cells in Northern blot experiments (n⫽ 6) is shown.
share the most related C-terminal ends among the AKR1B members of these two species (53% identity and 82% ho-mology), suggesting that they could assume overlapping
functions. Indeed, these C-terminal regions forming the ex-ternal folds for the substrate binding pocket (44) differ no-tably among members of the AKR1B subfamily and were FIG. 3. Effect of forskolin (Fsk) treatment on isocaproaldehyde reductase activity (ICR) in human adrenocortical cells. A, ICR activity (expressed as kcat) of recombinant AKR1B1 protein was measured in absence or presence of sorbinil, a specific aldose reductase inhibitor. The percentage
of ICR activity inhibition is indicated. B, ICR activity detected in cytosolic protein extracts from NCI-H295 cells cultured in absence (white bar) or presence of 10⫺5MFsk during 24 h (black bar) were determined, and AKR1B1-dependent activity was revealed by including sorbinil in the
reaction (the percentage of ICR activity inhibition is indicated). Fsk treatment induces a 33% increase of the ICR activity in cytosolic extracts. *, Significantly different from ICR activity in basal condition.
FIG. 4. Comparison of SF-1, DAX1, CYP11A, StAR, and AKR1B1 mRNA expression in adrenocortical tumors and Cushing’s hyperplasias. The
Northern blot was prepared with 25g of total RNA in each lane and transferred onto a nylon membrane, and the filter was sequentially hybridized with the indicated32P-labeled probes.
TABLE 2. Relative levels of AKR1B1, StAR, CYP11A, SF-1, and DAX1 mRNAs in adrenocortical tumors and hyperplasias
Tissue No. ID AKR1B1 StAR CYP11A SF-1 DAX1
ACC 5 ACC 1,2,4,c,b 0.9 (0.5–1.3) 0.7 (0.4 –1.0) 1.1 (0.5–2.2) 2.9 (1.0 – 4.6) 0.4 (0.1–1.0)
CPA 4 CPA 5–7,11 15.1 (8.7–21.0) 1.6 (0.5–2.9) 2.6 (1.1– 4.6) 6.4 (3.8 – 8.7) 0.5 (0.3– 0.6)
NFA 3 NFA 8,10,13 9.5 (3.8 –13.1) 1.4 (1.0 –2.0) 2.0 (1.1–2.7) 6.6 (3.0 – 8.9) 0.2 (0.1– 0.3)
APA 5 APA 15–19 4.5 (3.3– 6.8) 1.6 (0.8 –2.5) 1.3 (0.2–1.9) 4.7 (3.1– 8.9) 0.15 (0.1– 0.2)
Cushing’s disease 3 CD 21–23 3.9 (2.2–5.3) 1.8 (0.7–2.6) 1.7 (1.3–2.0) 2.4 (1.9 –2.8) 0.2 (0.1– 0.3) The values were calculated from phosphor imager scanning of the hybridization signals of Northern blots (samples were blotted twice, and the data were the average values). The filters were sequentially hybridized with the indicated probes and-actin probe. All the AKR1B1, StAR,
CYP11A, SF-1, and DAX1 signals were normalized with the respective-actin mRNA values. Means and ranges (in parentheses) are shown.
For each probe, the RNA signal obtained with the patient 1 adrenocortical carcinoma (ACC 1) was given the value 1. All the other RNA signals were expressed relative to this signal.
considered to be important for substrate specificity (36). Third, both proteins are major reductases for isocaproalde-hyde (Refs. 18,19 and present results). Fourth, gene expres-sion and isocaproaldehyde reductase activities of both AKR1B1 and AKR1B7 enzymes are significantly enhanced by forskolin. We conclude that AKR1B1 and akr1b7 genes may share conserved mechanisms with regard to their func-tion and regulated expression in adrenals.
