Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Internal Report (National Research Council of Canada. Division of Building Research), 1959-10-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=0de22aa9-6eab-4217-bd0a-248fe2c388f2 https://publications-cnrc.canada.ca/fra/voir/objet/?id=0de22aa9-6eab-4217-bd0a-248fe2c388f2
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20386766
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Cement-aggregate reaction in Kingston, Ontario
DIVISION OF BUILDING RESEARCH
OEMENT-AGGREGATE REAOTION IN KINGSTON, ONTARIO
by
E. G. Swenson
Report No. 182
of the
Division of Building Research
OTTAWA
PREFACE
The Division was asked, in 1955, to determine the causes of excessive expansion and cracking of a sidewalk at Kingston, Ontario. The extensive investigation which has grown out of this original request for assistance is now reported.· The difficulty has been found to be widespread and has been traced to crushed limestone commonly used at Kingston as coarse aggregate. A cement-aggregate reaction not previously
identified, unlike the known alkali-silica reactions, and not detected by the usual A.S.T.M. tests, has been shown to be the cause. The exact nature of the mechanism involved is still under study and a search' is being made for a quick method of detection.
The general nature of the phenomenon and the
precautions that can be taken to avoid expansion difficulties have now been established and it is possible to use the aggre-gate with reasonable confidence. This report has been prepared to summarize all the work carried out to date mainly for the private information and convenience of others engaged in con-crete research work. Reference is made, in the report, to co-operative studies by others; this interest thus shown, and the attention given to the problem by other laboratories is appreciated.
The author of this report is a chemist who, prior to joining the Division in 1950, worked with Dr. T. tィッセ。ャ、ウッョ
at the University of Saskatchewan on general concrete .problems as well as those peculiar to the Prairie Provinces; he is now responsible for the Division's studies on concrete and cement.
Ottawa,
Page 1
I INTRODUCTION
...
II THE EXTENT OF INTEREST IN THE PROBLEM ••.•.•••••••••••••• 2セii FIELD OBSERVATIONS AND CASES ••••.•••.•••••••••••••••••••
4
1. General Conditions •••.••••.•••.••••••••.•••••••••• 4
2. Special Cases 5
3.
Field Application of Remedial Measures •.•••••••••.7
4.
Local Limestone Quarries ••••••••••••.•••••.••••••• 85.
Results of Field Observations •••••••.•••••••.•••••9
IV RESUME OF PRELIMINARY STUDIES •.•••••••••••••.••••••••••. 101. Reported Studies of the Division of Building
Research 10
2. Petrographic Examination by the Bureau of
Reclamation 10
3. Examination by the Laboratory of the U. S. Anny
Corps of Engineers ••••.•••••セ ••.•••••••.•• セ • • •• 12
4.
Queen's University Thesis ••.•.••••••••...•••••••••13
5.
Studies by Other Laboratories •••••••••••••••••••••14
V STUDIES DIRECTED TO THE FIELD PROBLEM: EXPANSIONCHARACTERISTICS OF CONCRETE WITH KINGSTON LIMESTONES ••
14
1. Materials and Procedure·- General •••••••••••••••.. 15
2. Test of a Job-Mix Concrete .•••••••••••••••..•••••• 16
3.
Contribution to Expansion of Concrete Ingredients ••17
4.
Influence of Exposure Conditions •••••••••••••••••• 185.
Effectiveness of Inhibitors of Alkali-aggregateReaction and Reduction in Cement Content •••••••
19
6. Influence of Cement Alkalies and Added Alkalies ••• 20
7.
Contribution of SUlphide in Reactive Limestone •••.23
8. Influence of Initial Drying Period •••••••••••••••.• 24 9. Effect of Temperature ••••••••••••••••••.•.•••••••• 25 10. Influence of Other Possible Field Variables ••••••• 26 11. Variation in Reactivity of Quarry Horizons •••.••.• 29 VI . FURTHER DURABILITY STUDIES •••••••••••••..••••.•.•••••••.32
1. Results of Wetting-Drying Cycling Tests •••••••••••32
2. Results of Freeze-Tlmw Cycling Tests .•••••••••••••33
VII DISCUSSION OF STUDIES IN RELATION TO THE FIELD PROBLEM •• 361. Nature of the Reaction •••••••••••••••..••••••••••• 36 2. Relation of Laboratory and Field Observations
to Field Practice •••••••••••.•••••••••••••••••• 38
AC:KllOWLEDG.ENIENT ••••••••••••••••••••••••••••••••••••••••••••• 41
by
E. G. Swenson
I INTRODUCTION
. In 1955 the Division of Building Research was asked by Defence Construction (1951) Limited to investigate the cause of excessive expansion and map-cracking of a concrete sidewalk in the Barriefield area at Kingston, Ontario. The problem had occurred within a few montHs of placing and before winter had set in. The large volume of construction for which this federal agency was responsible in the Kingston area
justified the importance attached to this particular case. Preliminary laboratory tests made by the Division established that the argillaceous dolomitic limestone used as coarse aggregate was primarily responsible. Concrete prisms made with the same materials that had been used on the job expanded 0.1 per cent in about six weeks when subjected merely to near 100 per cent relative humidity conditions and a
temperature of 73°F.
The conditions under which the expansion occurred, and the symptoms of map-cracking, suggested that a type of cement-aggregate reaction was involved. Investigation showed, however, that the reactive limestone did not respond either to the recognized ASTM quick chemical test (C289-54T) or to the mortar bar test (C227-52T). Examination of samples of the limestone and of the affected concrete by
Dr.
R. C. Mielenz, a recognized authority on such phenomena, revealed certainmanifestations of alkali-aggregate reaction but failed to
detect any of the known alkali-reactive minerals or rock types. Preliminary field observations indicated that the problem has existed in the area for at least 30 years and has affected a large proportion of the concrete structures and elements in the area. The symptoms were particularly evident in such structures as sidewalks, curbs, floors, and foundation walls which are subjected to moist conditions.
Laboratory studies showed that the degree of expan-sion increased with increasing alkali content of the cement, and that a cement with sufficiently low alkali content could
be used with the potentially reactive limestone to produce field concrete free from excessive expansion. It appeared ,that a type of alkali-aggregate reaction was involved which
was somehow different from the well-known alkali-silica type of reaction.
Despite the preliminary nature of these first studies, the urgency of the situation made it necessary to issue information before the true nature of the problem was found. A general report was made to a meeting of the Kingston branch of the Engineering Institute of Canada in April 1957, and has been issued as DBR Internal Report No. 115 (1). An
off-the-record discussion was also presented at a meeting of the Highway Research Board in Washington, in January 1957 (2). A summary of the preliminary studies was published in the
December 1957 issue of ASTM Bulletin No. 226 (3).
The present report is a detailed presentation of
results of a continuing study and includes a summary of earlier work. It also includes a resume of field observations and
descriptions of some affected field concrete. The laboratory results are mainly those that have a direct bearing on the field problem. Studies concerning the nature of the problem are continuing and will be the subject of a separate report.
Although experimental and field studies indicate that concrete made with the reactive limestone and a cement of sufficiently low alkali content will not expand excessively, and can therefore be used with confidence in most cases of ordinary concrete construction, there are situations where even slightly more than normal expansion cannot be tolerated. Cases of this kind require further study.
II THE EXTENT OF INTEREST IN THE PROBLEM
During the course of this investigation many organiza-tions have become involved directly or indirectly. The studies were therefore not confined to the original case submitted by Defence Construction (1951) Limited.
Among the construction agencies other than Defence Construction (1951) Limited having an interest in the problem were: The Works Department of the Department of National Defence in connection with the large defence installations at Barriefield, the Ontario Department of Highways, the Hydro-Electric Power Commission of Ontario, and Central Mortgage and Housing Corporation. These organizations were concerned キゥエセ
building specifications and were in constant touch with the Division during the first part of the investigation.
Contractors who had had direct experience with the problem were vitally concerned with corrective measures.
Among these was the firm of McGinnis and O'Connor, Contracting Engineers, who were also the owners and operators of the
Pittsburgh quarry, one of the main sources of concrete lime-stone aggregate for the City of Kingston and the main source of the materials studied in this laboratory.