In accordance with a previous report (40), our data showed that expression of CYP11A and StAR genes, both involved in the rate-limiting step of steroidogenesis, did not correlate with the endocrine profiles of patients examined. In the same way, we confirmed that SF-1 mRNA levels did not reflect the hormonal status of the neoplasms (45, 46) and that low DAX1 expression seemed to be associated with mineralocorticoid-producing adenomas (42). The implication of SF-1 in the regulation of DAX1 has been first reported in adrenal cell transfection studies (47) and then formally established in vivo in the developing gonad (48). We did not observe such a positive link between the two orphan receptors expression in the adrenocortical tumors. Other mechanisms might prob-ably participate to DAX1 regulation in neoplastic tissues or as suggested by Hoyle et al. (48), the SF-1 requirement for DAX1 expression could vary during development, being essential during the embryonic period but not in the adult organ. Low expression of transcriptional repressors acting on SF-1 target genes seems to be a more general feature of APAs because low chicken ovalbumin upstream promoter tran-scription factor I expression was also found in these tumors
(46). However, mechanisms independent of SF-1 might also occur because CYP11B2 was shown to be not positively reg-ulated by SF-1 (49) [nor was overexpressed in APA (50)]. By contrast, cortisol hypersecretion in adenomas could result from disordered expression of SF-1 target genes encoding proteins acting at downstream steps, e.g. CYP17, rather than at initial steps of steroidogenesis (StAR or CYP11A). Indeed, excessive cortisol production in CPAs was shown to correlate with the overexpression of CYP17 in a manner reciprocal to that of its repressors DAX1 and COUP-TFs (51).
The most prominent finding of this study is that AKR1B1 gene is differentially expressed in benign vs. malignant tu-mors in adrenal cortex. AKR1B1 gene expression, measured at mRNA and protein levels, is strongly decreased in ACCs, compared with that in adenomas. Two reasons may account for low AKR1B1 expression in carcinomas. First, chromo-somal alterations, including mutations or rearrangements, could reduce expression of the gene. However, no chromo-somal abnormalities concerning the 7q35 region (the aldose reductase gene locus) (52) had ever been described in adrenal tumorigenesis (7). Alternatively, inhibition of AKR1B1 gene expression, in carcinomas, may be due to dysregulation of the mechanisms underlying the control gene expression. The mechanisms regulating expression of AKR1B1 in adrenals were unknown until the present report. Indeed, data re-ported herein suggest that cAMP is a regulator of AKR1B1 expression in human adrenocortical cells. Interestingly, the transcription factor cAMP-responsive element-binding tein (CREB) was shown to be strongly decreased at the pro-FIG. 5. Relative RNA levels of AKR1B1,
StAR, CYP11A, SF-1, and DAX1 in ACCs,
CPAs, NFAs, APAs, and Cushing’s diseases (CD). The values (mean⫾SEM) are calculated
as described in Table 2. Only individual values from akr1b1 mRNA signals are illustrated. Differences in mRNA signals were assessed by the Student’s t test. Asterisks point values sig-nificantly different from those of ACC group: **, P⬍ 0,001; *, P ⬍ 0.05. a points values, significantly different from APA group: P⬍ 0.05
tein level in ACCs (53). A loss of expression of the ACTH receptor have been reported in adrenal cancer (54), and cAMP stimulated PKA activity is lower in adrenal cancers than adenomas (55). This could take part in the decreased expression of AKR1B1. However, the factors that induced this inhibition of AKR1B1 in ACCs remain to be more thor-oughly determined.
Changes in AKR1B1 mRNA level in response to cAMP, in parallel with steroidogenic genes, suggest that AKR1B1 may be considered a marker of adrenocortical cell differentiation. There are some evidences that members of the AKR super-family may be associated with cancer progression. A previ-ous report (56) has shown that an aldose reductase-like protein was induced during rat hepatocarcinogenesis. Ad-ditionally, AKR1B1 and AKR1B10 are overexpressed by 29 and 54%, respectively, in some human liver cancers (57). The physiological function of aldose reductase is still unclear, and recent data have suggested that its main role may be detox-ication of reactive aldehydes. Oxidative stress plays an im-portant role in various pathological states including cancer (58). HNE is believed to be responsible for the cellular patho-logical effects observed during oxidative stress in vivo (59), and HNE protein adducts have been detected in human renal cell carcinomas (60). HNE exhibited a wide range of biolog-ical activities, including stimulation of phospholipase C, stimulation of neutrophil migration, and reduction of gap-junction communication (61, 62). Interestingly, it has been reported that␣1-connexin 43 gap junctions were decreased in the human malignant adrenocortical tumors (63). AKR1B1 catalyzes the reduction of HNE, suggesting that this enzyme may be a part of the cellular defenses against oxidative stress in physiological and pathological conditions (19, 62). On the contrary, overexpression of aldose reductase and/or aldose
reductase-like proteins in hepatomas has been interpreted as a defense reaction against harmful metabolites produced by the growing cancer cells (56). Whatever the mechanisms that lead to down-regulation of AKR1B1 in adrenocortical carci-nomas, further studies are needed to elucidate whether the decrease in AKR1B1 expression in malignant tumors is merely a consequence of a general dedifferentiation of the tumor or whether it contributes to the pathogenesis of this disease by promoting, for instance, as a consequence of HNE accumulation, alterations in cell-cell communication through progressive loss of gap junctions.
There are no reliable criteria for accurately distinguishing between benign and malignant adrenocortical tumors, al-though certain markers have been proposed to be specific of adrenocortical carcinomas. For example, it has been reported that malignant adrenocortical tumors expressed low levels of StAR mRNA, compared with adenomas (40). Other reports, however, showed that StAR expression was about equal in adrenocortical adenomas and carcinomas (42, 64). Immuno-histochemical studies have shown that GATA-4 (a zinc finger transcription factor) and Ki-67 (a cell cycle-associated marker) were overexpressed in adrenal carcinomas (65, 66). The angiogenic factor, vascular endothelial growth factor, and the protein thrombospondin-1 may represent possible markers of the transition of ACCs toward malignancy. Vas-cular endothelial growth factor concentrations were in-creased in carcinomas, whereas those of thrombospondin-1 were decreased (67). Overexpression of IGF-II, IGF-binding protein-2, and IGF-I receptor has been associated with ma-lignancy (68, 69). However, there is no perfect marker, and the combined use of multiple indicators of malignancy is required to advance our understanding of both normal and pathological adrenocortical physiology. Although the use-FIG. 6. Western blot analysis of AKR1B1 and-actin protein accumulation in normal adrenals, ACCs, CPAs, NFAs, APAs, and Cushing’s
hyperplasias. A, The Western blots were prepared using 15 mg total soluble proteins per lane, transferred onto a nitrocellulose membrane, and incubated with L3 antibody or anti--actin antibody as described in Materials and Methods. B, Relative levels of AKR1B1 signals in normal and pathological adrenals. The Western blot signals were quantitated by densitometric analysis as described in Materials and Methods, and the values were normalized with the respective-actin signal values. Individual values and means (horizontal bar) are shown. Differences in AKR1B1 signals were assessed by the Student’s t test. Asterisks point values significantly different from those of ACC group: **, P⬍ 0,001; *, P⬍ 0.01.
fulness of AKR1B1 has to be validated on a larger cohort and compared with standard criteria of histological diagnosis, several evidences point out that it could be chosen as a good candidate marker for further studies. Firstly, 88% of the carcinomas exhibit AKR1B1 protein concentrations below the lowest value measured in normal tissues and benign neoplasms. Second, AKR1B1 is a very stable soluble protein whose detection is easy to carry out simply using a Western blot analysis, although more sensitive quantitative analysis could be easily improved by development of an AKR1B1-based RIA or ELISA. Finally, induction of AKR1B1 mRNA levels by forskolin suggests that its expression is under ACTH control. Because AKR1B1 is a major reductase for reactive aldehydes formed during steroidogenesis and lipid peroxidation (19, 62), it will be worth investigating whether maintaining a high capacity of AKR1B1-dependent detoxi-cation could impair or delay malignant transformation pro-cess in adrenal cortex. It would be interesting to further evaluate the value of AKR1B1 for differential diagnosis be-tween adenomas and carcinomas and its prognosis value on a larger cohort and during prospective studies.
Acknowledgments Received October 21, 2003. Accepted March 10, 2004.
Address all correspondence and requests for reprints to: Dr. A. Mar-tinez, UMR6547 CNRS-Universite´ Blaise Pascal Clermont II, Ge´ne´tique des Eucaryotes et Endocrinologie Mole´culaire, Complexe Universitaire des Ce´zeaux, 24 avenue des Landais, 63177 Aubie`re Cedex, France. E-mail: antoine.martinez@univ-bpclermont.fr.
This work was supported by the Centre National de la Recherche Scientifique, Universite´ Blaise Pascal, Association de la Recherche contre le Cancer (Grant ARC 4471), and Cortico et Medullosurre´nale: Etude des Tumeurs Endocrines network.