Several cement manufacturers with possible outlets in the area have followed closely research progress. The Canada Cement Company, which has supplied the bulk of the cement used at Kingston from its Belleville plant, has begun to produce a low alkali cement at the Port Colborne plant specifically for the Kingston area. Other manufacturers have indicated interest in providing a similar service.
Several large industrial firms operating in the area have had direct experience with the problem and have consulted the Division regarding their ーイッ「ャ・ュセN Municipal engineers with the Kingston City Works Department, and Public utilities have consulted the Division about a number of concrete problems related to abnormal expansion and map-cracking. Some of these cases will be mentioned under field studies.
Many research organizations have become interested in the problem since it was first reported by the Division. Some have carried out extensive studies, for example, the Portland Cement Association, whose findings have been in
close agreement with those from this laboratory. The petrology laboratory of the Bureau of Reclamation carried out a detailed petrographic evaluation at the request of the Division, and independent expert opinion was also obtained from the labora-tory of the U. S. Army Corps of Engineers and other U. S. and Canadian authorities. Details of tllese reports are given later in this report. Samples for study were also requested by and obtained for the Danish National Institute for Building Research.
In order that subsequent local problems could be investigated at Kingston, the Division loaned the necessary research equipment to the Civil Engineering Department of Queen's University and to the Royal Military College. The Geology Department at Queen's University and the Federal
Bureau of Mines have begun some field surveys in an effort to determine the nature and extent of the reactive limestones in the area. Testing has also been initiated by the Ontario . Department of Highways laboratory at Toronto.
More recently, interest has spread セッ the many areas where carbonate rock, used as concrete coarse aggregate, has given trouble. Studies by Iowa State College (4, 5) have indicated a problem with Iowa limestone. Problems have
apparently been encountered in Michigan, New York, and Indiana. The Portland Cement Laboratories have begun a general study of the problems connected with the use of carbonate rocks as concrete aggregate. Continuing work in this laboratory is directed along the same lines.
III FIELD OBSERVATIONS AND CASES
From the beginning of the investigation the Division has made extensive studies of field cases and has obtained considerable information on sources of material and concrete
ーQセ」エゥ」・N In some cases examinations have been made indepen-dently; others have been carried out at the request of indivi-duals or agencies faced with the. problem.
1. General Conditions
The most evident cases of excessive expansion and map-cracking have occurred in exposed elements in contact with
ground moisture or highly humid conditions. The direct result of the reaction is excessive expansion, the map or
pattern-cracking occurring only where differential movement takes place. In a sidewalk, for example, the lower side is SUbjected to a continuous humid atmosphere whereas the top side is subjected to partial drying (Fig. 1). The lower side therefore expands more rapidly and causes cracking in the top section. . The depth
of penetration of cracks depends on the drying conditions and the age of the concrete. Where humidity or moisture conditions are uniform around a concrete element map-cracking does not occur, and excessive expansion will ultimately result in gross cracking only.
In many sidewalks the abnormal expansion has resulted in the closing of expansion joints causing extrusion of mastic
joint filler and, in some cases, the buckling of slaba and curbs (Fig. 2).
The uncracked areas defined by the pattern-cracking are about 2 to 4 inches across and the concrete in these
sections appears to be relatively sound and durable. For this reason, particularly where reinforcing is present, affected structures usually remain more or less intact and continue to perform satisfactorily.
In many cases of apparently unaffected exposed concrete and in cases where only gross cracks were present, the reaction had been obscured or partially retarded through the practice of two-course construction in which the upper course contained either no coarse aggregate or small amounts of small maximum-size coarse aggregate. This practice, as will be explained later, could have prevented detection of the reaction in some field cases, particularly sidewalks, leading to varied opinions as to the prevalence of the difficulty.
Concrete floors in garages, shops, or basements were usually found to be affected but the pattern-cracking was often obscured or hidden by a covering of dust and dirt. It was possible to examine many basement floors in Kingston through the courtesy of the late Mr. J. A. Compton, a contractor for 45 years in that city, whose inspection duties gave him access to many homes. Through this and other sources, it was possible to accumulate evidence indicating that the problem had existed for at least 35 years.
2. Special Oases
The following cases are cited to illustrate the extent and severity of the problem.
(a) Sidewalks.- Affected sidewalks showing varying degrees of map-cracking were observed on many streets throughout the city. In addition to those at Barriefield, the following examples are mentioned: Montreal street, Rideau street, Division Street, Upper Princess Street, Bagot Street, Lower Union Street, and sidewalks on the grounds of Queen's Univer-sity. These were examined from 1955 through 1958.
(b) R.O.E. Stores and Workshops Floor, Barriefield.- Examined
in June
1956.
Less than two years old and very badly cracked. Built on a 4-foot crushed rock fill and given special care as to mix design, composition and curing through previousexperience with the problem by the contractor. Joints
(c) Private Home, Lake Loughborough.- Built in 1953;
examined in June
1956.
A particularly serious case where basement walls have expanded and caused large cracks or separations in the brick masonry superstructure. Pattern cracking extensive on parged basement walls, retaining walls, steps and floors. The coarse aggregate was obtained from the Pittsburgh quarry.(d) Fort h・セ Housing Area School.- Built in
1955;
examined in o」セ・イ
1956.
EXpanding concrete foundation had produced large cracks in the brick masonry superstructure. Pittsburgh rock reported to have been used.(e) Machinery Bases near Kingston.- Examined in m。セ 1957; concrete about six months old. Large concrete blocks made
with Pittsburgh rock and Belleville cement. Unusual difficulty of machine shaft alignment probably caused by abnonnal "growth" of concrete base.
(f) Concrete Footings of Water Tower.- Examined in May
1957;
about two years old. Badly affected by pattern cracking(Fig.
3).
Covered later by concrete envelopes which developed radial cracking.(g) Filtration Plant, kゥョセウエッョNM Examined in May
1957;
concrete about four years01.
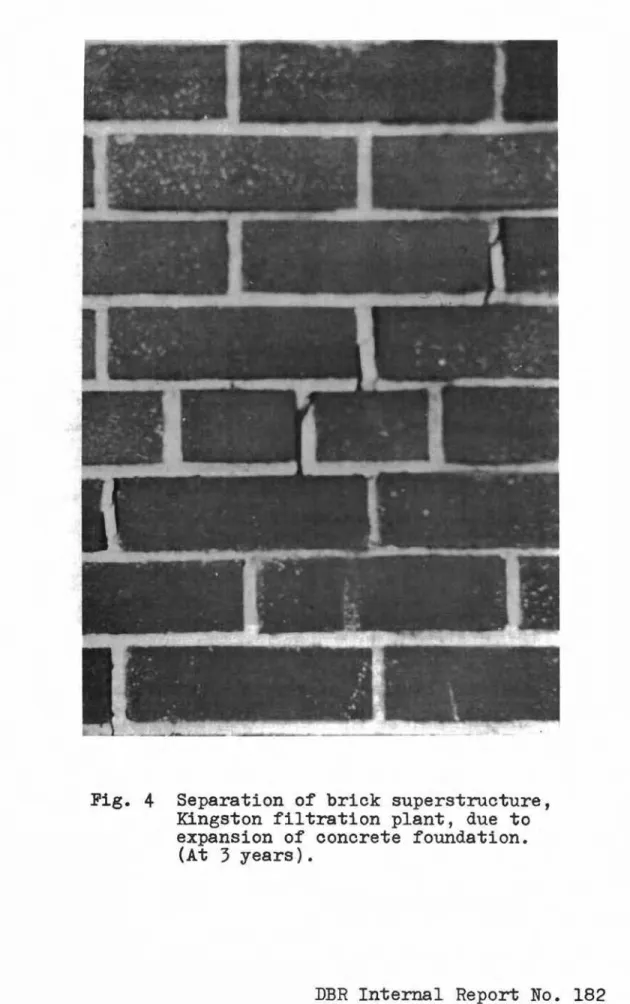
EXcessive expansion in earth-banked concrete walls had produced considerable separations in brick masonry superstructure (Fig.4).