References
1. Latronico AC, Chrousos GP 1997 Extensive personal experience: adrenocor-tical tumors. J Clin Endocrinol Metab 82:1317–1324
2. Kopf D, Goretzki P, Lehnert H 2001 Clinical management of malignant adrenal tumors. J Cancer Res Clin Oncol 127:143–155
3. Gicquel C, Bertagna X, Schneid H, Francillard-Leblond M, Luton JP, Girard
F, Le Bouc Y1994 Rearrangements at 11p15 locus and overexpression of insulin like growth factor II gene in sporadic adrenocortical tumors. J Clin Endocrinol Metab 78:1444 –1453
4. Liu J, Kahri AI, Heikkila P, Voutilainen R 1997 Ribonucleic acid expression of the clustered imprinted genes, p57KIP2, insulin-like growth factor II, and H19, in adrenal tumors and cultured adrenal cells. J Clin Endocrinol Metab 82:1766 –1771
5. Gicquel C, Raffin-Sanson ML, Gaston V, Bertagna X, Plouin PF,
Schlum-berger M, Louvel A, Luton JP, Le Bouc Y1997 Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in spo-radic adrenocortical tumors: study on a series of 82 tumors. J Clin Endocrinol Metab 82:2559 –2565
6. Bourcigaux N, Gaston V, Logie A, Bertagna X, Le Bouc Y, Gicquel C 2000 High expression of cyclin E and G1 CDK and loss of function of p57KIP2 are involved in proliferation of malignant sporadic adrenocortical tumors. J Clin Endocrinol Metab 85:322–330
7. Bornstein S, Stratakis C, Chrousos G 1999 Adrenocortical tumors: recent advances in basic concepts and clinical management. Ann Intern Med 130: 759 –771
8. Lin S, Lee Y, Tsai J 1994 Mutations of the p53 gene in human functional adrenal neoplasms. J Clin Endocrinol Metab 78:483– 491
9. Gicquel C, Bertagna X, Le Bouc Y 1995 Recent advances in the pathogenesis of adrenocortical tumours. Eur J Endocrinol 133:133–144
10. Heppner C, Reincke M, Agarwal SK, Mora P, Allolio B, Burns AL, Spiegel
AM, Marx SJ1999 MEN1 gene analysis in sporadic adrenocortical neoplasms. J Clin Endocrinol Metab 84:216 –219
11. Gortz B, Roth J, Speel EJ, Krahenmann A, De Krijger RR, Matias-Guiu X,
Muletta-Feurer S, Rutmann K, Saremaslani P, Heitz PU, Komminoth P1999 MEN1 gene mutation analysis of sporadic adrenocortical lesions. Int J Cancer 80:373–379
12. Schulte KM, Mengel M, Heinze M, Simon D, Scheuring S, Kohrer K, Roher
HD2000 Complete sequencing and messenger ribonucleic acid expression analysis of the MEN I gene in adrenal cancer. J Clin Endocrinol Metab 85: 441– 448
13. Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E, Bertherat J,
Chapuis Y, Duclos JM, Schlumberger M, Plouin PF, Luton JP, Le Bouc Y2001 Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res 61:6762– 6767
14. Hyndman D, Bauman DR, Heredia VV, Penning TM 2003 The aldo-keto reductase superfamily home page. Chem Biol Interact 143–144:621– 631 15. Kinoshita J, Nishimura C 1988 The involvement of aldose reductase in diabetic
complications. Diabetes Metab Rev 4:323–337
16. Lee AY, Chung SK, Chung SS 1995 Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci USA 92:2780 –2784 17. Grimshaw CE, Mathur EJ 1989 Immunoquantitation of aldose reductase in
human tissues. Anal Biochem 176:66 –71
18. Matsuura K, Deyashiki Y, Bunai Y, Ohya I, Hara A 1996 Aldose reductase is a major reductase for isocaproaldehyde, a product of side chain cleavage of cholesterol, in human and animal adrenal glands. Arch Biochem Biophys 328:265–288
19. Lefranc¸ois-Martinez A, Tournaire C, Martinez A, Berger M, Daoudal S,
Tritsch D, Veyssiere G, Jean C1999 Product of side-chain cleavage of cho-lesterol, isocaproaldehyde, is an endogenous specific substrate of mouse vas deferens protein, an aldose reductase-like protein in adrenocortical cells. J Biol Chem 274:32875–32880
20. Aigueperse C, Martinez A, Lefranc¸ois-Martinez AM, Veyssiere G, Jean C 1999 Cyclic AMP regulates expression of the gene coding for a protein related to the aldo-keto reductase superfamily (MVDP) in human and murine adre-nocortical cells. J Endocrinol 160:147–154
21. Martinez A, Lefrancois-Martinez AM, Manin M, Guyot S, Jean-Faucher C,
Veyssiere G, Kahn A, Jean C1999 5⬘-Flanking and intragenic sequences confer
androgenic and developmental regulation of mouse aldose reductase-like gene in vas deferens and adrenal in transgenic mice. Endocrinology 140:1338 –1348 22. Aigueperse C, Val P, Pacot C, Darne C, Lalli E, Sassone-Corsi P, Veyssie`re
G, Jean C, Martinez A2001 SF-1 (steroidogenic factor-1), C/EBP ( CCAAT)
enhancer binding protein, and ubiquitous transcription factors NF1 (nuclear factor 1) and SP1 (selective promoter factor 1) are required for regulation of the mouse aldose reductase-like gene (AKR1B7) expression in adrenocortical cells. Mol Endocrinol 15:93–111
23. Val P, Martinez A, Sahut-Barnola I, Jean C, Veyssiere G,
Lefrancois-Mar-tinez AM2002 A 77-base pair LINE-like sequence elicits androgen-dependent
mvdp/akr1– b7 expression in mouse vas deferens, but is dispensable for adrenal
expression in rats. Endocrinology 143:3435–3448
24. Chinn AM, Ciais D, Bailly S, Chambaz E, LaMarre J, Feige JJ 2002 Identi-fication of two novel ACTH-responsive genes encoding manganese-dependent superoxide dismutase (SOD2) and the zinc finger protein TIS11b [tetrade-canoyl phorbol acetate (TPA)-inducible sequence 11b]. Mol Endocrinol 16: 1417–1427
25. Simpson E, Waterman M 1988 Regulation of the synthesis of steroidogenic enzymes in adrenocortical cells by ACTH. Annu Rev Physiol 50:427– 440 26. Jefcoate C, McNamara B, Artemenko I, Yamazaki T 1992 Regulation of
cho-lesterol movement to mitochondrial cytochrome P450scc in steroid hormone synthesis. J Steroid Biochem Mol Biol 43:751–767
27. Stocco D, Clark B 1997 The role of the steroidogenic acute regulatory protein in steroidogenesis. Steroids 62:29 –36
28. Parker K, Schimmer B 1997 Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 18:361–377
29. Zanaria E, Muscatelli F, Bardoni B, Strom T, Guioli S, Guo W, Lalli E, Moser
C, Walker A, McCabe E, Meitinger T, Monaco A, Sassone-Corsi P, Camerino G1994 An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635– 641 30. Luton JP, Martinez M, Coste J, Bertherat J 2000 Outcome in patients with
adrenal incidentaloma selected for surgery: an analysis of 88 cases investigated in a single clinical center. Eur J Endocrinol 143:111–117
31. Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, Laudat
MH, Louvel A, Chapuis Y, Blondeau P, Bonnin A, Bricaire H1990 Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med 322:1195–1201
32. Streeter G 1920 Weight, sitting height, head size, foot length and menstrual age of the human embryo. Contrib Embryol 11:143–179
33. Pailhoux E, Martinez A, Veyssie`re G, Jean C 1990 Androgen-dependent protein from mouse vas deferens. J Biol Chem 265:19932–19936
34. Morel L, Brochard D, Manin M, Simon AM, Jean C, Veyssiere G 2001 Mouse seminal vesicle secretory protein of 99 amino acids (MSVSP99): characteriza-tion and hormonal and developmental regulacharacteriza-tion. J Androl 22:549 –557 35. Staels B, Hum DW, Miller WL 1993 Regulation of steroidogenesis in
NCI-H295 cells: a cellular model of the human fetal adrenal. Mol Endocrinol 7:423– 433
36. Bohren K, Grimshaw C, Gabbay K 1992 Catalytic effectiveness of human aldose reductase. J Biol Chem 267:20965–20970
37. Sahut-Barnola I, Lefrancois-Martinez AM, Jean C, Veyssiere G, Martinez A