Pattern cracking in exposed wall sections, walks, and retaining walls. No pattern cracking on inside concrete, probably because of uniformmoisture conditions.
(i) C.N.R. Bridge at By-Pass, Highways
15
and 401.- Examined in February1958;
concrete about two years old. Definitedevelopment of map-cracking, particularly on hand rails. Reported use of Frontenac stone and Belleville cement.
(j) Canal Swing-Bridge, Kingston Mills.- Examined in February
1958;
concrete about two years old. Ready-mixed concrete used. Considerable evidence of excessive expansion and map-cracking.(k) Collins Bay Penitentiary.- Visited in June 1958.
Workshop floors and other concrete elements exhibited evidence of map-cracking and abnormal expansion of concrete. Coarse aggregate from penitentiary limestone quarry.
3. Field Application of Remedial Measures
Since the first reports were issued establishing the problem as a type of alkali-aggregate reaction, the matter of the application of corrective measures has been the source of considerable disagreement among interested local individuals and agencies. In the case of the alkali-silica type of
reaction, recommendations have usually involved one of three
セイッ」・、オイ・ウZ (1) procurement of a non-reactive aggregate; (2) use of a cement with an alkali content below 0.60; or
(3)
use of a proven pozzolanic material as a partial replace-ment for cereplace-ment. Since laboratory studies by the Division have shown that pozzolans or lithium inhibitors are not effec-tive in the Kingston case, and that .the use of a cement with alkali content below about 0.40 to 0.45 per cent is effective, only the first two procedures need be considered.The initial approach has been usually to find a
source of non-reactive aggregate. Two ready-mix plants in the area are understood to have changed to outside aggregate, one using a crushed ァイ。ョゥエセ from a source near Kingston, and the other using a gravel aggregate from Brighton. Other construc-tion agencies have indicated their intenconstruc-tion to use outside aggregate. Since research work in this laboratory has shown that the use of a cement with a sufficiently low alkali
content can be used safely with the most reactive of the local limestones, some use has been made of this procedure. At the date of writing (February 1959) some concrete thus made is 15 months old and has so far shown no sign of distress. The three most important cases known to the Division are cited below and will continue to be test cases which should have a bearing on the future use of local limestones with low alkali cements.
(a) New eョヲゥョ・・イゥョセ Building Queen's
University.-Examined in deta
1
in Fe ruary1958
when about five months old; and in June 1958 when about eight and a half months old.Examined again in February 1959. Concrete footings, columns, floors, and beams made with Pittsburgh limestone coarse
aggre-gate and a low-alkali cement epecially produced at the Port Colborne plant of the Canada Cement Company. Detailed
examination, particularly of footings and other elements where access of moisture was possible, revealed no trace of pattern-cracking or excessive expansion.
Following almost complete enclosing of the concrete, subsequent examination has shown no evidence of cracking or other distortions due to excessive expansion of the concrete. Some shrinkage cracking has since occurred on some exposed
floors but this is clearly associated with a lack of joints, and may also be due in part to the two-course construction employed.
(b) Men's Residence
t
Queen's オョゥカ・イウゥエセNM Concreted aboutJuly 1957; examined in Fe mary
1959.
No in ication of abnormal expansion of the concrete which was also made with Port Colborne low-alkali cement and Pittsburgh limestone coarse aggregate. Some exposed floor areas show shrinkage cracking as in (a).(c) Curbs at Polsen Park Kingston.- Examined in October 1957 and in lI'ebruary
1958
(eigflt months old). Made with Port Colborne low-alkali cement and Pittsburgh limestone coarse aggregate. Examined again in February 1959. No sign of abnormal expansion or map-cracking. (Fig. 5).4. Local Limestone Quarries
Three limestone quarries in the Kingston area have been the main sources of limestone coarse aggregate for
concrete. The Pittsburgh quarry, the one most intensively studied by the Division, lies just east of the Cataraqui River two and a half miles north of Kingston on Highway 15.
Across the river is the Frontenac quarry. The general relation-ship between these two quarries is shown in Fig. 6, the
characteristic "green" bed at the 24- to 30-foot horizon in the Pittsburgh quarry corresponding to the floor of the
Frontenac quarry (6). Also shown in the figure are the natural or operating horizons in each quarry.
The Pittsburgh quarry was opened in 1948 and the only horizon used for concrete since then has been the 0- to 24-foot series. This horizon was subsequently found to be the most reactive limestone tested to date. It consists of a number of distinct strata ·of varying composition (6) but the carbonate rock can be generally termed argillaceous
dolomitic limestone, containing a 5 to 15 per cent clay-silica fraction and about 35 to 40 per cent dolomite, the remainder being calcite.
The 6-foot "green" bed at 24 to 30 feet was originally condemned for use in concrete by a government agency,
apparently beoause of its high absorption property, and care has been taken to exclude this material from such use. Its
olay-silica fraction is very high, approaching 40 per cent, with a dolomite content of about 50·per cent. It is therefore a
At the 30- to 36-foot level there is an even more absorptive argillaceous calcareous dolomite with about 70 per cent dolomite and about 10 per cent calcite. This series and the ones below it have not been used for concrete. The 36-to 48-foot horizon reverts again 36-to a dolomitic limes36-tone with about 50 per cent calcite and about 30 per cent dolomite. No information is at present available on the 48- to 60-foot horizon.
In the Frontenac quarry the whole 50-foot face has been quarried for concrete aggregate. At the 12- to 13-foot level, however, a shallow green band is present which appears to be similar to the green bed in the Pittsburgh quarry.
The Frontenac quarry has produced aggregate for at least twenty years whereas the Pittsburgh quarry was opened in 1948. In view of the extensive evidence of affected
concrete dating back at least thirty years, the inference is clear that the responsibility for the problem does not lie with any one quarry.
A third quarry has supplied limestone aggregate to the penitentiaries at Oollins Bay and Kingston. This material has not been investigated but reference has already been made to evidence of distress in concrete because of this material. No other sources of carbonate rock in the area have so far been studied in this laboratory.
5. Results of Field Observations
A proper evaluation of the problem can only be made by taking field evidence in conjunction with laboratory
results; this is considered in a later section of this report. Field observations alone have indicated, however, the wide-spread occurrence of this problem in the Kingston area. They have also shown that no one source of limestone can be singled out as the responsible factor. It has also been noted that milder cases have been readily obscured and are not always noticeable. Recent field evidence has shown that the use of low-alkali cement would appear to be a satisfactory solution. for normal concreting work. Exceptions may be cases of extreme moisture and temperature conditions, or cases where even slight expansion of concrete cannot be tolerated.
IV RESUME OF PRELIMINARY STUDIES
1. Reported Studies by the Division of Building Research
Published results obtained in preliminary work in this laboratory have already been noted in the Introduction.
References already given present a condensation of results
which will not be repeated in this report except where additional pertinent results have been obtained.
In the early studies it was found that the abnormal expansion of concrete observed in the field could be reproduced in the laboratory with concrete prisms, made with セェ「 materials, subjected merely to near 100 per cent relative humidity
conditions and 73°F. By using various combinations of Kingston limestones and sands and corresponding reference materials, along with high- and low-alkali cements , it was shown that the reactive combination was the Kingston limestone and a high-alkali cement. The degree of expansion was found to vary with the alkali content of the cement, and that concrete made with a cement of sufficiently low-alkali content would perform satisfactorily for normal concrete construction.
It was also shown that the use of pozzolans known to be effective in cases of alkali-silica type reactions were not effective in this case. Lithium chloride was also found to be ineffective. Wetting-drying cycling produced expansions of the same order as high humidity curing whereas freeze-thaw cycling and outside exposure produced a lower degree of expan-sion.
Despite the evidence that the phenomenon was a type of alkali-aggregate reaction, the recognized ASTM tests did not give a セッウゥエゥカ・ response. Petrographic examination,
summarized in the next section, was also inconclusive. Never-theless, the results provided positive evidence that the use of a suitable low-alkali cement would provide a satisfactory field solution.
2. Petrographic Examination by the Bureau of Reclamation
Samples of a land-mined sand used in the area, lime-stones from the Pittsburgh quarry, and field and laboratory concretes which had exhibited abnormal expansion and cracking were submitted to the laboratories of the Bureau of Reclamation for detailed evaluation. The results are summarized as follows:
Aggregates.- The land-mined sand consisted of rock and mineral types common to many sands. About 10 per cent of the sand was found to be physically unsound but this was not regarded as excessively high. About 2 per cent of the sand consisted of chalcedonic cherts known to be deleteriously reactive with high-alkali cement but this quantity was not regarded as
evidence that the sand as a whole was deleteriously reactive. The limestone from the 0- to 24-foot series was described as a fine-grained calcareous dolomite or dolomitic limestone, about 13 per cent of which was insoluble in hydro-chlorio acid. The aoid insoluble portion consisted predomi-nantly of illite-type clay, degraded micas, a smaller propor-tion of finely divided quartz, and a very small amount of feldspar. Physically, the crushed material was regarded as satisfactory.
The green limestone was described as a light grey, fine-grained dolomitic limestone, about 40 per cent of which was insoluble in hydrochloric acid •. The acid insoluble portion consisted of moderate amolU1ts of silt-size and very fine sand-size particles of quartz and somewhat smaller amo1U1ts of
degraded micas, illite-type clay and feldspars. Less than 5 per cent of the particles were regarded as physically unsound.
The ottawa Valley reference limestone was described as a grey-coloured, moderately firm, fine-grained, somewhat fosilliferous and argillaceous limestone, about 11 per cent of which was insoluble in hydrochloric acid. The acid
insoluble residue consisted chiefly of degraded micas, illite clay, a moderate amount of quartz, and small amounts of
miscellaneous minerals. Only 3 per cent of the particles were physically unsound.
Concretes.- In the laboratory and field samples of concrete there was unmistakable evidence of cement-aggregate reaction involving the crushed-stone particles, many of which exhibited darkened reaction rims (Fig.
7).
Analyses of the rim material suggested that for this aggregate, development of visiblereactive rims is dependent upon the proportion of acid insoluble material. The nature of the reaction and the
reactive constituent or constituents was not established with certainty. The rather finely divided quartz, present in
significant amounts, was a possibility but the varieties
which can be expected to be deleteriously reactive were present only in insignificant amounts in the acid-insoluble residue. The micaceous material was also a possibility but its role as a reactive material to cement alkalies has not beerl established.
It was significant that only traces of alkalic-silica gel were detected in the affected concretes. Deposits of both calcium carbonate and calcium sulpho-aluminate were found on fracture surfaces. Fractures were abundant and penetrated crushed aggregate particles as well as the cement mortar. There was considerable micro-fracturing also.
A significant number of contact surfaces between the cement paste and aggregate particles had been disrupted by large fractures and micro-fractures. Freshly broken mortar surfaces had a slightly chalky appearance.
In a private communication Dr. R. C. Mielenz added that organic matter may have played u role in the action since examination of the sockets left by breaking away of particles of limestone from the affected concrete revealed a characteristic brown staining.
Conclusions by the Bureau of Reclamation
Some sort of cement-aggregate reaction in the concrete samples was definitely indicated by the ッ」」セョ」・ of reaction rims on the coarse aggregate particles, but the exact nature of this reaction was not established. Alkali-aggregate
reaction was considered a possibility but the absence of
significant gel deposits was baffling. It was suggested that expansion data on mortars and concretes would determine
whether a type of alkali-aggregate reaction was the cause. It was considered possible that the porosity of the aggregate or cement paste was such that accumulation of gel deposits would not occur. The constituents of the crushed limestone aggregate were regarded as not normally deleteriously reactive with high-alkali cements.
3.
Examination by the Laboratory of the U.S. Army Corps of ErigineersOn freshly cut surfaces from affected concrete, faint, dark rims were found by stereomicroscope around the periphery of quite a few of the coarse aggregate particles. More than the usual amount of calcium sulfo-aluminate was
found unevenly distributed as rosettes and groups. In some of the older cracks and voids the deposits looked to be a mixture of sulpho-aluminate and dried gel.
In thin sections, the coarse aggregate appeared to be a carbonate rock that ranged from fine-grained, silty dolo-mitic limestone, to medium-grained dolodolo-mitic limestone, some of it containing clay and possibly chalcedony, to limestone apparently not dolomitic. '
Patches appear to be lower than normal in calcium hydroxide; the paste had the cloudy look found in concrete with alkali-aggregate reaction. Some of the coarse aggregate was bordered by a highly carbonated deposit of the type
thought to be old gel.
The concrete was considered to be similar to that which has been exposed to the Scholer test
(7,
8) in that there were rather obscure signs of cement-aggregate reaction(rims on some of the 」セャウィ・、 coarse aggregate, cracks through coarse aggregate; alteration of the paste in thin section around certain aggregate constituents), and quite a lot of calcium sUlpho-aluminate.
The general conclusion was that this may be a case where the less than optimal properties of the material may, through gross errors in workmanship or utilization of the material, have been the deciding factors.
4. Queen's University Thesis
A thesis submitted to the Department of Geological Science in 1955 (6) sought to determine the relationship between the mineralogical and chemical composition of the limestone and their physical properties. The following is a brief summary from this source of infonnation which is pertinent to this investigation.
The limestones in the vicinity of Kingston are "in these Black River group of the Ordovician". The thickness of the beds is approximately 130 feet; at Ottawa it is 125 feet and at Montreal 50 feet. In the Pittsburgh quarry it is generally a fine-textured, stratified rock that lies in beds 6 to 18 inches thick. Interbedded with this limestone are thin pelitic layers. On exposure, the limestone weathers into alternating grey and bro\vn bands.
The green members present show a surprisingly high clastic quartz content. Combined alumina and silica contents vary from
5
per cent for the upper layers to about 40 per cent for the green material, generally increasing from the top to the green level at 24 to 30 feet, followed by a decrease below this level. Both the silica and alumina follow the same trend.Correspondingly the total carbonates generally decrease from about 95 per cent at the top to about 60 per cent at the green level. In the top 24 feet the carbonate averages about 57 per cent calcite and 37 per cent dolomite,
whereas in the green bed the values are about 4 per cent
calcite and 50 per cent dolomite. At the 30- to 36-foot level the calcite is approximately 8 per cent and the dolomite 70 per cent. The 24- to 30-foot beds are green and the 30- to 36-foot beds are therefore dolomitic. Below this, the 36-to 48-foot level is again essentially a dolomitic limes36-tone with about 50 per cent calcite and about 30 per cent dolomite.
On the basis of petrographic evaluation, abrasion
loss, and absorption, the top 24 feet were considered relatively sound material whereas the green layer was rated as unsatis-factory. The high absorption of the 24- to 30-foot (green) and the 30- to 36-foot materials was attributed to the high dolomite content and the high clay fraction. These carbonates were described as "rough-textured" compared with the top material.
5. Studies by Other Laboratories
Petrographic examinations and some testing have been carried out by other laboratories but are not reported here as the work was carried out independent of any request by the
Division. Extensive studies by the Portland Cement Association have largely paralleled the work done in the DBR laboratory; free exchange of information has indicated very close agreement in all phases of study.
V STUDIES DIRECTED TO THE FIELD PROBLEM: EXPANSION CHARACTERISTICS OF CONCRETE WITH KINGSTON LIMESTONES
Since preliminary studies had shown that the type of alkali or cement-aggregate reaction occurring with Kingston limestone was apparently outside the normal experience of experts in the field, and that therefore the nature of the reaction might prove elusive, the first efforts in subsequent investigations were designed to provide information primarily for practical use and guidance in the field. This report is therefore devoted to this end and concurrent studies haVing to do with the mechanisms of the reaction will be considered in a subsequent report.
Some previously reported results are discussed again since additional information was obtained at later ages of test.
1. Materials and Procedure - General
The main purpose of this part of the investigation was to determine the influence of certain factors that may be encountered in the field on the expansive characteristics of concretes made with Kingston limestones. For this reason reference materials were used in each test series so that normal, non-expansive concretes could be compared with the potentially reactive samples. The reference concretes were made at the same time and with the same grading, mix design, and water-cement ratios as the test samples.