2000 Adrenal tumorigenesis targeted by the corticotropin-regulated promoter of the aldo-keto reductase AKR1B7 gene in transgenic mice. Endocr Res 26: 885– 898
38. Mesiano S, Jaffe RB 1997 Developmental and functional biology of the pri-mate fetal adrenal cortex. Endocr Rev 18:378 – 401
39. Rainey WE, Bird IM, Sawetawan C, Hanley NA, McCarthy JL, McGee EA,
Wester R, Mason JI1993 Regulation of human adrenal carcinoma cell (NCI-H295) production of C19 steroids. J Clin Endocrinol Metab 77:731–737 40. Zenkert S, Schubert B, Fassnacht M, Beuschlein F, Allolio B, Reincke M 2000
Steroidogenic acute regulatory protein mRNA expression in adrenal tumours. Eur J Endocrinol 142:294 –299
41. Grundmann U, Bohn H, Obermeier R, Amann E 1990 Cloning and prokary-otic expression of a biologically active human placental aldose reductase. DNA Cell Biol 9:149 –157
42. Reincke M, Beuschlein F, Lalli E, Arlt W, Vay S, Sassone-Corsi P, Allolio B 1998 DAX-1 expression in human adrenocortical neoplasms: implications for steroidogenesis. J Clin Endocrinol Metab 83:2597–2600
43. Lau E, Cao D, Lin C, Chung S, Chung S 1995 Tissue-specific expression of two aldose reductase-like genes in mice: abundant expression of mouse vas de-ferens protein and fibroblast growth factor-regulated protein in the adrenal gland. Biochem J 312:609 – 615
44. Wilson DK, Tarle I, Petrash JM, Quiocho FA 1993 Refined 1.8 A structure of human aldose reductase complexed with the potent inhibitor zopolrestat. Proc Natl Acad Sci USA 90:9847–9851
45. Sasano H, Shizawa S, Suzuki T, Takayama K, Fukaya T, Morohashi K,
Nagura H1995 Ad4BP in the human adrenal cortex and its disorders. J Clin Endocrinol Metab 80:2378 –2380
46. Shibata H, Ando T, Suzuki T, Kurihara I, Hayashi K, Hayashi M, Saito I,
Murai M, Saruta T1998 COUP-TFI expression in human adrenocortical ad-enomas: possible role in steroidogenesis. J Clin Endocrinol Metab 83:4520 – 4523
47. Vilain E, Guo W, Zhang YH, McCabe ER 1997 DAX1 gene expression up-regulated by steroidogenic factor 1 in an adrenocortical carcinoma cell line. Biochem Mol Med 61:1– 8
48. Hoyle C, Narvaez V, Alldus G, Lovell-Badge R, Swain A 2002 Dax1 expres-sion is dependent on steroidogenic factor 1 in the developing gonad. Mol Endocrinol 16:747–756
49. Bassett MH, Zhang Y, Clyne C, White PC, Rainey WE 2002 Differential regulation of aldosterone synthase and 11-hydroxylase transcription by ste-roidogenic factor-1. J Mol Endocrinol 28:125–135
50. Fallo F, Pezzi V, Barzon L, Mulatero P, Veglio F, Sonino N, Mathis JM 2002 Quantitative assessment of CYP11B1 and CYP11B2 expression in aldosterone-producing adenomas. Eur J Endocrinol 147:795– 802
51. Shibata H, Ikeda Y, Mukai T, Morohashi K, Kurihara I, Ando T, Suzuki T,
Kobayashi S, Murai M, Saito I, Saruta T2001 Expression profiles of COUP-TF, DAX-1, and SF-1 in the human adrenal gland and adrenocortical tumors: possible implications in steroidogenesis. Mol Genet Metab 74:206 –216 52. Graham A, Brown L, Hedge P, Gammack A, Markham A 1991 Structure of
the human aldose reductase gene. J Biol Chem 266:6872– 6877
53. Rosenberg D, Groussin L, Jullian E, Perlemoine K, Medjane S, Louvel A,
Bertagna X, Bertherat J2003 Transcription factor 3⬘,cyclic adenosine
5⬘-monophosphate-responsive element-binding protein (CREB) is decreased dur-ing human adrenal cortex tumorigenesis and fetal development. J Clin En-docrinol Metab 88:3958 –3965
54. Reincke M, Mora P, Beuschlein F, Arlt W, Chrousos GP, Allolio B 1997 Deletion of the adrenocorticotropin receptor gene in human adrenocortical tumors: implications for tumorigenesis. J Clin Endocrinol Metab 82:3054 –3058 55. Bertherat J, Groussin L, Sandrini F, Matyakhina L, Bei T, Stergiopoulos S,