The Kingston limestones studies included samplings from each of the operating horizons in both the Pittsburgh and Frontenac quarries. Most of the work was done with the most reactive material found, the 0- to 24-foot series in the Pittsburgh quarry. Several samplings were taken of this material, some crusher-run and some quarried by hand and
crushed in the laboratory. This mus:t be taken into account in comparing the rate and degree of expansion of samples in different series.
The reference limestone is a highly calcitic carbonate rock referred to as Ottawa Valley limestone. It does not produce abnormal expansion in concrete exposed
merely to high humidity conditions. Certain other limestones were also included in some test series and are described
therein.
In the preliminary work a land-mined sand was used, representing a type used extensively in Kingston up to about
1955
or1956.
In later work, a lake sand was used which has recently been used for much of the concrete work in theKingston area. The main reference sand is called Arnprior sand which has a history of sound performance in concrete.
The cements used in these tests were selected on the basis of alkali contents which are given in Table I. Air-entrainment was used in a few cases only, since it was estab-lished early in the work that its influence on abnormal
expansion owing to cement-aggregate イ・。」セゥッョ was not signifi-cant. Where pozzolans were used, replacement of cement was by weight in some cases and by volume in others.
Concrete mixes were designed to apprOXimate good quality field concrete although water-cement ratios and slumps were kept low to avoid a possible extraneous influence of
bleeding and segregation. The selection of maximum-size stone and proportions of stone sizes was varied somewhat for different studies and is indicated in each case.
The large concrete batches were mixed in a Lancaster machine for a total of
5
minutes. The water and cement were first mixed with part of the stone. The sand was then added and mixed, followed by the remainder of the stone. Water-cement ratios were kept constant for most mixes in a series but in some cases these were varied and slumps were maintained constant. The smaller mixes were hand mixed, followingessentially the same procedure.
Concrete prisms were made in specially designed moulds so that measuring studs were set the same distance apart in
each sample (Fig. 8). The inside surfaces were covered with vinyl sheeting to avoid greased surfaces.
The freshly moulded samples were stored for 24 hours in a curing room maintained at near 100 per cent relative humidity and 73.4°F and then removed from the moulds, marked, measured for length, and weighed .in preparation for test
conditioning.
In most cases 3- by 4- by QVMセョ」ィ prisms were used but, when certain materials were in short supply, 3- by 3- by 10-inch prisms were made. Measurement of length change was made to the nearest 0.0001 inch using a special comparator with reference bar (Fig. 9). Resonant frequency moduli were obtained for some series in accordance with ASTM requirements. In some cases the pulse velocities were measured by soniscope. Since it was determined that water-cement ratios had only a small influence on rate and degree of expansion of
affected concrete, compressive strengths were not determined except for special purposes.
2. Test of a Job-Mix Concrete
Duplicate 3- by 4- by 16-inch concrete prisms were made with materials from the same sources as those used in the sidewalk which the Division first investigated for Defence Construction (1951) Limited (Fig. 1). The mix proportions were the same as those used on the job: 1 part cement, 1.95 parts sand, 3.07 parts stone, and a water-cement ratio of 0.44. One sample セッョエ。ゥョ・、 entrained air and the other did not. Corresponding reference concrete prisms were made using ottawa Valley limestone and Arnprior sand, with exactly the same mix proportions. The samples were stored in the curing room and measured periodically for length change.
Figure 10 shows the record of abnormal expansion of the job-mix samples compared with the normal behaviour of the reference concretes. Cracking was first observed in the
former at about 0.08 to 0.10 per cent expansion. The side-walk was reported to have exhibited map-cracking within 6 months of placing.
It should be noted that air-entrainment has no significant influence up to the point where cracking begins. The difference between the air-entrained and non-airentrained samples beyond the point would obviously have little signifi-cance since the contin11ed expansion of cracked concrete would be influenced by physical factors.
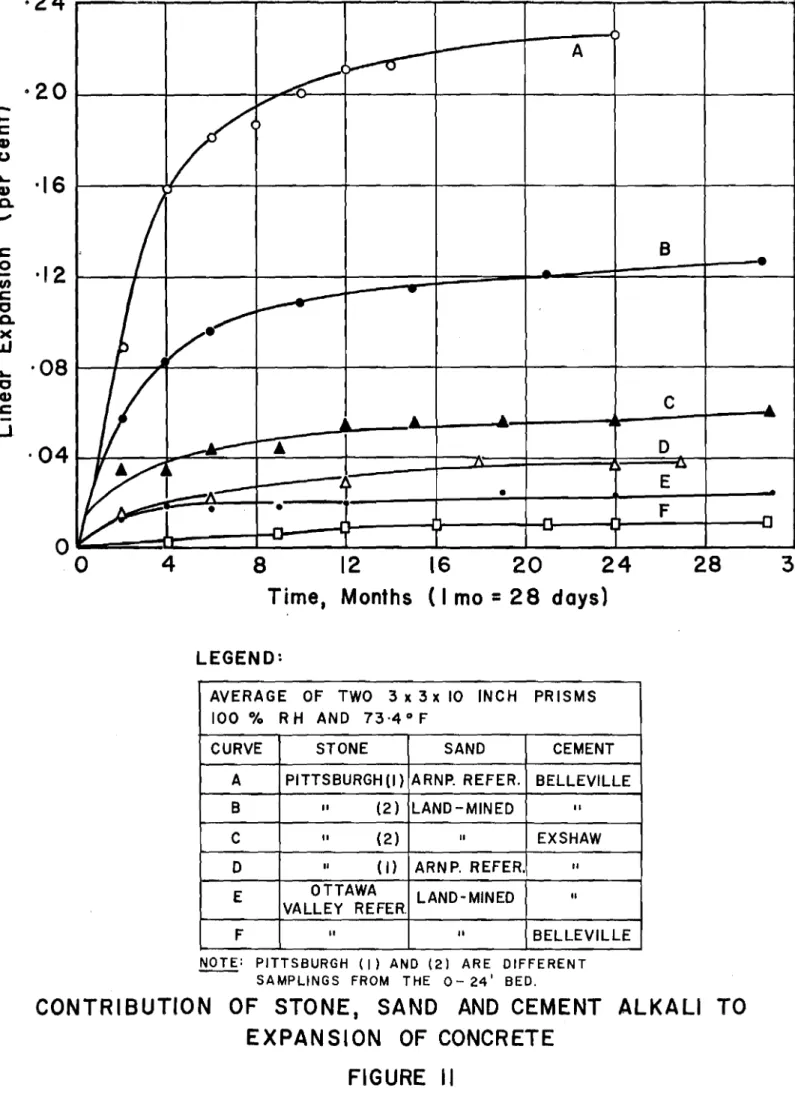
3. Contribution to Expansion of Concrete Ingredients
Experiments were made to show which of the ingredients, stone, sand, or cement, was responsible for the abnormal expan-sion of concrete at Kingston. Duplicate 3- by 3- by 10-inch prisms were made using various combinations including two samplings of Pittsburgh 0- to 24-foot limestone, Kingston
land-mined sand, ottawa Valley limestone (reference), Arnprior sand (reference), Belleville cement (high alkali) and Exshaw cement (low alkali). The mix proportions were: 1 part cement,
2i
parts sand and 2-1/3 parts stone, with a water-cement ratio of 0.47.The concrete prisms were stored continuously in a humid room at near 100 per cent relative humidity and 73.4°F and measured periodically for length and weight change over a period of about three years. The records of expansion are shown in Fig. 11. Weight changes were not considered signifi-cant and are not recorded here.
The concretes made with the Pittsburgh limestones and Belleville high-alkali cement produced excessive expansions with
cracking first observed at about 0.08 to 0.10 per cent expan-sion. The concretes made with the Pittsburgh limestones and Exshaw low-alkali cement (0.45 per cent alkali calculated as sodium oxide) showed much reduced expansions and no evidence of cracking after more than two and a half years under test.
The contribution of the sands appeared to be
negligible in this test although the land-mined sand sample showed potential unsoundness in the Conrow test (ASTM Designa-tion C342-55T). This sand is, however, reported to be no longer used in the Kingston area for concrete work. The slight
expan-sions observed for the reference concretes made with ottawa Valley limestone, with both cements, is considered normal under continuously moist conditions.
4.
Influence of Exposure ConditionsEarly in the investigation experiments were carried out to determine whether the abnormal expansion and cracking of concretes made with Kingston materials were due entirely to an "internal" instability or whether such agencies as
freezing and thawing were contributing factors. Accordingly, concrete prisms made with a reactive and a non-reactive lime-stone and high-alkali cements were subjected to the following four conditions:
(a) Continuous conditioning in the curing room at near 100 per cent relative humidity and 73.4°F. This condition had already been shown to reproduce expansions observed in the field.
(b) Wetting-drying cycling: 6 hours wetting at room tempera-ture and 6 hours drying at 130 ! 3°F. These two cycles a day represented a modification of the Scholer method
(7,8). Specimens were SUbjected to 'test at the age of 7 days and measurements for length, weight, and resonant frequency were made periodically, always near the end of the wetting part of a cycle. The cycle changes were made automatically without the handling of specimens.
(c) Freeze-thaw cycling: 6 hours freezing in air to 10°F and 6 hours thawing in water at room temperature. This
exposure was selected to approximate the relatively mild winters at Kingston. Specimens were subjected to test at the age of 28 days, having first been cured 14 days and then subjected to laboratory air drying for 14 days.
Measurements were made near the end of the thawing part of a cycle. The cycle changes were adlleved by means of an automatic apparatus.
(d) Outside exposure to weather. This began in early summer; age of specimens at start of exposure was 7 days.
The Kingston limestone used in this series was
laboratory crushed rock sampled from the 0- to 24-foot series in the Pittsburgh quarry. It was used with the local
land-mined sand and two high-alkali cements, Belleville and Montreal. The control specimens were made with ottawa Valley limestone, Ainprior sand and the same two cements. Both sands were first separated into six size fractions and reblended in the same
way to conform with ASTM grading requirements. Equal parts of
t-
to 3/8-inch and 3/8- to セMゥョ」ィ stone were used. All aggre-gates were in a laboratory-dried state before incorporation in the concrete. The mix proportions and water-cement ratio aregiven in Table II. Slumps ranged from セ inch to 2 inches. Duplicate concrete specimens, 3 by 4 by 16 inches were taken from the same batch for each test condition.
The grade of the concrete may be judged from the dynamic modulus values at 28 days given in Table II. The
flexural strengths at 28 days were taken on companion specimens stored in the curing room. The batch numbers and the flexural strengths (psi) were: 1 (1548), 2 (none taken),
3
(1614),4 (2094), 5 (2064), and 6 (2165).
The results of length measurements are given in Table II. Concretes made with the reactive limestone and either of the two high-alkali cements expanded excessively under all four conditions, with cracking becoming visible at about 0.08 to 0.10 per cent expansion (Fig. lla). Volume changes for the reference specimens are not abnormal.
The rate and degree of expansion were greatest for specimens subjected to moist curing and to wetting and drying cycling. The lower values observed in the freeze-thaw and outside exposure tests could be attributed to a combination of lower average temperatures and moisture availability. These results could be interpreted as indicating an internal reaction, probably of a chemical nature.
It was evident again that air-entrainment has no significant influence on this expansion phenomenon.
5.
Effectiveness of Inhibitors on aャォ。ャゥM。セRイ・ァ。エ・ Reaction and Reduction in Cement ContentThe possibility of finding a suitable pozzolanic material for inhibiting the abnormal expansion of concretes made with high-alkali cements and reactive limestones was
investigated as soon as it became clear that a type of cement-aggregate reaction was involved. The results are summarized in Table III.
In series I several materials were used to replace
25 per cent of the cement by weight and the resulting concretes were compared with a concrete with no replacement and one in which the cement content was reduced by 25 per cent. Slumps were maintained at approximately 1 inch.
In series II lithium chloride was added. This compound has been ウィッセュ to be an effective inhibitor of the
In series III three locally available materials were tested, using 25 per cent replacement of the cement by volume. A different sample of the reactive Pittsburgh limestone was
used in this case. All concrete specimens were kept continuously in the curing room.
None of the materials used was effective in inhibiting abnormal expansion and cracking of the concrete. The California calcined shale, a very effective pozzolan in alkali-silica
reactions (10), produced an initial reduction in the rate and degree of expansion but in two years its effectiveness was zero. This is illustrated graphically in Fig. 12. The rice hull ash, consisting mainly of cristobalite, not only was ineffective in reducing expansion, but had an adverse influence on the strength of the concrete.
The failure of the proven inhibitors of alkali-silica reaction was further evidence that the Kingston phenomenon was basically different in nature from the 。セォ。ャゥMウゥャゥ」。 type of reaction.
The reduction of cement content is seen to reduce the rate and degree of abnormal expansion. This is compatible
with the characteristics of cement-aggregate reactions generally. The final convergence of the curves in Fig. 12 for the normal concretes with different cement contents occurs after cracking has begun and therefore is not considered signifi-cant.
6. Influence of Cement Alkalies and Added Alkalies
Although previous experiments had shown the definite role played by the cement alkali, the apparently unique
character of the reaction in relation to the alkali-silica
type suggested an inherent danger in applying the same criteria. Practical and theoretical considerations made necessary
information as to the influence of source, composition, and
other properties of the cement, the safe limit of cement alkali, and relative aggressiveness of sodium and potassium OXides,
and other factors. For these reasons a rather extensive series of additional tests was carried out.
(a) Three U.S. cements, varying in alkali content from high to very low (Table I) were uspd in conjunction with the Pittsburgh 0- to 24-foot stone as coarse aggregate. These cements differed from the Canadian cements in that the sodium oxide contents were greater than the potassium oxide contents, and the cements were also more finely ground. The other
ingredients of the concrete and the mix proportions were exactly the same as for the concrete made with Belleville cement, the plot for which is shown as curve B in Fig. 11. The linear expansions are shown in Fig. 13. The concrete
specimens were maintained continuously at 100 per cent relative humidity and 73°F.
These plots show that the rate and degree of expan-sion decreases with decreasing alkali content of tIle cement, and that the sources and compositional differences of the cements are probably of little significance. The role of fineness is not clear but it apparently has little influence on expansion. Comparing curve B of Fig. 11 with the top curve of Fig. 13, and considering the difference in total cement alkali (1.19 per cent カウセPNYY per cent, Table I), it would appear that the soda component of the cement alkali is more aggressive than the potash component. This !las been found to be generally true of alkali-silica reactions. Of practical significance is the middle curve of ,Fig. 13 (total cement alkali = 0.52 per cent as soda) which, when compared with curves C and D in Fig. 11 (Exshaw cement, total alkali = 0.45 per cent as soda), suggests that the safe limit of total
alkali for cements to be used with Kingston limestone should be considerably lower than the 0.6 per cent limit recommended for alkali-silica type reactives (11).
(b) When the influence of the alkali content of the cement was definitely established, the Canada Cement Company undertook to produce a low-alkali cement at their Port Colborne plant to supply the Kingston requirements. This plant was
presumably selected rather than Belleville because the alkali content was already fairly low, approaching the 0.6 per cent limit normally recognized for alkali-aggregate reaction. The alkali content of this cement, as in most eastern Canadian cements, is mainly potash which can be much more readily
decrea,sed than soda because of the relative ease of volatiliza-tion during clinkering. The method used involved the addition of calcium chloride to the slurry before burning and subse-quently the discarding of the flue dust.
Three trial cements with alkali contents reduced by increasing calcium chloride increments were obtained by the Division and used in concretes made with reactive Pittsburgh 0- to 24-foot stone. The amounts of calcium chloride added were in the proportions: 1 part, 2 parts and Rセ parts by
weight. The corresponding alkali contents are given in Table I.
The concrete mix proportions were 1 part cement, 2 parts local lake sand, and 3 parts crusher-run sample of Pittsburgh 0- to 24-foot limestone. The water-cement ratio was 0.475 and the slumps were approximately,l inch.