Papageorgiou T, Bourdeau I, Kirschner LS, Vincent-Dejean C, Perlemoine K, Gicquel C, Bertagna X, and Stratakis CA2003 Molecular and functional analysis of PRKAR1A and its locus (17q22–24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res 63:5308 –5319
56. Zeindl-Eberhart E, Jungblut P, Otto A, Kerler R, Rabes H 1997 Further characterization of a rat hepatoma-derived aldose-reductase-like protein. Eur J Biochem 247:792– 800
57. Cao D, Fan S, Chung S 1998 Identification and characterization of a novel human aldose reductase like gene. J Biol Chem 273:11429 –11435
58. Stadman E, Berlett B 1997 Reactive oxygen mediated protein oxidation in aging and disease. Chem Res Toxicol 10:485– 494
59. Esterbauer H, Schaur R, Zollner H 1991 Chemistry and biochemistry of 4-hydroxynonenal malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
60. Okamoto K, Toyokuni S, Uchida K, Ogawa O, Takenewa J, Kakehi Y,
Kinoshita H, Hattori-Nakakuki Y, Hiai H, Yoshida O1994 Formation of 8-hydroxy-2⬘-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer 58:825– 829
61. Radu A, Moldovan N 1991 4-Hydroxynonenal reduces junctional communi-cation between endothelial cells in culture. Exp Cell Res 196:121–126 62. Vander Jagt D, Kolb N, Vander Jagt T, Martinez F, Mills R, Young S, Vander
Jagt D1995 Substrate specificity of human aldose reductase: identification of 4-hydroxynonenal as an endogenous substrate. Biochim Biophys Acta 249: 117–126
63. Murray S, Davis K, Fishman L, Bornstein S 2000␣1 Connexin 43 gap junctions are decreased in human adrenocortical tumors. J Clin Endocrinol Metab 85: 890 – 895
64. Liu J, Heikkila P, Kahri A, Voutilainen R 1996 Expression of the steroidogenic acute regulatory protein mRNA in adrenal tumors and cultural adrenal cells. J Endocrinol 150:43–50
65. Kiiveri S, Siltanen S, Rahman N, Bielinska M, Lehto VP, Huhtaniemi IT,
Muglia LJ, Wilson DB, Heikinheimo M1999 Reciprocal changes in the ex-pression of transcription factors GATA-4 and GATA-6 accompany adreno-cortical tumorigenesis in mice and humans. Mol Med 5:490 –501
66. Terzolo M, Boccuzzi A, Bovio S, Cappia S, De Giuli P, Ali A, Paccotti P,
Porpiglia F, Fontana D, Angeli A2001 Immunohistochemical assessment of Ki-67 in the differential diagnosis of adrenocortical tumors. Urology 57:176 – 182
67. De Fraipont F, El Atifi M, Gicquel C, Bertagna X, Chambaz EM, Feige JJ 2000 Expression of the angiogenesis markers vascular endothelial growth factor-A, thrombospondin-1, and platelet-derived endothelial cell growth factor in hu-man sporadic adrenocortical tumors: correlation with genotypic alterations. J Clin Endocrinol Metab 85:4734 – 4741
68. Boulle N, Logie´ A, Gicquel C, Perin L, Le Bouc Y 1998 Increased levels of insulin-like growth factor II (IGF-II) and IGF-binding protein-2 are associated with malignancy in sporadic adrenocortical tumors. J Clin Endocrinol Metab 83:1713–1720
69. Weber M, Auernhamma C, Kiess W, Engelhardt D 1997 Insulin-like growth factor receptors in normal and tumorous adult human adrenocortical glands. Eur J Endocrinol 136:296 –303
70. Donohue P, Alberts O, Hampton B, Winkles J 1994 A delayed-early gene activated by fibroblast growth factor-1 encodes a protein related to aldose reductase. J Biol Chem 269:8604 – 8609
71. Hyndman D, Flynn J 1998 Sequence and expression levels in human tissues of a new member of the aldo-keto reductase family. Biochim Biophys Acta 1399:198 –202
JCEM is published monthly by The Endocrine Society (http://www.endo-society.org), the foremost professional society serving the endocrine community.