The linear expansions of the concretes, condittoned continuously at 100 per cent relative humidity and 73°F, are . shown in Fig. 14. The per cent expansion decreases with
decreasing total alkali, which, in turn, corresponds with increasing amounts of calcium chloride used in manufacture. Comparison of these values with the unmodified cement produced during this time was not possible since a sample was not
available. The sample of regular Port Colborne cement shown in Table I, with a total alkali content of 0.54, had beeD.;
obtained several years previously and kept in a sealed container. It is evident from Fig. 14, as observed previously, that the safe limit for cement alkali in the Kingston case should be not higher than some value in the neighbourhood of 0.40 or 0.45 per cent. It may also be observed that there would presumably be some difficulty in achieving such a limit by attempting to reduce the alkali of cements with initially high-alkali contents.
(c) The addition of sodium or potassium hydroxide to mortar mixes containing a cement of low-alkali content and an alkali-reactive siliceous aggregate has been used in studies of alkali influence in alkali-silica reactions (12).
Five concrete mixes were made using the same
Pittsburgh 0- to 24-foot stone as in the previous section (b), and the Port Colborne modified cement of 0.31 per cent total alkali (as Na20). The proportions were 1 part cement, 2 parts sand and 3* parts stone, with a fixed water-cement ratio of 0.475 and a low slump of apprOXimately * inch. Duplicate 3-by 4- 3-by 16-inch prisms were made for each series, some of which contained added alkali. They were continuously condi-tioned at 100 per cent relative humidity and 73°F and periodic length measurements were made.
The first mix had no added alkali; the low expansions of the concrete prisms are represented by the lowest curve in Fig. 15. In the next two mixes, sodium hydrOXide was added to the mixing water in one and an equimolecular amount of potassium hydroxide to the other, so that the total alkali (calculated as soda) in each case was 0.71 per cent. The expansions of these concretes are represented by the two intermediate curves in Fig. 15 which are practically identical in position. The last two mixes were made in the same way with the two alkalies again in equimolecular proportions so that the total alkali was 1.11 per cent (as soda). These are represented by the two top curves in Fig. 15.
It is observed that the influence of the alkali, whether it originated entirely in the cement, or whether part of it was added, was of the same order as for previous experi-ments. The increase in total alkali COlltent produced a
corresponding increase in the expansion of this concrete as in the previous two cases. Where the エッエセセ alkali was about
0.11 per cent (calculated as soda) no significant difference was produced between the sodium hydroxide and the potassium hydroxide. At a higher concentration of alkali, of about 1.11
per cent (calculated as soda), the sodium hydroxide produced a higher rate and degree of expansion of the concrete tl1an the potassium hydroxide. This has also been noted for alkali-silica reactions for later ages of concrete (13). In the
latter instance it has also been observed that the initial effect is the opposite, the potash component being the more aggressive. At 7 days, this effect was noticeable only in the mixes with total alkali content of cement equivalent to
0.71 per cent.
In general, the results of these experiments tend to accentuate the similarities between the Kingston phenomenon and the alkali-silica type of reaction.
1.
Contribution of Sulphide in Reactive LimestoneThroughout the investigation mechanisms other than alkali-aggregate reactions were kept in mind as possible causes of expansion. One was the possibility of an internal sulphate reaction based on the appearance of a larger than normal
amount of calcium sulphoaluminate in voids in the affected concrete (referred to in Part IV of this report), and the presence of a high concentration of hydrogen sulphide in distilled water used in leaching the reactive limestone. It was also reported that the water in many wells in the Kingston area is polluted with hydrogen sulphide. Oxidation of the sulphide to the sulphate would occur quite readily in the concrete. Although this source of sulphate is limited its
possible contributing influence led to some preliminary studies. Two concrete mixes were made from the same sample of Pittsburgh 0- to 24-foot rock (3/4 inch maximum size) used in preceding studies with added alkalies. In one a sulphate-resistant cement was used (Winnipeg Ealicrete) with a total alkali content of 0.50 per cent (calculated as Na20). To the second mix, the same cement was added but with additional sodium hydroxide so that the total alkali was 0.73 per cent
(calculated as Na20). The same mix proportions and
3- by 4- by 16-inch prisms were stored in the curing room and measured periodically for length and キ・ゥヲセエ change. The
records of expansion are plotted in Fig. 16.
Comparing these values with those of concrete prisms with comparabLe total alkalies in Fig. 15, it is evident that the reaction is not inhibited by a reduced tricalcium silicate content in the alkali-resistant cement, and, therefore, an internal sulphate attack can be ruled out. The effect of
added alkali is the same as in Fig. 15 but the actual expansion of the concrete with the sulphate-resistant cement is higher than for the concretes with the Belleville cement. The latter cement bas a somewhat lower specific surface area than the former.
For the sulphate-resistant cement, the calculated C3A is about 2 per cent and the C4AF content about 13.3 per cent. The corresponding approximate values for the Belleville cement are: cセ
=
11.5 per cent and C4AF=
8.4 per cent.Concurrently with the above experiment, two mixes were made using the Ottawa Valley reference limestone and Arnprior sand, but with the limestone vacuum-saturated before fabrication with a saturated solution of hydrogen sUlphide. It was assumed that if an internal sulphate reaction can occur owing to the presence of a SUlphide, it should accur with the reference limestone as well as with the Kingston material. Exactly the same mix proportions were used as in the previous series and the concrete prisms were conditioned and measured in the same manner. One pair was made with Belleville cement and one with modified Port Colborne cement No. 4 (total alkali as Na20 = 0.36 per cent).
At the end of 15 months no abnormal expansions were observed, the per cent expansion for the Belleville cement concrete befng 0.017 per cent, and for the other, 0.006 per cent. It was concluded that sulphate reaction due to the
presence of sulphide in the coarse aggregate was not·the cause of abnormal expansion in concretes made with the Kingston
limestone and a high-alkali cement. 8. Influence of Initial dAケゥョセ Period
Previous results had ウィッセョ that moisture and tempera-ture influenced the rate and d0gree of expansion, suggesting a chemical or physical-chemical reaction. The effect of a protracted initial drying period should prOVide evidence relating to the probable chemical nature of the reaction and
also show whether relatively dry periods encountered in the field would reduce or retard the reaction.
The method used was similar to that employed in the laboratories of the Portland Cement Association in studies of this type of reaction. Pittsburgh limestone from the same 0- to 24-foot series was used as in the previous experiments. Belleville cement and the modified Port Golborne low-alkali cement No. 4 were used with lake sand. The reference limestone was ottawa Valley. The mix was 1 : ,2 : 3t, water-cement ratio of 0.50, with an average slump of
It
inch. Maximum size stone wasi
inch.Duplicate 3- by 4- by l6-inch concrete prisms for each mix were cured for 7 days, then dried at 50 per cent relative humidity and 73°F to 70 days of age, and finally stored in water at 73°F. The results of periodic length measurements are shown graphically in Fig. 17.
It will be observed that drying at 50 per cent relative humidity effectively retards the reaction (compare with the 2-month and 3-month values for similar mixes in previous tables and graphs). Subsequent immersion in water, however, results in abnormal expansion at a rate which
indicates that the drying period has no permanent effect on the reaction.
9.
Effect of TemperaturePrevious experiments had indicated that the reaction of Kingston limestone and high-alkali cement was affected by temperature. Additional studies were made to compare this characteristic with that of the alkali-silica type of reaction and to prOVide practical information for field use.
The same sample of pゥエエウ「uQセァィ 0- to 24-foot limestone used in the preceding work was used as coarse aggregate. Two mixes were made, one with Belleville cement (high alkali) and one with the modified Port Colborne No.3 cement (low alkali). The local lake sand was used. The same mix proportions,
maximum size stone, and wa"Ger-cement ratio were used as in section 7 above. From the first mix duplicate
3-
by4-
byl6-inch prisms were subjected to 100 per cent relative humidity at each of three temperatures: 73, 100 and 130°F. Similar specimens from the second mix were conditioned at 73 and 100°F. The records of expansion are plotted in Fig. 18.
The increase in rate and degree of expansion with increase in temperature from 73 to 100°F and the reversal at 130°F show a remarkable similarity to experiences with alkali-silica reactions (14). The expansion at 100°F of the concrete made with the low-alkali cement is excessive and suggests that under wet or humid conditions, combined with high temperatures, the use of a low-alkali cement is not a safe remedy. However, the same effect occurs with the alkali-silica reactions and the likelihood of such a combination of high humidity and high temperature occurring in a field situation is extremely
improbable. Nevertheless, the possibility of such an occur-rence should be kept in mind.
10. Influence of Other Possible Field Variables
During the course of the investigation the Division was frequently consulted by builders and other interested parties in the Kingston area with reference to the influence of certain variables which could occur in" the field on the expansion characteristics of local concretes. Although the general nature of the reaction made it possible to predict the answers to most of these questions, individual experiments were carried out in each case. It was particularly important to obtain results which might be used to indicate similarites and differences between this reaction and the alkali-silica type. In general these are single experiments and, although the results may be used as a guide, the conclusions must be considered as tentative.
(a) Maximum stone Size.- Two mixes were made with the same Pittsburgh 0- to 24-foot sample used in series I with pozzolans. The stone size in one was
i
to 3/8 inch only, and in the otheri
to セ inch only. Belleville cement and land-. mined sand were used. Proportions were 1 : 2.25 : 2.33, with a water-cement ratio of 0.47 and a slump of 1 inch. Triplicate3-
by 4- by l6-inch specimens were kept continuously in the curing room and measured periodically for length of change. The records of expansion are shown in Fig. 19.It will be observed that the specimens with the
larger stone size expand more rapidly and to a greater degree. These results, taken together with early results on mortar bars (3) in which expansions were relatively slight, show
that the rate and degree of expansion decrease with decreasing size of reactive aggregate. This appears to agree with
theoretical concepts developed for alkali-silica reactions (15). It may in part also account for the apparent absence of
reaction in topping mixes and concrete blocks where the maximum size of stone was small.
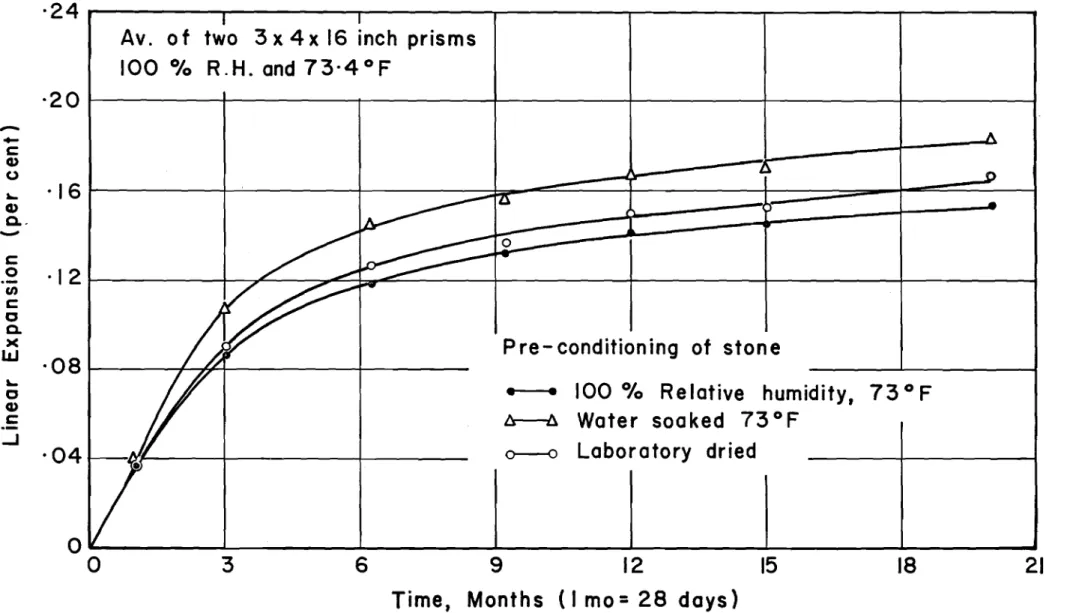
(b) Initial Moisture Condition of Stone.- Samples of the same reactive limestone used in series III with pozzolans were preconditioned in three ways: (1) 100 per cent relative
humidity and 73°F for 24 hours, (2) immersed in water at 73°F for 24 hours, and (3) laboratory dried for several weeks. Proportions were 1 : 2.25 : 2.75 with a maximum size stone of
セ inch, a water-cement ratio of 0.50 and a slump of ャセ inches. Belleville cement and lake sand were used. Duplicate 3- by
4- by 16-inch specimens of each mix were kept continuously
moist in the curing room. Records of expansion of the concretes are shown in Fig. 20.
It will be observed that initial expansions up to the critical limit of about 0.08 per cent where cracking has begun are practically the same. A slightly increased expansion occurs with specimens made with pre-soaked stone. It may be concluded that the moisture condition of the aggregate prior to mixing is not an important influence in this reaction.
(c) Water-cement Ratio.- In preceding experiments
differences in water-cement ratio appeared to have no signifi-cant influence on the rate and degree of expansion of concretes made with the reactive limestones. To check this point two mixes were made which were identical except for water-cement ratio. The proportions were 1 : 2 : 3t of Belleville cement, Arnprior sand and the same reactive limestone as used in
section
9
above. In one mix the water-cement ratio was 0.475 and in the other 0.600. The latter had a slump of 8 inches. Duplicate 3- by 4- by 16-inch concrete prisms of each mix were stored in the curing room. Records of expansion are plotted in Fig. 21.It is observed that the higher water-cement ratio produces lower expansion but the difference up to the critical limit of cracking is small and probably not an important
factor.
(d) Sample Size.- During these studies it was sometimes necessary to make
3-
by 3- by 10-inch prisms instead of the usual 3- by4-
by l6-inch size in order to conserve certain samples of materials. Although the difference in size of specimen appeared to have no significant influence, this factor was checked by making the two sizes from the samebatch and recording length changes. The ingredients and their proportions were the same as in the preceding section; slumps were セ inch.
Records of expansion, plotted in Fig. 22, show no significant difference in rate and degree of growth for the two sizes of prisms. This is true only for specimens kept
continuously moist. In the field it is to be expected that small or thin elements would dry out more rapidly and the reaction would be slower than for massive elements.
(e) Mix Proportions.- In previous as well as in subse-quent experiments, mix proportions were varied, usually to take care of different stone sizes available. Cement-sand ratios varied from 1 : 2 to 1 : 2.25 and cement-stone ratios from 1 : 2.33 to 1 : 3.75. Stone grading was varied from a ウゥョセャ・ size, to equal parts of two sizes, to a 1 : 2.25 : 3.25 of 4 to 3/8, 3/8 to セ and
i
toi
inch. As far as it waspossible to compare expansion results of these different
concretes, the variations in mix proportions as described did not materially affect the rate and degree of expansion. It is to be expected, of course, that the reactive stone content, for any given size, would have a direct relationship to expan-sion. For normal concretes, however, the range of stone
contents would be similar to that used in these studies.
(f) Continuous Water-soakin s vs.Hi h Humidit,.- The near 100 per cen re a lve urn 1 y 0 e curlng room was considered as an unlimited source of moisture for the reaction which
produced excessive expansion of concrete. Since, however, certain field concretes might be subjected to continuous water soaking, the effect of continuous immersion in water as
compared with high humidity conditioning was investigated early in these studies.
Four 3- by 4- by 16-inch concrete prisms were made from a single batch made from the same materials and exactly in the same way as the mixes used in part (b) of this section. Two prisms were stored in the curing room (near 100 per cent relative humidity) and two prisms immersed in water after demoulding. Over a two-year period the percent expansions were, respectively, 0.036 and 0.034 at one month, 0.118 and 0.111 at 6 months, 0.136 and 0.142 at 12 months, and 0.158 and 0.164 at 24 months. These results indicate that no significant difference in the rate and degree of expansion occurs under these two different conditions.
(g) Sand.- The three sands used in these studies, the local ャ。ョ、セ・、 and lake sands, and the Arnprior reference sand, appeared to have no significant influence on the
expan-sion properties of the concrete containing reactive limestone. A possible exception was the land-mined sand which appeared to contribute slightly to the expansion owing to some reactivity of its own which was mentioned in part 3 of section V.